Abstract
Microglia are unique cells of the central nervous system (CNS) with a distinct ontogeny and molecular profile. They are the predominant immune resident cell in the CNS. Recent studies have revealed a diversity of transient and terminal physical interactions between microglia and neurons in the vertebrate brain. In this review, we follow the historical trail of the discovery of these interactions, summarize their notable features, provide implications of these discoveries to CNS function, emphasize emerging themes along the way and peak into the future of what outstanding questions remain to move the field forward.
Keywords: Microglia, Neurons, two photon imaging, physical interactions, P2Y12, NMDAR
1. Introduction: A brief history of microglial-neuronal physical interactions
In vivo imaging opened up new avenues for visualizing brain cells beginning with neurons [19, 22] then astrocytes [40, 59] and more recently microglia [9, 39]. These initial studies have expanded to include the elucidation of microglial phagocytosis in the developing brain and in the neurogenic niche as well as various types of microglial physical contact with neuronal elements. It is now established that microglia interact physically with neuronal somata, axons, axon initial segments and dendrites. In this review, we consider these findings and highlight the field of microglial-neuronal physical interactions as a bona fide area of neuroglial and neuroimmune research.
1.1. Microglial-neuronal physical interactions discovered from 2008-2012
In 2008, Peri and Nusslein-Volhard documented microglial phagocytosis of apoptotic neurons in the developing zebrafish making the zebrafish a powerful system to understand microglial phagocytic mechanisms. In 2009, Wake et al. uncovered transient microglial physical interactions with murine synaptic spines and boutons. In 2010, Tremblay et al. confirmed experience-dependent interactions between microglia and neurons using combined electron microscopic and two-photon imaging approaches. In the same year, Sierra et al. showed that microglia engage in phagocytic clearance of apoptotic neurons in the homeostatic regulation of the neurogenic mouse dentate gyrus. In 2011, Paolicelli et al. first provided experimental support for synaptic elimination by microglia in the developing hippocampus. In 2012, this hypothesis was further developed with the report of complement-dependent synaptic pruning in the mouse visual system. In the same year, two studies in the zebrafish showed further microglial-neuronal physical interactions. First, a novel microglial sensing mechanism for neuronal injury was discovered by Sieger et al. Then, the functional consequence of microglial contact on neuronal excitability was revealed by Li et al.
1.2. Microglial-neuronal physical interactions discovered from 2014-2018
In 2014, back-to-back studies revealed an N-Methyl-D-aspartate (NMDA)R-mediated control of microglial process outgrowth [12, 17]. In 2015, two studies emerged highlighting novel physical interaction . First, Baalman et al. uncovered axon-initial segment (AXIS) microglia. Then, a microglial process convergence phenomenon whereby microglial processes spontaneously focus on neuronal dendrites following epileptiform activity was revealed [14]. In 2016, two fascinating studies from the same lab revealed a neuroprotective targeting of axonal damage in response to excess depolarization [26] and the induction of spines following physical contact with dendritic shafts [33]. In 2017, microglia were shown to interact with dendrites in human epilepsy tissue [66] and in 2018, cerebellar microglia were first observed in vivo to dynamically interact with Purkinje neurons [57]. Finally, Weinhard et al. [63] failed to observe microglial phagocytosis of pre- or post-synaptic elements. Rather, more modest remodeling of these elements by microglia was reported. Therefore, a diversity of physical interactions between microglia and neurons haven been documented. These will now be discussed in detail in two categories: transient interactions and terminal interactions.
2. Transient interactions
2.1. Engaging synaptic elements: dendrites, spines and boutons
Wake et al. 2009 [61] first identified synaptic elements as targets of microglial processes. Microglia made contacts with pre-synaptic boutons and post-synaptic spines. Furthermore, contact duration increased about 15-fold in the ischemic penumbra where 25% of contacted boutons were subsequently lost raising the possibility that microglia interact with synapses in a functionally-relevant manner [61]. Later, electron microscopy showed that microglial processes contact only ~3.5% of synapses in the healthy brain [55]. Few (~1.5%) labeled hippocampal spines were contacted briefly (~1.5 mins) by microglial processes [46]. Interestingly, contacts were rarely repeated towards the same spine during an 80-minute imaging period. Therefore, over time, microglial processes could sample a significant number of synapses. Two-photon imaging and detailed ultrastructural electron microscopy revealed that microglial processes preferentially contacted smaller spines that increased in size following contact. Furthermore, microglial-contacted spines were three times more likely to be eliminated within 48 hours than non-contacted ones. Following sensory deprivation, microglia preferentially contacted larger spines that reduced in size following contact. [60]. Together, these results provide further evidence for putative microglial process regulation of synaptic stability.
The mechanisms underlying microglial-neuronal physical interactions include neuronal NMDAR and microglial P2Y12R signaling [12, 17]. Using mainly pharmacological approaches, these studies showed that neuronal NMDAR activation elicited microglial process outgrowth (where microglial processes radiate outward from their somata in all directions) towards several regions on nearby dendrites and increased microglial process contact of neurons. These responses were recapitulated during kainic acid-induced seizures [17]. In addition, a different phenomenon (where microglial processes converged focally to dendritic hotspots, rather than radiating outward on neuronal dendrites) was discovered following global glutamatergic activation or kainic acid induced seizures [16]. Both phenomena depend specifically on the GluN2a NMDAR subunit [13]. Knocking out P2Y12Rs abolished both phenomena and corresponded with increased seizure intensities [16, 17] suggesting that these interactions may serve to downregulate neuronal activity.
Long term potentiation (LTP) is thought to be a cellular basis for neuronal plasticity underlying learning and memory [30]. At least two studies combining electrophysiological and imaging approaches have investigated microglial physical responses to high frequency stimulation (HFS) used to trigger LTP. In an initial study, microglial motility was unchanged following LTP [65]. However, a subsequent study examined doubly labelled microglial and neuronal elements at physiological temperatures and showed that following HFS, microglia increased their surveillance density but contacted fewer spines for longer periods of time. NMDARs were required for this increased surveillance [46]. Although the functional significance of these post-HFS contacts are currently unclear, microglia participate in regulating synaptic plasticity [48, 54] and these contacts may thus function to monitor synapses in this context. Microglia have also been implicated in some version of long-term depression (LTD), where synaptic strength is weakened [69].
GluN2a expression and function is upregulated in latter postnatal development. Because NMDAR-triggered microglial process interactions with dendrites require GluN2a function, they are absent in the developing brain. However, microglia still interact physically with neurons in the postnatal brain [33]. In a narrow window between P8 and P10, microglia frequently induced filopodia, precursors to spines, on dendritic shafts in a contact-dependent manner. Moreover, microglial depletion correlated with reduced spine densities and reduced functional synapses suggesting that microglia promote synaptogenesis in the developing cortex [13]. Interestingly, microglia promote learning-dependent synapse formation in adults in a brain derived neurotrophic factor (BDNF)-dependent manner [43]. These findings may imply that microglia employ similar mechanisms during critical synaptogenic periods either in development or during experience-dependent learning.
Microglial physical interactions with neurons also occur in the human brain e.g. in refractory epileptic brain tissue [66]. Here, complement signaling was suggested to be an underlying mechanism for these contacts. Future work will have to assess the extent to which findings in experimental rodent seizures recapitulate these findings of human seizure disorders.
2.2. Engaging with axons and somata
Microglia engage in physical interactions with axons e.g. following repeatedly evoked action potentials in neurons [26]. Here, axons swelled and attracted microglial processes through an ATP-dependent mechanism. This study strongly correlated microglial process contact of swollen axons with a rescue from the evoked hyperactivity. When microglial process contact of axons was blocked, hyperactive neurons failed to be rescued suggesting a paramount neuroprotective role for microglial contact during hyperactivity [26].
Microglia also make contacts with axons in physiology. At least one of such contacts have recently been identified where microglial somata were localized to the axon initial segment of excitatory neurons and are thus termed AXIS (i.e. AXon Initial Segment-associated) microglia [3]. These interactions continually increased from early postnatal development into maturity and are lost in mild traumatic brain injury suggesting relevant homeostatic functions [3]. Other microglia are localized to the neuronal cell body sometimes denoted (perineuronal) “satellite microglia”. Satellite microglia display unique spontaneous electrical activity that is neither displayed by non-satellite microglia nor coupled to their adjacent neuronal partners [64]. Satellite microglia increase in number following microglial activation induced by LPS treatment [8] or axotomy injury [28, 51, 52]. Increased microglial association with neuronal somata in cortical layers III and V reduce inhibitory synapses following LPS treatment [8]. Such LPS treatment and the corresponding elimination of somal inhibitory synapses was neuroprotective in traumatic brain injury [8].
Similar microglial-neuronal interactions were recently reported in the mouse cerebellum [57] and are conserved in the zebrafish. Using in vivo two-photon imaging of fluorescently labeled microglia and Ca2+-loaded neuronal somata in the developing zebrafish, [29] showed that microglial processes make repeated contacts with neuronal somata much like in the mouse [60, 61]. Here, localized excitatory neuronal activity was sufficient to attract microglial processes in a purinergic-dependent manner. Upon microglial process contact, neuronal hyperactivity was reduced suggesting once more that microglia function in a neuroprotective homeostatic manner [29]
In closing this consideration of the transient microglial-neuronal physical interactions elucidated to date (see Fig. 1), three crucial themes emerge. First, these interactions are intimate involving all structural elements of these cells. Second, these interactions serve mainly neuroprotective functions supporting the hypothesis that microglia participate in the homeostatic regulation of brain physiology. Finally, neuronal NMDAR activation is coupled to microglial P2Y12R signaling indicating that this signaling axis is paramount for regulating microglial-neuronal physical interactions.
Fig. 1: Transient Microglial-Neuronal Physical Interactions.
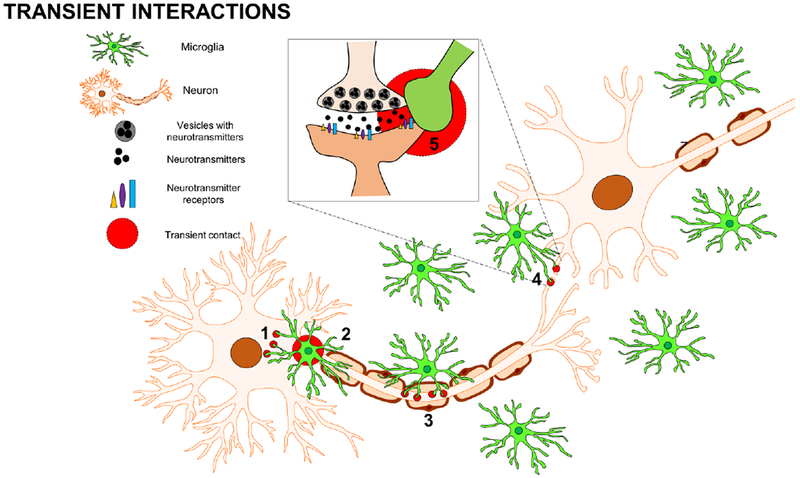
Microglia physically interact with neurons in a variety of ways. Some of these interactions are transient and can involve: (1) microglial processes contacting neuronal somata, (2) microglial cell bodies aligning along the axon initial segment (3) microglial processes contacting the neuronal axon, and (4) microglial processes contacting the neuronal synapse including (5) pre- and post-synaptic elements.
3. Terminal interactions
In the brain, excess neurons are produced, many of which are systematically eliminated during development [11, 67]. Microglia are the predominant brain phagocyte during development and disease. Significant progress has been made into documenting and understanding microglial phagocytic clearance of neurons. We will now consider these in the context of development, the neurogenic niche and in the engulfment of viable neurons.
3.1. Phagocytosis in development
Microglia clear dead cells in the developing mouse cortex [2], hippocampus [15, 45, 62] and cerebellum [31]. However, the zebrafish has recently been used to elucidate the underlying mechanisms for microglial phagocytosis. Here, microglia exhibit profound efficiency in the phagocytic clearance of apoptotic debris [44, 58]. Specifically, phosphatidylserine receptors BAI1 and TIM-4 were required for phagosome formation and stabilization, respectively [32], while the v0-ATPase a1 subunit was required for the fusion of phagosomes into lysosomes [44]. Whether these mechanisms are conserved in mammals and/or recapitulated in disease should be a focus of future studies.
3.2. Phagocytosis in the neurogenic niche
After development, new neurons are only generated in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) along the lateral wall of the ventricle [27]. In the DG, majority of the newborn neurons die by apoptosis following their birth and are efficiently cleared by microglia [53]. Microglial clearance efficiency in the DG is maintained in aging, systemic inflammation and excitotoxic injury but is impaired following seizures and in epilepsy [1, 53]. The proposed mechanism for this impairment involved abolishing purinergic gradients generated by the production of excessive purines following seizures. Since purinergic mechanisms regulate microglial sensing and phagocytic dynamics [6, 18, 35], microglia fail to sense and adequately phagocytose apoptotic cells [1].
Microglia also regulate clearance in the SVZ with great efficiency [20]. Here, the TAM (Tyro3, Axl and Mer) family of receptor tyrosine kinases regulate microglial phagocytosis. However, the functional significance of these interactions also remains to be determined. Interestingly, in a transgenic Parkinson’s Disease (PD) mouse model, TAM proteins were upregulated and correlated with reduced disease survival [20]. Thus, at least in a PD context, microglial phagocytosis aberrantly targeted live neurons (phagoptosis, see below) resulting in poorer outcomes.
3.3. Phagoptosis.
Microglia are also known to induce the killing and subsequent clearance of viable neurons. For example, in the developing cerebellum, microglia induced the demise of Purkinje neurons through the release of superoxide ions [31]. Similarly, apoptosis of hippocampal neurons during development was facilitated by microglial DAP12 and CR3 signaling [62]. Microglial phagocytosis of otherwise viable neurons also occurs during inflammation. Microglia in co-culture with neurons phagocytosed otherwise healthy neurons when exposed to inflammatory agents including amyloid beta. Here, neuronal viability was preserved when microglial phagocytic processes were inhibited [36, 38]. In this inflammation-induced phagocytosis, microglia triggered the expression of calreticulin on neurons that was subsequently recognized by the microglial low-density lipoprotein receptor-related protein to trigger phagocytosis of otherwise viable neurons. This form of phagocytosis is now referred to as phagoptosis where microglial phagocytosis is the primary inducer of cell death as distinct from traditional phagocytosis where microglial phagocytosis occurs secondary to cell death [4, 5].
3.4. Synaptic pruning
During brain development, extranumerary synapses are eliminated [37, 47] presumably by microglial synaptic pruning. In support of this hypothesis, microglia took up both pre- and post-synaptic material in the developing hippocampus [42]. Fractalkine signaling (CX3CL1-CX3CR1) where CX3CL1 is expressed by neurons and CX3CR1 is solely expressed by microglia in the brain [21, 41] is a unique avenue for microglial-neuronal communications. Mice lacking microglial CX3CR1 exhibited increased synaptic engulfment, transiently reduced spine density and delayed functional neuronal maturation. These aberrations were correlated with a transient reduction in microglial density in CX3CR1-deficient hippocampi [25, 42]. Although these aberrations were restored in adult mice, CX3CR1-deficient mice still exhibited global brain under-connectivity suggesting that transient aberrations in microglial pruning could have long lasting cognitive and social effects [68].
Hoxb8 is also exclusively expressed by a subset of microglia in the brain [7]. Genetic ablation of Hoxb8 resulted in a pathological grooming phenotype in mice reminiscent of obsessive compulsive behavior in humans [7]. These mice exhibit aberrant spine densities compared to wildtype mice [34]. The altered synaptic density in Hoxb8-deficient mice suggests synaptic pruning deficits. Since synaptic pruning is identical in Hoxb8 and non-Hoxb8 microglia [10], these results imply that in the absence of Hoxb8, synaptic pruning by the Hoxb8 microglia is dysregulated.
Microglial complement signaling has also been implicated in synaptic pruning. The first suggestion of complement involvement in synaptic pruning was provided by evidence of C1q upregulation in developing neurons. Furthermore, C1q-deficient mice showed deficient synaptic pruning [56]. Because microglia express C3 receptors (C3R), which are activated downstream of C1q activation, they became a focus of study. Genetic abrogation of C3 or C3R reduced microglial presynaptic engulfment and increased synaptic density [49]. A similar upregulation of C1q, C3 and C3R-dependent microglial synaptic pruning has been reported for glaucoma [56] epilepsy [50, 66] and Alzheimer’s Disease [23] indicating that these developmental mechanisms may be re-instituted during neurodegeneration [24].
The above-mentioned studies, while documenting microglial uptake of synaptic material, simply presumed a phagocytic mechanism. However, detailed correlated light and electron microscopy coupled with ex vivo imaging was used to test this hypothesis [63]. Remarkably, microglia were not actively engulfing whole synapses. Rather, they refined synapses by partial “nibbling” through a process known as trogocytosis. However, molecular mechanisms underlying the process are not known [63].
In closing this consideration of the terminal interactions employed by microglia to physically interact with neurons (see Fig. 2), the zebrafish has been developed as an adequate model to elucidate microglial phagocytic clearance mechanisms during development and novel terminal interactions between microglia and neurons have been discovered including phagoptosis (the phagocytosis of live neurons) and trogocytosis (the refinement of synapses).
Fig. 2: Terminal Microglial-Neuronal Physical Interactions.
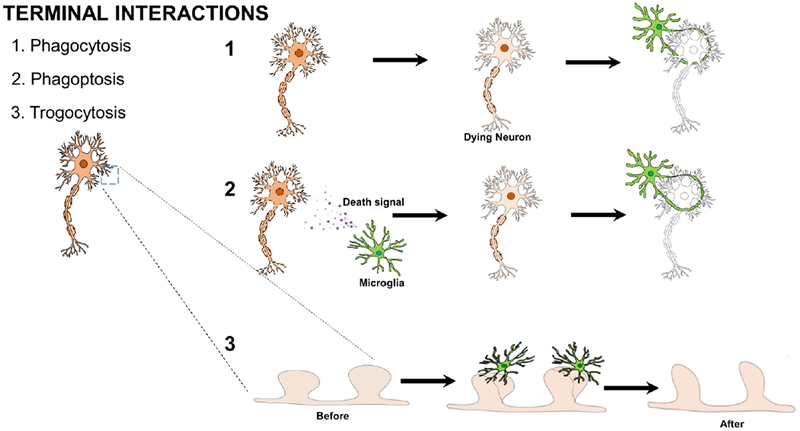
Microglia physically interact with neurons during or in response to the permanent elimination of the neuron or neuronal element. These include: (1) microglial phagocytic clearance of dead neurons during development or in the neurogenic niche; (2) microglial phagoptotic induction of neuronal death and subsequent clearance and (3) microglia trogocytic remodeling of synapses in synaptic pruning.
4. Concluding Remarks
It has been an exciting decade of research into the transient and terminal physical interactions between microglia and neurons. Three themes emerge from these findings. First, the varied forms of engagement of neuronal elements by microglial somata and processes indicate that microglial-neuronal physical interactions are robust and intimate. Second, with the existence of such interactions in the zebrafish and mouse, these interactions have been conserved across vertebrate species. Finally, these interactions tend to be beneficial. These themes are consistent and when taken in reverse order, it is reasonable that these beneficial interactions would be highly conserved across species and could thus be expected to be robust.
Outstanding questions remain for future research. First, research so far have been mostly descriptive rather than mechanistic. What molecular factors regulate microglial contact-induced filopodia formation in development, AXIS-associated microglial alignment with the axon initial segment, trogocytic refinement of synapses or phagocytic clearance in the neurogenic niche? Moreover, the downstream by which microglia may elicit neuroprotective activity is not known. Second, the functional significance of several of these phenomena are not clear. What is the function of AXIS microglia or of phagocytic clearance in the neurogenic niche? It has been an exciting decade of research on this subject but it has also raised further questions that should be the focus of future research in the next decade.
HIGHLIGHTS.
Transient microglia-neuronal physical interactions are intimate, homeostatic, and largely dependent on NMDAR activation - microglial P2Y12R coupling.
Microglia-neuronal interactions could be terminal, for example during phagocytosis and the recently discovered interactions, phagoptosis and trogocytosis.
Microglia-neuronal physical interactions appear to be beneficial especially the transient interactions, and could be conserved across vertebrate species.
Acknowledgements
We would like to thank Madhuvika Murugan with help with the schematic drawings.
Funding
This work is supported by a grant from the NIH: K22NS104392 to U.B.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors declare no competing interests.
References
- [1].Abiega O, Beccari S, Diaz-Aparicio I, Nadjar A, Laye S, Leyrolle Q, Gomez-Nicola D, Domercq M, Perez-Samartin A, Sanchez-Zafra V, Paris I, Valero J, Savage JC, Hui CW, Tremblay ME, Deudero JJ, Brewster AL, Anderson AE, Zaldumbide L, Galbarriatu L, Marinas A, Vivanco M, Matute C, Maletic-Savatic M, Encinas JM, Sierra A, Neuronal Hyperactivity Disturbs ATP Microgradients, Impairs Microglial Motility, and Reduces Phagocytic Receptor Expression Triggering Apoptosis/Microglial Phagocytosis Uncoupling, PLoS biology 14 (2016) e1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahlers KE, Karacay B, Fuller L, Bonthius DJ, Dailey ME, Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration, Glia (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN, Axon initial segment-associated microglia, J Neurosci 35 (2015) 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brown GC, Neher JJ, Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’, Trends Biochem Sci 37 (2012) 325–332. [DOI] [PubMed] [Google Scholar]
- [5].Brown GC, Neher JJ, Microglial phagocytosis of live neurons, Nat Rev Neurosci 15 (2014) 209–216. [DOI] [PubMed] [Google Scholar]
- [6].Castellano B, Bosch-Queralt M, Almolda B, Villacampa N, Gonzalez B, Purine Signaling and Microglial Wrapping, Adv Exp Med Biol 949 (2016) 147–165. [DOI] [PubMed] [Google Scholar]
- [7].Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR, Hematopoietic origin of pathological grooming in Hoxb8 mutant mice, Cell 141 (2010) 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD, Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4, J Neurosci 32 (2012) 11706–11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB, ATP mediates rapid microglial response to local brain injury in vivo, Nat Neurosci 8 (2005) 752–758. [DOI] [PubMed] [Google Scholar]
- [10].De S, Van Deren D, Peden E, Hockin M, Boulet A, Titen S, Capecchi MR, Two distinct ontogenies confer heterogeneity to mouse brain microglia, Development 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].De Zio D, Giunta L, Corvaro M, Ferraro E, Cecconi F, Expanding roles of programmed cell death in mammalian neurodevelopment, Semin Cell Dev Biol 16 (2005) 281–294. [DOI] [PubMed] [Google Scholar]
- [12].Dissing-Olesen L, LeDue JM, Rungta RL, Hefendehl JK, Choi HB, MacVicar BA, Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth, J Neurosci 34 (2014) 10511–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eyo UB, Bispo A, Liu J, Sabu S, Wu R, DiBona VL, Zheng J, Murugan M, Zhang H, Tang Y, Wu LJ, The GluN2A Subunit Regulates Neuronal NMDA receptor-Induced Microglia-Neuron Physical Interactions, Sci Rep 8 (2018) 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eyo UB, Gu N, De S, Dong H, Richardson JR, Wu LJ, Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium, J Neurosci 35 (2015) 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eyo UB, Miner SA, Weiner JA, Dailey ME, Developmental changes in microglial mobilization are independent of apoptosis in the neonatal mouse hippocampus, Brain Behav Immun 55 (2016) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eyo UB, Peng J, Murugan M, Mo M, Lalani A, Xie P, Xu P, Margolis DJ, Wu LJ, Regulation of Physical Microglia-Neuron Interactions by Fractalkine Signaling after Status Epilepticus, eNeuro 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ, Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus, J Neurosci 34 (2014) 10528–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Farber K, Kettenmann H, Purinergic signaling and microglia, Pflugers Arch 452 (2006) 615–621. [DOI] [PubMed] [Google Scholar]
- [19].Fetcho JR, O’Malley DM, Imaging neuronal networks in behaving animals, Curr Opin Neurobiol 7 (1997) 832–838. [DOI] [PubMed] [Google Scholar]
- [20].Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagorska A, Rothlin CV, Nimmerjahn A, Lemke G, TAM receptors regulate multiple features of microglial physiology, Nature 532 (2016) 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L, Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia, Proc Natl Acad Sci U S A 95 (1998) 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Helmchen F, Denk W, Deep tissue two-photon microscopy, Nat Methods 2 (2005) 932–940. [DOI] [PubMed] [Google Scholar]
- [23].Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B, Complement and microglia mediate early synapse loss in Alzheimer mouse models, Science 352 (2016) 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hong S, Dissing-Olesen L, Stevens B, New insights on the role of microglia in synaptic pruning in health and disease, Curr Opin Neurobiol 36 (2016) 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E, Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex, J Neurosci 32 (2012) 15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kato G, Inada H, Wake H, Akiyoshi R, Miyamoto A, Eto K, Ishikawa T, Moorhouse AJ, Strassman AM, Nabekura J, Microglial Contact Prevents Excess Depolarization and Rescues Neurons from Excitotoxicity, eNeuro 3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kazanis I, Neurogenesis in the adult mammalian brain: how much do we need, how much do we have?, Curr Top Behav Neurosci 15 (2013) 3–29. [DOI] [PubMed] [Google Scholar]
- [28].Kreutzberg GW, Microglia: a sensor for pathological events in the CNS, Trends Neurosci 19 (1996) 312–318. [DOI] [PubMed] [Google Scholar]
- [29].Li Y, Du XF, Liu CS, Wen ZL, Du JL, Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo, Dev Cell 23 (2012) 1189–1202. [DOI] [PubMed] [Google Scholar]
- [30].Lynch MA, Long-term potentiation and memory, Physiol Rev 84 (2004) 87–136. [DOI] [PubMed] [Google Scholar]
- [31].Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M, Microglia promote the death of developing Purkinje cells, Neuron 41 (2004) 535–547. [DOI] [PubMed] [Google Scholar]
- [32].Mazaheri F, Breus O, Durdu S, Haas P, Wittbrodt J, Gilmour D, Peri F, Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia, Nat Commun 5 (2014) 4046. [DOI] [PubMed] [Google Scholar]
- [33].Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J, Microglia contact induces synapse formation in developing somatosensory cortex, Nat Commun 7 (2016) 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nagarajan N, Jones BW, West PJ, Marc RE, Capecchi MR, Corticostriatal circuit defects in Hoxb8 mutant mice, Mol Psychiatry (2017). [DOI] [PubMed] [Google Scholar]
- [35].Neher JJ, Neniskyte U, Hornik T, Brown GC, Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo, Glia 62 (2014) 1463–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC, Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death, J Immunol 186 (2011) 4973–4983. [DOI] [PubMed] [Google Scholar]
- [37].Neniskyte U, Gross CT, Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders, Nat Rev Neurosci 18 (2017) 658–670. [DOI] [PubMed] [Google Scholar]
- [38].Neniskyte U, Neher JJ, Brown GC, Neuronal death induced by nanomolar amyloid beta is mediated by primary phagocytosis of neurons by microglia, J Biol Chem 286 (2011) 39904–39913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nimmerjahn A, Kirchhoff F, Helmchen F, Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo, Science 308 (2005) 1314–1318. [DOI] [PubMed] [Google Scholar]
- [40].Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F, Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo, Nat Methods 1 (2004) 31–37. [DOI] [PubMed] [Google Scholar]
- [41].Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M, Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia?, FEBS Lett 429 (1998) 167–172. [DOI] [PubMed] [Google Scholar]
- [42].Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT, Synaptic pruning by microglia is necessary for normal brain development, Science 333 (2011) 1456–1458. [DOI] [PubMed] [Google Scholar]
- [43].Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB, Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor, Cell 155 (2013) 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peri F, Nusslein-Volhard C, Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo, Cell 133 (2008) 916–927. [DOI] [PubMed] [Google Scholar]
- [45].Petersen MA, Dailey ME, Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices, Glia 46 (2004) 195–206. [DOI] [PubMed] [Google Scholar]
- [46].Pfeiffer T, Avignone E, Nagerl UV, Induction of hippocampal long-term potentiation increases the morphological dynamics of microglial processes and prolongs their contacts with dendritic spines, Sci Rep 6 (2016) 32422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Riccomagno MM, Kolodkin AL, Sculpting neural circuits by axon and dendrite pruning, Annu Rev Cell Dev Biol 31 (2015) 779–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C, CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity, J Neurosci 31 (2011) 16241–16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner, Neuron 74 (2012) 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schartz ND, Wyatt-Johnson SK, Price LR, Colin SA, Brewster AL, Status epilepticus triggers long-lasting activation of complement C1q-C3 signaling in the hippocampus that correlates with seizure frequency in experimental epilepsy, Neurobiol Dis 109 (2018) 163–173. [DOI] [PubMed] [Google Scholar]
- [51].Schiefer J, Kampe K, Dodt HU, Zieglgansberger W, Kreutzberg GW, Microglial motility in the rat facial nucleus following peripheral axotomy, J Neurocytol 28 (1999) 439–453. [DOI] [PubMed] [Google Scholar]
- [52].Schoen SW, Graeber MB, Kreutzberg GW, 5’-Nucleotidase immunoreactivity of perineuronal microglia responding to rat facial nerve axotomy, Glia 6 (1992) 314–317. [DOI] [PubMed] [Google Scholar]
- [53].Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M, Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis, Cell Stem Cell 7 (2010) 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE, Majewska AK, Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex, Nat Commun 7 (2016) 10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sogn CJ, Puchades M, Gundersen V, Rare contacts between synapses and microglial processes containing high levels of Iba1 and actin--a postembedding immunogold study in the healthy rat brain, Eur J Neurosci 38 (2013) 2030–2040. [DOI] [PubMed] [Google Scholar]
- [56].Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA, The classical complement cascade mediates CNS synapse elimination, Cell 131 (2007) 1164–1178. [DOI] [PubMed] [Google Scholar]
- [57].Stowell RD, Wong EL, Batchelor HN, Mendes MS, Lamantia CE, Whitelaw BS, Majewska AK, Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo, Dev Neurobiol 78 (2018) 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Svahn AJ, Graeber MB, Ellett F, Lieschke GJ, Rinkwitz S, Bennett MR, Becker TS, Development of ramified microglia from early macrophages in the zebrafish optic tectum, Dev Neurobiol 73 (2013) 60–71. [DOI] [PubMed] [Google Scholar]
- [59].Takano T, Han X, Deane R, Zlokovic B, Nedergaard M, Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease, Ann N Y Acad Sci 1097 (2007) 40–50. [DOI] [PubMed] [Google Scholar]
- [60].Tremblay ME, Lowery RL, Majewska AK, Microglial interactions with synapses are modulated by visual experience, PLoS biology 8 (2010) e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J, Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals, J Neurosci 29 (2009) 3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A, Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor, J Neurosci 28 (2008) 8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT, Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction, Nat Commun 9 (2018) 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wogram E, Wendt S, Matyash M, Pivneva T, Draguhn A, Kettenmann H, Satellite microglia show spontaneous electrical activity that is uncorrelated with activity of the attached neuron, Eur J Neurosci 43 (2016) 1523–1534. [DOI] [PubMed] [Google Scholar]
- [65].Wu LJ, Zhuo M, Resting microglial motility is independent of synaptic plasticity in mammalian brain, Journal of neurophysiology 99 (2008) 2026–2032. [DOI] [PubMed] [Google Scholar]
- [66].Wyatt SK, Witt T, Barbaro NM, Cohen-Gadol AA, Brewster AL, Enhanced classical complement pathway activation and altered phagocytosis signaling molecules in human epilepsy, Exp Neurol 295 (2017) 184–193. [DOI] [PubMed] [Google Scholar]
- [67].Yamaguchi Y, Miura M, Programmed cell death in neurodevelopment, Dev Cell 32 (2015) 478–490. [DOI] [PubMed] [Google Scholar]
- [68].Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT, Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior, Nat Neurosci 17 (2014) 400–406. [DOI] [PubMed] [Google Scholar]
- [69].Zhang J, Malik A, Choi HB, Ko RW, Dissing-Olesen L, MacVicar BA, Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase, Neuron 82 (2014) 195–207. [DOI] [PubMed] [Google Scholar]


