Abstract
Background.
Smoking constitutes a significant public health risk. Alcohol and methamphetamine use disorders are also highly co-morbid with smoking, further increasing negative health outcomes. An important question in determining the underlying neurobiology of nicotine poly-drug use is understanding whether having a positive history with nicotine effects alters later drug-taking behavior.
Methods.
The current experiments sought to elucidate whether having an appetitive nicotine conditioning history would affect later alcohol or methamphetamine self-administration. Adult male and female Long-Evans rats were first trained on a discriminated goal-tracking task in which the interoceptive effects of nicotine predicted sucrose reinforcement. As a control, pseudo-conditioned groups were included that had equated nicotine and sucrose experience. Rats were then shifted to either alcohol self-administration or methamphetamine self-administration.
Results.
Nicotine conditioning history had no effect on acquisition or maintenance of alcohol self-administration in males or females. In contrast, an appetitive nicotine conditioning history decreased methamphetamine self-administration in female rats, but not males.
Conclusions.
In female, but not male rats, an appetitive conditioning history with nicotine decreases methamphetamine, but not alcohol, self-administration. This dissociation suggests that the effects may be due to a specific increase in the reinforcing value of methamphetamine. This may have implications for better understanding the progression of drug use from nicotine to methamphetamine.
Introduction
Smoking continues to be the leading cause of preventable death, carrying not only a significant health risk to individuals but also a tremendous public health cost annually (CDC 2014). Compounding this issue is evidence that smoking is highly co-morbid with other drugs of abuse including alcohol and methamphetamine. Indeed, as many as 80% of adults with an alcohol use disorder (AUD) and 97% of methamphetamine users smoke (Brecht et al. 2004; Chatterjee and Bartlett 2010). Despite high rates of tobacco use, nicotine alone has been shown to have weak primary reinforcing properties (Caggiula et al. 2009; Rose 2006). However, previous studies suggest that nicotine may act to enhance other reinforcers that in turn maintain nicotine use. For example, nicotine enhances responding to cues related to presentation of drug rewards, non-drug rewards, and brain stimulation (Arregui-Aguirre et al. 1987; Barrett et al. 2017; Chaudhri et al. 2006; Kenny et al. 2009; Olausson et al. 2004; Palmatier et al. 2007a; Paterson et al. 2008). This leads to the question of the role that experience with nicotine may play in initiating the use of other drugs such as alcohol or methamphetamine.
There is ample evidence in both humans and preclinical animal models that drug-seeking behavior is influenced by drug-associated cues. While these cues are often external/contextual, there is a great deal of interest in the role of interoceptive cues. That is, the interoceptive effects of a drug that become associated with other rewarding events (Bevins and Besheer 2014). Indeed, previous work from our laboratories have demonstrated that reward-seeking behavior can come under the control of drug interoceptive cues (Charntikov et al. 2014; Charntikov et al. 2017b; Murray and Bevins 2007a; b; 2009; Pittenger and Bevins 2013; Randall et al. 2016).
The purpose of the present work was to assess the impact of appetitive nicotine conditioning history on initiating self-administration of alcohol (Experiment 1) or methamphetamine (Experiment 2). To do so, a discriminated goal-tracking task was used in which the interoceptive effects of nicotine signaled whether sucrose would be presented non-contingently throughout the session. While there is a rich literature showing that reward-related cues can influence later drug taking behavior, these studies tend to depend on external cues, not internal drug-states. However, one such study by Cortright and colleagues (2012), demonstrated that male rats pre-exposed to nicotine through either contextual conditioning or through drug discrimination training, enhanced later self-administration of amphetamine in the absence of nicotine. Based on this, we hypothesized that an explicitly appetitive conditioning history with nicotine would enhance subsequent acquisition and maintenance of drug self-administration beyond that of rats without this conditioning history but with equal exposure to nicotine. Additionally, the current experiments were conducted in male and female rats in parallel. Given that females have been shown to self-administer more alcohol and methamphetamine than males (Randall et al., 2017; Roth and Carroll, 2004), we hypothesized that any enhancement from nicotine conditioning history would be evident in female, not male rats.
Materials and Methods
Subjects
Adult Long-Evans rats (Envigo-Harlan) were used in these experiments. Experiment 1: n=42 males / 42 females. Experiment 2: n = 22 males / 22 females. Rats were approximately 7 weeks old upon delivery and were food restricted to maintain ~90% body weight. Water was available ad libitum in the home cage. The vivariums were maintained on a 12-h light/dark cycle, and experiments were conducted during the light cycle. Experiment 1 was conducted at the University of North Carolina – Chapel Hill and Experiment 2 was conducted at the University of Nebraska – Lincoln. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the respective institution and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Apparatus
All experiments were conducted in standard operant conditioning chambers (31 × 32 × 24 cm; Med Associates, Georgia, VT) located within light-attenuating cubicles equipped with an exhaust fan that provided both ventilation and masking of external sounds. Chambers were fitted with a house light to provide general illumination. In both experiments, a liquid dipper receptacle was centered on the right wall for nicotine interoceptive conditioning phase. A 0.1-ml metal cup was attached to the dipper arm. For the alcohol self-administration phase of Experiment 1, chambers were fitted with a retractable lever on the left wall and a white cue light (2.54 cm diameter; 28V, 100mA) was centered 7-cm above the lever. A liquid receptacle was centered on that wall and located to the left of the lever. Lever responses activated a syringe pump (Med Associates) that delivered 0.1 ml of solution into the receptacle during a 1.66-s period. The white cue light located above the lever was illuminated during pump activation. During the initial nicotine interoceptive conditioning phase, a metal panel was placed to cover the entire left wall of the chamber to block entry into the liquid receptacle where alcohol self-administration would later be trained. Conversely, during the alcohol self-administration phase, a metal panel covered the entire right side of the chamber to block entry into the liquid dipper receptacle. In Experiment 2, the chambers were fitted with two retractable levers on either side of the centered liquid dipper receptacle on the right wall. A white cue light (2.54 cm diameter; 28V, 100mA) was centered 7-cm above each lever, 14.6 cm above the rod floor, and 3.5 cm from the nearest polycarbonate wall. The outside of each chamber was fitted with a balanced metal arm and liquid swivel. An attached spring leash hung into the chamber through the center of the ceiling. Tygon tubing extended through the leash and was connected to a 20-ml syringe mounted on an infusion pump (Med Associates) located outside of the sound-attenuating cubicle. During the nicotine interoceptive conditioning phase, levers were retracted (i.e., not available within the chamber); during the methamphetamine self-administration phase, a metal panel was inserted to block entry into the liquid dipper receptacle. For both experiments, all chambers were interfaced to a computer and data collection and presentation of experimental events was controlled with Med Associates Interface and Software.
Drugs
(−)-Nicotine hydrogen tartrate (Sigma, St. Louis, MO, USA) and dissolved in 0.9% saline and brought to a pH of 7.4±0.2. Nicotine (0.4 mg/kg) was administered subcutaneously (SC) at a volume of 1 ml/kg. Alcohol (95%, Pharmaco-AAPER, Shelbyville, KY) was diluted (v/v) in distilled water along with sucrose (w/v) to achieve the desired concentration. D-methamphetamine hydrochloride (Sigma) was dissolved in 0.9% sterile saline and administered intravenously (IV) at a dose of 0.05 mg/kg/infusion and a rate of 0.04 ml/second based on each rat’s individual weight.
Nicotine Interoceptive Conditioning (Phase 1 for Experiments 1 and 2)
In both experiments, rats were randomly assigned to the nicotine conditioned stimulus (CS) trained group or the pseudoconditioning CS (pseudo-CS) control group. For both groups, rats received nicotine (0.4 mg/kg) or saline injections 5 min prior to each 20-min session. The CS group was trained such that on nicotine sessions there were 36 sucrose presentations (26% w/v, 0.1 ml, 4-s access), with no sucrose presentation occurring less than 90 seconds from the start of the session. There were no sucrose presentations on saline sessions. The pseudo-CS group received sucrose presentations on half of the nicotine sessions and half of the saline sessions. For both groups, training sessions were randomly assigned for each rat with the limitation that no more than two of the same session type (sucrose; no-sucrose) or drug injection (nicotine; saline) were presented in a row. The rate of head entries into the sucrose receptacle before the first sucrose delivery (dipper entries per second) was the measure for conditioning. Rate of entries into the sucrose receptacle during the equivalent interval were also measured on sessions in which no sucrose was delivered. Training proceeded for 32 sessions (16 nicotine/16 saline; 16 sucrose/16 no-sucrose).
Experiment 1: Alcohol Self-Administration (Phase 2)
At the conclusion of nicotine interoceptive conditioning, rats began the alcohol self-administration phase. For alcohol self-administration, all reinforcers are delivered in the liquid receptacle on the left side of the chamber (opposite from the liquid dipper used for the interoceptive conditioning phase). A sucrose-fading procedure was used to train alcohol self-administration in which alcohol was gradually added to a 10% (w/v) sucrose solution. The exact order of fading was as follows: 10% (w/v) sucrose (10S), 2% (v/v) alcohol/10% (w/v) sucrose (2A/10S), 5A/10S, 10A/10S, 10A/5S, 10A/2S, 10A. There was one session at each concentration. Following sucrose fading, a 10% alcohol (v/v) solution was the reinforcer for the remainder of the study. Alcohol self-administration sessions (30 min) were conducted 5 days per week (M-F). The alcohol lever was maintained on a fixed ratio 2 (FR2), such that every 2nd response on the lever resulted in the activation of a syringe pump (Med Associates) that delivered 0.1 ml of alcohol. Rats underwent 14 sessions of alcohol self-administration.
Experiment 2: Methamphetamine Self-Administration (Phase 2)
At the conclusion of nicotine interoceptive conditioning, rats underwent surgery to implant a jugular catheter for intravenous methamphetamine self-administration. Rats were anaesthetized with a 1 ml/kg intramuscular injection of a ketamine hydrochloride (100 mg/ml): xylazine hydrochloride (20 mg/ml) cocktail (Midwestern Veterinary Supply, Des Moines, IA, USA). Tubing (SAI Infusion Technologies, Lake Villa, IL) of a silastic catheter constructed in house was implanted into the right external jugular vein. The tubing was threaded subcutaneously over the shoulder and connected to a metal cannula fitted within a polycarbonate back plate (Plastics One, Roanoke, VA) implanted under the skin just below the scapula. Buprenorphine hydrochloride (0.1 mg/kg; Sigma) was injected subcutaneously immediately following surgery and once per day for two more days for pain management. Catheters were flushed daily with 0.1 ml sterile saline mixed with heparin (30 U/ml; Midwest Veterinary Supply) and Baytril (5.0 mg/ml; Midwest Veterinary Supply; Lakeville, MN). Rats were allowed 7 days of recovery before beginning methamphetamine self-administration.
Methamphetamine Self-Administration Training.
Following surgical recovery, all rats entered the methamphetamine self-administration phase. At the start of each 1-hr session, the house light was on and both levers were available. Lever assignment of ‘active’ and ‘inactive’ was counterbalanced across rats. Methamphetamine (0.05 mg/kg/infusion) was available under continuous reinforcement (fixed ratio schedule 1; FR1) in which one active lever press resulted in an infusion and initiated a 20-sec time out period; inactive lever pressing had no programmed consequence. During the time out, both levers were retracted, the house light was extinguished, and the cue light above the active lever was illuminated. After three FR1 sessions, the contingency was switched to a variable ratio 3 (VR3) reinforcement schedule in which, on average, every third lever press (range = 1-5) resulted in the drug and time out. Rats received 12 sessions on the VR3 schedule.
Statistical Analyses
For the nicotine interoceptive conditioning phase, the dependent variable for all sessions was head entries per second prior to the first sucrose presentation. For sessions without sucrose presentations, the program measured head entry rate during an equivalent amount of time. For sucrose fading and maintenance of self-administration, the dependent variables were alcohol lever responses and alcohol intake (g/kg) in Experiment 1 and methamphetamine infusions for Experiment 2. Sex and each phase of training were analyzed separately, and all experiments were analyzed using repeated measures analysis of variance (RM-ANOVA) with nicotine treatment, conditioning group, and session as factors. Where interactions were present, post-hoc analysis (Tukey) was used to determine differences between specific points and conditions. Significance was set at p<0.05.
Results
Experiment 1: Nicotine conditioning history does not affect alcohol self-administration
Nicotine interoceptive conditioning (Phase 1)
As shown in Figure 1 A, head entry rate on nicotine sessions was consistently greater than on saline sessions in the male nicotineCS group but not the male pseudoCS group. For the 3-way ANOVA, there was a main effect of Session (F[15,600] = 12.086, p < 0.001, ηp2 = .232), Drug (F[1,40] = 196.299, p < 0.001, ηp2 = .831), Drug by Session interaction (F[15,600] = 9.808, p < 0.001, ηp2 = .197) and Drug by Session by Conditioning History interaction (F[15,600] = 3.540, p < 0.001, ηp2 = .081). In the male nicotineCS group, there was a main effect of Session (F[15,300] = 6.451, p < 0.001, ηp2 = .244), Drug (F[1,20] = 174.353, p < 0.001, ηp2 = .897) and Drug by Session interaction (F[15,300] = 10.203, p < 0.001, ηp2 = .338). Post hoc analysis found that head entry rate was greater on nicotine sessions 3-16 compared to saline in the male nicotineCS group. In the male pseudoCS group, there was a main effect of Session (F[15,300] = 6.850, p < 0.001, ηp2 = .255), Drug (F[1,20] = 35.524, p < 0.001, ηp2 = .640) and Drug by Session interaction (F[15,300] = 3.107, p < 0.001, ηp2 = .134). Post hoc analysis found that head entry rate was only greater on nicotine sessions 4, 6-11, 14 and 16 compared to saline in the male pseudoCS group suggesting inconsistent and overall considerably less conditioning occurred compared to the male nicotineCS group.
Figure 1.
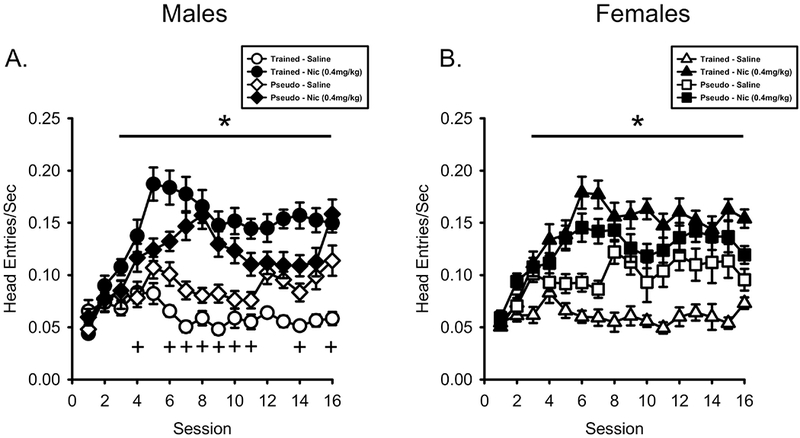
Anticipatory head entry rate was greater on nicotine sessions compared to saline sessions. A) Mean(±SEM) head entries/sec prior to the first sucrose presentation (PreUS) for male nicotineCS and pseudoCS rats. NicotineCS trained males showed higher head entry rates on nicotine sessions than saline sessions, more consistently than pseudoCS males. B) Mean(±SEM) head entries/sec PreUS for the female nicotineCS and pseudoCS rats. NicotineCS trained females showed higher head entry rates on nicotine sessions compared to saline sessions. PseudoCS trained females did not differ between nicotine and saline sessions. *p< 0.05, nicotine session higher than saline session in nicotineCS group, +p < 0.05, nicotine session higher than saline session in pseudoCS group.
As shown in Figure 1B, head entry rate on nicotine sessions was greater than on saline sessions in the female nicotineCS group. In contrast, there was no difference in head entry rate between nicotine and saline sessions in the female psuedoCS group. In the 3-way ANOVA, there was a main effect of Session (F[15,600] = 10.977, p < 0.001, ηp2 = .215), Drug (F[1,40] = 182.322, p < 0.001, ηp2 = .820), Drug by Session interaction (F[15,600] = 5.637, p < 0.001, ηp2 = .124) and Drug by Session by Conditioning History interaction (F[15,600] = 2.551, p = 0.001, ηp2 = .060). In the female nicotineCS group, there was a main effect of Session (F[15,300] = 6.317, p < 0.001, ηp2 = .240), Drug (F[1,20] = 518.168, p < 0.001, ηp2 = .963), and Drug by Session interaction (F[15,300] = 8.247, p < 0.001, ηp2 = .292). Post-hoc analysis found that head entry rate was greater nicotine sessions 3-16 compared to saline. In the female pseudoCS group, there was a main effect of Session (F[15,300] = 5.755, p < 0.001, ηp2 = .223) and Drug (F[1,20] = 13.115, p = 0.002, ηp2 = .396), but no Drug by Session interaction (F[15,300] = 1.010, p = 0.445, ηp2 = .048).
Sucrose Fading
As shown in Figure 2A, males in both training groups showed an initial increase in lever responses as alcohol was added to the solution and decreased as sucrose was removed from the solution. There was a main effect of alcohol/sucrose Concentration (F[5,200] = 63.922, p < 0.001, ηp2 = .615) but no Concentration by Conditioning History interaction (F[5,200] = 0.532, p = 0.752, ηp2 = .013). Alcohol intake (g/kg) showed a similar pattern to lever responses (Figure 2B). There was a main effect of Concentration (F[5,200] = 122.130, p < 0.001, ηp2 = .753) but no Concentration by Conditioning History interaction (F[5,200] = 0.285, p = 0.921, ηp2 = .007).
Figure 2.
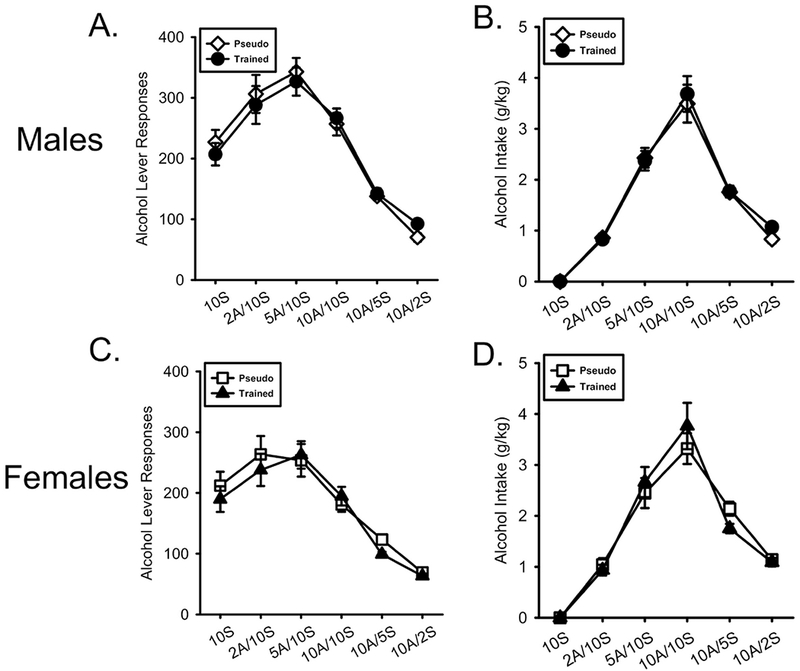
Nicotine conditioning history did not affect acquisition of alcohol self-administration. A/B) Mean(±SEM) alcohol lever responses and alcohol intake (g/kg) respectively in male nicotineCS and pseudoCS groups. C/D) Mean(±SEM) alcohol lever responses and alcohol intake (g/kg) respectively in the female nicotineCS and pseudoCS groups. Alcohol intake did not differ between any group.
As shown in Figure 2C, similar to males, females in both training groups showed an initial increase in lever responses as alcohol concentration increased and then decreased as sucrose concentration decreased. There was a main effect of alcohol/sucrose Concentration (F[5,200] = 47.636, p < 0.001, ηp2 = .544) but no Concentration by Conditioning History interaction (F[5,200] = 0.622, p = 0.683, ηp2 = .015). Similar effects were observed with alcohol intake (Figure 2D) with a main effect of Concentration (F[5,200] = 94.774, p < 0.001, ηp2 = .703) but no Concentration by Conditioning History interaction (F[5,200] = 1.212, p = 0.305, ηp2 = .029).
Maintenance of Alcohol Self-administration
As shown in Figure 3A, alcohol lever responses increased across sessions in both groups of males. There was a main effect of Session (F[13,520] = 4.927, p < 0.001, ηp2 = .110) but not Session by Conditioning History interaction (F[13,520] = 0.570, p = 0.878, ηp2 = 0.014). Similarly, alcohol intake (Figure 3B) increased across sessions but did not differ between training conditions. There was a main effect of Session (F[13,520] = 4.470, p < 0.001, ηp2 = .103) but no Session by Conditioning History interaction (F[13,520] = 0.545, p = 0.896, ηp2 = .014).
Figure 3.
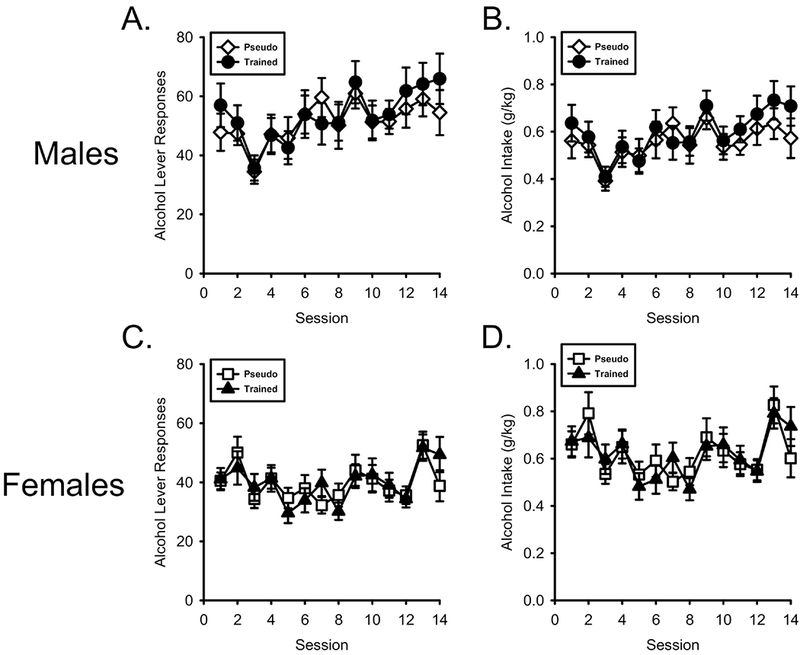
Nicotine conditioning history did not affect alcohol self-administration. A/B) Mean(±SEM) alcohol lever responses and alcohol intake (g/kg) respectively in male nicotineCS and pseudoCS groups. C/D) Mean(±SEM) alcohol lever responses and alcohol intake (g/kg) respectively in the female nicotineCS and pseudoCS groups. Alcohol intake did not differ between any group.
As shown in Figure 3C, similar to males, alcohol lever responses increased across sessions in both female groups. There was a main effect of Session (F[13,520] = 4.397, p < 0.001, ηp2 = .099) but no Session by Conditioning History interaction (F[13,520] = 0.772, p = 0.690, ηp2 = .019). Alcohol intake in females (Figure 3D) was similar was a main effect of Session (F[13,520] = 4.637, p < 0.001, ηp2 = .104) but no Session by Conditioning History interaction (F[13,520] = 0.692, p = 0.772, ηp2 = .017).
Experiment 2: Nicotine conditioning history has sex-dependent effects on methamphetamine self-administration
Nicotine interoceptive conditioning (Phase 1)
As shown in Figure 4A, head entry rate on nicotine sessions was greater in the male nicotineCS group compared to saline whereas there was no difference in the male pseudoCS group. There was a main effect of Session (F[15,300] = 4.105, p < 0.001, ηp2 = .170), Drug (F[1,20] = 45.834, p < 0.001, ηp2 = .696), Drug by Session interaction (F[15,300] = 3.811, p < 0.001, ηp2 = .160), Drug by Conditioning History interaction (F[1,20] = 37.636, p < 0.001, ηp2 = .653), and Drug by Session by Conditioning History interaction (F[15,300] = 2.502, p = 0.002, ηp2 = .111). For the nicotineCS group, there were higher head entries on sessions 4-16 [p < .05; Main effect of Drug (F[1,10] = 118.181, p < .001, ηp2 = .922); Main effect of Session (F[15,150] = 2.848, p = .001, ηp2 = .222); Drug by Session interaction (F[15,150] = 4.984, p < .001, ηp2 = .333). For the pseudoCS group, there was only a main effect of Session (F[15,150) = 2.459, p = .003, ηp2 = .197). There was no Drug effect (F<1) or Drug by Session interaction (F[15,150] = 1.240, p = .248).
Figure 4.
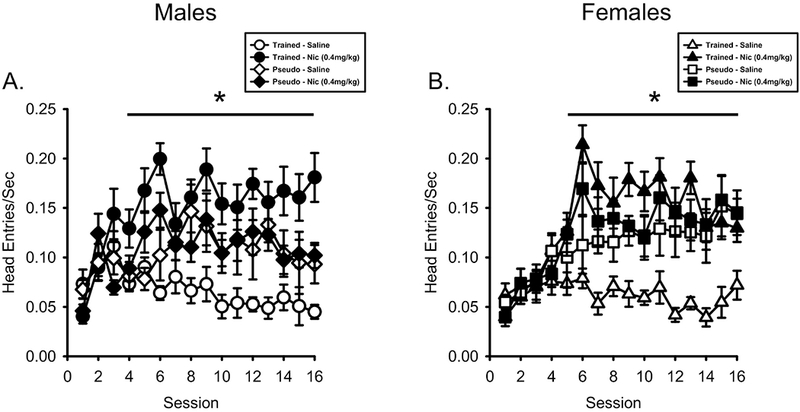
Anticipatory head entry rate was greater on nicotine sessions compared to saline sessions. A) Mean(±SEM) head entries/sec prior to the first sucrose presentation (PreUS) for male nicotineCS and pseudoCS rats. NicotineCS trained males showed higher head entry rates on nicotine sessions than saline sessions. PseudoCS trained males did not differ between nicotine and saline sessions. B) Mean(±SEM) head entries/sec PreUS for the female nicotineCS and pseudoCS rats. NicotineCS trained females showed higher head entry rates on nicotine sessions compared to saline sessions. PseudoCS trained females did not differ between nicotine and saline sessions. * p< 0.05, nicotine session higher than saline session in nicotineCS group.
As shown in Figure 4B, similar to males, head entry rate on nicotine sessions in the female nicotineCS group was greater than saline sessions. There was a main effect of Session (F[15,300] = 10.302, p < 0.001, ηp2 = .340), Drug (F[1,20] = 99.975, p < 0.001, ηp2 = .833), Drug by Session interaction (F[15,300] = 5.792, p < 0.001, ηp2 = .225), Drug by Conditioning History interaction (F[1,20] = 52.489, p < 0.001, ηp2 = .724), and Drug by Session by Conditioning History interaction (F[15,300] = 2.522, p = 0.002, ηp2 = .112). The nicotineCS females had higher head entries evoked by nicotine compared to saline on sessions 5-16 [p < .05; Main effect of Drug (F[1,11] = 119.501, p < .001, ηp2 = .916); Main effect of Session (F[15,165] = 6.102, p < .001, ηp2 = .357); Drug by Session interaction (F[15,165] = 8.580, p < .001, ηp2 = .438)]. For the pseudoCS females, there were main effects of Session (F[15,135) = 5.414, p < .001, ηp2 = .376) and Drug (F[1,9] = 6.248, p = .034, ηp2 = .410) with nicotine evoking greater head entries than saline when collapsed across sessions (Means: nicotine = 0.124±.013; saline = 0.112±.014). There was no Drug x Session interaction (F<1).
Methamphetamine Self-administration (Phase 2)
As shown in Figure 5A, previous training with nicotine as an appetitive CS did not affect methamphetamine self-administration in males. There was a main effect of Session (F[13,260] = 2.930, p = 0.001, ηp2 = .128), however there was no Session by Conditioning History interaction (F[13,260] = 0.413, p = 0.965, ηp2 = .020). In contrast, in the females, nicotine conditioning history attenuated methamphetamine self-administration (Figure 5B). In the female groups, there was a main effect of Session (F[13,260] = 2.410, p = 0.004, ηp2 = .108) and Conditioning History (F[1,20] = 15.905, p = 0.001, ηp2 = .443), but no Conditioning History by Session effect, F(13,260)=1.572, p=.093, ηp2=.073
Figure 5.
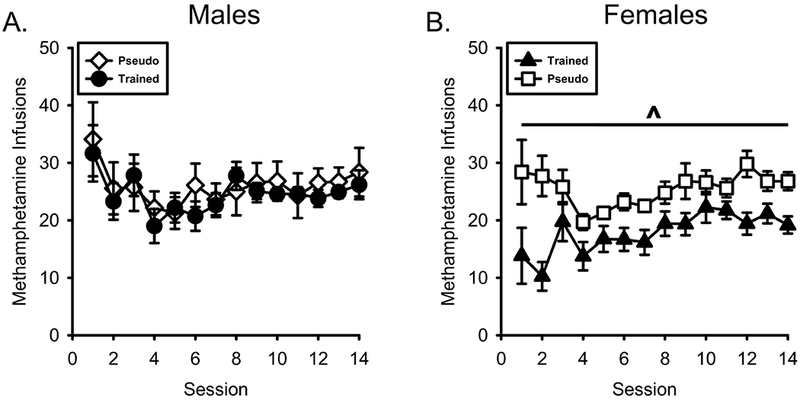
Prior appetitive conditioning history with nicotine as a CS selectively reduced methamphetamine intake in females. A) Mean(±SEM) methamphetamine infusion taken during self-administration in the male nicotineCS and pseudoCS groups. B) Mean(±SEM) methamphetamine infusion taken during self-administration in the female nicotineCS and pseudoCS groups. NicotineCS trained females self-administered less methamphetamine than the pseudoCS females. ^ - indicates significant main effect of Conditioning History, p = 0.001.
Discussion
Drug experience plays a role in subsequent drug taking. Understanding the nuances of this interaction will be crucial to understanding how drug abuse and dependence progress. However, as the current experiments demonstrate, this is not a generalizable phenomenon. As shown in Experiment 1, having a previous appetitive conditioning history with nicotine had no impact on later acquisition or maintenance of alcohol self-administration in male or female rats. In contrast, Experiment 2 found that this same nicotine conditioning history led to an overall attenuation of methamphetamine self-administration in female, but not male rats. This finding identifies an intriguing sex effect and implicates the nature of the nicotine conditioning history – rather than the nicotine itself – in the subsequent methamphetamine use.
In both Experiments 1 and 2, reward-seeking behavior readily came under control of the interoceptive stimulus effects of nicotine in the nicotineCS group. That is, rats showed significantly greater rate of anticipatory head entries on nicotine sessions compared to saline because nicotine was a salient predictor of reward. By contrast, in the pseudoCS group, neither nicotine nor saline consistently predicted reward (Charntikov et al. 2012) which resulted in most rats showing no difference in head entry rate between nicotine and saline sessions. This acquired control of head entries into the dipper receptacle in nicotineCS trained rats was evident in both males and females, and, consistent with a recent paper from our lab (Charntikov et al. 2017a).
Following the nicotine interoceptive conditioning phase, rats began alcohol (Experiment 1) or methamphetamine (Experiment 2) self-administration, and at this point, a diverging pattern emerged between rats trained to self-administer alcohol and those trained to self-administer methamphetamine. Having a nicotine conditioning history did not affect the sucrose fading phase or maintenance of alcohol self-administration in female or male rats. This finding is consistent with a previous study assessing nicotine exposure in adolescence that found that nicotine exposure alone did not enhance alcohol self-administration (Madayag et al. 2017). However, several studies have shown that nicotine administered immediately prior to alcohol self-administration sessions increases alcohol-seeking (Larraga et al. 2017; Le et al. 2014; Smith et al. 1999), suggesting that proximity of nicotine treatment to self-administration sessions is important when assessing nicotine effects on alcohol self-administration.
In contrast to the alcohol self-administration study, female, but not male rats with the nicotine conditioning history (i.e., nicotineCS group) showed an overall reduction in methamphetamine self-administration relative to their same sex cohorts without this conditioning history (pseudoCS). These results suggest that the nicotine conditioning history had a selective and significant impact on the reinforcing value of methamphetamine in the females. That is, nicotine may have enhanced the reinforcing value of methamphetamine, causing a leftward shift in the demand curve for methamphetamine in females. As such, given an increased reward value, female rats required less methamphetamine overall. Similar findings have been observed in studies of brain stimulation in which nicotine decreases frequency threshold for reinforcement (Bozarth et al. 1998a; b; Ivanova and Greenshaw 1997). Alternatively, the reduction in self-administration in the females could instead be interpreted as decreased reinforcement efficacy of methamphetamine, perhaps due to enhanced anxiogenic effects of methamphetamine (Beirami et al. 2017; Schutova et al. 2009). It will be interesting for future work to replicate and investigate these ideas of altered reinforcing function of methamphetamine. Moreover, it has been previously suggested that the ability of nicotine to enhance reinforcement may depend on the initial reinforcing value of the stimulus being moderate (as opposed to weak) which may explain why methamphetamine, but not alcohol, was affected (Palmatier et al. 2007b). Furthermore, nicotine and methamphetamine have overlapping discriminative stimulus effects (Desai and Bergman 2010; Gatch et al. 2008), which may, in part, explain differences observed between Experiments 1 and 2. However, more experiments are needed to parse out this question.
The present study was designed to examine the impact of the nicotine conditioning history on subsequent methamphetamine (or alcohol) self-administration, and as such all rats received nicotine and saline injections. Therefore, we cannot determine whether nicotine history alone impacted methamphetamine (or alcohol) self-administration as there were no groups that were nicotine-naive (i.e., received saline only throughout the experiment). For example, it is possible that methamphetamine self-administration was enhanced in the pseudoCS group. This outcome would be interesting and would suggest that the reinforcing function of methamphetamine was changed following the unpredictable nicotine-sucrose conditioning history. Indeed, other studies have focused on that question and found that nicotine exposure can enhance self-administration of both alcohol and methamphetamine (Larraga et al. 2017; Le et al. 2014; Neugebauer et al. 2010; Smith et al. 1999). Our study contributes to that literature by demonstrating that beyond nicotine exposure, an explicitly appetitive conditioning history with nicotine can reduce subsequent methamphetamine self-administration in female rats while having no consequences on subsequent alcohol self-administration in male or female rats.
Highlights.
Nicotine gains control over reward-seeking behavior in both male and female rats
Nicotine conditioning history does not affect alcohol self-administration
Nicotine conditioning history decreases methamphetamine SA in female rats
Conditioning history, not simply nicotine exposure, affects reward-seeking
Acknowledgements:
This work was funded by NIH grant DA039356 to RAB and JB. The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arregui-Aguirre A, Claro-Izaguirre F, Goni-Garrido MJ, Zarate-Oleaga JA, Morgado-Bernal I (1987) Effects of acute nicotine and ethanol on medial prefrontal cortex self-stimulation in rats. Pharmacol Biochem Behav 27: 15–20. [DOI] [PubMed] [Google Scholar]
- Barrett ST, Geary TN, Steiner AN, Bevins RA (2017) Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology (Berl) 234: 187–198. [DOI] [PubMed] [Google Scholar]
- Beirami E, Oryan S, Seyedhosseini Tamijani SM, Ahmadiani A, Dargahi L (2017) Intranasal insulin treatment alleviates methamphetamine induced anxiety-like behavior and neuroinflammation. Neurosci Lett 660: 122–129. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J (2014) Interoception and learning: import to understanding and treating diseases and psychopathologies. ACS Chem Neurosci 5: 624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Pudiak CM, KuoLee R (1998a) Effect of chronic nicotine on brain stimulation reward. I. Effect of daily injections. Behav Brain Res 96: 185–8. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Pudiak CM, KuoLee R (1998b) Effect of chronic nicotine on brain stimulation reward. II. An escalating dose regimen. Behav Brain Res 96: 189–94. [DOI] [PubMed] [Google Scholar]
- Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD (2004) Methamphetamine use behaviors and gender differences. Addict Behav 29: 89–106. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009) The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv 55: 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, deWit NR, Bevins RA (2014) Interoceptive conditioning with nicotine using extinction and re-extinction to assess stimulus similarity with bupropion. Neuropharmacology 86: 181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Falco AM, Fink K, Dwoskin LP, Bevins RA (2017a) The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats. Neuropharmacology 113: 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Swalve N, Li M, Bevins RA (2017b) Double dissociation of the anterior and posterior dorsomedial caudate-putamen in the acquisition and expression of associative learning with the nicotine stimulus. Neuropharmacology 121: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Tracy ME, Zhao C, Li M, Bevins RA (2012) Conditioned response evoked by nicotine conditioned stimulus preferentially induces c-Fos expression in medial regions of caudate-putamen. Neuropsychopharmacology 37: 876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE (2010) Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets 9: 60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF (2006) Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 189: 27–36. [DOI] [PubMed] [Google Scholar]
- Cortright JJ, Sampedro GR, Neugebauer NM, Vezina P (2012) Previous exposure to nicotine enhances the incentive motivational effects of amphetamine via nicotine-associated contextual stimuli. Neuropsychopharmacology 37: 2277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Bergman J (2010) Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther 335: 807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Flores E, Forster MJ (2008) Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depend 93: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova S, Greenshaw AJ (1997) Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology (Berl) 134: 187–92. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA Jr., Markou A (2009) NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology 34: 266–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraga A, Belluzzi JD, Leslie FM (2017) Nicotine Increases Alcohol Intake in Adolescent Male Rats. Front Behav Neurosci 11: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Lo S, Coen K (2014) Operant self-administration of alcohol and nicotine in a preclinical model of co-abuse. Psychopharmacology (Berl) 231: 4019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag AC, Czarnecki KS, Wangler LM, Robinson DL (2017) Chronic Nicotine Exposure Initiated in Adolescence and Unpaired to Behavioral Context Fails to Enhance Sweetened Ethanol Seeking. Front Behav Neurosci 11: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA (2007a) Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol 561: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA (2007b) The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol 18: 707–16. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA (2009) Acquired appetitive responding to intravenous nicotine reflects a Pavlovian conditioned association. Behav Neurosci 123: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Harrod SB, Bardo MT (2010) Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend 106: 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR (2004) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171: 173–8. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF (2007a) The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology 32: 1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF (2007b) The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend 89: 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A (2008) Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res 10: 995–1008. [DOI] [PubMed] [Google Scholar]
- Pittenger ST, Bevins RA (2013) Interoceptive conditioning in rats: effects of using a single training dose or a set of 5 different doses of nicotine. Pharmacol Biochem Behav 114-115: 82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Cannady R, Besheer J (2016) The nicotine + alcohol interoceptive drug state: contribution of the components and effects of varenicline in rats. Psychopharmacology (Berl) 233: 3061–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Stewart RT, Besheer J (2017) Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol Biochem Behav 156: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE (2006) Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 184: 274–85. [DOI] [PubMed] [Google Scholar]
- Schutova B, Hruba L, Pometlova M, Slamberova R (2009) Impact of prenatal and acute methamphetamine exposure on behaviour of adult male rats. Prague Med Rep 110: 67–78. [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, Amit Z (1999) Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl) 142: 408–12. [DOI] [PubMed] [Google Scholar]


