Abstract
In this case-control study, the influence of waterpipe tobacco smoking on the plasma and saliva levels of cadmium, lead, and zinc was examined in participants who were waterpipe tobacco smokers (WS) or never-smokers (NS). The concentration of metals was higher in WS relative to NS. The mean (SEM) cadmium concentration in plasma was 3.3 (0.18)μg/dL in WS versus 0.82 (0.09)μg/L in NS (P < 0.001) and in saliva was 5.1 (0.36)μg/L in WS versus 0.64 (0.2)μg/L in NS (P < 0.001). The mean (SEM) lead concentration in plasma was 5.2 (0.25) μg/dL in WS versus 3.4 (0.41) μg/dL in NS (P < 0.01) and in saliva was 4.8 (0.58) μg/L in WS versus 2.8 (0.27) μg/L in NS (P < 0.05). Similarly, a significant difference in zinc concentration was observed, with a mean of 2.0 (0.17) μg/mL in WS plasma versus 1.49 (0.16) μg/mL in NS (P < 0.05) and a mean 0.94 (0.07) μg/mL in WS saliva versus 0.45 (0.06) μg/mL in NS (P < 0.01). In conclusion, waterpipe tobacco smoking is associated with elevated levels of metals in body fluids. These results provide another demonstration of how waterpipe tobacco smoking exposes smokers to harmful toxicants.
Keywords: Tobacco, waterpipe, smoking, toxicants, metals, saliva, serum
1. INTRODUCTION:
Waterpipe tobacco smoking has spread globally and can be found across the Arab world, the Indian subcontinent, as well as across Europe, North America, and Australia (Ward et al. 2015; Hammal et al. 2016; Lopez et al. 2017; Ramji et al. 2017; Jawad et al. 2018). Recently, rates of waterpipe tobacco smoking have been rising relative to cigarette smoking (Maziak et al. 2017; Jawad et al. 2018). Waterpipe smoking is associated with several health problems (Ali and Jawad 2017; Waziry et al. 2017), including cardiovascular disease (Alomari et al. 2014; Haddad et al. 2016; Hammal et al. 2016), respiratory disease (Khabour, Alzoubi, Bani-Ahmad, et al. 2012), and cancer (Mamtani et al. 2017).
Numerous studies have demonstrated that waterpipe tobacco smoke contains many toxicants, including the dependence-producing drug nicotine, carcinogenic polycyclic aromatic hydrocarbons (PAHs), pulmonary disease-causing volatile aldehydes, and carbon monoxide (CO) that contributes to cardiovascular disease (Shihadeh 2003). Many of these toxicants have been found in the body fluids of waterpipe tobacco smokers (Blank et al. 2011), and waterpipe-induced exposure to nicotine and CO has clear physiological effects in the short-term (Blank et al. 2011; Retzky 2017). Metals are another class of toxicants that have been found in waterpipe tobacco smoke, and they include cobalt, chromium, nickel, cadmium and lead (Shihadeh 2003; Apsley et al. 2011; Schubert et al. 2015). For example, lead has been found in waterpipe smoke, in the range of 0.2–6.8 μg/session (Shihadeh 2003; Apsley et al. 2011). This observation is alarming due to the fact that lead is neurotoxic and long-term exposure to it via waterpipe tobacco smoking could result in cognitive impairments and other neurological disorders (Liu et al. 2013; Caito and Aschner 2017). Cadmium also has been found in waterpipe (0.10 – 0.27 μg/session) smoke (Apsley et al. 2011) and long-term exposure to this toxic element can cause kidney disease, fragile bones, hypertension, anemia, cancer, and cardiovascular disease (Rafati Rahimzadeh et al. 2017; Rinaldi et al. 2017). Unlike other toxicants such as PAHs, nicotine, and CO, there have been no studies examining if metals found in waterpipe tobacco smoke are also found in the body fluids of waterpipe tobacco smokers. Thus, the current study was conducted to examine the concentration of three metals (cadmium, lead, and zinc) in the blood and saliva of a group of waterpipe tobacco smokers, with a group of never smokers serving as a control. Based on analyses of waterpipe smoke toxicant content, we hypothesized that higher concentrations of metals would be observed in participants with a history of waterpipe tobacco smoking, relative to those who had no history of tobacco smoking. Results revealed the extent to which waterpipe tobacco smokers are exposed to these toxicants and serve as a baseline for future research measuring metal exposure among waterpipe smokers in areas where this form of tobacco use is common.
2. METHODS:
2.1. Subjects:
In this case-control study, we examined the influence of waterpipe tobacco smoking on the plasma and saliva levels of three metals in 88 Jordanian waterpipe smokers as compared to 40 Jordanian never-smokers. Waterpipe smokers were defined as those who smoke only waterpipe and did not use any other form of tobacco consumption. Never-smokers were those who did not use tobacco product during their life. The study protocol was approved by institutional review board of Jordan University of Science and Technology. A validated questionnaire (Khabour, Alzoubi, Eissenberg, et al. 2012) was used to collect participants’ demographics and waterpipe use. Waterpipe dependence was assessed using the Lebanese Waterpipe Dependence Scale (LWDS-11) scale as previously described (Primack et al. 2014). Informed consent was obtained from all subjects after verbal and written explanation of the study objectives and procedures.
2.2. Samples Collection:
A total of 5 milliliter of venous blood was drawn from an antecubital vein in EDTA tubes. Tubes were mixed immediately and then centrifuged at 1500 xg for 5 minutes to obtain plasma. Saliva samples were collected as described previously (Azab et al. 2015). In brief, participants rinsed their mouth three times with distilled water and, 5 minutes after, about 2 mL of saliva was collected. It is worth to mention that distilled water was not evaluated for trace metal contamination, however, the same source of water was used for smokers and never-smokers. Aliquots of plasma and saliva samples were transferred to 1 mL tubes, sealed and then stored at −20°C for later analysis.
2.3. Analysis of metals content:
Plasma samples were processed as previously described (Massadeh et al. 2017; Massadeh and Al-Massaedh 2018). Briefly, each sample was thawed at room temperature, and was treated with 3 mL HNO3 and 1 mL HClO4 in a cleaned porcelain evaporating dish. The mixture was heated at 105°C until dry. Next, 5 mL of 1% HNO3 was added to the dry mixture and subsequently filtered through 0.45-μm filter paper. The filtrate was completed to 25 mL with deionized water resulting in a clear colorless solution. The solution was kept in a polyethylene bottle and stored at 4°C until analysis. Lab blank underwent the same procedure without blood sample. Analysis of metals was carried out using coupled plasma optical emission spectrometry (ICP-OES, VISTA-MPX, CCD simultaneous ICPOES, VARIAN, nebulizer type: glass concentric with pressure of 200 kPa) at the Department of Chemistry at Yarmouk University, Jordan. Wavelength used were Cadmium: 214.439 nm, Lead: 220.353 nm and Zinc: 213.857 nm, with plasma argon flow rate of 12 L/min, auxiliary argon flow rate of 0.6 L/min, nebulizer argon flow rate of 0.4 L/min, integration time 100 s, read delay 20 s, and peristaltic pump flow rate of 1 mL/min (Massadeh et al. 2017; Massadeh and Al-Massaedh 2018).
2.4. Statistical analysis:
Two group comparisons were performed using the Student’s t test. All analysis and graphics were done using the GraphPad Prism software (version 5, La Jolla, CA). A threshold of p < 0.05 was used to indicate significant differences.
3. RESULTS:
The study included 88 waterpipe tobacco smokers with no other smoking history (WS) and 40 never-smokers (NS). The mean (SD) age of WS group and NS group was 31.7 (9.3) years and of the NS was 32.9 (13.2) years (P > 0.50, Table 1). The majority of both groups were men (60% WS, 57.5% NS; (P > 0.20). About 50% of the WS used waterpipe daily, while 36.3% used it weekly. The mean (SD) LWDS-11 score of the WS group was 12.26 (4.3), out of a possible 33 maximum score (Table 1).
Table 1:
Demographics of waterpipe users and never-smokers (controls)
| variable | Smokers (N=88) |
Never-smokers (N=40) |
|---|---|---|
| Age(mean± SD) | 31.7± 9.3 | 32.9 ± 13.2 |
| Gender (n)% | ||
| Male | 51(60.0) | 23(57.5) |
| Female | 37(40.0) | 17(42.5) |
| Waterpipe smoking | ||
| Daily | 45(51.2) | |
| Weekly | 32(36.3) | NA |
| Occasionally (1–3 times/month) | 11 (12.5) | |
| LWDS 11 score (mean ± SD) | 12.26 ± 4.3 | NA |
LWDS: Lebanon Waterpipe Dependence Scale, SD: Standard Deviation
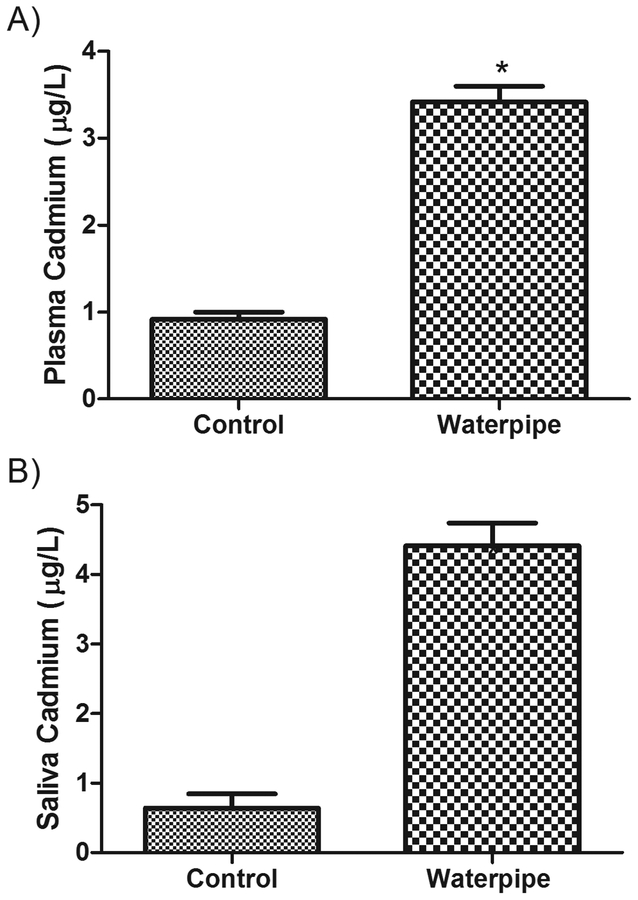
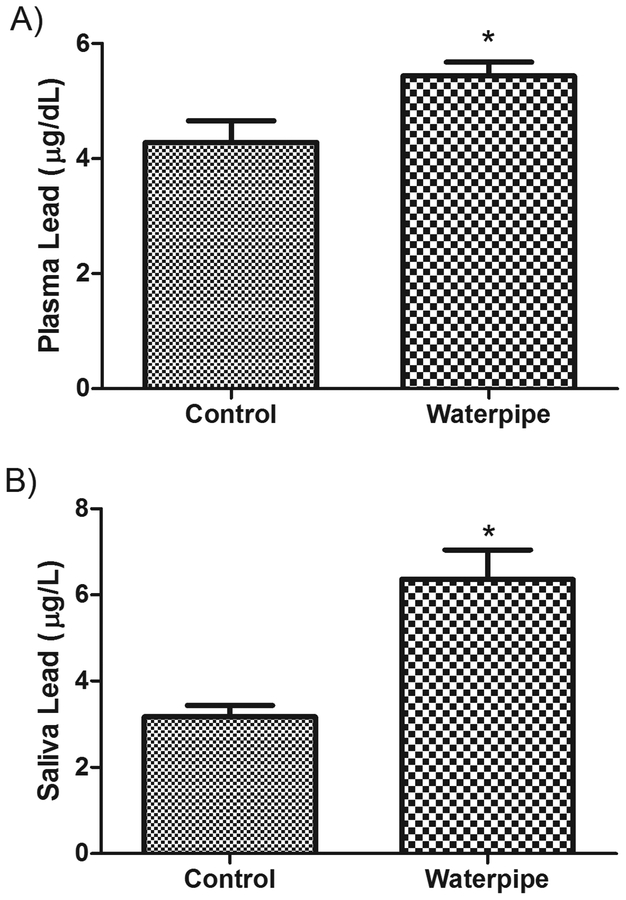
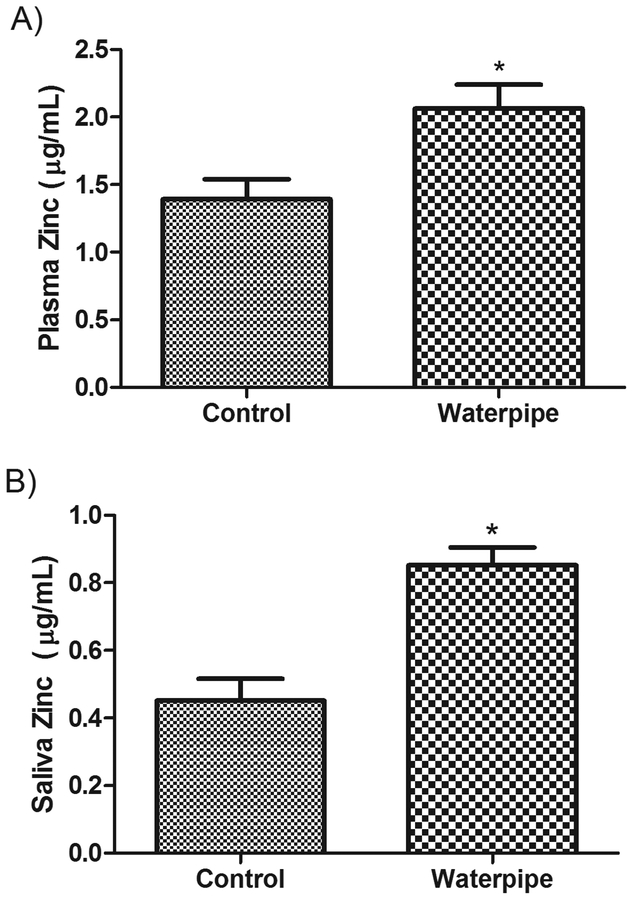
The levels of three metals were compared between WS and NS groups in both plasma and saliva samples. The concentration of metals was higher in WS relative to NS. The mean (SEM) cadmium concentration in plasma was 3.3 (0.18) μg/dL in WS versus 0.82 (0.09) μg/L in NS (P < 0.001, Figure 1 A) and in saliva was 5.1 (0.36) μg/L in WS versus 0.64 (0.2) μg/L in NS (P < 0.001, Figure 1B). The mean (SEM) lead concentration in plasma was 5.2 (0.25) μg/dL in WS versus 3.4 (0.41) μg/dL in NS (P < 0.01, Figure 2A) and in saliva was 4.8 (0.58) μg/L in WS versus 2.8 (0.27) μg/L in NS (P < 0.05, Figure 2B). Similarly, a significant difference in zinc concentration was observed, with a mean of 2.0 (0.17) μg/mL in WS plasma versus 1.49 (0.16) μg/mL in NS (P < 0.05, Figure 3A) and a mean 0.94 (0.07) μg/mL in WS saliva versus 0.45 (0.06) μg/mL in NS (P < 0.01, Figure 3B).
Figure 1: Levels of Cadmium in the body fluids of waterpipe smokers.
Cadmium levels were measured in plasma (A) and saliva (B) samples obtained from waterpipe smokers and never smokers. Significant elevations in Cadmium levels were observed in both preparations. Data are expressed as Mean ± SEM. *indicates significant difference.
Figure 2: Levels of Lead in the body fluids of waterpipe smokers.
Lead levels were measured in plasma (A) and saliva (B) samples obtained from pure waterpipe smokers and never smokers. Significant elevations in Lead levels were observed in both preparations. Data are expressed as Mean ± SEM. *indicates significant difference.
Figure 3: Levels of Zinc in the body fluids of waterpipe smokers.
Zinc levels were measured in plasma (A) and saliva (B) samples obtained from pure waterpipe smokers and never smokers. Significant elevations in Zinc levels were observed in both preparations. Data are expressed as Mean ± SEM. *indicates significant difference.
Levels of metals in the waterpipe group were correlated with LWDS. Positive correlations were found between LWDS and cadmium plasma levels (r2 = 0.060, P = 0.04), lead plasma levels (r2= 0.142, P = 0.01) and lead saliva levels (r2 = 0.062, P = 0.03). However, no correlations were detected between LWDS and cadmium saliva levels r2 = 0.034, P = 0.17), zinc plasma levels (r2 = 0.011, P = 0.50) and zinc saliva levels (r2 = 0.008, P = 0.62).
4. DISCUSSION
The current study was based on the observation that waterpipe tobacco smoke contains metals (Shihadeh 2003; Apsley et al. 2011; Schubert et al. 2015) and was conducted to determine if waterpipe tobacco smoking is associated with elevated concentration of cadmium, lead, and zinc in plasma and saliva of waterpipe tobacco smokers. Results demonstrate that, relative to never-smokers, there is a greater concentration of these metals in the blood and saliva of waterpipe tobacco smokers. These findings from a Jordanian sample are consistent with those from a study of Saudi Arabian waterpipe tobacco smokers that showed, using inductive coupled plasma mass spectrometry (ICP-MS), significant elevations in Pb, Cr, Zn, Ni and Hg in the nails of waterpipe smokers (AL-Ramadi et al. 2017).
That waterpipe tobacco smoking was associated with higher concentrations of lead in body fluids is alarming due to the documented toxicity of lead to different body organs including brain (Liu et al. 2013; Caito and Aschner 2017). Similarly, cadmium is a toxic element and prolonged exposure to it can cause kidney and bone damage, cardiovascular diseases and cancer (Rafati Rahimzadeh et al. 2017; Rinaldi et al. 2017). The magnitude of the increase in body fluid metals was between 300–700% for cadmium, 50–70% for lead, and 25–55% for zinc. The mean lead concentration in plasma of waterpipe smokers was 5.2 μg/dL. This means that approximately, 50% of waterpipe smokers have high levels of lead according to the National Institute for Occupational Safety and Health (NIOSH), which designated 5 μg/dL in whole blood as the reference blood lead level for adults (Weiss et al. 2017). Previous studies indicated that lead exposure at low doses can lead to adverse cardiovascular and kidney effects, cognitive dysfunction, and adverse reproductive outcomes (Liu et al. 2013; Caito and Aschner 2017). Impaired renal function was associated with blood lead levels at 5 μg/dL or lower, and increased risk of hypertension and essential tremor at blood lead levels below 10 μg/dL (ul Haq et al. 2013). With respect to cadmium, it was several fold higher in the waterpipe smokers than never-smokers. This is expected since smoking was considered as a major source of cadmium exposure in general population. Once cadmium enters the body, it is usually accumulated in the body and then slowly excreted in urine (Satarug 2018). Diet and occupational exposure are also additional sources of cadmium exposure (Moon et al. 2016). Exposure to low levels of cadmium over long periods of time was associated with kidney and lung diseases (Rafati Rahimzadeh et al. 2017; Rinaldi et al. 2017). The observed slight elevation in zinc levels in the waterpipe smokers is of less clinical significance as health consequences of zinc exposure are observed at much higher levels than that observed in the current study. It is worth to mention that some studies in the literature have reported significantly low zinc levels in blood samples in smokers than non-smokers (Suarez-Varela et al. 2015). However, in the current study, waterpipe smoking is associated with slightly elevated plasma and saliva zinc levels. This could be explained by the use of charcoal that contains zinc (Baker et al. 2011) to burn tobacco in the waterpipe.
The results showed weak positive correlations between LWDS and cadmium, and lead plasma and saliva levels, whereas no correlation was detected between LWDS and zinc plasma/saliva levels or cadmium saliva levels. Such weak or no correlations could be explained by the wide diversity in the types of “moassel” and charcoals used in waterpipe smoking (Shihadeh 2003; Apsley et al. 2011; Schubert et al. 2015). Al these findings need to be confirmed in a larger sample size study that needs to consider types of “moassel” and charcoal used by the participants.
These results should be used to inform public awareness campaigns regarding the health effects of waterpipe tobacco smoking. They also can guide policy related to waterpipe tobacco smoking, keeping in mind that waterpipe tobacco smoking may involve multiple sources of metal exposure, including the charcoal used to heat the tobacco, aluminum that separates the charcoal from the tobacco, and the tobacco itself. Thus, relevant policy may include requiring manufacturers to provide regulators with the metal content of waterpipe charcoal and tobacco, and, potentially, regulating the amount of metals that can be in these products if they are to be sold to consumers.
Current findings indicate that waterpipe tobacco smoking was associated with higher concentrations of cadmium, lead, and zinc in the body fluids of waterpipe tobacco smokers. Regular intake of these toxicants could lead to serious disease. These findings should encourage the development interventions and the enforcement of policies to control the waterpipe smoking epidemic through health education programs targeting all age groups.
Among the limitations of the current study is that only three metals were evaluated. In addition, levels of metals in urine were not investigated. Furthermore, confounding factors such as diet, occupational exposure, and second hand smoke exposure to metals or smoke containing metals were not included in the study design. Therefore, future research with a more extensive design that measures levels of other metals, include all body fluids, and accounts for second hand smoke exposure in a larger sample of waterpipe smokers is strongly recommended.
ACKNOWLEDGMENTS:
Authors thank Jordan University of Science and Technology for providing support to conduct the study (grant number 16/2016 to OK, and grant number 79/2013 to KA). The study was also supported by fund National Cancer Institute (NCI) of the National Institutes of Health (NIH) under Award Number PAR-15-55 (JUST: 319-2015). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the NCI.
Footnotes
CONFLICT OF INTEREST:
Authors have no conflict of interest to declare
REFERENCES:
- AL-Ramadi MA, AL-Askar NA, Mostafa GA. 2017. Simultaneous determination of some heavy metals in nail samples of Saudi Arabian smokers by inductive coupled plasma mass spectrometry. Biomedical Research. 28(10):4568–4574. [Google Scholar]
- Ali M, Jawad M. 2017. Health Effects of Waterpipe Tobacco Use: Getting the Public Health Message Just Right. Tobacco use insights. 10:1179173X17696055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomari MA, Khabour OF, Alzoubi KH, Shqair DM, Eissenberg T. 2014. Central and peripheral cardiovascular changes immediately after waterpipe smoking. Inhalation toxicology. 26(10):579–587. [DOI] [PubMed] [Google Scholar]
- Apsley A, Galea KS, Sánchez-Jiménez A, Semple S, Wareing H, van Tongeren M. 2011. Assessment of polycyclic aromatic hydrocarbons, carbon monoxide, nicotine, metal contents and particle size distribution of mainstream Shisha smoke. Journal of Environmental Health Research. 11(2):93–103. [Google Scholar]
- Azab M, Khabour OF, Alzoubi KH, Mahmoud SA, Anabtawi M, Quttina M. 2015. Assessment of genotoxicity of waterpipe smoking using 8-OHdG biomarker. Genetics and molecular research : GMR. 14(3):9555–9561. [DOI] [PubMed] [Google Scholar]
- Baker LL, Strawn DG, Rember WC, Sprenke KF. 2011. Metal content of charcoal in mining-impacted wetland sediments. The Science of the total environment. 409(3):588–594. [DOI] [PubMed] [Google Scholar]
- Blank MD, Cobb CO, Kilgalen B, Austin J, Weaver MF, Shihadeh A, Eissenberg T. 2011. Acute effects of waterpipe tobacco smoking: a double-blind, placebo-control study. Drug and alcohol dependence. 116(1–3):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito S, Aschner M. 2017. Developmental Neurotoxicity of Lead. Advances in neurobiology. 18:3–12. [DOI] [PubMed] [Google Scholar]
- Haddad L, Kelly DL, Weglicki LS, Barnett TE, Ferrell AV, Ghadban R. 2016. A Systematic Review of Effects of Waterpipe Smoking on Cardiovascular and Respiratory Health Outcomes. Tobacco use insights. 9:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammal F, Wild TC, Finegan BA. 2016. Knowledge About the Waterpipe (Hookah), a Qualitative Assessment Among Community Workers in a Major Urban Center in Canada. Journal of community health. 41(4):689–696. [DOI] [PubMed] [Google Scholar]
- Jawad M, Charide R, Waziry R, Darzi A, Ballout RA, Akl EA. 2018. The prevalence and trends of waterpipe tobacco smoking: A systematic review. PloS one. 13(2):e0192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Bani-Ahmad M, Dodin A, Eissenberg T, Shihadeh A. 2012. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhalation toxicology. 24(10):667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Eissenberg T, Mehrotra P, Azab M, Carroll MV, Afifi RA, Primack BA. 2012. Waterpipe tobacco and cigarette smoking among university students in Jordan. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 16(7):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Hao JH, Zeng Y, Dai FC, Gu PQ. 2013. Neurotoxicity and biomarkers of lead exposure: a review. Chinese medical sciences journal = Chung-kuo i hsueh k’o hsueh tsa chih. 28(3):178–188. [DOI] [PubMed] [Google Scholar]
- Lopez AA, Eissenberg T, Jaafar M, Afifi R. 2017. Now is the time to advocate for interventions designed specifically to prevent and control waterpipe tobacco smoking. Addictive behaviors. 66:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamtani R, Cheema S, Sheikh J, Al Mulla A, Lowenfels A, Maisonneuve P. 2017. Cancer risk in waterpipe smokers: a meta-analysis. International journal of public health. 62(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massadeh AM, Al-Massaedh AAT. 2018. Determination of heavy metals in canned fruits and vegetables sold in Jordan market. Environmental science and pollution research international. 25(2):1914–1920. [DOI] [PubMed] [Google Scholar]
- Massadeh AM, El-Khateeb MY, Ibrahim SM. 2017. Evaluation of Cd, Cr, Cu, Ni, and Pb in selected cosmetic products from Jordanian, Sudanese, and Syrian markets. Public health. 149:130–137. [DOI] [PubMed] [Google Scholar]
- Maziak W, Ben Taleb Z, Jawad M, Afifi R, Nakkash R, Akl EA, Ward KD, Salloum RG, Barnett TE, Primack BA et al. 2017. Consensus statement on assessment of waterpipe smoking in epidemiological studies. Tobacco control. 26(3):338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon CS, Yang HR, Nakatsuka H, Ikeda M. 2016. Time trend of cadmium intake in Korea. Environmental health and preventive medicine. 21(3):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primack BA, Khabour OF, Alzoubi KH, Switzer GE, Shensa A, Carroll MV, Azab M, Eissenberg T. 2014. The LWDS-10J: reliability and validity of the Lebanon Waterpipe Dependence Scale among university students in Jordan. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 16(7):915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. 2017. Cadmium toxicity and treatment: An update. Caspian journal of internal medicine. 8(3):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji R, Arnetz BB, Nilsson M, Wiklund Y, Jamil H, Maziak W, Arnetz J. 2017. Waterpipe use in adolescents in Northern Sweden: Association with mental well-being and risk and health behaviours. Scandinavian journal of public health.1403494817746534. [DOI] [PubMed] [Google Scholar]
- Retzky SS. 2017. Carbon Monoxide Poisoning from Hookah Smoking: An Emerging Public Health Problem. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 13(2):193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi M, Micali A, Marini H, Adamo EB, Puzzolo D, Pisani A, Trichilo V, Altavilla D, Squadrito F, Minutoli L. 2017. Cadmium, Organ Toxicity and Therapeutic Approaches: A Review on Brain, Kidney and Testis Damage. Current medicinal chemistry. 24(35):3879–3893. [DOI] [PubMed] [Google Scholar]
- Satarug S 2018. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics. 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J, Muller FD, Schmidt R, Luch A, Schulz TG. 2015. Waterpipe smoke: source of toxic and carcinogenic VOCs, phenols and heavy metals? Archives of toxicology. 89(11):2129–2139. [DOI] [PubMed] [Google Scholar]
- Shihadeh A 2003. Investigation of mainstream smoke aerosol of the argileh water pipe. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 41(1):143–152. [DOI] [PubMed] [Google Scholar]
- Suarez-Varela MM, Llopis-Gonzalez A, Gonzalez Albert V, Lopez-Izquierdo R, Gonzalez-Manzano I, Chaves J, Biosca VH, Martin-Escudero JC. 2015. Zinc and smoking habits in the setting of hypertension in a Spanish populations. Hypertension research : official journal of the Japanese Society of Hypertension. 38(2):149–154. [DOI] [PubMed] [Google Scholar]
- ul Haq N, Tabassum S, Anjum R, Fatima B. 2013. Lead, hypertension, and chronic renal failure. Journal of Ayub Medical College, Abbottabad : JAMC. 25(1–2):96–99. [PubMed] [Google Scholar]
- Ward KD, Siddiqi K, Ahluwalia JS, Alexander AC, Asfar T. 2015. Waterpipe tobacco smoking: The critical need for cessation treatment. Drug and alcohol dependence. 153:14–21. [DOI] [PubMed] [Google Scholar]
- Waziry R, Jawad M, Ballout RA, Al Akel M, Akl EA. 2017. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. International journal of epidemiology. 46(1):32–43. [DOI] [PubMed] [Google Scholar]
- Weiss D, Tomasallo CD, Meiman JG, Alarcon W, Graber NM, Bisgard KM, Anderson HA. 2017. Elevated Blood Lead Levels Associated with Retained Bullet Fragments - United States, 2003–2012. MMWR Morbidity and mortality weekly report. 66(5):130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]