Abstract
Background:
Exposure to endocrine-disrupting chemicals (EDCs) during sensitive developmental windows, such as in utero, may influence disease later in life but direct measurement of fetal hormones is not feasible. The ratio of the length of the second finger digit to the fourth digit (2D:4D), a sexually dimorphic trait, is a biomarker of androgen levels and the androgen/estrogen balance in utero. However, it is unclear whether in utero EDC exposure might alter 2D:4D ratio.
Aims:
We examined 2D:4D ratio in Seveso children in relation to in utero exposure to a potent EDC, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) using linear regression.
Study design:
The Seveso Women’s Health Study (SWHS) is a historical cohort study, following the health of women exposed to TCDD during a 1976 explosion in Seveso, Italy. Individual-level TCDD was measured for SWHS in serum collected soon after the accident. In 2014, the SWHS children born after the explosion were enrolled in the Seveso Second Generation Study.
Subjects:
594 SWHS children born post-explosion to 397 mothers.
Outcome Measures:
Right hand 2D:4D ratio.
Results:
On average, 2D:4D ratio for males was significantly lower than for females (p<0.05). Overall, in utero TCDD exposure, either as maternal initial serum TCDD concentration or as TCDD extrapolated to pregnancy was not significantly associated with 2D:4D ratio in Seveso children. Results from all adjusted sensitivity analyses remained non-significant.
Conclusions:
Our results suggest in utero exposure to TCDD is not associated with alteration in 2D:4D ratio.
Keywords: Digit ratio, Dioxin, TCDD, in utero exposure, endocrine disruptors
Introduction
Endocrine-disrupting chemicals (EDCs) can interact with or mimic endogenous endocrine compounds (1). Exposure to EDCs in utero when the human fetus develops organs and neural systems is hypothesized to play a causal role in female and male reproductive disorders and some cancers (2, 3). Although direct measurement of fetal hormone concentrations is not feasible due to risks to the pregnant woman and her fetus (4–6), the ratio of the length of the second finger digit to that of the fourth digit (2D:4D) of a child has been hypothesized to be a marker of in utero androgen exposure and the androgen/estrogen balance (5, 7, 8). In humans and other animals, the 2D:4D ratio is sexually dimorphic with females having a higher mean ratio than males (4, 7, 9). This ratio is thought to be fixed after about two years of age (10). Furthermore, the 2D:4D ratio has been associated with several health outcomes in humans, including coronary heart disease in males and breast cancer in females (11, 12).
Human and animal studies provide support for the hypothesis that 2D:4D ratio is a biomarker of fetal androgen levels (13–18). Two of three case-control studies of children with congenital adrenal hyperplasia (CAH) found a significantly lower 2D:4D ratio compared to controls of the same sex (15–17). Another case-control study observed that males with complete androgen insensitivity syndrome (CAIS) had 2D:4D ratios significantly higher than male controls, but not significantly different from female controls (18). Thus, there is evidence to support the hypothesis that 2D:4D ratio is a biomarker of fetal androgen levels.
There is a scarcity of research on the association between in utero exposure to exogenous EDCs and 2D:4D ratio (14, 19, 20). In mice, perinatal exposure to estrogenic or anti-androgenic compounds including bisphenol A (BPA) alone or combined with vinclozolin or genistein was related to a higher digit ratio in males as well as in their male offspring. Wainstock et al. (20) examined 2D:4D ratio in children of Michigan residents exposed to polybrominated biphenyls (PBB), an EDC. Estimated in utero PBB exposure was non-significantly associated with increased digit ratio in females but not males. However, the sample size included only 19 males and 32 females (20). To our knowledge, no other epidemiologic studies have examined in utero exposure to EDCs and 2D:4D ratio.
In 1976, an explosion at a chemical plant near Seveso, Italy released an aerosol cloud containing 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), exposing the residents to some of the highest levels of human exposure (21–24). TCDD is an endocrine disruptor that can interfere with estrogen and androgen signaling (25–27). It is lipophilic, has a half-life of 7 to 9 years (28), and can cross the placenta (29). In the present study, we used data from the Seveso Women’s Health Study and the Seveso Second Generation Study, a prospective cohort of the female residents and their children born after the accident (30, 31). We assessed whether increased maternal exposure to TCDD, based on concentrations measured in blood collected soon after the accident and extrapolated to the time of pregnancy, is associated with altered digit ratio of male and female Seveso children.
Materials and methods
Study Design and Participants
Details of the Seveso Women’s Health Study (SWHS) cohort have been described previously (30–32). Briefly, in 1996, women aged 0–40 years old at the time of the explosion, with an adequate stored serum sample collected soon after the explosion, and who lived in 1976 in Zones A or B (30) were eligible to participate in the SWHS. A total of 981 women participated. In 2014, the Seveso Second Generation Study was initiated, including the SWHS women and their children age 2 years or older (31). Of 920 children alive and eligible, 611 (66.4%) born to 402 mothers completed the 2014 study visit. Of these children, we excluded 7 sets of twins (14 children) to remove a potential high source of correlation within the data, as we did not have information on whether they were dizygotic of monozygotic twins. We further removed two children who were missing 2D:4D ratio data, and one with a health condition related to abnormal hormone levels in utero (CAH by self-report), leaving a final analytic sample of 594 children (288 males, 306 females) born to 397 mothers.
Interview
The study was approved by the Institutional Review Boards of the participating institutions. Informed consent was obtained from all adult participants (mothers and adult children). Mothers of children <18 years provided written consent for their child; children 7 to 12 years provided oral assent and children 12 to 17 years provided written assent.
Details of the study procedures have been described previously (31). Briefly, interviews were conducted by trained nurse-interviewers who were blind to maternal exposure information, the zone of residence and serum TCDD concentrations (31, 33). Information collected from interviews of all adult participants included sociodemographic questions, personal habits, medical history, pregnancy history and pregnancy specific information (mothers and female children). Mothers of children age 2–17 years provided sociodemographic information and medical histories for their children (31).
Digit Ratio
The study outcome is the ratio of the lengths of the second and fourth finger digits (2D:4D). We measured these two digits for the right hand for all participants. In addition, we measured these digits on the left hand for participants who reported that they were left handed. Photocopies were made of the ventral surface (palm) of participants’ hands. Digit length was measured from the basal crease to the central tip of each finger, starting with the second finger, using Vernier calipers to the nearest 0.01mm, as done previously (34). If two creases were visible at the digit base, the crease closest to the palm was used. Two measurements were taken of each digit and the average of each digit measurement was used to calculate 2D:4D for each hand. For the whole sample (n=594), the correlation between the repeated measurements was high (0.87). A random sample of 57 participants had repeat measurements made of the photocopy by a second rater; the intraclass correlation coefficient was 0.93.
TCDD Serum Concentrations and Total TEQ Serum Concentrations
Archived maternal serum samples collected by venipuncture near the time of the explosion were stored at −20°C until analyzed at the Centers for Disease Control (CDC). TCDD was measured using high-resolution gas chromatography/mass spectrometry (35). A “summation” method was used to measure the total lipid content of each sample (36). Measurements were lipid adjusted and reported in parts per trillion (ppt) (33). For women with TCDD measurements below the limit of detection (average LOD = 18.8 ppt), a TCDD level of half the LOD was assigned (n=40, 10%) (37).
For a subset of women with a child born after January 1, 1994, total dioxin toxic equivalents (TEQ), a more comprehensive exposure measure to dioxins and dioxin-like chemicals, was also measured in serum collected in 1996 and 2008 (38). Serum samples were analyzed for seven polychlorinated dibenzo-p-dioxins (PCDDs), 10 polychlorinated dibenzofurans (PCDFs), and 12 polychlorinated biphenyls (PCBs) using high-resolution gas chromatography/isotope-dilution high resolution mass spectrometry (39, 40). Total TEQ was calculated using the 2005 WHO-Toxicity Equivalency Factor (TEF) system (41). Individual compounds where < 50% of samples were above the LOD were excluded from the total TEQ calculations (5 PCDFs, 2 PCBs).
As previously described, maternal TCDD and total TEQ were estimated at pregnancy based on a first-order kinetic model that varied with initial dose, age at explosion and additional covariates (31, 38). Maternal serum TCDD and total TEQ at pregnancy was estimated using the mother’s most recent serum sample measurement prior to the pregnancy (1976, 1996, 2008). For women with TCDD measurements below the limit of detection (1996 LOD 1.0 ppt, n=13; 2008 LOD 0.3 ppt, n=16), we assumed the most proximal TCDD concentration was equal to the LOD (35, 38). Total TEQ estimated at pregnancy was available for a subset of the sample (n=161 overall, 81 males and 80 females of 121 mothers).
Statistical Analysis
We examined exposure in three ways: 1) initial maternal serum TCDD collected near 1976; 2) TCDD level extrapolated to the time of pregnancy; and 3) total TEQ level extrapolated to time of pregnancy. All exposure measures (1976 serum TCDD, TCDD estimated at pregnancy, total TEQ estimated at pregnancy) were right skewed and log10 transformed to create an approximately normal distribution. The transformed exposure variables were then analyzed as continuous variables.
We assessed normality of digit measurements and 2D:4D ratio using a Shapiro-Wilk test. In order to compare digit measurements and 2D:4D ratio between males and females, we used ANOVA, with a significance level of 0.05.
In order to assess the relationship between maternal serum levels of TCDD or total TEQ and child right hand 2D:4D ratio, we performed linear regression analyses overall and stratified by child sex. In the overall models, we included a variable for child sex and an interaction term for child sex and exposure (1976 TCDD, TCDD estimated at pregnancy, total TEQ estimated at pregnancy) in order to test for interaction between exposure and child sex. To account for siblings in the main analyses, we used a clustered Huber-White sandwich estimator to account for non-independence. For any sensitivity analyses that did not include siblings, we used the robust Huber-White sandwich estimator to estimate standard errors (42, 43).
Potential confounders were identified a priori from the literature and using a directed acyclic graph (DAG). Maternal age at explosion, child age, and maternal smoking during pregnancy were identified as potential confounders (20, 44). Based on the DAG, we selected a minimally sufficient set of covariates to block all backdoor paths. Therefore, we adjusted for child age and maternal smoking during pregnancy (yes/no). No participants were missing data on child age, and for participants missing data on maternal smoking during pregnancy (n=4), values were imputed with “no” since the majority were non-smokers.
We performed a number of sensitivity analyses. First, we excluded participants with conditions that may affect finger measurement, such as a history of broken fingers (n=51) and syndactyly at birth (n=1). We also excluded participants with conditions that may indicate abnormal exposure to hormones in utero unrelated to TCDD exposure such as children with hypospadias/epispadias (n=2) (13). Additionally, we limited the analysis to each woman’s first post explosion live birth (n=363), since maternal body burden of TCDD would be highest for the pregnancy closest to the explosion. Due to our exclusion of twins from the main analysis, we performed a sensitivity analysis including 13 children, since one child was missing 2D:4D ratio. As the main analyses were for right hand 2D:4D, we performed analyses limiting the sample to those who were right hand dominant (n=559). We also performed analyses substituting the left hand 2D:4D measures for the n=35 left hand dominant participants. Finally, we repeated final models excluding n=3 observations with studentized residuals > 3 or < −3. All analyses were conducted using STATA 15.0 (45).
Results
Maternal and Child Characteristics
About half of the Seveso children were female (51.5%) and 94.1% were right-hand dominant (Table 1). Most were 18 years or older at the time of the interview (70.9%), with an average age of 23.8 (standard deviation 9.3) years. About 60% of children were the first born after the explosion. Median (interquartile range [IQR]) for 1976 maternal serum TCDD was 54.3 (25.7, 125.5) ppt among males and 70.2 (31.4, 180.0) ppt among females. Median maternal serum TCDD levels estimated at pregnancy were 11.3 (5.9, 29.5) ppt for males and 15.8 (6.4, 35.7) ppt for females. TCDD measures did not differ significantly by child sex or age. Total TEQ estimated to the pregnancy also did not differ between males and females: 22.3 (18.4, 29.9) ppt and 23.3 (17.1, 32.4) ppt, respectively.
Table 1.
Select maternal and child characteristics by maternal 1976 serum TCDD levels and maternal TCDD estimated at pregnancy, Seveso Second Generation Study, Seveso, Italy 1976–2016.
| Characteristic | n (%) | 1976 TCDD (ppt) Median (IQR) | Pregnancy TCDD (ppt) Median (IQR) | |||
|---|---|---|---|---|---|---|
| Total Children | 594 | (100.0) | 63.3 | (29.1, 160.0) | 13.6 | (6.2, 32.6) |
| Child sex | ||||||
| Male | 288 | (48.5) | 54.3 | (25.7, 125.5) | 11.3 | (5.9, 29.5) |
| Female | 306 | (51.5) | 70.2 | (31.4, 180.0) | 15.8 | (6.4, 35.7) |
| Child age at interview (years)*† | ||||||
| 2–6 | 18 | (3.0) | 191.6 | (86.7, 389.0) | 4.4 | (1.5, 10.0) |
| 7–17 | 155 | (26.1) | 95.8 | (44.4, 247.0) | 4.5 | (2.7, 9.5) |
| 18–29 | 224 | (37.7) | 64.2 | (29.1, 122.5) | 14.2 | (7.9, 28.9) |
| 30–39 | 197 | (33.2) | 41.9 | (21.3, 98.9) | 32.0 | (16.6, 69.4) |
| Primary wage earner’s education | ||||||
| < Required | 174 | (29.3) | 64.7 | (29.9, 164.0) | 12.1 | (6.4, 27.4) |
| Required/high school | 219 | (36.9) | 64.4 | (33.2, 191.0) | 13.8 | (5.9, 34.9) |
| University | 201 | (33.8) | 52.9 | (23.9, 129.0) | 15.5 | (6.4, 36.2) |
| Dominant hand*† | ||||||
| Left | 35 | (5.9) | 111.0 | (50.5, 315.0) | 22.5 | (8.9, 108.8) |
| Right | 559 | (94.1) | 61.2 | (28.6, 157.0) | 13.3 | (6.1, 32.0) |
| Post explosion birth order† | ||||||
| 1 | 363 | (61.1) | 65.3 | (30.6, 167.0) | 16.4 | (7.4, 40.9) |
| 2 | 200 | (33.7) | 58.6 | (26.9, 138.5) | 9.9 | (4.9, 24.1) |
| ≥ 3 | 31 | (5.2) | 49.9 | (19.5, 105.0) | 8.6 | (3.2, 19.5) |
| Total Mothers | 397 | (100.0) | 63.2 | (29.1, 162.0) | ||
| Maternal characteristics at explosion (n=397 mothers) | ||||||
| Age at explosion (years) * | ||||||
| 0–10 | 90 | (22.7) | 166.5 | (57.6, 348.0) | -- | |
| 11–20 | 200 | (50.4) | 54.0 | (24.3, 114.5) | -- | |
| 21–30 | 99 | (24.9) | 43.7 | (23.9, 98.9) | -- | |
| 31–40 | 8 | (2.0) | 53.3 | (29.6, 125.0) | -- | |
| Maternal characteristics at pregnancy (n=594 pregnancies) | ||||||
| Age at pregnancy (years) *† | ||||||
| < 25 | 106 | (17.8) | 44.0 | (20.7, 109.0) | 25.5 | (11.7, 56.5) |
| 25–30 | 245 | (41.2) | 59.4 | (29.9, 136.0) | 16.0 | (7.7, 33.3) |
| 31–34 | 133 | (22.5) | 85.1 | (35.5, 238.0) | 9.2 | (4.4, 26.2) |
| ≥ 35 | 110 | (18.5) | 70.1 | (33.2, 162.0) | 6.6 | (3.4, 19.6) |
| Smoking during pregnancy †a | ||||||
| No | 532 | (89.6) | 63.7 | (29.9, 164.0) | 12.8 | (5.9, 32.4) |
| Yes | 62 | (10.4) | 55.2 | (21.9, 109.0) | 18.7 | (8.9, 37.5) |
| Weight gain during pregnancy (kg)b | ||||||
| <10 | 123 | (20.7) | 72.6 | (38.2, 130.0) | 16.1 | (5.6, 38.7) |
| 10–14 | 287 | (48.3) | 53.3 | (26.1, 135.0) | 13.6 | (6.5, 32.4) |
| 15–29 | 118 | (19.9) | 57.6 | (21.6, 191.0) | 11.6 | (5.0, 27.7) |
| ≥20 | 66 | (111) | 78.9 | (40.1, 223.0) | 10.7 | (6.4, 31.9) |
| Maternal characteristics at time of interview (n=397 mothers) | ||||||
| Marital status c | ||||||
| Married/cohabitating | 332 | (87.4) | 63.4 | (29.4, 158.5) | -- | |
| Not married/cohabitating | 48 | (12.6) | 71.5 | (31.1, 175.8) | -- | |
| Maternal education d | ||||||
| < Required | 171 | (45.5) | 61.2 | (25.2, 122.0) | -- | |
| Required/high school | 82 | (21.8) | 63.7 | (33.2, 179.0) | -- | |
| University | 123 | (32.7) | 67.5 | (30.2, 199.0) | -- | |
ANOVA p-value<0.05 for 1976 TCDD.
ANOVA p-value<0.05 for TCDD estimated at pregnancy.
Missing data on maternal smoking during pregnancy for 4 pregnancies, imputed with 0 (no).
Missing data on pregnancy weight gain for 11 children, imputed with median (12 kg).
Missing marital status for 17 mothers.
Missing maternal education for 21 mothers.
At the time of the explosion, 22.7% of Seveso mothers were age 0 to 10 years, and 50.4% were age 11 to 20 years. At the time of pregnancy, most were 30 years or younger (59.0%), and 10.4% of mothers smoked during their pregnancy.
Digit Ratios
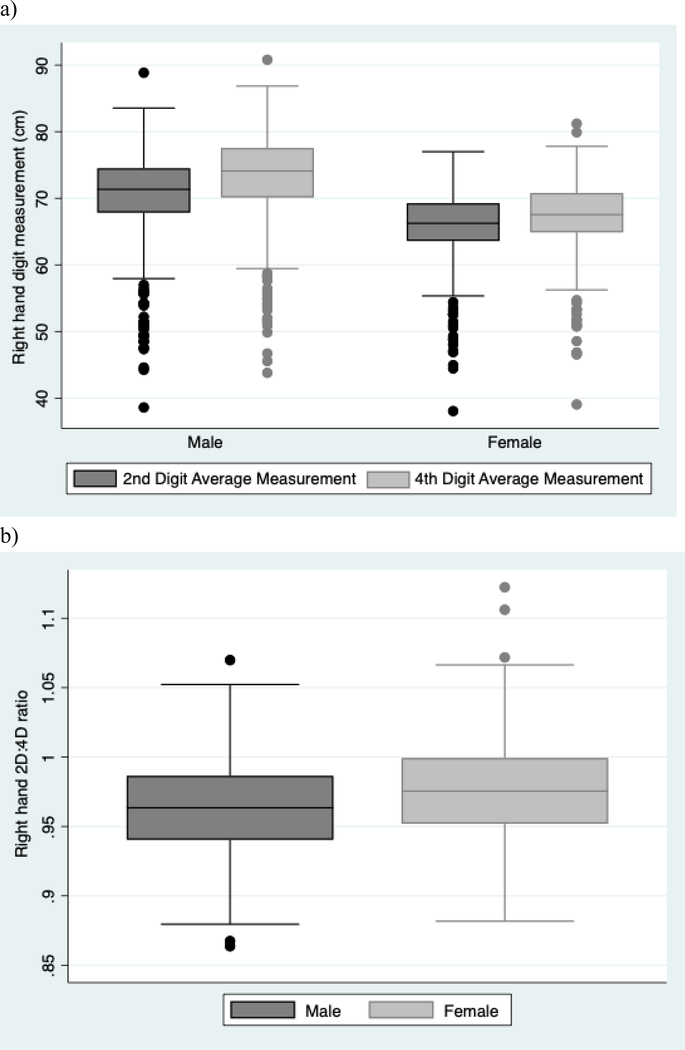
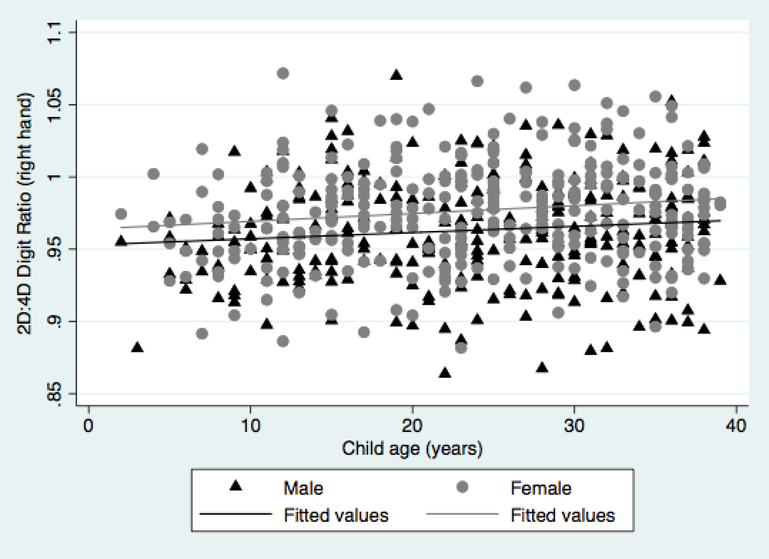
Figure 1 presents boxplots of digit measurements and 2D:4D ratio by child sex. The mean 2D:4D digit ratio for males (mean=0.963, SD=0.036) was significantly lower than for females (mean=0.977, SD=0.036) (p < 0.01). For the 35 participants with both left and right hand 2D:4D ratios measured, the mean difference between the right and left hand 2D:4D ratio was −0.020. Figure 2 presents 2D:4D ratio by child age. Among the full group, 2D:4D ratio was significantly correlated with age (n=594, r = 0.125, p=0.002); results were similar for both females (r = 0.140, p=0.01) and males (r = 0.113, p=0.06).
Figure 1.
Boxplots of right hand a) digit measurements and b) 2D:4D ratio stratified by child sex, Seveso Second Generation Study, Seveso, Italy, 2014–2016.
Abbreviations: 2D, 2nd digit; 4D, 4th digit.
Figure 2.
Scatterplot of right hand 2D:4D ratio by child age, Seveso Second Generation Study, Seveso, Italy, 2014–2016.
2D:4D Ratios and TCDD concentrations
In crude and adjusted models, there were no significant associations between maternal 1976 serum concentrations of TCDD and right hand 2D:4D ratio for Seveso children overall (adj-β per 10-fold increase in TCDD=0.000, 95% CI: −0.005, 0.005) or for either sex (females: adj-β=0.000, 95% CI: −0.007, 0.007; males: adj-β=0.000, 95% CI: −0.008, 0.008) (Table 2). Similarly, there were no significant associations of maternal TCDD estimated at the time of the pregnancy and 2D:4D ratio (for a 10-fold increase in maternal serum TCDD estimated at pregnancy adj-β=−0.002, 95% CI: −0.008, 0.005 overall; adj-β=−0.003, 95% CI: −0.011, 0.005 for males; adj-β=0.000, 95% CI: −0.009, 0.008 for females). Results were likewise null for total TEQ estimated at pregnancy and 2D:4D ratio. The interaction between child sex and exposure (maternal 1976 serum TCDD, maternal TCDD estimated at pregnancy, maternal total TEQ estimated at pregnancy) was not significant for any of the models.
Table 2:
Results of adjusted linear regression models for in utero TCDD exposure (log10) and child right hand 2D:4D ratio, overall and stratified by child sex, Seveso Second Generation Study, Seveso, Italy, 1976–2016.
| Overall | Female | Male | |||||
|---|---|---|---|---|---|---|---|
| Exposure (ppt)c | N | Adj.a β (95% CI) | N | Adj.b β (95% CI) | N | Adj.b β (95% CI) | p-int |
| 1976 TCDD | 594 | 0.000 (−0.005, 0.005) | 306 | 0.000 (−0.007, 0.007) | 288 | 0.000 (−0.008, 0.008) | 0.90 |
| Pregnancy TCDD | 594 | −0.002 (−0.008, 0.005) | 306 | 0.000 (−0.009, 0.008) | 288 | −0.003 (−0.011, 0.005) | 0.56 |
| Pregnancy total TEQ | 161 | −0.007 (−0.029, 0.015) | 80 | −0.010 (−0.036, 0.016) | 81 | 0.000 (−0.046, 0.046) | 0.61 |
Adjusted for: child sex, child age, maternal smoking during pregnancy.
Adjusted for: child age, maternal smoking during pregnancy.
Results are for a 10-fold increase in exposure.
Abbreviations: 2D, 2nd digit; 4D, 4th digit; CI, confidence interval; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TEQ, toxic equivalent.
In sensitivity analyses (see Supplementary Tables 1–3), results were not meaningfully different when we excluded participants with conditions that may affect finger measurement (n=52) or those with conditions that may indicate abnormal exposure to hormones in utero (n=2). Limiting the analysis to first post-explosion livebirth (n=363), or including twins with non-missing exposure and outcome data (n=13) did not meaningfully change the results. When we limited the analysis to participants who were right hand dominant (n=559) or substituted left-hand measures for participants who were left hand dominant (n=35), the results were not different. Finally, excluding participants with studentized residuals >3 or <−3 (n=3) did not change results meaningfully.
Discussion
In the Seveso Second Generation study, consistent with previous literature, we found the 2D:4D ratio among Seveso children is sexually dimorphic with females having a higher mean ratio than males. In this investigation of children born to a highly TCDD-exposed population, we did not find any significant associations between in utero TCDD exposure either based on maternal initial serum TCDD concentration or TCDD extrapolated to pregnancy, and 2D:4D digit ratio in Seveso children in crude and adjusted analyses. When we assessed exposure to dioxin-like compounds using total TEQ, we also did not find any significant associations. Additionally, we found no evidence of associations in sensitivity analyses.
While literature on this topic is sparse, our results are consistent with the null findings in the only other paper in humans assessing in utero exposure to EDCs, albeit to a non-dioxin like compound, PBB-153, and 2D:4D ratio (20). In addition, our finding of a significantly higher 2D:4D digit ratio in females than males agrees with the literature (4, 6, 7, 9).
There is some debate about the relationship between digit ratio and age in the literature. Some suggest that digit ratio is fixed at age 2, while others suggest that digit ratio may increase slightly with age (5, 13, 14, 20). Two studies have longitudinally assessed 2D:4D and found that while 2D:4D increased slightly with age, the rank order of digit ratios remained the same (46, 47). In our large study, we also found a small positive correlation albeit significant between child age and 2D:4D. During puberty and adolescence, there are large changes in growth rates and bone lengths in the hand. Under the hypothesis that 2D:4D ratios reflect fetal hormone levels of androgens and estrogens, these sex hormones may influence growth during puberty and adolescence. This may contribute to the variation in finger lengths and therefore finger ratios across time (46, 47).
One cross-sectional study found an association between maternal smoking during pregnancy and a lower right hand 2D:4D ratio in boys age 5 to 11 years (44). In our study, although median TCDD estimated at pregnancy was higher among mothers who smoked during pregnancy, maternal smoking during pregnancy (cigarettes/day) was not significantly correlated with 2D:4D ratio in either sex (females: r = 0.08, p=0.17; males: r = 0.00, p=0.99), and we controlled for maternal smoking during pregnancy. Because there is little known about other potential influences on 2D:4D ratio, it is possible we did not include all appropriate confounders.
A limitation of the study is that 66.4% of eligible children participated and females (72.1% of eligible) were more likely to participate than males (61.3% of eligible). However, there was no difference between participants and non-participants in child age at follow up or measures of in utero TCDD exposure (maternal initial serum TCDD or maternal TCDD estimated at pregnancy).
This study also has several strengths. To our knowledge, our study has the largest sample size (n=594) investigating exposure to an EDC and 2D:4D ratio, compared to the sample size of Wainstock (n=51) (20). Furthermore, we have TCDD measured in serum collected close to the time of the 1976 explosion, and thus capture the women’s highest lifetime dose. These serum measures were used to estimate maternal serum TCDD exposure during pregnancy, and we would expect any non-differential exposure misclassification to be towards the null. While TCDD is only one congener, we also investigated a more comprehensive exposure measure of dioxins and dioxin like compounds (total TEQ) during pregnancy in a subset of participants and results remained null.
The Seveso Second Generation study measured digits indirectly, using a standardized method of measurement from photocopies. There is some evidence that 2D:4D ratios measured indirectly are lower than 2D:4D ratios measured directly, and that the sex difference from indirect methods is larger than the sex difference from direct methods (9, 34). As those performing the measurements were blind to exposure information, we would expect any bias from measurement error to be non-differential.
The population of Seveso is relatively ethnically homogenous, with all women and children in our sample self-reporting as Caucasians of European descent. As studies have found greater variability of 2D:4D among different ethnic groups, the ethnic homogeneity of the Seveso study population will help mitigate these problems of ethnicity confounding the associations with exposure measurements (4).
In summary, although we observed a difference in digit ratio by sex in the Seveso Second Generation study our results suggest in utero exposure to TCDD is not associated with alteration in 2D:4D ratio. While the difference in 2D:4D ratio between males and females gives support for the hypothesis that digit ratio reflects androgen levels and the balance between androgen and estrogen levels in utero, it is unclear whether exogenous chemical exposures during pregnancy can influence 2D:4D ratio. Human studies that have found differences in digit ratio have all been in study populations where the endogenous hormonal disturbance in utero from genetic conditions is quite large (16–18, 48). The SWHS mothers experienced a wide range of TCDD exposures from the initial 1976 accident, and median maternal TCDD levels at pregnancy remained higher compared to background levels of TCDD (around 1.3 ppt worldwide for 1989–2010) (49). Despite maternal exposure to a potent endocrine disruptor, we found no evidence of an association between in utero TCDD exposure and 2D:4D ratio. It is possible in utero exposure to other EDCs may influence digit ratio.
Supplementary Material
Highlights.
A 1976 explosion in Seveso, Italy exposed residents to high levels of TCDD.
Children of Seveso female residents were enrolled in a second-generation study.
Examined digit ratio (2nd:4th digit length) in relation to in utero TCDD exposure.
Digit ratio among Seveso children was sexually dimorphic (females > males).
No associations between in utero TCDD exposure and digit ratio were found.
Acknowledgements
The authors would like to acknowledge the SWHS field staff, study participants and their families, colleagues at CDC for specimen analysis, and Stephen Rauch for his statistical support.
Formatting of funding sources
This work was supported by Grant Numbers F06 TW02075-01 from the National Institutes of Health, R01 ES07171 and 2P30-ESO01896-17 from the National Institute of Environmental Health Sciences, R82471 from the U.S. Environmental Protection, and #2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy.
Abbreviations
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- CAIS
complete androgen insensitivity syndrome
- CAH
congenital adrenal hyperplasia
- EDCs
endocrine-disrupting chemicals
- PBB
polybrominated biphenyl
- 2D:4D
second to fourth digit ratio
- SWHS
Seveso Women’s Health Study
Footnotes
Declarations of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Global assessment of the state-of-the-science of endocrine disruptors. Damastra T SB, Bergman A, Kavlock R VanDerKraak G, editor. Geneva, Switzerland: WHO; 2002. 180 p. [Google Scholar]
- 2.Scsukova S, Rollerova E, Bujnakova Mlynarcikova A. Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone-related cancer. Reproductive biology. 2016;16(4):243–54. [DOI] [PubMed] [Google Scholar]
- 3.Bonde JP, Flachs EM, Rimborg S, Glazer CH, Giwercman A, Ramlau-Hansen CH, et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update. 2016;23(1):104–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollier LP, Keelan JA, Jamnadass ES, Maybery MT, Hickey M, Whitehouse AJ. Adult digit ratio (2D:4D) is not related to umbilical cord androgen or estrogen concentrations, their ratios or net bioactivity. Early human development. 2015;91(2):111–7. [DOI] [PubMed] [Google Scholar]
- 5.Abbo O, Ferdynus C, Kalfa N, Huiart L, Sauvat F, Harper LH. Male infants with hypospadias and/or cryptorchidism show a lower 2D/4D digit ratio than normal boys. Archives of disease in childhood. 2015;100(7):643–7. [DOI] [PubMed] [Google Scholar]
- 6.Lawrance-Owen AJ, Bargary G, Bosten JM, Goodbourn PT, Hogg RE, Mollon JD. Genetic association suggests that SMOC1 mediates between prenatal sex hormones and digit ratio. Hum Genet. 2013;132(4):415–21. [DOI] [PubMed] [Google Scholar]
- 7.Manning JT, Stewart A, Bundred PE, Trivers RL. Sex and ethnic differences in 2nd to 4th digit ratio of children. Early human development. 2004;80(2):161–8. [DOI] [PubMed] [Google Scholar]
- 8.Dean A, Sharpe RM. Clinical review: Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013;98(6):2230–8. [DOI] [PubMed] [Google Scholar]
- 9.Honekopp J, Watson S. Meta-analysis of digit ratio 2D:4D shows greater sex difference in the right hand. American journal of human biology : the official journal of the Human Biology Council. 2010;22(5):619–30. [DOI] [PubMed] [Google Scholar]
- 10.Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early human development. 2004;77(1–2):23–8. [DOI] [PubMed] [Google Scholar]
- 11.Hong L, Zhan-Bing M, Zhi-Yun S, Xiao-Xia S, Jun-Li Z, Zheng-Hao H. Digit ratio (2D:4D) in Chinese women with breast cancer. American journal of human biology : the official journal of the Human Biology Council. 2014;26(4):562–4. [DOI] [PubMed] [Google Scholar]
- 12.Lu H, Ma Z, Zhao J, Huo Z. Second to fourth digit ratio (2D:4D) and coronary heart disease. Early human development. 2015;91(7):417–20. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci U S A. 2011;108(39):16289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auger J, Le Denmat D, Berges R, Doridot L, Salmon B, Canivenc-Lavier MC, et al. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proceedings Biological sciences. 2013;280(1768):20131532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd to 4th digit length ratio-comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum Reprod. 2003;18(5):976–9. [DOI] [PubMed] [Google Scholar]
- 16.Brown WM, Hines M, Fane BA, Breedlove SM. Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Hormones and behavior. 2002;42(4):380–6. [DOI] [PubMed] [Google Scholar]
- 17.Okten A, Kalyoncu M, Yaris N. The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early human development. 2002;70(1–2):47–54. [DOI] [PubMed] [Google Scholar]
- 18.Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150(11):5119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber SE, Lenz B, Kornhuber J, Muller CP. Prenatal androgen-receptor activity has organizational morphological effects in mice. PLoS One. 2017;12(11):e0188752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wainstock T, Pearce B, Barr DB, Marder ME, Terrell M, Marcus M. Exposure to PBB-153 and Digit Ratio. Early human development. 2016;103:33–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.di Domenico A, Silano V, Viviano G, Zapponi G. Accidental release of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) at Seveso, Italy. II. TCDD distribution in the soil surface layer. Ecotoxicol Environ Saf. 1980;4(3):298–320. [DOI] [PubMed] [Google Scholar]
- 22.Mocarelli P, Needham LL, Marocchi A, Patterson DG Jr., Brambilla P, Gerthoux PM, et al. Serum concentrations of 2,3,7,8-tetrachlorodibenzo-p-dioxin and test results from selected residents of Seveso, Italy. J Toxicol Environ Health. 1991;32(4):357–66. [DOI] [PubMed] [Google Scholar]
- 23.Mocarelli P, Marocchi A, Brambilla P, Gerthoux P, Beretta C, Colombo L, et al. Human data derived from the Seveso accident- relevance for human risk assessment. Toxic Subst J. 1992;12:51–73. [Google Scholar]
- 24.Needham LL, Gerthoux PM, Patterson DG Jr., Brambilla P, Turner WE, Beretta C, et al. Serum dioxin levels in Seveso, Italy, population in 1976. Teratog Carcinog Mutagen. 1997;17(4–5):225–40. [PubMed] [Google Scholar]
- 25.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111(4):389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghotbaddini M, Powell JB. The AhR Ligand, TCDD, Regulates Androgen Receptor Activity Differently in Androgen-Sensitive versus Castration-Resistant Human Prostate Cancer Cells. Int J Environ Res Public Health. 2015;12(7):7506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, Winneke G, Wilhelm M, Wittsiepe J, Lemm F, Furst P, et al. Environmental exposure to dioxins and polychlorinated biphenyls reduce levels of gonadal hormones in newborns: results from the Duisburg cohort study. International journal of hygiene and environmental health. 2008;211(1–2):30–9. [DOI] [PubMed] [Google Scholar]
- 28.Pirkle JL, Wolfe WH, Patterson DG, Needham LL, Michalek JE, Miner JC, et al. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam Veterans of Operation Ranch Hand. J Toxicol Environ Health. 1989;27(2):165–71. [DOI] [PubMed] [Google Scholar]
- 29.Schecter A, Papke O, Ball M. Evidence for transplacental transfer of dioxins from mother to fetus: chlorinated dioxin and dibenzofuran in te livers of stillborn infants. Chemosphere. 1990;21(8):1017–22. [Google Scholar]
- 30.Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, et al. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40(9–11):1247–53. [DOI] [PubMed] [Google Scholar]
- 31.Eskenazi B, Warner M, Brambilla P, Signorini S, Ames J, Mocarelli P. The Seveso accident: A look at 40years of health research and beyond. Environ Int. 2018;121(Pt 1):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2011;119(12):1700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Samuels S, et al. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112(1):22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allaway HC, Bloski TG, Pierson RA, Lujan ME. Digit ratios (2D:4D) determined by computer-assisted analysis are more reliable than those using physical measurements, photocopies, and printed scans. American journal of human biology : the official journal of the Human Biology Council. 2009;21(3):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson DG, Hampton L, Lapeza CR, Belser WT, Green V, Alexander L, et al. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal Chem. 1987;59(15):2000–5. [DOI] [PubMed] [Google Scholar]
- 36.Akins JR, Waldrep K, Bernert JT Jr. The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184(3):219–26. [DOI] [PubMed] [Google Scholar]
- 37.Eskenazi B, Mocarelli P, Warner M, Chee WY, Gerthoux PM, Samuels S, et al. Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ Health Perspect. 2003;111(7):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warner M, Mocarelli P, Brambilla P, Wesselink A, Patterson DG, Turner WE, et al. Serum TCDD and TEQ concentrations among Seveso women, 20 years after the explosion. J Expo Sci Environ Epidemiol. 2014;24(6):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson DG, Turner WE. Method 28: Measurement of PCDDs, PCDFs, and Coplanar PCBs in Serum by HRGC/ID-HRMS. Atanta, GA: National Center for Enviromental Health, CDC; 2005. [Google Scholar]
- 40.Patterson DG, Turner WE. Method 28: Measurement of PCBs and Persistent Pesticides in Serum by HRGC/ID-HRMS. Atlanta, GA: National Center for Enviromental Health, CDC; 2005. [Google Scholar]
- 41.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber PJ. The behavior of maximum likelihood estimates under non-standard conditions In: LeCam LM, Neyman J, editors. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. 1. Berkeley, CA: University of California Press; 1967. p. 221–33. [Google Scholar]
- 43.White H A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica: Journal of the Econometric Society. 1980:817–38. [Google Scholar]
- 44.Rizwan S, Manning JT, Brabin BJ. Maternal smoking during pregnancy and possible effects of in utero testosterone: evidence from the 2D:4D finger length ratio. Early human development. 2007;83(2):87–90. [DOI] [PubMed] [Google Scholar]
- 45.Stata Corp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 46.Trivers R, Manning J, Jacobson A. A longitudinal study of digit ratio (2D:4D) and other finger ratios in Jamaican children. Hormones and behavior. 2006;49(2):150–6. [DOI] [PubMed] [Google Scholar]
- 47.Kralik M, Ingrova P, Koziel S, Hupkova A, Klima O. Overall trends vs. individual trajectories in the second-to-fourth digit (2D:4D) and metacarpal (2M:4M) ratios during puberty and adolescence. American journal of physical anthropology. 2017;162(4):641–56. [DOI] [PubMed] [Google Scholar]
- 48.Manning JT, Kilduff LP, Trivers R. Digit ratio (2D:4D) in Klinefelter’s syndrome. Andrology. 2013;1(1):94–9. [DOI] [PubMed] [Google Scholar]
- 49.Consonni D, Sindaco R, Bertazzi P. Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: A review, 1989–2010. Environ Int. 2012;44:151–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.