Abstract
Bone marrow derived stromal cells or mesenchymal stromal cells (BMSCs or MSCs, as we will call them in this work) are multipotent progenitor cells that can differentiate into osteoblasts, adipocytes and chondrocytes. In addition, MSCs have been shown to modulate the function of a variety of immune cells. Donor age has been shown to affect the regenerative potential, differentiation, proliferation and anti-inflammatory potency of MSCs; but the impact of donor age on their immunosuppressive activity is unknown. In this study, we evaluated the ability of MSCs derived from very young children and adults on T cell suppression and cytokine secretion by monocytes/macrophages. MSCs were obtained from extra digits of children between 10–21 months and adults between 28 and 64 years of age. We studied cell surface marker expression, doubling time, lineage differentiation potential, and immunosuppressive function of the MSCs. Young MSCs double more quickly and differentiate into bone and fat cells more efficiently than those from older donors. They also form more and dense colonies of fibroblasts (CFU-F). MSCs from both young and adult subjects suppressed T cell proliferation in a mitogen induced assay at 1:3 and 1:30 ratios. At a 1:30 ratio, however, MSCs from adults did not, but MSCs from infants did suppress T cell proliferation. In the mixed lymphocyte reaction assay, MSCs from infants produced similar levels of suppression at all three MSC/T cell ratios, but adult MSCs only inhibited T cell proliferation at a 1:3 ratio. Cytokine analyses of cocultures of MSCs and macrophages showed that both adult and young MSCs suppress TNF-α and induce IL-10 production in macrophage co-culture assay in a similar manner. Overall, this work shows that developing MSCs display a higher level of immunosuppression than mature MSCs.
Keywords: Immunomodulation, MSCs, IL-10, TNF-α, T-cells
Introduction
Bone marrow derived mesenchymal stromal cells are a heterogeneous [1] population of self-renewing, multi-potent progenitor cells that are easily separated from bone marrow by their adherence to plastic with potential to differentiate into osteoblasts, adipocytes and chondrocytes [2]. This has led to their use in tissue engineering. Interestingly, MSCs also have immune modulatory effects [3] that were exploited clinically [4] years before they were studied in detail. The immune modulatory function first was used in the treatment graft-versus-host disease (GvHD) [4, 5] and later in other autoimmune diseases [6, 7]. Many studies have since focused on the immunomodulatory potential of MSCs in both animal models and humans [8]. The cells seemed beneficial in several animal models of inflammatory and immune disorders including systemic lupus erythematosus [9], multiple sclerosis [10], autoimmune type I diabetes [11], asthma [12], sepsis [13], pulmonary fibrosis [14], primary biliary cirrhosis [15], autoimmune myasthenia gravis [16] and stroke [17]. MSCs are being tested as treatments for GvHD [5, 18, 19], SLE [20, 21], and multiple sclerosis [22, 23].
There have been many studies exploring the mechanism of MSC-driven immune modulation, yet the phenomenon is still incompletely understood. MSCs have immunosuppressive and anti-inflammatory effects that likely depend on several different mechanisms including cell contact-dependent secretion of soluble factors [24, 25]. Several of these factors suppress the production of proinflammatory cytokines (e.g., interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-1α or IL-1β) and shift the immune system to an anti-inflammatory status. The factors secreted by MSCs that induce these changes include TGFβ1 [12, 26], prostaglandin E2 (PGE2) [13], hepatocyte growth factor (HGF), indoleamine-pyrrole 2,3-dioxygenase (IDO) [26], nitric oxide (NO) [27] and interleukin-10 (IL-10) [6, 26]. In addition to secreting soluble factors, the immune modulatory function of MSCs is also mediated by extracellular vesicles (EVs) [28]. EVs contain proteins, peptides, mRNA, microRNA and lipids which all can play a role in immune-regulation. EVs administration in GvHD [29] and sepsis [30] have been shown to alleviate symptoms. The MSCs’ immune-modulatory function is exerted at the sites of inflammation and is also regulated by cells and factors present in the local microenvironment. In acute inflammatory conditions in the tissue high concentrations of local inflammatory cytokines are present and these stimulate the MSCs’ immune-modulatory activities. However, in chronic inflammation the cytokine levels are lower and may not be sufficient to induce the immune-modulatory functions of MSCs [31].
MSCs have been isolated from a variety of sources and many species. There are many similarities and some differences among these MSCs, but there is no indication that the immune suppressive properties of bone marrow derived MSCs would be dependent on the different skeletal origin of the BM (see[32, 33]). A recent equine study suggested that in an inflammatory environment MSCs from different sources are likely to respond similarly [34].
MSCs can inhibit T cell proliferation, reduce their survival, and induce Treg production [35]. MSC driven T cell suppression seems to result from a combination of cell contact and release of soluble factors [6]. It has been reported that the number of MSCs in the bone marrow decreases with age [36] along with their ability to proliferate, differentiate, and produce cytokines [37–39]. Changes in gene expression were described in the MSC population during aging [40] in the adult population (17–84 years). We wanted to study the impact of age on the immunomodulatory function of MSCs derived from bones in extra digits of infants 10–21 months of age (called “young MSCs”) compared to those isolated from adults. We also compared their cell surface marker expression, lineage differentiation potential and immune suppressive activity. Young MSCs appear to divide more quickly, differentiate more efficiently, and suppress immune function more effectively than adult MSCs do.
Materials and Methods
Reagents
All reagents used in this study were obtained from Sigma‐Aldrich (St Louis, MO) unless noted. Carboxyfuorescein succinimidyl ester (CFSE) and Concanavalin A were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CD3‐APC antibody and 7‐ AAD were purchased from eBioscience. All other flow cytometry antibodies are from BD Biosciences, USA (Supplementary Table 1).
Isolation and culture of bone marrow stromal cells
MSCs are usually separated from the aspirate or tissue by their preferential adherence to plastic. Infant and adult MSCs were isolated from surgically resected polydactylous (i.e. extra) digits or from iliac crest biopsies, respectively. Adult MSCs from healthy donors were obtained from the Bone Marrow Stromal Cell Transplantation Center of the NIH and cultured as described earlier [41] (IRB approved protocol NCT01071577). These MSCs were grown from marrow aspirated from the iliac crest. Young human MSCs were obtained from extra digits removed electively from healthy infants between 10–21 months of age with polydactyly of the hands or feet (approved by IRB (P00006257). All samples are de-identified. Four different donor samples were obtained (two toes and two fingers) from patients clinically diagnosed with isolated, non- syndromic polydactyly (i.e. extra digits were identified, but no other pathology was found). The bones were mechanically cleaned of all fibrous tissue and cut into small pieces before being digested in collagenase type IV for 30 minutes. Cells and the digested bone fragments from the digest were then collected and plated in separate dishes. Cells from both culture dishes were combined, this combined fraction is used as one “young” MSC donor. Each donor sample is kept separate. The culture medium used was α-MEM with 20% FBS, 1% Pen/Strep, 1% Glutamine.
Population doubling
To determine the population doubling time MSCs were initially seeded at 3000 cells/cm2 in a 100-mm plate and counted after 96 hours. Cell population doubling time was calculated using the formula DT = t (log2)/(logNt − logNo), where 𝑡 represents the culture time, and No and Nt are the initial and final cell numbers before and after seeding, respectively [42].
Immunophenotypic analysis
MSCs at passage 5 underwent immunophenotypic analyses based on flow cytometry with accepted markers. The following antibodies were used: CD13-APC, CD34-FITC, CD44-PE, CD45-APC, CD73-PE, CD90-PE, CD105-FITC, HLA-I-APC, HLA-II-PE. Isotype antibodies were used as controls (for details, see Suppl. Table 1). A minimum of 50,000 events were acquired using a BD AccuriC6 flow cytometer (BD Biosciences, San Jose, CA). The data collected were analyzed using FlowJo software (BD, Franklin Lakes, NJ). Normalized median fluorescence intensity (nMFI) were calculated using FlowJo software as previously published [43]
Lineage differentiation
Osteogenic differentiation
MSCs were cultured in α-MEM with 10% FBS, 1% Pen/Strep, 1% Glutamine. Cells were plated at 25,000 cells per well in 6-well plates. When the cells reached confluency osteogenic differentiation media was added containing 10nM dexamethasone, 50 μg/ml of ascorbate-2-phosphate and 2 mM of β- glycerophosphate. The medium was changed every three days for 21 days at which time the cultures were stained with alizarin red to identify bone formation [44].
Adipogenic differentiation
MSCs are cultured in DMEM high glucose with 10% FBS. Cells were plated as above and at confluency they were differentiated to adipocytes as previously described [45] with modifications. The differentiation was achieved by adding 5 μM piglitazone for 4 days, removing it and culturing the cells for 6 additional days. The culture medium was changed every two days. On day 10, the cells were stained with Oil Red O.
CFU-F assay
CFU-F (colony forming unit-fibroblast) assay was performed by seeding 500 cells per 100-mm plate. The cells were cultured in α-MEM with 20% FBS, 1% Pen/Strep, 2 mM Gluta-MAX, 1 mM sodium pyruvate and 55 μM β-mercaptoethanol. The medium was changed on day 6, and on day 14 the cells were fixed with 10% neutral buffered formalin and stained with 0.5% crystal violet. Colony counting was performed using Image J (National Institutes of Health, Bethesda, MD) after the plates were all scanned. Colony size and density were quantified as previously described using Image J (V1.34i, NIH) [46].
T-cell proliferation assays
Mitogen induced T-cell proliferation assay
PBMCs used in this study are frozen. We obtained fresh isolated PBMCs from the NIDCR Flow cytometry core facility and froze the cells for later use in freezing media containing IMDM with 40% FBS and 10% DMSO. The viability after thawing is 92–96%. MSCs and PBMCs were co-cultured in RPMI‐1640 media with 10% heat‐inactivated FBS, 2 mM glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 1× nonessential amino acids, and 1% penicillin‐streptomycin (T-cell media). At first, young and adult MSCs (at passage 5) were seeded at 1×105, 1×104, cells/well in T-cell medium. After an overnight incubation, 3×105 CFSE-labelled PBMCs per well were added to reach a final MSC:PBMC ratio of 1:3 or 1:30, respectively. The co-cultures were stimulated with 5 μg/ml of concanavalin A (ConA) [47] for 96 hours at which time T‐cell proliferation was determined by CFSE dye dilution of CD3‐positive cells using an AccuriC6 flow cytometer. The gating strategy has been described previously [47].
Mixed lymphocyte reaction (MLR) assay
PBMCs from three random donors were pooled and stained with CFSE, the pooled cells were used as responder cells [48]. For stimulator cells one donor PBMCs were used without CFSE stain. The MSC:PBMC ratios were the same as in the mitogen assay. Flow cytometry analysis was done using CD3 as a T cell marker and CFSE for monitoring proliferation on day 7, without additional stimulus.
TNF-α and IL-10 cytokine secretion assay
MSCs for cytokine assay were cultured in α-MEM, supplemented with 10% FBS, 1% GlutaMax, and 1% penicillin‐streptomycin (MSC medium). THP monocytes were obtained from ATCC. RPMI-1640, supplemented with 10% FBS, 1% penicillinstreptomycin and 50 μM β-mercaptoethanol (macrophage medium). For the cytokine production assay, 10,000 adult and young MSCs (passage 5), were seeded with 100,000 THP-1 cells per well in 200 μl of macrophage medium. A large portion of the THP1 cells adhered to the plate and spontaneously took on macrophage characteristics [49]. The ELISA assay was done in quadruplicates. Cells were allowed to adhere overnight, and then stimulated with 1μg/ml of LPS. For TNF-α production assay, the culture media were collected at six hours after stimulation with LPS. For the IL-10 production assay, the culture media was collected after twenty-four hours of stimulation. The collected culture media was either stored temporarily at −20°C or used immediately for ELISA to determine IL-10 and TNF-α levels using DuoSet ELISA (R&D Systems, Minneapolis, MD); used according to the manufacturer’s instructions). The co-cultures of both the young and adult MSCs were performed using 4–4 donors with and without LPS stimulation. With no LPS stimulation the cytokines were not detectable at all (TNFα) or were just below or at the lowest detectable standard (IL-10).
RNA-sequencing and analysis
For RNA-sequencing the cultured cells (young and adult MSCs) were prepared by TRIzol extraction (Fisher Scientific, 15–596-018) following the recommendations of the manufacturer. RNA integrity was assessed using a Fragment Analyzer (Advanced Analytical, Ankeny, IA). RNA was converted into full length cDNA using the SMART-seq UltraLow v4 kit (Clontech, Mountain View, CA) and Illumina cDNA sequencing libraries were prepared using the Nextera XT kit (Illumina, San Diego, CA). Libraries were sequenced on an IlluminaHiSeq 1500 sequencer, on 126bp paired-end mode. Raw sequences underwent initial QC analysis and were then aligned to the mouse GRCm38 genome version with STAR v2.5.2a. Raw gene read counts produced by STAR were filtered to remove low expressing genes and further processed in R (R Development Core Team, 2011) using the EdgeR package [50] and the START application [51]. A subset of genes involved in hematopoietic- and neural crest development was examined with both RNA sequencing and RT-qPCR to yield multiple, cross-supporting data sets.
RNA isolation and quantitative PCR
To confirm RNAseq results we used QPCR to detect genes of interest. Total RNA was isolated from cells and purified using the Arcturus PicoPure RNA isolation kit (ThermoFisher) according to the manufacturer`s recommendation. On-column DNA digestion was performed using Qiagen DNase (RNase free DNase kit, Qiagen) at room temperature for 30 minutes. Isolated total RNA was reverse transcribed using oligo-dT primers and Moloney-murine leukemia virus reverse transcriptase according to the instructions of the manufacturer (Promega). The resultant cDNA was amplified with QuantiTect SYBR Green RT-PCR kit (Qiagen) using the StepOnePlus Real Time PCR System (Applied Biosystems). The expression data were normalized to the housekeeping gene (HPRT). Relative gene expressions were calculated as the average of three (n=3) threshold cycle numbers (Ct) compared to GAPDH expressions: calculated as ΔΔCt. For primer sequences and target gene bank accession numbers, see Supplementary Table 2
Statistical analysis
All values are expressed as standard error of the mean (SEM) of four donors for each young and adult MSCs. Statistical significance was assessed by either student t test or one-way ANOVA followed by Tukey’s post hoc testing using Prism 7 (GraphPad Software, La Jolla, CA). p values are as follows: *p < 0.05, **p< 0.01, ***p < 0.001, ****p <0.0001.
Results
Immunophenotype, differentiation, proliferation, and clonogenic potential of MSCs
Flow cytometric analysis of young and adult MSCs confirmed that both types of MSCs were negative for hematopoietic markers CD45 and CD34. More than 95% of the cells in both populations expressed MSCs markers CD73, CD13, CD44, CD105 and CD90. HLA-I expression in young MSCs was about half that in adult MSCs. The percentage of cells which express these markers was not different among donors (Figure.1A,B). It has been reported that measuring normalized median fluorescence intensity (nMFI) is more accurate in describing the expression of markers in the population [43]. The nMFI of MSC specific positive surface markers are shown in Figure.1C. The expression of CD44 and CD73 are significantly increased in the young compared to the adult MSCs. Both young and adult MSCs were differentiated into osteoblast and adipocytes and young cells differentiated more efficiently than those from adults did (Figure.1D). To analyze the proliferative capacity of the cells, population doubling times were calculated. Young MSCs mean population doubling time is 24 hours compared to 34 hours to that of adult MSCs (Figure.1E), indicating increased proliferative potential of the young MSCs. To assess the clonogenic potential of the cells, CFU-F assays were done [2]. As seen in Figure. 2A, B the total number of CFU-F colonies was higher in young MSCs compared to adult ones. The CFU-F colonies varied in size and morphology. Large, dense (LD) colonies were typical of cells with high proliferation rates and low mobility [46]. The number of LD CFU-F were significantly more in young than adult MSC cultures (Figure. 2C). Overall, these results suggest that young MSCs have higher proliferation and differentiation capacity than adult MSCs.
Figure 1: Phenotypic, expansion and lineage differentiation differences between adult and young MSCs.
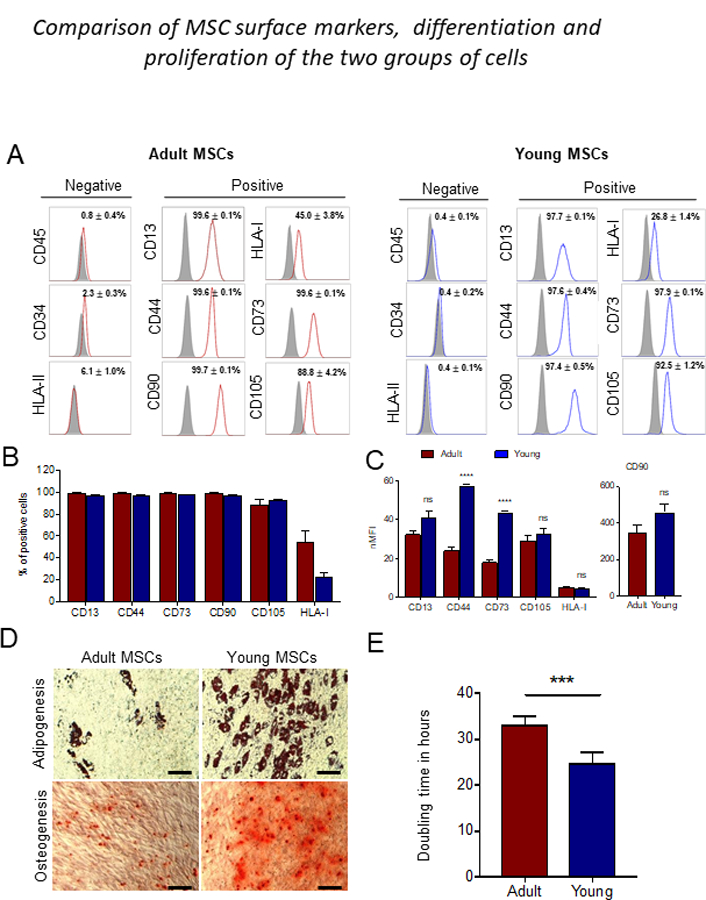
(A) Flow cytometry analysis of adult and young MSCs at passage 5. Cells were labeled with negative markers-CD45, CD34 and HLA-II, and positive markers-CD13, CD44, CD90, CD73, CD105 and HLA-I. The values represent the mean percentage of all four donors, and graphs were done with these values. Red line (adult MSCs), blue line (young MSCs), filled black indicate isotype control. (B) Graph represent mean percentage of positive marker from all four donors. (C) Normalized median fluroscence intensities (nMFI) quantification of the positive surface markers. (D) Adipogenic differentiation was indicated by neutral lipid accumulation stained with Oil Red O staining. Osteogeneic differentiation was indicated with calcium deposition stained with Alizarin red stain of adult and young MSCs. Young MSCs show higher adipogenic and osteogenic potential compared to adult MSCs. (E) Average doubling time of adult and young MSCs. Error bars represent mean ± SEM; n=4; ***p < 0.001; ns-not significant; scale bar equals 100 μm.
Figure 2: CFU-F assay.
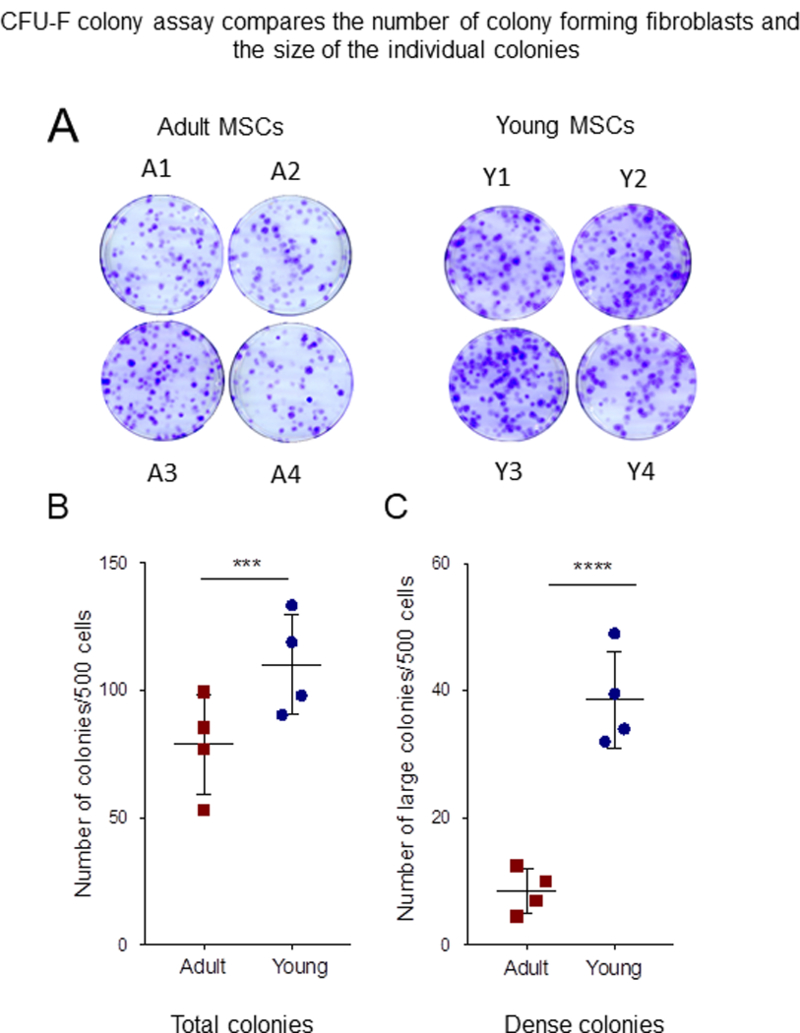
(A) 500 cells of adult and young BMSCs were seeded per 100-mm plate. After 10 days, the plates were stained with crystal violet. Pictures are crystal violet stained plate of CFU-F assay performed on four donors, adult BMSCs (A1-A4) and young (Y1-Y4). (B) Quantification of total colony number. There is significant donor variation in between the samples (C) Colony morphology was assessed by size and density. Large diameter (> 2.5 mm) and dense cell growth (> 80% confluency), the graph shown is quantification of large and dense (LD) colony number. The young cells perform significantly better than the adult cells. Error bars represent mean±SEM; n=4; *p < 0.05; **p < 0.01.
Young MSCs are more immunosuppressive than adult MSCs.
To test the potential of adult and young MSCs to suppress T-cell proliferation, we used peripheral blood mononuclear cells (PBMCs) in mitogen stimulation and mixed lymphocyte reaction (MLR) assays. PBMCs were labelled with CFSE and the cell division was measured by CFSE dye dilution in gated T cell population [39], in the presence of young and adult MSCs from different donors at 1:3 and 1:30 (MSCs:PBMCs) ratio. For mitogen stimulation assay, the cocultures were stimulated with concanavalin A (Con A) [38]. After 72 hours, flow cytometric analysis of T cell proliferation showed that adult MSCs at ratio of 1:3 significantly inhibited T cell proliferation compared to cultures without MSCs. However, at a concentration of 1:30 no suppression of T cells was observed (Figure. 3A; supplementary Figure 1A). Young MSCs at a concentration of 1:3 also significantly inhibited T cell proliferation compared to cultures without MSCs. In contrast to adult MSCs, Y1 and Y3 MSCs at 1:30 ratio was able to suppress T cell proliferation (Figure. 3B; supplementary Figure 1B).
Figure 3: Young BMSCs are more potent in inhibiting mitogen induced T cell proliferation.
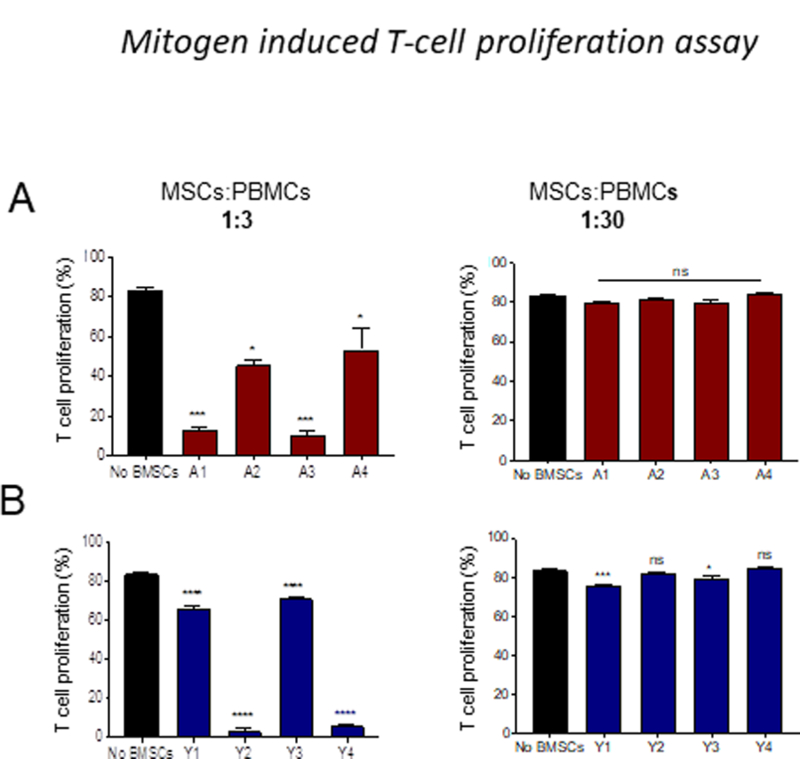
CD3 T cell proliferation was evaluated using CFSE dilution method. CFSE-labelled PBMC were stimulated with Con A (5 ug/ml) in the presence or absence of BMSCs. BMSCs to PBMCs ratio used are 1:3, 1:10 and 1:30. The data presented is the percentage of CFSE+CD3+ cells. (A) Adult BMSCs (B) Young BMSCs. Error bars represent mean±SEM; n=4; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns-not significant.
In the MLR assay, A1, A2, and A3 adult MSCs donors (Figure. 4A; supplementary Figure 2A) at a 1:3 ratio significantly inhibited T cell proliferation, but A4 donor did not inhibit T cell proliferation at this ratio. However, at a ratio of 1:30, A1 and A2 donors did not suppress T cell proliferation; A3 and A4 significantly suppressed T cell proliferation. Young MSCs inhibited T cell proliferation at both 1:3 and 1:30 ratio (Figure. 4B; supplementary Figure 2B). A significant donor variation observed with in both young and adult MSCs in both mitogen and MLR assays. Together, these results indicate that young MSCs are more potent in suppressing T cell proliferation than adult MSCs.
Figure 4: Young BMSCs show strong immunosuppressive capacity in MLR assay.
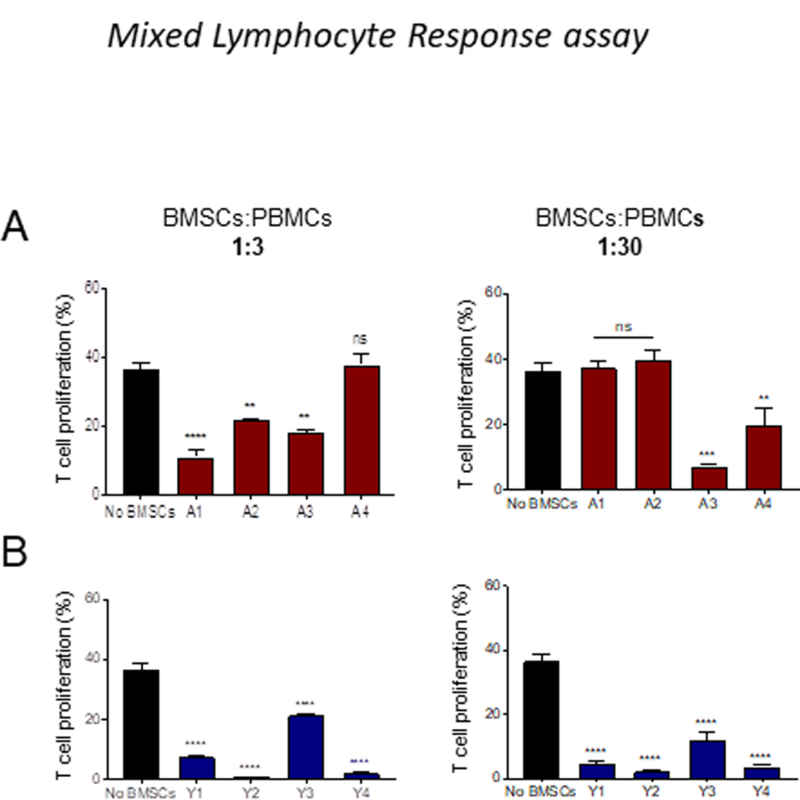
CD3 T cell proliferation was evaluated using CFSE dilution method in the MLR assay, in the presence or absence of BMSCs at various concentrations of BMSCs. Effect of BMSCs on T cell proliferation was measured on day 7 (A) Adult BMSCs (B) Young BMSCs. Error bars represent mean±SEM; n=4; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns-not significant.
Effect of age on secretion of IL-10 and TNF-α by co-cultured macrophages
We next examined the potential of adult and young MSCs to regulate macrophage effector (TNF-α) and regulatory (IL-10) cytokine production following LPS stimulation[13]. THP-1 (monocytic) cells were co-cultured with MSCs and stimulated with LPS. Analysis of the co-culture supernatant showed that the THP-1 cell derived IL- 10 production significantly increased (3–4 fold) when MSCs were in the culture (Figure. 5A). At the same time, TNF-α (pro-inflammatory cytokine) production was significantly suppressed (4 fold) by both adult and young MSCs compared to the no MSCs condition. In this assay both young and adult MSCs performed equally well (Figure. 5B), suggesting that the mechanism underlying this effect is not affected by aging.
Figure 5: Both young and adult BMSCs are equipotent in inhibiting TNFα secretion, and promoting the IL-10 production.
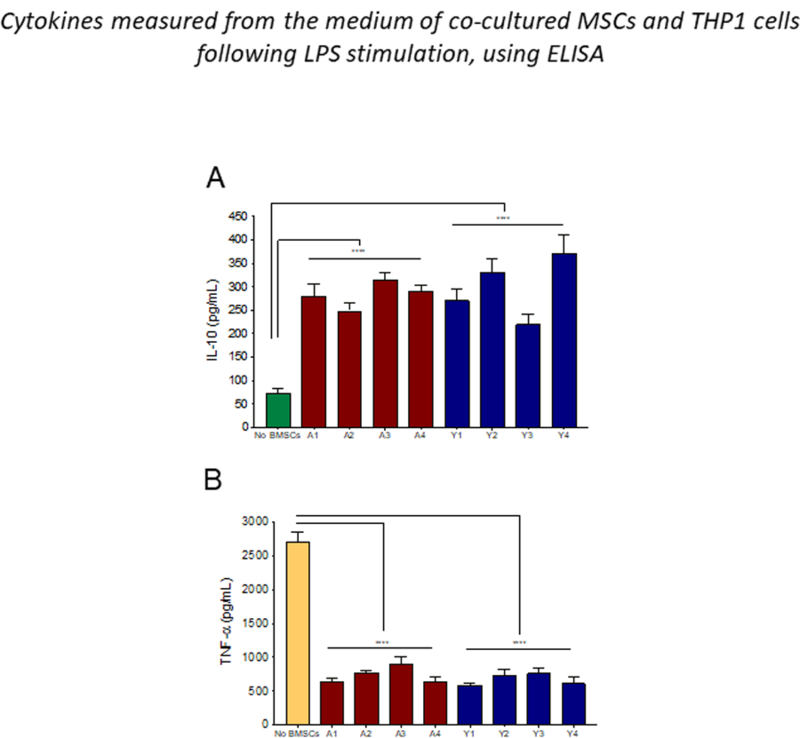
Adult and young BMSCs were directly co-culture with LPS stimulated THP-1 cells. Cell culture media were collected and ELISA is done for IL-10 (A), and TNFα (B). Error bars represent mean±SEM; n=4; ****p < 0.0001.
Differences in gene expression profiles
We used RNAseq to look for differences in gene expression between young and old donors by using two samples from each age group. The initial characterization of the RNASeq dataset (by principal component analysis, multidimensional scaling analysis, sample to sample correlation analysis and unsupervised clustering) revealed that most of the variance across samples could be explained by donor-to-donor variation. Analysis of differential gene expression returned a large number of differentially expressed (DE) genes (Suppl. Table 3). Most of these have roles in differentiation, migration, and growth based on enrichment and gene ontology analyses. This was not unexpected as there were significant age differences among the donors. It precluded more in-depth analyses because the dataset was heavily biased by this fact. We might have been able to reduce the impact of noise by increasing the number of samples studied, but this was not possible because it is difficult to obtain many samples from affected children. With more samples, process-relevant variation will persist, but irrelevant genes will vary across samples and this variation will diminish their significance yielding a cleaner dataset with a smaller number of more relevant genes across conditions. Since we could not easily obtain additional samples to study, we decided to focus our analysis on qPCR data instead of the RNASeq results. Based on the RNAseq results we used quantitative PCR to confirm the observed differences indicated by RNAseq (Figure. 6). The graph shows fold change differences compared to average of adult MSCs. The highest upregulated gene was TSG-6 (TNAIP6). Four out of five young donors showed an over 20-fold upregulation of this gene (Fig.6). Two additional genes that are upregulated 6–7-fold in young donors are hepatic growth factor (HGF) and pro- enkephalin (PENK), while COX1, COX2 and SOCS3 showed a 2–3-fold increase in the young cells. Down-regulated genes included prostaglandin E synthase (PTGES), pregnancy specific glycoprotein (PSG4) and LIF-1. No change was detected in the expression of HEMOX1, HEMOX2, PDL1, IDO, C3AR1 (C3a anaphylatoxin chemotactic receptor), IL-6 and HLADMB genes. These results confirm that there are donor variations in the immunosuppressive reported genes.
Figure 6: Q-PCR analysis of genes known to be involved in the immune modulatory effect of MSCs.
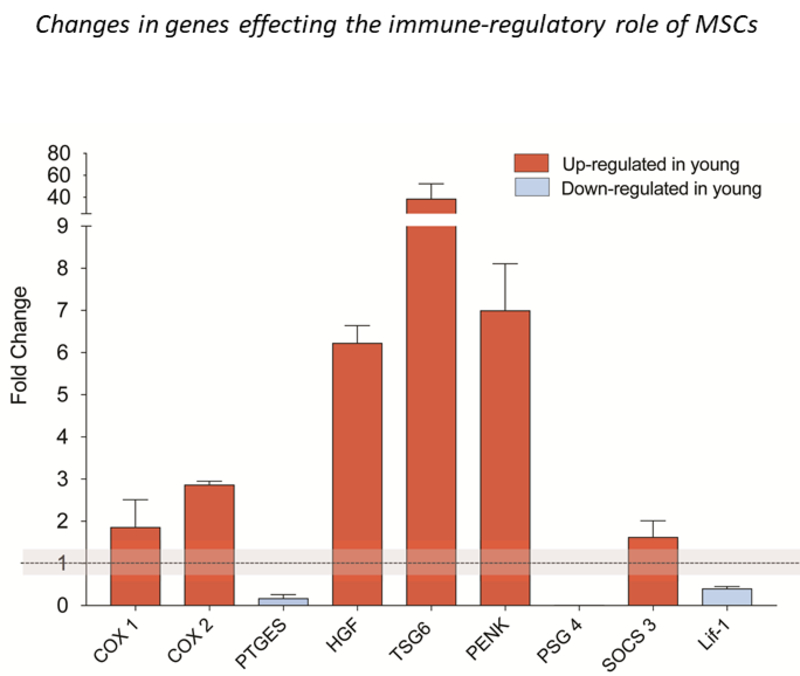
All data are expressed as fold increase/decrease of young MSCs vs. adult MSCs. Two adult and two young donors were compared and showed identical trend in COX1, COX2, HGF, PENK, and PSG4. The qPCR was run at least twice in triplicates. When there was discrepancy between the two samples, we used 3 additional donors (TSG6, SOCS3, Lif-1) and show the average of 4–5 donors. These qPCRs were also run multiple times, in duplicates. Data are average ± SEM. Adult average is considered 1 and the grey area covers the SEM of adult data (largest is shown among all genes). qPCR was run, but no change was detected in the expression of HEMOX1, HEMOX2, PDL1, IDO, C3AR1, IL-6 and HLADMB genes (not shown in the graph). Primers are shown in Supplementary Table 2.
Discussion
In the first of our studies we found that young MSCs proliferate and differentiate into osteoblasts and adipocytes more efficiently than adult MSCs do. This was not unexpected; aging correlates a decrease in stem cell numbers in a variety of tissues as well as a reduction in their capacity to proliferate [36]. The high proliferative potential of young MSCs along with a reduction in their migratory behavior was reflected in their formation large size CFU-F colonies compared to adult MSCs. The LD-CFU-F colonies represent high proliferation and less migratory potential of the young MSCs. These colonies have been reported to make less alkaline phosphatase (AP) [46], an osteoblast marker, but we observed that young MSCs differentiate efficiently into both osteoblasts and adipocytes. This is in agreement with a previous description of adipose derived stem cells, cells with low AP activity that also readily differentiate into osteoblasts and adipocytes [52]. Bone marrow stromal cells have been in clinical use for over a decade as immune-modulators [5, 7, 53]. Immunomodulation by MSCs may be related to their differentiation potential. MSCs injected into wounds during healing modulated immune responses and tissue regeneration [54], minimizing tissue damage due to inflammation and facilitating reparative and regenerative processes.
Next, we compared the immunomodulatory functions of young MSCs with adult. We examined the immunomodulatory potential of young vs adult MSCs using mitogen stimulated T cell-, MLR-, and cytokine production-assays. MSCs obtained from 10–21 months old children were more efficient in suppressing immune functions than adult MSCs. This is in agreement with previous reports that donor age influences stem cell proliferative capacity, differentiation potential and cytokine production [36, 37, 39] though Landgraf et al. found that the inhibitory effect of MSCs on T cell proliferation is preserved in old age [55]. Unlike the authors cited above, wanted to compare the ability of MSCs from developing bone marrow of very young children with those from adults.
In order to try to mimic physiological state as much as possible, we chose an assay to study immunosuppressive effects where no irradiation of MSCs or PBMCs has to be used. In mitogen induced T cell proliferation assay, we observed that both adult and young MSCs show significant inhibition of T cell proliferation at MSCs:PBMCs ratio of 1:3 and 1:30. However, we did not see dose dependent changes (i.e. higher suppression with 1:3 vs. 1:30 ratio) in either age most likely due to the fact that without irradiation the proliferation of MSCs might cause an over-crowding affecting our results. The interesting fact is that at a ratio of 1:30 (when this is not a problem anymore due to the relative rarity of MSCs) two donors of young MSCs still showed a significant immunosuppression, indicating a higher potency of young MSCs in this assay.
Our MLR assay was a two-way MLR assay with minimal cell manipulation to make it as close to physiological conditions as possible. The true potential of young MSCs was more obvious in this MLR assay than the previously described mitogen induced assay. We do not believe that the observed effect on T cell proliferation was falsely diminished due to T cell competition for nutrients, since the color of the culture medium did not show exhaustion at any point during the assay.
In contrast to the differences seen in T cell suppression between young and adult MSCs, our cytokine assay showed no difference between young and adult MSCs. In this assay we cultured THP-1 cells together with MSCs and measured the cytokine levels in the media following LPS stimulation. The assay has been used in earlier studies in mice [13] to show that MSCs can change the character of macrophages from pro-inflammatory (M1) to anti-inflammatory (M2). Human MSCs have also been shown to alter the character of cultured human peripheral blood monocyte derived macrophages that increase IL-10 and decrease TNF-α production [56]. In our assay, the LPS stimulated THP1 cells (monocytic cell line) increase their TNF-α production which increase is significantly reduced by the presence of MSCs in the culture. Reversely, THP1 cells make no or very little IL-10, but when placed in culture with MSCs, they increase their production of IL-10, a well-known anti-inflammatory cytokine. We observed these effects in all of the adult and young donors, however, there seemed to be no difference in the efficacy of MSCs between the two groups (adult vs. young) in this assay. This might be due to several differences between the above assays. In one assay the targeted immune cells are T cells, while in the other they are monocytes/macrophages. It is very possible that the MSC derived factors involved in suppressing T cell proliferation are differentially expressed between young and adult cells, while those regulating the cytokine switch in macrophages are not. Furthermore, the timing of the co-cultures is also different.
We have to take into consideration that although we were focusing on the age differences in this study, there might be other factors that differ between the two groups of cells. The young MSCs were derived from bone marrow of digits, while the adults were from iliac crest biopsies. When we used all accepted surface markers and FACS, the two populations were indistinguishable. Also, there are no data suggesting that MSCs are different depending on the source of bone marrow from which they are derived. In a recent report [57] the authors found differences between BMSCs derived from mandible and limbs (tibia) with regards to osteogenic differentiation and in vitro proliferation, but did not study the effect of MSCs on T- cell proliferation and/or their effect on inflammatory macrophages. Also, in the above-mentioned work the developmental origin of the two sources are different (the mandible has a significant neural crest contribution while the tibia does not), while our samples were all derived from limbs of both the young and the adult patients. We should also mention the fact that our young donors had non-syndromic polydactyly as did their parents. Two donors had extra digits outside the thumb; and two had an extra digit outside of the big toe. These forms of polydactyly are “preaxial”. Many different genetic alterations may underlie these developmental abnormalities [58], and our donors may not have had genetic mutations in common. We do not know whether or how such genetic changes might have affected the experimental results we obtained, but since they are likely to have differed from subject to subject, we prefer the hypothesis that the age of the donors was more important than their genotypes.
Our preliminary gene expression analyses based on RNAseq data from two of the young and two of the adult donors revealed significant young/adult differences in transcript levels of several genes that have been suggested to play a role in T cell proliferation and in immunomodulation. Among these were members of the PGE2 pathway (COX1 and COX2) that we reported earlier [13] and genes that affect innate immunity (HGF) [59–61]. Interestingly, the most upregulated gene in the young donors (in 4 out of five donors) was TSG-6 (TNFα stimulated gene 6), described by Prockop’s group in 2014 [62] as a biomarker to predict efficacy in human MSCs ability to alleviate inflammation. We also looked for differences in programmed death-1 ligands (PD-L1) that was shown to be secreted by MSCs and regulate T cell mediated immune suppression [59] and found that only in one of five samples was upregulated while the others did not change. During screening for secreted proteins from MSCs that affect immune function Milwid et al. found that proenkephalin (PENK) and microfibrillar associated protein 5 (MFAP5) contribute to increased IL-10 concentration in the serum of septic mice. We found a 6–8-fold increase in PENK in the young samples suggesting that the above findings might be relevant in humans as well. Interestingly, in our samples MFAP5 did not differ between the two groups which might be explained by looking at mRNA as opposed to protein as Milwid et al. did [63]. A difference in translation might account for the discrepancy. Kilpinen et al. [39] were studying differences in membrane glycerophospholipid composition in MSCs derived from young and older adults and found differences in a number of genes that are also known to affect innate immune function. Among these genes were C3AR1 (Complement component 3a receptor 1), SOCS3 (suppressor of cytokine signaling) and HLA-DMB (Major histocompatibility complex); only SOCS3 was found upregulated in our younger MSC samples. The most downregulated gene was PSG4, a member of the human specific glycoprotein family, originally isolated from human placental library and suggested to participate in regulation of immune-suppression [64, 65]. In addition, PTGES and LIF-1 [66] have also been down-regulated in the young samples.
Authors of a recent paper [67] suggested that the inhibition of T cell proliferation depends on phagocytosis of apoptotic MSCs in vivo. This is not the case in our cell culture models. From our results it seems that MSCs may exploit a variety of mechanisms to modulate immune function. Genes that are known to encode agents participating in the process of immune suppression seem to be differentially regulated between very young and adult patients’ MSCs. The combination of these differences might be responsible for the significantly more efficient immune suppression observed by young MSCs. There is still a lot of work to do before we understand how they do this. It is important work to do though because the more we learn, the better able we will be to select cells that work optimally in patients.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDCR. The authors would like to thank Dr. Michael J. Brownstein for advice, and for critical reading and editing the manuscript. We want to thank Ms. Carolyne Pike for her help with preparation of samples.
The NIDCR Combined Technical Research Core (ZIC DE000729‐09) for providing the PBMCs. RNAseq and biometrics were performed by the Genomics & Computational Biology core of NIDCD and NIDCR. The authors would like to thank Dr. Robert Morell for his guidance in designing the RNAseq experiment.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- [1].Phinney DG, Functional heterogeneity of mesenchymal stem cells: implications for cell therapy, Journal of cellular biochemistry 113(9) (2012) 2806–12. [DOI] [PubMed] [Google Scholar]
- [2].Bianco P, Robey PG, Simmons PJ, Mesenchymal stem cells: revisiting history, concepts, and assays, Cell stem cell 2(4) (2008) 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM, Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli, Blood 99(10) (2002) 3838–43. [DOI] [PubMed] [Google Scholar]
- [4].Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O, Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells, Lancet (London, England) 363(9419) (2004) 1439–41. [DOI] [PubMed] [Google Scholar]
- [5].Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O, Developmental B Committee of the European Group for, T. Marrow, Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study, Lancet (London, England) 371(9624) (2008) 1579–86. [DOI] [PubMed] [Google Scholar]
- [6].Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q, Mesenchymal stem cells and immunomodulation: current status and future prospects, Cell death & disease 7 (2016) e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nauta AJ, Fibbe WE, Immunomodulatory properties of mesenchymal stromal cells, Blood 110(10) (2007) 3499–506. [DOI] [PubMed] [Google Scholar]
- [8].Nemeth K, Mezey E, Bone marrow stromal cells as immunomodulators. A primer for dermatologists, J Dermatol Sci 77(1) (2015) 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S, Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans, Stem cells (Dayton, Ohio) 27(6) (2009) 1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A, Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy, Blood 106(5) (2005) 1755–61. [DOI] [PubMed] [Google Scholar]
- [11].Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R, Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes, Journal of immunology (Baltimore, Md. : 1950) 183(2) (2009) 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E, Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma, Proceedings of the National Academy of Sciences of the United States of America 107(12) (2010) 5652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E, Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production, Nature medicine 15(1) (2009) 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL, Bone marrow-derived mesenchymal stem cells in repair of the injured lung, American journal of respiratory cell and molecular biology 33(2) (2005) 145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang D, Zhang H, Liang J, Gu Z, Ma X, Huang J, Lin J, Hou Y, Lu L, Sun L, Effect of allogeneic bone marrow-derived mesenchymal stem cells transplantation in a polyI:C-induced primary biliary cirrhosis mouse model, Clinical and experimental medicine 11(1) (2011) 25–32. [DOI] [PubMed] [Google Scholar]
- [16].Yu J, Zheng C, Ren X, Li J, Liu M, Zhang L, Liang L, Du W, Han ZC, Intravenous administration of bone marrow mesenchymal stem cells benefits experimental autoimmune myasthenia gravis mice through an immunomodulatory action, Scandinavian journal of immunology 72(3) (2010) 242–9. [DOI] [PubMed] [Google Scholar]
- [17].Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L, Participants S, Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II, Stroke 42(3) (2011) 825–9. [DOI] [PubMed] [Google Scholar]
- [18].Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, Monroy R, Uberti J, Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease, Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 15(7) (2009) 804–11. [DOI] [PubMed] [Google Scholar]
- [19].von Bonin M, Stolzel F, Goedecke A, Richter K, Wuschek N, Holig K, Platzbecker U, Illmer T, Schaich M, Schetelig J, Kiani A, Ordemann R, Ehninger G, Schmitz M, Bornhauser M, Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium, Bone marrow transplantation 43(3) (2009) 245–51. [DOI] [PubMed] [Google Scholar]
- [20].Li X, Wang D, Liang J, Zhang H, Sun L, Mesenchymal SCT ameliorates refractory cytopenia in patients with systemic lupus erythematosus, Bone marrow transplantation 48(4) (2013) 544–50. [DOI] [PubMed] [Google Scholar]
- [21].Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L, Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience, Cell transplantation 22(12) (2013) 2267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S, Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis, Archives of neurology 67(10) (2010) 1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S, Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study, The Lancet. Neurology 11(2) (2012) 150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B, Immunomodulatory properties of human placental mesenchymal stem/stromal cells, Placenta (2017). [DOI] [PubMed] [Google Scholar]
- [25].Gao P, Zhou Y, Xian L, Li C, Xu T, Plunkett B, Huang SK, Wan M, Cao X, Functional effects of TGF-beta1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma, Journal of immunology (Baltimore, Md. : 1950) 192(10) (2014) 4560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ryan JM, Barry F, Murphy JM, Mahon BP, Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells, Clinical and experimental immunology 149(2) (2007) 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y, Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide, Cell stem cell 2(2) (2008) 141–50. [DOI] [PubMed] [Google Scholar]
- [28].Rani S, Ryan AE, Griffin MD, Ritter T, Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications, Mol Ther 23(5) (2015) 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B, MSC-derived exosomes: a novel tool to treat therapy- refractory graft-versus-host disease, Leukemia 28(4) (2014) 970–3. [DOI] [PubMed] [Google Scholar]
- [30].Zheng G, Huang R, Qiu G, Ge M, Wang J, Shu Q, Xu J, Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis, Cell and tissue research 374(1) (2018) 1–15. [DOI] [PubMed] [Google Scholar]
- [31].Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A, Treatment of inflammatory diseases with mesenchymal stem cells, Inflamm Allergy Drug Targets 8(2) (2009) 110–23. [DOI] [PubMed] [Google Scholar]
- [32].Bernardo ME, Fibbe WE, Mesenchymal stromal cells: sensors and switchers of inflammation, Cell stem cell 13(4) (2013) 392–402. [DOI] [PubMed] [Google Scholar]
- [33].Le Blanc K, Davies LC, MSCs-cells with many sides, Cytotherapy 20(3) (2018) 273–278. [DOI] [PubMed] [Google Scholar]
- [34].Cassano JM, Fortier LA, Hicks RB, Harman RM, Van de Walle GR, Equine mesenchymal stromal cells from different tissue sources display comparable immune-related gene expression profiles in response to interferon gamma (IFN)-gamma, Vet Immunol Immunopathol 202 (2018) 25–30. [DOI] [PubMed] [Google Scholar]
- [35].Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom HJ, Kim MG, Oh KH, Ahn C, Yang J, Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response, Transplant immunology 25(1) (2011) 7–15. [DOI] [PubMed] [Google Scholar]
- [36].Fibbe WE, Mesenchymal stem cells. A potential source for skeletal repair, Annals of the rheumatic diseases 61 Suppl 2 (2002) ii29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stolzing A, Jones E, McGonagle D, Scutt A, Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies, Mechanisms of ageing and development 129(3) (2008) 163–73. [DOI] [PubMed] [Google Scholar]
- [38].Ren J, Stroncek DF, Zhao Y, Jin P, Castiello L, Civini S, Wang H, Feng J, Tran K, Kuznetsov SA, Robey PG, Sabatino M, Intra-subject variability in human bone marrow stromal cell (BMSC) replicative senescence: molecular changes associated with BMSC senescence, Stem Cell Res 11(3) (2013) 1060–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kilpinen L, Tigistu-Sahle F, Oja S, Greco D, Parmar A, Saavalainen P, Nikkila J, Korhonen M, Lehenkari P, Kakela R, Laitinen S, Aging bone marrow mesenchymal stromal cells have altered membrane glycerophospholipid composition and functionality, Journal of lipid research 54(3) (2013) 622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alves H, van Ginkel J, Groen N, Hulsman M, Mentink A, Reinders M, van Blitterswijk C, de Boer J, A mesenchymal stromal cell gene signature for donor age, PLoS One 7(8) (2012) e42908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sabatino M, Ren J, David-Ocampo V, England L, McGann M, Tran M, Kuznetsov SA, Khuu H, Balakumaran A, Klein HG, Robey PG, Stroncek DF, The establishment of a bank of stored clinical bone marrow stromal cell products, J Transl Med 10 (2012) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ock SA, Jeon BG, Rho GJ, Comparative characterization of porcine mesenchymal stem cells derived from bone marrow extract and skin tissues, Tissue engineering. Part C, Methods 16(6) (2010) 1481–91. [DOI] [PubMed] [Google Scholar]
- [43].Chan LY, Yim EK, Choo AB, Normalized median fluorescence: an alternative flow cytometry analysis method for tracking human embryonic stem cell states during differentiation, Tissue engineering. Part C, Methods 19(2) (2013) 156–65. [DOI] [PubMed] [Google Scholar]
- [44].Taylor SE, Shah M, Orriss IR, Generation of rodent and human osteoblasts, BoneKEy reports 3 (2014) 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Myneni VD, Melino G, Kaartinen MT, Transglutaminase 2--a novel inhibitor of adipogenesis, Cell death & disease 6 (2015) e1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gothard D, Dawson JI, Oreffo RO, Assessing the potential of colony morphology for dissecting the CFU-F population from human bone marrow stromal cells, Cell and tissue research 352(2) (2013) 237–47. [DOI] [PubMed] [Google Scholar]
- [47].Myneni VD, Mezey E, Immunomodulatory effect of vitamin K2: Implications for bone health, 24(1–2) (2018) 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ketterl N, Brachtl G, Schuh C, Bieback K, Schallmoser K, Reinisch A, Strunk D, A robust potency assay highlights significant donor variation of human mesenchymal stem/progenitor cell immune modulatory capacity and extended radio-resistance, Stem Cell Res Ther 6 (2015) 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chanput W, Mes JJ, Wichers HJ, THP-1 cell line: an in vitro cell model for immune modulation approach, Int Immunopharmacol 23(1) (2014) 37–45. [DOI] [PubMed] [Google Scholar]
- [50].McCarthy DJ, Chen Y, Smyth GK, Differential expression analysis of multifactor RNA- Seq experiments with respect to biological variation, Nucleic Acids Res 40(10) (2012) 4288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nelson JW, Sklenar J, Barnes AP, Minnier J, The START App: a web-based RNAseq analysis and visualization resource, Bioinformatics 33(3) (2017) 447–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ock SA, Baregundi Subbarao R, Lee YM, Lee JH, Jeon RH, Lee SL, Park JK, Hwang SC, Rho GJ, Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue, Stem cells international 2016 (2016) 9581350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mezey E, Nemeth K, Mesenchymal stem cells and infectious diseases: Smarter than drugs, Immunol Lett 168(2) (2015) 208–14. [DOI] [PubMed] [Google Scholar]
- [54].Cerqueira MT, Pirraco RP, Marques AP, Stem Cells in Skin Wound Healing: Are We There Yet?, Advances in Wound Care 5(4) (2016) 164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Landgraf K, Brunauer R, Lepperdinger G, Grubeck-Loebenstein B, The suppressive effect of mesenchymal stromal cells on T cell proliferation is conserved in old age, Transplant immunology 25(2–3) (2011) 167–72. [DOI] [PubMed] [Google Scholar]
- [56].Kim J, Hematti P, Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages, Exp Hematol 37(12) (2009) 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lloyd B, Tee BC, Headley C, Emam H, Mallery S, Sun Z, Similarities and differences between porcine mandibular and limb bone marrow mesenchymal stem cells, Arch Oral Biol 77 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Biesecker LG, Polydactyly: how many disorders and how many genes? 2010 update, Dev Dyn 240(5) (2011) 931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Le Blanc K, Davies LC, Mesenchymal stromal cells and the innate immune response, Immunol Lett 168(2) (2015) 140–6. [DOI] [PubMed] [Google Scholar]
- [60].Bartosh TJ, Ylostalo JH, Bazhanov N, Kuhlman J, Prockop DJ, Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1), Stem cells (Dayton, Ohio) 31(11) (2013) 2443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Prockop DJ, Oh JY, Lee RH, Data against a Common Assumption: Xenogeneic Mouse Models Can Be Used to Assay Suppression of Immunity by Human MSCs, Mol Ther 25(8) (2017) 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, Oh JY, Prockop DJ, TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo, Proceedings of the National Academy of Sciences of the United States of America 111(47) (2014) 16766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Milwid JM, Elman JS, Li M, Shen K, Manrai A, Gabow A, Yarmush J, Jiao Y, Fletcher A, Lee J, Cima MJ, Yarmush ML, Parekkadan B, Enriched protein screening of human bone marrow mesenchymal stromal cell secretions reveals MFAP5 and PENK as novel IL-10 modulators, Mol Ther 22(5) (2014) 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chan WY, Zheng QX, McMahon J, Tease LA, Characterization of new members of the pregnancy-specific beta 1-glycoprotein family, Mol Cell Biochem 106(2) (1991) 161–70. [DOI] [PubMed] [Google Scholar]
- [65].Zheng QX, Tease LA, Shupert WL, Chan WY, Characterization of cDNAs of the human pregnancy-specific beta 1-glycoprotein family, a new subfamily of the immunoglobulin gene superfamily, Biochemistry 29(11) (1990) 2845–52. [DOI] [PubMed] [Google Scholar]
- [66].Najar M, Raicevic G, Boufker HI, Fayyad-Kazan H, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L, Adipose-tissue-derived and Wharton’s jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor, Tissue Eng Part A 16(11) (2010) 3537–46. [DOI] [PubMed] [Google Scholar]
- [67].Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F, Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation, Sci Transl Med 9(416) (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


