Abstract
The auditory mismatch field (MMF) is a pre-attentive processing component, reflecting neural discrimination and inhibitory processing. Abnormal MMFs have been reported in children with autism spectrum disorder (ASD) along with an association with abnormal language comprehension; however, relatively little is known about MMF abnormalities to contrasting vowel stimuli in adults with ASD. To better understand the neurophysiological mechanisms underlying auditory language discrimination of vowel stimuli in individuals with ASD, magnetoencephalography was used to measure MMFs during an auditory oddball paradigm with vowel stimuli (/a/ and /u/) in adults with ASD. MMFs arising from left and right superior temporal gyrus are reported from nine high-functioning right handed males with ASD (22.22±5.74yrs) and sixteen typically developing (TD) right handed males (27.25±6.63yrs). The MMF was delayed in adults with ASD (188.90±5.8ms) as compared to the TD participants (173.08±4.31, p<0.05). Replicating previous findings in children, the earlier M100 component to single stimulus tokens was also delayed in adults with ASD (108.59±4.1ms) compared to the TD participants (94.60±3.0, p<0.05). However, there was no correlation between delayed M100 latency and MMF latency. Furthermore, whereas TD participants showed a leftward lateralization of MMF amplitude, participants with ASD showed an opposite (rightward) lateralization. Findings suggest that adults with ASD have hemispherically- and temporally- abnormal auditory discrimination processing in addition to and distinct from abnormal neurophysiological mechanisms in earlier cortical responses.
Keywords: autism spectrum disorder, magnetoencephalography, adults, vowel mismatch fields, auditory language discrimination process and laterality
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impaired social and communication skills, and by repetitive and stereotyped behavior [1]. Magnetoencephalographic (MEG) studies have shown abnormal auditory cortical responses in superior temporal gyrus (STG) such as delayed auditory response components (e.g.M100) in children with ASD [2]. The STG, encompassing primary and secondary auditory cortex, is believed to be a substrate for underlying clinical features in individuals with ASD [3]. The auditory mismatch field (MMF) response reflects an individual’s ability to detect changes in auditory patterns (e.g. changes in phoneme tokens) by presenting regularly occurring “standard” stimuli, occasionally interrupted with rare “deviant” stimuli [4]. Most previous studies of the MMF have reported atypical auditory discrimination processing in individuals with social impairment or ASD [5-8]. For example, delayed MMF latencies to speech and non-speech sounds have been observed in children with ASD compared to typically developing peers (TD), suggesting that difficulty parsing transient differences in sounds may lead to impaired acoustic or phonological representations [9]. These impaired representations appear to be a marker of language impairment in ASD [9]. Furthermore, a previous study reported that children with ASD showed abnormal neural activation hemispheric laterality indices (LIs) to passive auditory vowel stimuli and suggested that children with ASD showed different maturational trajectories in language lateralization, indicative of atypical functional specialization in ASD [10]. In addition, Herbert et al., [11] reported children with autism showed rightward asymmetry in frontal language areas whereas controls showed leftward asymmetry. In addition, Gallagher et al., [12] reported that increased tuber burden was associated with decreased LIs (a lack of leftward lateralization) in participants with tuberous sclerosis and epilepsy, suggesting inter-hemispheric cerebral language reorganization predisposing to a decrease of left-hemispheric language dominance in patients with tuberous sclerosis complex.
The persistence of atypical STG MMF responses into adulthood for individuals with ASD remains unclear. To contribute to the understanding of the neurophysiological mechanisms of auditory language discrimination processing in adults with ASD, MEG was used to measure cortical responses in adults with ASD to an auditory oddball paradigm with vowel stimuli (/a/ and /u/) identical to those used in a prior study with children with ASD [13]. Based on previous reports, we hypothesized that delayed MMF latency and abnormal rightward lateralization would be observed in adults with ASD.
Methods
Participants
Twenty-two adult male participants with ASD were recruited from the Adult Autism Spectrum Program in the Department of Psychiatry at the Hospital of the University of Pennsylvania. Twenty-two adult male TD participants were recruited through local advertisements. All ASD participants had a prior diagnosis of ASD, made by an expert clinician according to DSM criteria [1]. At the time of study participation, adults with ASD were required to exceed established cut-offs on the Autism Diagnostic Observation Schedule Second Edition (ADOS-2) [14] as well as on either the Social Communication Questionnaire (SCQ, Lifetime) [15] or Social Responsiveness Scale, 2nd Edition (SRS-2), Adult-Informant Report [16]. Individuals for whom informant report was not available were included in the ASD group if they had a documented prior diagnosis of ASD and exceeded established cut-offs on the ADOS-2 as well as on both the SRS-2 Adult-Self Report and Broad Autism Phenotype Questionnaire [17]. Individuals 1 point below diagnostic cut-offs on the ADOS-2 were included if they exceeded cut-offs on two informant report questionnaires or on the Autism Diagnostic Interview-Revised [18]. The Wechsler Abbreviated Intelligence Scale-II (WASI-II) was administered for all participants [19]. Inclusion criteria for the TD adults included scoring below the cut-off for ASD on all domains of the ADOS-2 and below cut-offs on informant and self-report questionnaires, along with performance above the 16th percentile on the Clinical Evaluation of Language Fundamentals - Fourth Edition (CELF-4) [20] and WASI-II Verbal Comprehension Index.
All participants had English as a first language. Exclusion criteria included 1) claustrophobia, 2) metallic implanted prosthetic, stimulation device, excessive metallic dental work or other non-removal metal in the body, and 3) any known genetic syndromes or neurological or sensory impairments. TD adults also had no history of current psychiatric illness, documented by self and informant ratings on the Adult Behavior Checklist [21], and were taking no psychotropic medications. Four of the nine participants with ASD were taking medication; participant 1 (stimulants; Adderall™), participant 2 (stimulants; Concerta™, Focalin™ and Metadate™), participant 3 (stimulants; Concerta™), participant 4 (Antidepressants; Zoloft™). Previously, several researchers have reported delayed auditory latency either including or excluding children with ASD who were taking such medications with no apparent differences in findings secondary to any possible effect of drugs on cortical responses [22,23]. So we chose to retain these 4 subjects, noting their medication use. Data from these 4 subjects did not show evidence of forming an outlier cluster in terms of either M100 or MMF. The study was approved by the CHOP Institutional Review Board and all participants provided written informed consent.
Auditory stimuli
Prior to the MEG recording, each participant’s hearing threshold was determined, and auditory stimuli were presented at 45dB SL (above threshold). The auditory stimuli (/a/ and /u/) were presented using Eprime v1.1 (Psychology Software Tools Inc., Pittsburgh, PA), binaurally via sound pressure transducers and sound conduction tubing to the participant’s peripheral auditory canal via eartip inserts (ER3A, Etymotic Research, Illinois). Vowel stimuli were of 300 ms duration with each token used as the standard (85%, range 530-552 occurrences) or deviant (15%, range 94-105). Stimulus onset asynchrony was 700ms [24]. Two sessions with the vowels alternating as standard/deviant were conducted for matched token subtraction (i.e., deviant /u/–standard /u/ and deviant /a/-standard /a/).
MEG recording
MEG data were obtained in a magnetically shielded room using a 275-channel whole-cortex CTF magnetometer (CTF MEG, Coquitlam, Canada). At the start of the session, three head-position indicator coils were attached to the scalp to provide continuous specification of the position and orientation of the MEG sensors relative to the head. Foam wedges were inserted between the side of each subject’s head and the inside of the MEG dewar to increase subject comfort and ensure that the head remained in the same place across recording sessions [2,13]. To minimize fatigue and encourage an awake state, participants viewed a movie projected on to a screen positioned at a comfortable viewing distance. To aid identification of eye-blink activity, the electro-oculogram (EOG, bipolar oblique, upper right and lower left sites) was collected. Electrodes were also attached to the left and right collarbone for electrocardiogram (ECG) recording.
Data analysis
All analyses were performed blind to participant group [2,13]. After a band-pass filter (0.03-150Hz), EOG, ECG, and MEG signals were down sampled to 500Hz with third-order gradiometer environmental noise reduction implemented for the MEG data. Epochs 100msec pre-stimulus to 498msec post-stimulus were defined from the continuous recording. To correct for eye blinks, a typical eye blink was manually identified in the raw data including EOG for each participant. The pattern search function in BESA Research 6.1 (BESA GmbH, Germany) scanned the raw data to identify other blinks and computed an eye-blink average. An eye blink was modeled by its first component topography from principal component analysis (PCA), typically accounting for more than 99% of the variance in the eye-blink average. In addition to eye-blink activity, a heartbeat average was obtained and heartbeat activity was modeled by the first two PCA components topographies of a heartbeat average, typically accounting for more than 85% of the variance in the heartbeat average. Scanning the eye blink and heartbeat-corrected raw data, epochs with artifacts other than blinks and heartbeat were rejected by amplitude and gradient criteria (amplitude>300fT, gradients>25fT/cm).
Using all 275 channels of MEG data, determination of the strength and latency of auditory evoked field (AEF) sources in the left and right STG was accomplished by applying a standard source model to transform each participant’s raw MEG surface activity into brain space (MEG data coregistered to the MNI averaged brain) using a model with multiple sources [25,26,27]. The standard source model applied to each subject was constructed by including left and right STG dipole sources placed at Heschl’s gyrus as well as nine fixed regional sources that modeled brain background activity and served as probe sources for additional activity. The eye-blink and heartbeat source vectors derived for each participant were also included in each participant’s source model [28,29].
The final source model served as a source montage for the raw MEG [30,31]. The MEG sensor data was transformed from channel space into brain source space where the visualized waveforms represent the modeled source activities. This spatial filter disentangled the source activities of the different brain regions that overlapped at the sensor level. Of note, although the amplitude and latency of the ~100msec STG responses as well as the MMFs were obtained using a dipole source placed at a standard location, in each subject left- and right-hemisphere dipoles were individually oriented at the maximum of the M100 component. For the source analysis, a 1Hz (12dB/octave, zero phase) to 55Hz (48dB/octave, zero-phase) band-pass filter and notch filter at 60Hz (width 5Hz) were applied.
The M100 response was determined as the maximum intensity signal with appropriate scalp topography in the latency window 50-150ms. It was computed separately for standard and deviant occurrences of each token from source-space waveforms. The higher signal to noise ration of the deviant responses provides a superior estimate of the M100 latency than that of the standard response, although no systematic differences were resolved. MMF components were defined from the difference wave obtained by subtraction of the standard response from the deviant response for each token, with the MMF peak identified as the first peak following the residual M100 in the subtracted waveform (occurring ~250ms post stimulus onset). MMF responses were thus defined for each token and each hemisphere separately.
To evaluate hemisphere laterality; LIs was computed using the following formula, , where LH and RH represent MMF amplitude in the left and right hemispheres, respectively.
Potential effects of group, hemisphere and token on M100 and MMF latency and amplitude were evaluated with linear mixed models (LMMs) using these factors and age as a covariate (allowing group×age interaction). Potential influence of language ability (VIQ) and autism severity (SRS-2) was considered by incorporating these measures as additional covariates. LIs were assessed using a LMM with group and token as fixed factors and subject as a random effect. If there were significant main effects, we performed post-hoc tests. Bonferroni correction was applied for multiple comparison. We calculated effect size (r) using the F ratio and number of degrees of freedom (df). Potential associations between M100 and MMF latency were evaluated via a hierarchical linear regression including age. All statistical analyses were performed with SPSS Statistics Version 25 (IBM, Armonk, USA).
Results
Demographics
Forty-four male adults (26.05±7.12yrs) entered the study. Nineteen participants were excluded from final analysis: four (n=2 ASD; n=2 TD) participants who were left-handed assessed by Edinburgh Handedness Inventory [32] and fifteen participants who did not complete or have analyzable MEG (n=7 ASD; n=4 TD) or meet inclusion criteria on neurophysiological assessments (n=4 recruited as ASD but who did not meet ASD criteria). Characteristics of participants included in the final analysis are shown in Table 1. As expected, there were group differences in SCQ (t=8.27, p<0.01), SRS-2 total score (t=6.10, p<0.01) and ABCL total problem score (t=4.56, p<0.01). Group differences in age, FSIQ, VIQ and PIQ were not statistically significant (p’s>.05).
Table 1.
Characteristics of study participants.
| Typically Developing Control (Mean, SD) |
Autism Spectrum Disorder (Mean, SD) |
|
|---|---|---|
| Number of participants | 16 | 9 |
| Gender | Male | Male |
| Age | 27.25±6.63 | 22.22±5.74 |
| SCQ | 2.89±3.62 | 18.13±5.03 * |
| SRS - 2 Adult - informant | ||
| Report | 40.57±2.82 | 69.11±11.92 * |
| WASI - II | ||
| FSIQ | 114.10±16.48 | 106.22±15.12 |
| VIQ | 119.50±19.70 | 101.44±17.42 |
| PIQ | 105.70±12.71 | 109.89±13.33 |
| CELF 4 Core language index | - | 90.17±18.27 |
| ABCL Total problems | 41.00±3.42 | 59.00±7.67 * |
ASD: autism spectrum disorder. SCQ: social communication questionnaire. SRS-2 Adult-Informant Report: Social responsiveness scale −2nd Edition Adult - Informant Report. WASI-II: The Wechsler Abbreviated Scale of Intelligence – second edition. FIQ: full intelligence quotient. VIQ: verbal intelligence quotient. PIQ: performance intelligence quotient. CELF-4: Clinical Evaluation of Language Fundamentals – 4th edition. ABCL: Adult Behavior Checklist.
p < 0.05 for comparison between groups.
M100 / MMFs latencies and amplitude
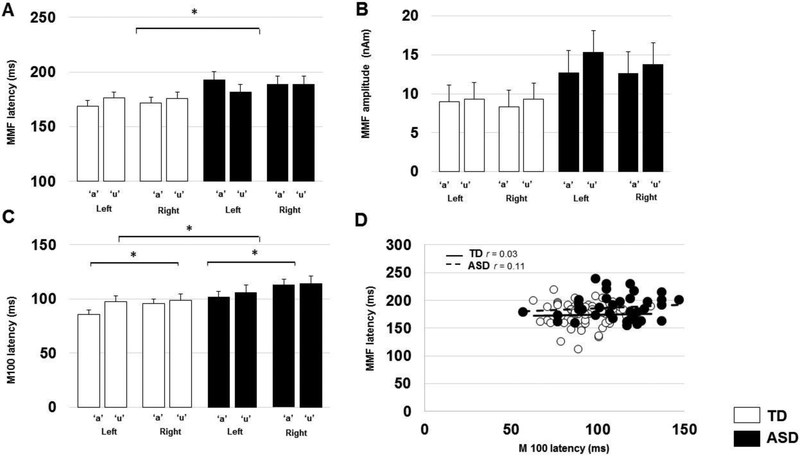
Example MMF waveforms are shown in Figure 1. A LMM with factors of group, hemisphere and token and age, as well as allowing an age×group interaction, showed a significant effect of group on MMF latency (TD=173.08+/−4.31ms; ASD=188.9+/−5.8 ms; p<0.05, r=.33), with no effect of hemisphere (LH:179.80+/−3.8ms; RH:182.18+/−3.8 ms; p>0.45,r=.06) or token (/a/: 181.36+/−3.8ms; /u/: 180.6+/−3.8ms; p>0.80,r=.02) and no interaction (p>0.20, r=.09), indicating ASD showed significantly delayed MMF latency across both hemisphere and token (p<0.05, Figure2-A).
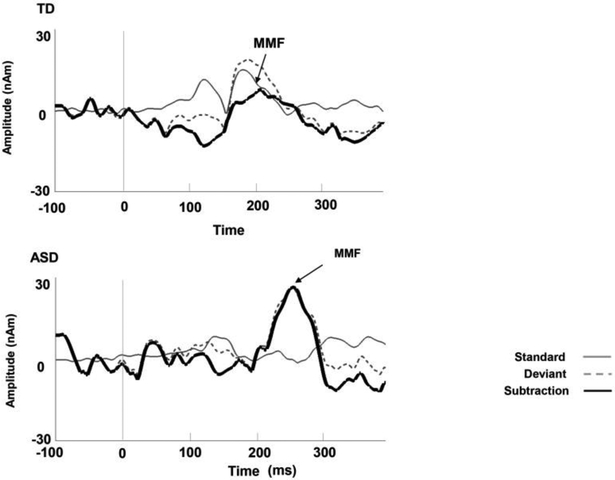
Figure 1.
Example waveforms from right superior temporal gyrus in representative individuals with (a) typical development, (b) ASD. Vertical lines on the waveform indicated stimulus onset (zero ms). Arrows indicated MMF peak (TD: MMF peak latency 190ms, amplitude 7.7nAm, ASD: MMF peak latency 224ms, amplitude 26.78nAm). As shown in right figure, gray line indicated standard, dashed gray line indicated deviant and black line indicated subtraction.
Figure 2.
(A) Estimated marginal means MMF latencies by group, hemisphere and token. Error bars represent one standard error of the marginal means. There is a significant (asterisk: p<0.05) main effect of group on MMF latency, indicating ASD showed significantly delayed MMF latency across both hemispheres and tokens (p<0.05). There were no main effects of hemisphere or token.
(B) Estimated marginal means of MMF amplitude by group and token. Error bars represent one standard error of the marginal means. Although MMF amplitude appears to tend to be greater in ASD compared to TD, there are in fact no statistically significant main effects of group, hemisphere or token.
(C) Estimated marginal mean M100 latencies by group, hemisphere and token. Error bars represent one standard error of the marginal means. There are significant main effects of group and hemisphere on M100 latency, indicating ASD showed significantly delayed M100 latency across hemispheres and tokens (p<0.05) as well as that, in general, for either group, right hemisphere responses were slightly later than left hemisphere responses in this study.
(D) Grouped scatter plot of MMF latencies vs M100 latencies, depicting no significant association within either TD or ASD groups across hemispheres and tokens (p<0.05). While both M100 and MMF latencies are in general delayed in ASD compared to TD, these delays appear to be decoupled from each other.
Adults with ASD tended to show increased MMF amplitude, however, there were no significant main effects of group (TD:9.6+/−1.5nAm; ASD:12.4+/−2.1nAm; p=0.11, r=.27, Figure 2-B) or hemisphere (LH:11.30+/−1.4nAm; RH: 10.8+/−1.4nAm; p>0.68, r=.04) or token (/a/: 10.5+/−1.4 nAm; /u/: 11.6+/−1.4nAm; p>0.41, r=.07) and no interaction between group, hemisphere and token (p>0.70,r=.03).
As shown in Figure 2-C, for M100 latency to deviant stimuli, there were also significant effects of group (TD=94.60+/−3.0ms; ASD=108.59+/−4.1ms; p<0.05, r=.47), hemisphere (LH:94.60+/− 2.7ms; RH:108.59+/−2.7ms; p<0.05, r=.24), and token (/a/:98.90+/− 2.7ms; /u/: 104.30+/− 2.7ms; p>0.05, r=.15) and no interaction on group, hemisphere and token (p>0.70,r=.07), indicating that adults with ASD showed delayed M100 latency across hemispheres and tokens (p<0.05). There was no association between MMF latency and M100 latency (to deviant token) across hemispheres (TD: r=0.03; ASD: r=0.11; p’s>0.5, Figure2-D). There were also no associations between MMF latency and dimensional scores of autism severity (SRS-2 total score) or language ability (CELF4,VIQ, p’s>0.05) in the ASD cohort.
Laterality Indices
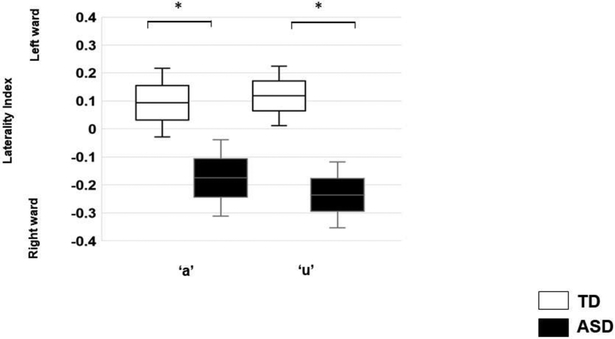
Adults with ASD showed right-ward LIs derived from hemispheric MMF amplitudes compared with TD, indicating a lack of typical left-ward lateralization (TD: +0.16+/−.076; ASD:−0.20+/− 0.10; p<0.05,r=.34, Figure 3). There was no effect of token (/a/:−0.04+/−0.08; /u/:−0.05+/− 0.08; p>0.05,r=.02) and no interaction (p>0.05, r=.05).
Figure 3.
Estimated marginal mean laterality indices derived from MMF amplitude, by group and token. Error bars represent one standard error of the marginal means. Adults with ASD showed rightward (-ve) LIs compared to TD (p<0.05).
Discussion
The main finding of this study is the observation of a delayed magnetic mismatch field in adults with ASD compared to typically-developing peers. MMF is a neurophysiological index of auditory sensory memory and discrimination processes [6,7]. Consequently, we interpret the delay in MMF observed in this study to reflect impaired processing of the auditory stream in adults with ASD. Similar results have been observed in studies of children with ASD [2,13,24] and this study suggests that these delays persist into adulthood and are not resolved during child-adolescent development. Whether the findings are unique to the auditory system or reflect an index of more generalized neural processing deficits is beyond the present scope, but at least it appears that neural activity in auditory cortex and related areas of superior temporal gyrus is compromised in these patients.
The mechanism underlying delay in the MMF remains unclear. However, several studies have reported analogous delays in earlier auditory processing responses (e.g. M50 and M100) in ASD [22,23,36]. For example, Roberts et al [22] reported delayed M100 STG latency in children with ASD, suggesting M100 latency delay indicated disruption of encoding simple sensory information and that maturation of cortical auditory systems in children with ASD may follow a differential path compared to typically developing children. We could therefore speculate that the delay in MMF observed in the present study in adults with ASD also arises from anomalies in the maturation of the underlying white matter pathways including not only the auditory sensory pathway, but all connections subserving subsequent processing. In fact, while both are delayed, the decoupling of delays in M100 latency and MMF latency in this study rather supports the additional anomalous neural activity in the circuitry subsequent to early sensory responses giving rise to MMF delays which are independent of earlier M100 delays.
Findings of increased MMFs amplitude (although not quite attaining significance, p=0.11), may be interpreted in light of knowledge that N methyl D -aspartate (NMDA) receptor-related neurotransmission is crucial for MMF activation [33]. Schmidts et al., [34] indicated that ketamine (which is an NMDA receptor antagonist) selectively reduces the normal increase in synaptic plasticity in STG in response to deviant tones. Based on the previous findings, dysfunction of NMDA receptors may also contribute to the tendency towards increased MMF amplitude in adults with ASD.
Another finding was a lack of leftward MMF amplitude lateralization in the adults with ASD. In fact a laterality index constructed from the hemispheric MMF amplitudes was significantly rightward in bias, opposite to that observed in typically developing adults. This rightward pattern is consistent with prior reports of abnormal hemispheric asymmetry in children with ASD with both structural [11,35] and functional [10] measures identifying anomalies in the right hemisphere.
Taken together, we speculate that abnormal MMF responses in adults with ASD and characterized by delayed MMF latency, increased MMF amplitude and rightward LIs in STG relate to atypical white matter maturational trajectories, perhaps not only implicating anterograde conduction but also interhemispheric inhibition [36]. Candidate anomalies of maturation could be atypical myelination, atypical axon diameter and/or atypical fiber density, leading to reduced conduction velocity, or impaired synaptic function. Whether increased rightward lateralization in ASD represents an attempt to achieve compensatory function remains unclear. To examine this speculative hypothesis, further studies are needed perhaps with longitudinal designs. The different relative contributions of MMF delay, MMF amplitude and MMF lateralization, while all pointing to atypically developing cortical/subcortical systems might be taken to characterize individual differences across the spectrum of adults with ASD and thus might provide a mechanistically-driven basis for stratification of subtypes. These measures could also provide objective markers of atypical brain development underlying auditory/language discrimination in ASD. Interestingly, unlike previous reports in children with ASD, we did not observe a correlation between delayed MMF latency and clinical assessment of language impairment (CELF-4 core language index). While this could be attributed to a small sample size, it could also be that during development and adulthood compensatory processes / strategies have evolved to improve language function, despite the neurophysiological (MMF) evidence of atypical vowel discrimination.
Some study limitations should be acknowledged. First, our evaluable participant pool was limited because of the problems of artifacts or incomplete recording and assessment. Therefore, sample size is insufficient to adequately probe associations with general cognitive function as well as dimensional assessments of ASD or language function. Second, we did not examine associations between MMF response and other modalities (for example diffusion tensor imaging and/or GABA magnetic resonance spectroscopy). Thirdly, in common with almost the entire literature on mismatch negativity [37], we conducted our experiments with the subjects not actively attending to the auditory stimuli, but rather watching a silent movie. There is considerable evidence for the MMN (and its magnetic counterpart the MMF) reflecting pre-attentive, obligate activity and thus not being influenced by the visual distraction. Certainly, the viewing of a silent movie reduces the participants’ symptoms of fatigue and helps maintain a steady head position, improving resultant data quality. Nonetheless we cannot rule out the possibility of some modulation of auditory cortex neural activity by the visual stimulus of the movie. That said, both ASD and typically-developing cohorts were scanned under identical movie-watching conditions and so the relative delays observed herein cannot likely be attributed to the movie itself.
Conclusion
This study demonstrated delayed MMF responses and a reversal (i.e. towards right-ward) of typical left-ward amplitude lateralization in adults with ASD. These MMF findings are largely independent of delays in earlier response components, as evidenced by a lack of correlation between M100 latency and MMF latency. These findings provide evidence of the adult consequences of atypical brain development in ASD.
Highlights:
Delayed vowel discrimination process in adults with autism spectrum disorder
Abnormal rightward lateralization in adults with autism spectrum disorder
Delayed MMF response was decoupled from the earlier auditory M100 response delay
Acknowledgements:
Dr Roberts and authors gratefully acknowledge all participants, their families and the Oberkircher family for the Oberkircher Family Chair in Pediatric Radiology. Excellent technical assistance was provided by Rachel Golembski, Peter Lam and the MEG lab team, Department of Radiology at CHOP.
Funding: This study was supported by a maturational human biology grant (TR/EB) from ITMAT at UPenn (supported by UL1RR024134) as well as NIH R01DC008871 (TR) and the institutional IDDRC (U54-HD 086984).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: Dr Roberts declares consulting/advisory board relationships with Prism Clinical Imaging, CTF, Ricoh and Spago Nanomedical. Additionally, he discloses intellectual property related to MEG as a biomarker for pharmaceutical therapy, under license to MEGIN. Dr. Berman declares a consulting relationship with McGowan Associates.
References
- [1].Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Association. Washington, DC: American Psychiatric Publishing, Inc; 947; (2013). [Google Scholar]
- [2].Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, Woldoff S, Qasmieh S, Gandal M, Schmidt GL, Zarnow DM, Levy SE, Edger JC. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 1 (2010) 8–18. 10.1002/aur.lll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berman JI, Edgar JC, Blaskey L, Kuschner ES, Levy SE, Ku M, Dell J, Roberts TP. Multimodal Diffusion-MRI and MEG Assessment of Auditory and Language System Development in Autism Spectrum Disorder. Front Neuroanat. 10 (2016) 30 10.3389/fnana.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Näätänen R, Gaillard AWK, Mäntysalo S, Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 42 (1978) 4, 313–329. 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz S, Shinn-Cunningham B, Tager–Flusberg H. Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci Biobehav Rev. 87 (2018) 106–117. 10.1016/j.neubiorev.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alho K, Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 16 (1995) 38–51. 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- [7].Näätänen R, Sussman ES, Salisbury D, Shafer VL. Mismatch negativity (MMN) as an index of cognitive dysfunction. Brain Topogr. 27 (2014) 451–466. 10.1007/s10548-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ford TC, Woods W, Crewther DP. Mismatch field latency, but not power, may mark a shared autistic and schizotypal trait phenotype. Int J Psychophysiol. 116 (2017) 60–67. 10.1016/j.ijpsycho.2017.02.008. [DOI] [PubMed] [Google Scholar]
- [9].Oram Cardy JE, Flagg EJ, Roberts EJW, Roberts TP. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 16:5 (2005) 521–525. 10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- [10].Flagg EJ, Oram Cardy JE, Roberts W, Roberts TP. Language lateralization development in children with autism: insights from the late field magnetoencephalogram. Neurosci Lett. 386: 2 (2005) 82–87. 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- [11].Herbert MR, Ziegler DA, Deutsch CK., et al. , Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 128 (2005), 213–226. 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- [12].Gallagher A, Tanaka N, Suzuki N., et al. , Decreased language laterality in tuberous sclerosis complex: A relationship between language dominance and tuber location as well as history of epilepsy. Epilepsy Behav. 2012:25:36–41. 10.1016/j.yebeh.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roberts TP, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF, Qasmieh S, Levy SE, Edge JCr. Auditory Magnetic Mismatch Field Latency: A Biomarker for Language Impairment in Autism. Biol Psychiatry. 70:3 (2011) 263–269. 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL, Luyster RJ, Guthrie W. Autism Diagnostic Observation Schedule, second edition. Los Angeles, CA: Western Psychological Services; (2012). [Google Scholar]
- [15].Rutter M, Bailey A, Lloyd C. SCQ: Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; (2003). [Google Scholar]
- [16].Constantino J, Gruber CP. Social Responsiveness Scale, second edition. Los Angeles, CA: Western Psychological Services; (2012). [Google Scholar]
- [17].Hurley RSE, Losh M, Parlier M, Reznick JSJS, Piven J. The broad autism phenotype questionnaire. J Autism Dev Disord. 37:9 (2007) 1679–90. 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- [18].Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview - Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24 (1994) 659–685. 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- [19].Wechsler D. Wechsler Abbreviated Scale of Intelligence – second edition. San Antonio, TX: NCS Pearson; (2011). [Google Scholar]
- [20].Semel E, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals - Fourth Edition. San Antonio, TX: The Psychological Corporation; (2003). [Google Scholar]
- [21].Achenbach TM, Rescorla LA. Adult Behavior Checklist. Burlington, VT: ASEBA; (2003). [Google Scholar]
- [22].Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, Woldoff S, Qasmieh S, Gandal M, Schmidt GL, Zarnow DM, Levy SE and Edger JC. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 3: (2010) 8–18. 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Matsuzaki J, Kagitani-Shimono K, Sugata H, Hanaie R, Nagatani F, Yamamoto T, Tachibana M, Tominaga K, Hirata M, Mohri I and Taniike M. Front. Hum. Neurosci 11 (2017) 446 10.3389/fnhum.2017.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roberts TP, Heiken K, Kahn SY, Qasmieh S, Blaskey L, Solot C, Parker WA, Verma R, Edger JC. Delayed magnetic mismatch negativity field, but not auditory M100 response, in Specific Language Impairment. Neuroreport. 23:8 (2012) 463–468. 10.1097/WNR.0b013e32835202b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scherg M. Fundamentals of dipole source potential analysis In: Gandori MHGLR, editor. Auditory evoked magnetic fields and electric potentials. Advances in audiology (6th ed.). Basel, Switzerland: Karger; (1990) pp. 40–69. [Google Scholar]
- [26].Scherg M, Berg P. New concepts of brain source imaging and localization. Electroencephalogr Clin Neurophysiol Suppl, 46 (1996) 127–137. [PubMed] [Google Scholar]
- [27].Scherg M, von Cramon D. A new interpretation of the generators of BAEP waves I–V: results of a spatio-temporal dipole model. Electroencephalogr Clin Neurophysiol. 62:4 (1985) 290–9. 10.1016/0168-5597(85)90006-1. [DOI] [PubMed] [Google Scholar]
- [28].Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 90 (1994), 229–241. 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- [29].Lins OG, Picton TW, Berg P, Scherg M. Ocular artifacts in recording EEGs and event-related potentials. II:source dipoles and source components. Brain Topogr. 6 (1993) 65–78. 10.1007/BF01234128. [DOI] [PubMed] [Google Scholar]
- [30].Scherg M, Ebersole JS. Brain source imaging of focal and multifocal epileptiform EEG activity. Neurophysiol Clin. 24 (1994) 51–60. 10.1016/S0987-7053(05)80405-8. [DOI] [PubMed] [Google Scholar]
- [31].Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: virtual source montages, wholehead mapping, correlation, and phase analysis. J Clin Neurophysiol, 19 (2002) 91–112. 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- [32].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:1 (1971) 97–113. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- [33].Ehrlichmann RS, Luminais SN, White SL, Rudnick ND, Ma N, Dow HC, Kreibich AS, Abel T, Brodkin ES, Hahn CG, Siegel SJ. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res. 1294 (2009) 116–127. 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schmidt A, Diaconescu AO, Kometer M, Friston KJ, Stephan KE, Vollenweider FX. Modeling ketamine effects on synaptic plasticity during the mismatch negativity. Cereb Cortex. 23 (2013) 2394–2406. 10.1093/cercor/bhs238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Herbert MR., Harris GJ, Adrien KT., Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, Takeoka M, Tager - Flusberg H. and Caviness VS Jr. Abnormal Asymmetry in Language Association Cortex in Autism. Ann Neuron. 25 (2002) 588–596. 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- [36].Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. The cellular basis of GABA (B) - mediated interhemispheric inhibition. Science. 335:6071 (2012) 989–993. 10.1126/science.1217276. [DOI] [PubMed] [Google Scholar]
- [37].Näätänen R. The mismatch negativity: a powerful tool for cognition neuroscience. Ear Hear. (1995) 16:1:6–18. [PubMed] [Google Scholar]