Abstract
BACKGROUND:
Neonatal abstinence syndrome (NAS) is one of the consequences at birth affecting the newborn after discontinuation of prenatal drug exposure to mainly opioids. The objective of this study was to determine the extent of the problem in the state of West Virginia (WV) using a real-time statewide surveillance system.
METHODS:
Project WATCH is a surveillance tool that since 1998 collects data on all infants born in the state of WV. NAS surveillance item was added to the tool in October 2016. This study examined all births (N = 23,667) in WV from October to December 2017. The data from six WV birthing facilities were audited for 1 month to evaluate how well this tool was capturing NAS data using κ-statistics.
RESULTS:
The 2017 annual incidence rate of NAS was 51.3 per 1000 live births per year for all births and 50.6 per 1000 live births per year for WV residents only. The κ-coefficient between the hospital medical records and Project WATCH data was 0.74 (95% confidence interval: 0.66–0.82) for NAS.
CONCLUSION:
The study provides justification to develop effective systems of care for the mother–infant dyad affected by substance use, especially targeting pregnant women in rural communities.
INTRODUCTION
Neonatal abstinence syndrome (NAS) is a multi-system withdrawal syndrome of the newborn that presents shortly after birth when in utero exposure to illegal or prescription drugs (classically opioids) is suddenly discontinued at delivery.1,2 Some of the clinical signs that may appear within the first few days after birth and may include excessive sweating, tremors, high-pitched cry, poor feeding, watery stools, and excessive weight loss.3,4 Negative long-term outcomes are also evident as these children have an increased risk of social and behavior abnormalities in early years,5 as well as poorer school performances than their peers.6
In the United States (US), the incidence rate of NAS in number of cases per 1000 live births per year has increased from 1.2 in 2000 to 3.4 in 20097 to 5.8 in 2012.8 In 2012, the estimated cost of admissions for infants diagnosed with NAS was nearly US$316 million nationally.9 The aggregate hospital charges for NAS totaled US$1.5 billion.8 Moreover, nearly 80% of the infants diagnosed with NAS are born to families whose health insurance coverage is through state-funded Medicaid programs,7,9 thus escalating the burden on the already strained healthcare system.10
The incidence of NAS is disproportionately higher in rural counties compared to urban counties and increasing much more rapidly in rural areas compared to urban areas.11 There are geographical disparities in NAS rates based on Appalachian mountain regional status as well; in the states of Kentucky and Tennessee, the rates of NAS were more in the Appalachian regions compared to the non-Appalachian regions.12,13
With the rise in the opioid epidemic nationally, the state of West Virginia (WV) has experienced a much higher rate of substance use due to the constellation of several socio-demographic factors that negatively impact health behaviors. WV is the only state that is entirely within the Appalachian mountain region and nearly half of the people in the state live in rural areas.14,15 WV also has the highest age-adjusted drug overdose death rate in the nation.16 A study in 2009 showed that 19% of mothers used drugs or drank alcohol during pregnancy in WV.17 Stabler et al.18 examined the 2007–2013 WV Health Care Authority and Uniform Billing Data for WV and found that between 2007 to 2013 the incidence of NAS increased from 7.74 per 1000 live birth per year to 31.56 per 1000 live birth per year.18
Most studies use administrative data based on billing and coding from medical documentation to obtain NAS estimates, which leads to a few years of lag in presenting current NAS estimates.18–21 With the steep increase of substance use in the state of WV, this lag may lead to misrepresentation of the current crisis. The objective of the manuscript was to present the most current rate of NAS for WV as well as evaluate how well Project WATCH surveillance tool was capturing NAS data in real time for the state. Given the rise in opioid epidemic and the parallel surge in infants with NAS nationally as well as in the state of WV, the results of this study have the potential to contribute to addressing this critical public health problem in one of the hardest hit states in the country by informing policy makers as to the true extent of the problem in real time.
METHODS
The study used data from Project WATCH (Working in Appalachia to identify at-risk infants, Critical congenital heart disease, and Hearing loss). This project as a statewide mandate since 1998 (House Bill 238822) focuses on collecting surveillance data on every infant born in WV birthing hospitals/facilities. More information about this project can be found elsewhere.23 In October 2016, Project WATCH collaborated with The West Virginia Perinatal Partnership and the WV Department of Health and Human Resources to expand its surveillance tool to include real-time information on substance use during pregnancy and presence of NAS at the time of infant discharge from the hospital. Given that Project WATCH has been used by all WV birthing hospitals for more than 20 years, the addition of a new surveillance item to this tool that captures real-time data appeared to be an ideal way to identify infants with NAS and accurately estimate the extent of the problem in WV.
The nurses at each WV birth facility completed the questionnaire form for Project WATCH before hospital discharge. Intrauterine substance exposure (IUSE) was assessed using several possible sources (self-report, documented in medical record, or/and positive drug screening test). In September 2014, WV neonatologists, pediatricians, hospital coders, and members of the West Virginia Perinatal Partnership met to develop a standardized definition for NAS as well as guidance for documenting exposure and withdrawal in newborns. The definition that was agreed upon was as follows: NAS is diagnosed when a baby has intrauterine exposure to a neuro-active substance, and exhibited clinical signs of withdrawal, regardless of whether or not pharmacological treatment is required. Training sessions on the standardized definition for NAS were conducted statewide at all birth facilities in 2015–2016. Following the statewide training efforts, the IUSE and NAS surveillance items were added to the Project WATCH surveillance tool on 1 October 2016. Infants who had IUSE were assessed for signs of NAS consistent with the agreed upon statewide definition
This study examined all births in WV over a 15-month time period (1 October 2016 to 31 December 2017). Nearly 16% of infants were born to mothers who live in the surrounding states of Kentucky, Maryland, Ohio, Pennsylvania, and Virginia, but gave birth in WV. The data were analyzed for all births as well as births for WV residents only. Within WV, the 55 counties were clustered into six regions. These regions are based on the sub-state regions defined by the 2012–2014 National Survey on Drug Use and Health (NSDUH) conducted by the Substance Abuse and Mental Health Services Administration (SAMHSA). These regions were created to understand the geographic variability of substance use within WV, which in turn provided vital information for policy efforts.24 Most substance use data are analyzed using these regions, rather than using county-level data since not all counties have the same level of resources and people cross county lines to access services. Regions were compared on both the prevalence of IUSE and the incidence of NAS.25,26
One-month data collection audit
Since Project WATCH started collecting data on substance use and NAS in October 2016, the research team wanted to assess the quality of data being recorded in Project WATCH database. Subsequently, a chart audit was performed on all births from six large birthing hospitals in WV for the month of January 2017. Detailed and standardized review instructions were provided to all hospital personnel who were to be responsible for data abstraction. Data abstraction was not blinded but data were deidentified before analysis. All births were reviewed to examine what was reported in the medical chart, which ICD-10 code was entered and what was reported by Project WATCH. The ICD-10-CM codes included P04.1–P04.4, P04.8, P04.9, and P96.1.27 The κ-coefficient was calculated to examine the level of agreement between the hospital medical records and the data from Project WATCH.
RESULTS
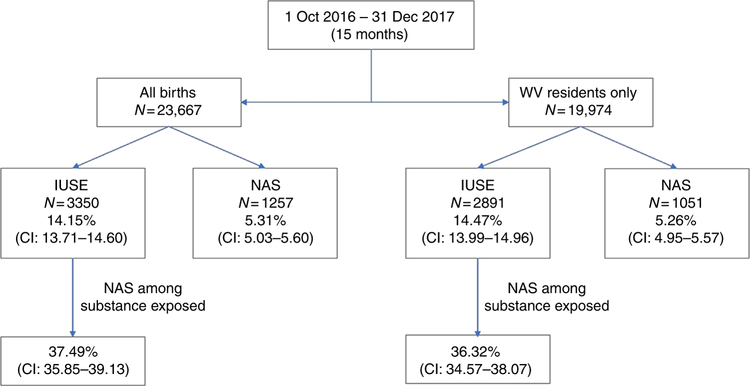
The results showed that in the select project period there were 23,667 births in the state of WV. The rate of IUSE and NAS for all births and for WV residents only (84%) is shown in Fig. 1. For the WV residents only, the incidence of NAS was 52.6 per 1000 live births per year (Fig. 1). The breakdown of the data by year 2016 (3 months) and 2017 (12 months) as well as the total for the 15-month period is shown in Table 1.
Fig. 1.
Prevalence of intra-uterine substance exposure (IUSE) and incidence of neonatal abstinence syndrome (NAS) in West Virginia from 1 October 2016 to 31 December 2017 for all births (N = 23,667) and for West Virginia residents only (N = 19,974)
Table 1.
Prevalence of intra-substance use exposure (IUSE) and incidence of neonatal abstinence syndrome (NAS) in West Virginia by year 2016 and 2017
| Total | 2016 Oct to Dec (3 months) (N=4,870) |
2017 Jan to Dec (12 months) (N= 18,787) |
Total Oct 2016 to Dec 2017 (15 months) (23,667) |
|||
|---|---|---|---|---|---|---|
| n | Percent (95% CI) | n | Percent (95% CI) | n | Percent (95% CI) | |
| IUSE | 720 | 14.78 (13.79 – 15.78) | 2630 | 13.99 (13.50 – 14.49) | 3350 | 14.15 (13.71 – 14.60) |
| NAS | 295 | 6.06 (5.39 – 6.73) | 962 | 5.12 (4.80 – 5.43) | 1257 | 5.31 (5.03 – 5.60) |
| West Virginia only | ||||||
| (N=4131) | (N=15,843) | (N= 19,974) | ||||
| IUSE | 626 | 15.15 (14.06–16.25) | 2265 | 14.30 (13.75–14.84) | 2891 | 14.47 (13.99–14.96) |
| NAS | 249 | 6.03 (5.30–6.75) | 820 | 5.06 (4.72–5.40) | 1051 | 5.26 (4.95–5.57) |
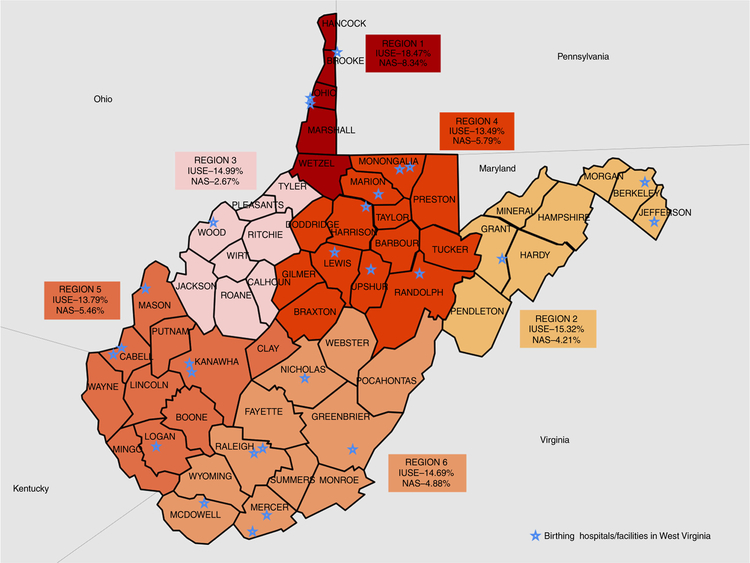
The results of IUSE and NAS rates according to the six predefined SAMHSA regions are displayed in Table 2 and Fig. 2. Region 1 had one of the highest prevalence of IUSE and the highest incidence of NAS in the state. Similar patterns were not found in other regions. For example, in region 4 the prevalence of the IUSE was the lowest, but the incidence of the NAS was the second highest in the state (Table 2).
Table 2.
Prevalence of intra-substance use exposure (IUSE) and incidence of neonatal abstinence syndrome (NAS) in West Virginia (October 2016 to 31 Dec 2017) by SAMHSA sub-state regional classification system, N = 19,974
| Regions | Population frequency (%) | IUSE % (95% CI) | Rank | NAS % (95% CI) | Rank | NAS % among IUSE (95% CI) | Rank |
|---|---|---|---|---|---|---|---|
| 1 | 1379 (6.92%) | 18.49 (16.44–20.54) | 1 | 8.41 (6.95–9.88) | 1 | 45.49 (39.38–51.60) | 1 |
| 2 | 1854 (9.30%) | 15.32 (13.68–16.96) | 2 | 4.21 (3.29–5.12) | 5 | 27.46 (22.27–32.66) | 5 |
| 3 | 1801 (9.04%) | 14.99 (13.34–16.64) | 3 | 2.67 (1.92–3.41) | 6 | 17.78 (13.22–22.34) | 6 |
| 4 | 4871 (24.44%) | 13.49 (12.53–14.45) | 6 | 5.79 (5.13–6.45) | 2 | 42.77 (38.99–46.55) | 2 |
| 5 | 6171 (30.96%) | 13.79 (12.93–14.65) | 5 | 5.46 (4.89–6.03) | 3 | 39.60 (36.31–42.89) | 3 |
| 6 | 3853 (19.33%) | 14.69 (13.57–15.81) | 4 | 4.88 (4.20–5.56) | 4 | 33.22 (29.34–37.10) | 4 |
| Total | 19,929 | 14.47 (13.98–14.95) | 5.26 (4.95–5.57) | 36.35 (34.60–38.11) | |||
| Missing | 45 | 45 | 45 | 8 |
Fig. 2.
Project WATCH surveillance data on prevalence of intra-uterine substance exposure (IUSE) and neonatal abstinence syndrome (NAS) in West Virginia by SAMHSA sub-state regional classification system, N = 20,002
The information on IUSE was gathered from multiple sources and could include more than one source per person. Of those with IUSE (n = 3350), nearly two-thirds (65.76%; confidence interval (CI): 64.15–67.37) had a positive drug screen during their labor and delivery hospital admission and nearly half of the women self-reported (52.72%; CI: 51.03–54.14) or had a positive substance use information in their past medical records (50.90%; CI: 49.20–52.59).
One-month data collection audit
There were 789 births in the six hospitals where the chart audits were performed. This represented 52% of 1521 births in Project WATCH database for all birth in January 2017 in WV. Out of 133 IUSE cases identified in the hospital medical records, 107 (81%) were identified as IUSE cases by the Project WATCH surveillance data tool (κ-coefficient = 0.85 (CI: 0.83–0.91)). For the NAS data, out of 79 cases identified in the hospital medical records, 56 (71%) were identified as NAS cases by the Project WATCH surveillance tool (κ-coefficient = 0.74 (CI: 0.66–0.82). (Table 3). The ICD-10-CM code for NAS was recorded by less than half (47.25%) of the infants diagnosed with NAS in the combined data for the six hospitals.
Table 3.
One-month (January 2017) audit data for six hospitals in the state of WV comparing the project WATCH data with the hospital medical records and the ICD-10-CM codes (N = 789)
| Data source | Frequency | Percent (95% CI) |
|---|---|---|
| Intrauterine substance exposure | ||
| Hospital data | 134 | 17.07 (14.36–19.60) |
| Birth Score data | 111 | 14.14 (11.64–16.49) |
| ICD-10-CM code in chart—code P04. x | 57 | 7.23 (5.42–9.03) |
| Neonatal abstinence syndrome | ||
| Hospital data | 79 | 10.1 (7.92–12.11) |
| Birth Score data | 67 | 8.55 (6.55–10.44) |
| ICD-10-CM code in chart—code P96.1 | 43 | 5.46 (3.87–7.03) |
DISCUSSION
The study used a real-time surveillance system to assess the extent of NAS in a state that has been significantly impacted by the current opioid crisis. The rate of NAS was 53 cases per 1000 live births per year, which was much higher than rate of NAS reported in earlier studies in 2013, that is, nearly 30 per 1000 live births per year.18,19 This rise was expected, as national trends show a significant increase in NAS diagnoses as well. However, based on the discordance between coding data and Project WATCH data, there is a level of concern that the earlier rates may have been grossly underestimated. WV is a rural Appalachian state and the results from this study showed that NAS rate was seven times higher in WV than what was found in rural regions of the country in 2013 (i.e., nearly 7 per 1000 live births per year).11 Moreover, this study demonstrated that the rate of NAS in WV is one of the highest in the country and nearly 10-fold the national estimate of5.8 per 1000 births live births per year in 2012.8
The rate of IUSE in WV was 14%, which is consistent with a study conducted in 2009.17 Stitely et al.17 examined umbilical cord tissues of women who gave birth in 1 month in eight birth hospitals in WV and found 14% of mothers were positive for drug use. Based on the rising opioid epidemic in the state, we hypothesized that in 2017 the prevalence would be much higher than what was observed in 2009. One of the likely reasons for the underestimated prevalence in our study is the fact that data on IUSE was gathered through self-report, previous documentation, or drug testing at certain hospitals compared to the earlier study that used universal umbilical cord tissue samples.17 WV does not have a mandated maternal drug-screening program.
Geographical disparities in the rates of IUSE and NAS were also observed. The northern panhandle (region 1) has one of the highest rates of IUSE as well as NAS incidence. The northern central region 4 has the lowest prevalence of IUSE, but the second highest NAS rates. We postulate that the inconsistency in this particular region likely arises from a combination in obtaining accurate drug history data from mother as well as the possibility that physicians in region 4 may be more conservative in diagnosing NAS. Interestingly, our regional results are not consistent with the results from Stabler et al.,18 as they found the southern region 4 and region 5 had the highest rates of NAS in 2013.18 Some of the reasons for this discrepancy may be, (1) the use of administrative data source (ICD-9-CM codes) compared to the current study that uses the diagnosis made at the hospital and recorded by the nurses before hospital discharge, and (2) the likely steep increase of incidence between 2013 and 2017. Furthermore, in 2017 the high-intensity drug trafficking areas (HIDTAs) in WV include regions 1, 5, and 6, which may explain the high prevalence of IUSE and NAS in region 1, but nonetheless fails to explain the lower incidence in regions 5 and 6 in the current study.28
The results of the audit found that Project WATCH was capturing the prevalence of IUSE and incidence of NAS reasonably well. However, the agreement between the hospital chart review and the Project WATCH data for IUSE was higher than the agreement between the Project WATCH data and NAS. One likely explanation for this observation is that one of the six hospitals, which incidentally had one of the highest contributions of birth data to the audit, ultimately contributed a higher rate of NAS, which may be reflective of biases from the chart reviewers. Project WATCH had already used this audit data for quality improvement of its data collection process by training nurses on data entry and report. The audit data also revealed that only half of the infants diagnosed with NAS were identified utilizing the ICD-10-CM codes. Other studies have also shown that administrative data diagnostic codes under-represent the actual number of NAS cases diagnosed in the hospital.20
Based on our results, Project WATCH appears to capture IUSE and NAS diagnoses via a novel, accurate, and cost-effective electronic surveillance tool. Although these data does not include births delivered outside the hospital (~0.01%), Project WATCH collects data on every infant born in WV birthing hospitals/facilities. This new data source shows improved ability to capture NAS cases compared to the traditional method of utilizing electronic claims data via ICD codes. Additionally, Project WATCH data are captured in real time allowing for timely surveillance of a leading public health issue in the state of WV. WV is one of a few states that collects surveillance data on NAS; four additional states (Florida, Georgia, Kentucky, and Tennessee) have legally mandated NAS as a reportable condition and have a passive surveillance system in place.29 States without a specific surveil-lance system for NAS report the condition via traditional methods such as diagnostic codes and hospital billing records (e.g., the State Hospital Discharge Database or State All Payer Claims data). Traditional methods used to survey the burden of NAS may have several months of lag from data collection to analysis.18–21 A real-time estimation of NAS has the potential to guide targeted resources and interventions in a timely manner.
Some of the limitations include the fact that Project WATCH does not gather information on the type(s) of specific substances that the infant is exposed to in utero. It is likely that many of the infants in this study were exposed to multiple substances simultaneously. Although the effect of polysubstance use on the occurrence of NAS is controversial,30 results from a large population-based cohort study showed significant increases in the risk of NAS associated with opioid use along with co-exposure to antidepressants, benzodiazepines, and gabapentin compared with opioids alone.31 However, given the main purpose of the study was to document the incidence of NAS, this was not felt to be a significant limitation. Moreover, despite a standardized statewide definition of NAS, there is a degree of subjectivity and individual variability in approaches when making NAS diagnosis and these differences in identification approaches are difficult to assess.32 Data collection for Project WATCH started in October 2016 and therefore cannot be retrospectively compared to traditional hospital billing data. Future research comparing Project WATCH and hospital billing data utilizing ICD codes is warranted. Moreover, non-punitive policies that enable early identification of NAS, improve access to comprehensive prenatal care and opioid replacement therapy, and increase funding for child welfare systems are also warranted. Additional resources are needed, especially to those sub-state regions with the highest rates of substance use and NAS. In addition, other states may benefit from a similar NAS surveillance system used by Project WATCH in WV.
ACKNOWLEDGEMENTS
The West Virginia WATCH/Birth Score Program is funded under an agreement with the West Virginia Department of Health and Human Resources, Bureau for Public Health, Office of Maternal, Child, and Family Health.
Footnotes
Competing interests:
The authors declare no competing interests.
Publisher’s note:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Kocherlakota P Neonatal abstinence syndrome. Pediatrics 134, e547–e561 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Convertino I et al. Neonatal adaptation issues after maternal exposure to prescription drugs: withdrawal syndromes and residual pharmacological effects. Drug Saf 39, 903–924 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Logan BA, Brown MS & Hayes MJ Neonatal abstinence syndrome: treatment and pediatric outcomes. Clin. Obstet. Gynecol 56, 186–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQueen K & Murphy-Oikonen J Neonatal abstinence syndrome. N. Engl. J. Med 375, 2468–2479 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Klinger G et al. Long-term outcome following selective serotonin reuptake inhibitor induced neonatal abstinence syndrome. J. Perinatol 31, 615–620 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Oei JL et al. Neonatal abstinence syndrome and high school performance. Pediatrics 139, e20162651 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Patrick SW et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA 307, 1934–1940 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Patrick SW, Davis MM, Lehmann CU & Cooper WO Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J. Perinatol 35, 650–655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corr TE & Hollenbeak CS The economic burden of neonatal abstinence syndrome in the United States. Addiction 112, 1590–1599 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Ridic G, Gleason S & Ridic O Comparisons of health care systems in the United States, Germany and Canada. Mater. Sociomed 24, 112–120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM & Patrick SW Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatr 171, 194–196 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Brown JD, Goodin AJ & Talbert JC Rural and appalachian disparities in neonatal abstinence syndrome incidence and access to opioid abuse treatment. J. Rural Health 34, 6–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren MD et al. Implementation of a statewide surveillance system for neonatal abstinence syndrome—Tennessee, 2013. Morb. Mortal. Wkly Rep 64, 125–128 (2015). [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser Family Foundation KFF Medicaid’s Role in West Virginia (Kaiser Family Foundation, Washington, 2018). [Google Scholar]
- 15.Griffith BN, Lovett GD, Pyle DN & Miller WC Self-rated health in rural Appalachia: health perceptions are incongruent with health status and health behaviors. BMC Public Health 11, 229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedegaard H, Warner M & Minino AM Drug overdose deaths in the United States, 1999–2015. NCHS Data Brief 1–8 (2017). [PubMed] [Google Scholar]
- 17.Stitely ML, Calhoun B, Maxwell S, Nerhood R & Chaffin D Prevalence of drug use in pregnant West Virginia patients. W V Med. J 106, 48–52 (2010). [PubMed] [Google Scholar]
- 18.Stabler ME et al. Neonatal abstinence syndrome in West Virginia Substate Regions, 2007–2013. J. Rural Health 33, 92–101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko JY et al. Incidence of neonatal abstinence syndrome—28 States, 1999–2013. Morb. Mortal. Wkly Rep 65, 799–802 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Burns L & Mattick RP Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug Alcohol Rev 26, 487–492 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Traylor J, Miller A & Warren MD Evaluation of the web-based neonatal abstinence syndrome surveillance system in Tennessee In Conference: 2014 Council of State and Territorial Epidemiologists Annual Conference (2014). https://www.wvdhhr.org/birthscore/. [Google Scholar]
- 22.Article 22B. Birth Score Program (1998). https://www.wvdhhr.org/birthscore/.
- 23.Mullett MD, Britton CM, John C & Hamilton CW WV birth score: maternal smoking and drugs of abuse. W V Med. J 106, 16–18, 20 (2010). [PubMed] [Google Scholar]
- 24.Zhang Z, Infante A, Meit M & English N An Analysis of Mental Health and Substance Abuse Disparities and Access to Treatment Services in the Appalachian Region. CHAPTER 2: Substance Use, Mental Disorders, and Access to Treatment Services in Household Surveys (2008). [Google Scholar]
- 25.The Governor’s Initiative on Substance Abuse (2017). http://governorssubstanceabusetaskforceswv.com.
- 26.SAMHSA 2016 State Reports From the 2014 National Survey on Drug Use and Health (NSDUH) https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2016-NSDUH.
- 27.ICD-10-CM Codes (2018). https://www.icd10data.com/ICD10CM/Codes.
- 28.HIDTA 2017 High Intensity Drug Trafficking Areas Program (HIDTA), ONDCP Available at https://www.dea.gov/ops/HIDTA_2017_map.pdf. Accessed on 21 Jan 2018.
- 29.Ko JY et al. CDC grand rounds: public health strategies to prevent neonatal abstinence syndrome. Morb. Mortal. Wkly Rep 66, 242–245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Academy of Pediatrics Committee on Drugs. Neonatal drug withdrawal. Pediatrics 101,1079–1088 (1998). [PubMed] [Google Scholar]
- 31.Huybrechts KF et al. Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: cohort study. BMJ 358, j3326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar S & Donn SM Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J. Perinatol 26, 15–17 (2006). [DOI] [PubMed] [Google Scholar]