ABSTRACT
Background
Minimizing consumption of added sugars is recommended to prevent excessive weight gain among pregnant women. A common approach to lowering sugar intake is the use of low-calorie sweeteners (LCSs), yet little is known about LCS use during pregnancy or its effects on infant weight and health.
Objective
The aim of the study was to investigate temporal trends in LCS consumption by source (foods, beverages, or packets) among pregnant women in the United States from 1999 to 2014 and to compare recent LCS consumption patterns across sociodemographic subgroups and product categories.
Methods
Data were collected from pregnant women aged 20–39 y (n = 1,265) who participated in the NHANES from 1999–2000 through 2013–2014. Prevalence of LCS consumption was assessed using two 24-h dietary recalls. Analytical procedures for complex survey design were used, and sampling weights were applied to estimate national prevalence of LCS use. Rao–Scott modified chi-square tests were used to compare consumption prevalence across sociodemographic subgroups, and logistic regression was used to examine trends in LCS use over time.
Results
The prevalence of LCS consumption among pregnant women increased by approximately 50% rising from 16.2% in 1999–2004 to 24.0% in 2007–2014, P = 0.04, with the highest prevalence observed in 2005–2006 (38.4%). This trend was driven predominantly by increases in LCS beverage use (9.9% in 1999–2004 compared with 18.3% in 2007–2014, P = 0.02). Prevalence of LCS consumption was highest among non-Hispanic white women and increased with education and income. No differences were observed based on prepregnancy weight status or trimester of pregnancy.
Conclusions
Approximately one-quarter of pregnant women in the United States reported consumption of LCS during at least 1 of 2 dietary recalls. Given the widespread LCS consumption during pregnancy, research to elucidate potential effects of early life LCS exposure on taste preferences, weight trajectory, and risk of later metabolic disease is needed.
Keywords: diet soda, artificial sweeteners, gestational weight gain, soft drinks, children
Introduction
Low-calorie sweeteners (LCSs) provide sweetness without calories and are commonly used as replacements for added sugars by food manufacturers and consumers. Consumption of LCSs is widespread in US children (25%) and nonpregnant adults (41%) and has increased over the past decade (1, 2). In nonpregnant adults, LCS use is associated with obesity, diabetes, and other unfavorable health consequences in epidemiologic studies (3, 4), yet randomized controlled trials demonstrate beneficial (5) or neutral (3) effects on body weight. LCS use below the acceptable
daily intake level established by the US FDA is considered safe during pregnancy (6); meanwhile, recommendations for child LCS consumption are inconsistent (7).
It was recently reported that maternal LCS use during pregnancy is associated with higher infant body weight at 1 year of age (8). A similar study demonstrated that maternal LCS consumption predicted higher birth weight and increased risk of childhood obesity at 7 y of age (9). However, in other studies, no associations were observed between maternal LCS consumption and birthweight (10) or weight gain during childhood (11). Although a causal relation between in utero LCS exposure and future weight and health has not been established, maternal behaviors during pregnancy are known to influence offspring weight and health (12). In addition, it is well established that the development of taste and flavor preferences begins prenatally (13, 14). Particular concern has been raised with respect to excessive sweetness exposure during critical developmental periods (15), because this may predispose children to overconsume sweetened foods and beverages, which often contain excess sugar and calories. Given the ubiquity of LCSs in the food and beverage supply (16, 17), assessing LCS consumption patterns and determining whether LCS exposure in utero may impact fetal and infant weight trajectory and future metabolic health (18, 19) are of paramount importance. However, to our knowledge, the prevalence of LCS consumption among pregnant women in the United States has not been previously investigated. The purpose of this study was to describe temporal trends in the prevalence of LCS consumption in a nationally representative sample of pregnant women in the United States and to compare estimates by LCS source (foods, beverages, packets) and across sociodemographic subgroups. Importantly, the present study was not designed to evaluate effects of LCSs on fetal or infant health.
Subjects and Methods
The national health and nutrition examination survey (NHANES) is a cross-sectional survey of the United States population, which is conducted in 2-y cycles. Details of the NHANES methods and sampling methodology are described elsewhere (20). The current analysis included data collected during 8 NHANES survey cycles: 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, and 2013–2014. All NHANES survey procedures were reviewed and approved by the National Center for Health Statistics (NCHS).
The present analysis included women 20–39 y of age (n = 1265) determined to be pregnant by pregnancy status at exam (positive laboratory pregnancy test) or self-reported pregnant status at the mobile examination center. Females 19 y of age and younger were excluded because in survey cycles following 2007, the NCHS only released pregnancy data for females aged 20–44 y. Owing to the small number of pregnant women 40 y of age or older (n = 21), the age range for our sample was capped at 39 y old (21). Only those with complete data for at least 1 dietary recall (reliable and met minimum criteria) were included in the analysis. Pregnant women with missing data for any sociodemographic characteristic were excluded only from the specific subgroup comparison for which the data were missing, but were included in all other analyses. This resulted in a final analytic sample of 1265 pregnant women (Table 1).
TABLE 1.
Sample characteristics of US pregnant women ages 20–39 y in NHANES 1999–20141
| N | Percentage (95% CI) | |
|---|---|---|
| Age at screening, y | ||
| 20–24 | 410 | 29.6 (25.9, 33.3) |
| 25–29 | 415 | 31.6 (28.0, 35.1) |
| 30–34 | 310 | 24.9 (20.9, 29.0) |
| 35–39 | 130 | 13.9 (10.7, 17.1) |
| Race/ethnicity | ||
| Non-Hispanic white | 565 | 54.1 (48.8, 59.5) |
| Mexican American | 336 | 14.3 (11.5, 17.0) |
| Non-Hispanic black | 195 | 15.9 (12.6, 19.2) |
| Other | 169 | 15.7 (11.5, 19.9) |
| Poverty level | ||
| Missing | 87 | 7.4 (4.9, 9.8) |
| PIR <130% | 391 | 24.3 (20.8, 27.7) |
| PIR 130 to <350% | 418 | 32.9 (28.7, 37.2) |
| PIR ≥350% | 369 | 35.4 (30.2, 40.7) |
| Education level | ||
| Missing | 1 | <1 (0, 0.9)2 |
| Less than high school | 325 | 18.5 (15.3, 21.6) |
| HS diploma or GED | 268 | 18.8 (15.3, 22.2) |
| Some college | 359 | 31.8 (27.8, 35.9) |
| College degree | 312 | 30.9 (26.7, 35.1) |
| Marital status | ||
| Missing | 40 | 3.7 (1.9, 5.5) |
| Married | 811 | 63.6 (59.2, 68.1) |
| Not married | 414 | 32.6 (28.4, 36.9) |
| Trimester | ||
| Missing | 177 | 20.6 (16.5, 24.7) |
| 1st (1–3 mo) | 257 | 24.1 (20.7, 27.5) |
| 2nd (4–6 mo) | 426 | 28.0 (24.3, 31.6) |
| 3rd (7–10 mo) | 405 | 27.3 (23.6, 31.0) |
| Prepregnancy BMI2 | ||
| Missing | 25 | 2.1 (0.7, 3.6)2 |
| Underweight | 68 | 5.9 (3.8, 8.0) |
| Normal weight | 658 | 50.3 (45.9, 54.7) |
| Overweight | 287 | 23.4 (19.9, 26.9) |
| Obese | 227 | 18.3 (15.1, 21.5) |
| Physician-diagnosed diabetes | 15 | 1.3 (0.2, 2.4)2 |
n = 1265. HS, high school; PIR, poverty to income ratio.
Relative SE >30%. Column percentages are survey-weighted.
Prevalence of LCS consumption was assessed using data collected during 1 (n = 604) or 2 (n = 661) 24-h dietary recalls (depending on the survey cycle), the first of which was conducted in person, whereas the second was conducted by telephone. When available, data from both dietary recalls were used, although participants with only 1 reliable recall were also included in the analysis (n = 604). Frequency of LCS consumption (times per day) was assessed using the 1 in-person dietary recall only. Consistent with our prior studies (1, 2, 21), LCS-containing foods and beverages were identified using food descriptions provided in the Food and Nutrient Database for Dietary Studies (FNDDS). The FNDDS version used corresponded to each of the respective 2-y cycles in our analysis (1999–2000 through 2013–2014). The FNDDS includes all foods and beverages consumed by NHANES participants and is based on detailed food-composition data from the USDA National Nutrient Database for Standard Reference (22). Food codes were evaluated for terms including “diet,” “dietetic,” “low-calorie,” “no sugar added,” “light,” “sugar-free,” “sugar substitute,” “low-calorie sweetener,” or “no-calorie sweetener.” Each code was then categorized as an LCS beverage, LCS food, or LCS packet. A total of 2874 unique food and beverage items were reported in either of 2 recalls by our sample of 1265 pregnant women in NHANES 1999–2014. Of these unique items, 79 contained LCSs. The 79 LCS-containing foods and beverages were grouped into 3 mutually exclusive categories: beverages (44 types), foods including condiments (31 types), and packets (4 types).
Subgroup analyses were conducted to assess differences in the prevalence of LCS consumption during pregnancy based on sociodemographic characteristics, prepregnancy weight status, and trimester of gestation. Age was categorized as 20–24, 25–29, 30–34, or 35–39 y. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, or other. Income was categorized using the poverty to income ratio (PIR) (low PIR <100% to 185%, middle 130% to 350%, or high >350%) and education level as less than high school, high school diploma, or GED, some college, or college degree and above. Marital status was assessed dichotomously as married or nonmarried, and prepregnancy BMI was categorized using standard BMI cutoffs for underweight, normal weight, overweight, and obese (23).
Statistical analysis
All analyses were performed using SAS 9.4, using complex survey procedures to account for the NHANES survey design and were weighted to generate nationally representative estimates (20). Prevalence estimates were determined using frequency procedures and expressed as percentage of consumers (95% CI). Rao–Scott modified chi-square tests were used to compare prevalence of LCS consumption across sociodemographic, weight status, and pregnancy trimester subgroups and across product categories. Trends in prevalence of consumption over time were assessed using chi-square tests for trend and F tests. Logistic regression models were used to test for trends in prevalence across survey cycles. Tukey-adjusted pairwise differences were also conducted to compare prevalence over time. Relative SEs were checked and reported if >30%. P values of <0.05 were considered statistically significant.
Results
Characteristics of the sample of US pregnant women participating in NHANES 1999–2014 are summarized in Table 1. Pregnant women were oversampled in the 4 earliest cycles, and thus sample sizes are larger for 1999–2000, 2001–2002, 2003–2004, and 2005–2006 than for the later survey cycles (Supplemental Table 1). No differences in sociodemographic, pregnancy, or health characteristics were observed across the 8 survey cycles (Supplemental Table 1).
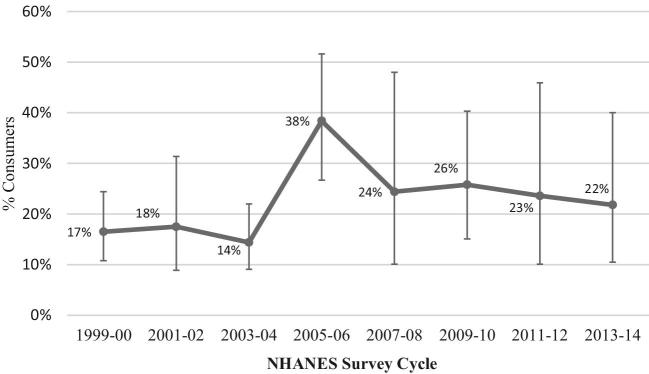
As shown in Figure 1 and Supplemental Table 2, prevalence estimates for LCS consumption in 1999–2000, 2001–2002, and 2003–2004 were similar, as were estimates in the 2007–2008, 2009–2010, 2011–2012, and 2013–2014 survey cycles. We therefore combined the cycles as follows: 1999–2004 and 2007–2014 survey years because of the small sample of pregnant women in the later cycles and high (>30%) relative SEs (Table 2). The percentage of US pregnant women consuming LCSs (from any source) increased by approximately 50%, rising from 16.2% in 1999–2004 to 24.0% in 2007–2014, with a marked peak in consumption prevalence observed (38.4%) in 2005–2006 (P trend = 0.04). A parallel increase in the percentage of US pregnant women reporting LCS beverage use was observed, rising from 9.9% in 1999–2004 to 18.3% in 2007–2014 (P trend = 0.02), with peak prevalence in 2005–2006 (29.3%). A trend toward increasing LCS packet use was also observed (P trend = 0.11), increasing from 2.9% in 1999–2004 to 5.7% in 2007–2014, with no differences in the prevalence of LCS-containing food consumption. Across LCS product categories, the highest prevalence of consumption was consistently observed in the 2005–2006 survey cycle (38.4%, 29.3%, 11.6%, and 8.0% for any LCS, LCS beverages, LCS foods, and LCS packets, respectively), consistent with reports among nonpregnant individuals (2). As shown in Table 3, increases in the prevalence of LCS consumption among pregnant women were of a considerably smaller magnitude (16.5% in 1999–2000 to 21.8% in 2013–2014) than the increases observed among nonpregnant women 20–39 y of age (24.4% in 1999–2000 to 31.0% in 2013–2014) and in the general US population (24.7% in 1999–2000 to 38.5% in 2013–2014).
FIGURE 1.
Prevalence of low-calorie sweetener consumption among US pregnant women by NHANES Survey Cycle, NHANES 1999–2014. The percentage of US pregnant women consuming low-calorie sweeteners (from any source) increased by approximately 50%, rising from 16.2% in 1999–2004 to 24.0% in 2007–2014, with a marked peak in consumption prevalence observed (38.4%) in 2005–2006 (P trend = 0.04).
TABLE 2.
Prevalence (percentage of consumers) of LCS consumption among US pregnant women NHANES 1999–2004, 2005–2006, and 2007–2014
| 1999–2004 | 2005–2006 | 2007–2014 | P trend1 | |
|---|---|---|---|---|
| N = 726 | N = 313 | N = 226 | ||
| Percentage (95% CI) | Percentage (95% CI) | Percentage (95% CI) | ||
| Any LCSs | 16.2 (11.9, 21.6)a | 38.4 (26.7, 51.6)b | 24.0 (17.1, 32.7) | 0.0419* |
| LCS beverages | 9.9 (6.6, 14.6)a | 29.3 (20.1, 40.7)b | 18.3 (12.0, 26.9) | 0.0181* |
| LCS foods | 5.6 (3.1, 10.1)2 | 11.6 (5.6, 22.5)2 | 4.3 (2.0, 8.9)2 | 0.282 |
| LCS packets | 2.9 (1.3, 6.0)2 | 8.0 (3.0, 19.5)2 | 5.7 (2.5, 12.4)2 | 0.113 |
P trend: 1-sided P value for a linear trend across survey cycles using survey-weighted logistic regression. Significant Tukey-adjusted pairwise differences (P < 0.05) between cycles are indicated by different superscript letters. *Statistically significant, P < 0.05. LCS, low-calorie sweetener.
Relative SE >30%.
TABLE 3.
LCS consumption among pregnant women, similar-age nonpregnant women, and US general population
| Overall | 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | P trend1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pregnant women 20–39 y | ||||||||||
| N | 1265 | 241 | 279 | 206 | 313 | 55 | 65 | 47 | 59 | – |
| Percentage of LCS consumers (95% CI) | 19.5 (15.0, 24.0) | 16.5 (10.8, 24.4) | 17.5 (8.9, 31.4) | 14.4 (9.1, 22.0) | 38.4 (26.7, 51.6) | 24.4 (10.1, 48.0) | 25.8 (15.1, 40.3) | 23.6 (10.1, 45.9) | 21.8 (10.5, 40.0) | 0.1249 |
| Non-pregnant women 20–39 y | ||||||||||
| N | 6031 | 607 | 716 | 621 | 674 | 836 | 958 | 785 | 834 | – |
| Percentage of LCS consumers (95% CI) | 34.7 (32.7, 36.7) | 24.4 (19.6, 30.0) | 24.6 (20.1, 29.7) | 35.0 (29.2, 41.4) | 41.6 (36.3, 47.1) | 39.3 (35.0, 43.8) | 38.7 (34.4, 43.3) | 41.3 (36.3, 46.5) | 31.0 (24.9, 37.9) | 0.0001 |
| US general population 2+ y | ||||||||||
| N | 67,502 | 8074 | 9033 | 8273 | 8579 | 8529 | 9042 | 7935 | 8067 | – |
| Percentage of LCS consumers (95% CI) | 36.1 (35.2, 37.1) | 24.7 (23.2, 26.4) | 25.2 (22.3, 28.3) | 33.7 (31.1, 36.5) | 39.8 (37.4, 42.2) | 40.2 (38.4, 42.0) | 42.9 (40.5, 45.2) | 40.1 (37.3, 42.9) | 38.5 (35.4, 41.7) | <0.0001 |
P trend: 1-sided P value for a linear trend across survey cycles using survey-weighted logistic regression. LCS, low-calorie sweetener.
Among pregnant women reporting LCS consumption, the majority reported LCS consumption once per day (69.0%). Twenty-two (22.4%) percent indicated consumption of a LCS-containing food or beverage twice, and 8.6% reported LCS consumption 3 or more times, respectively, during their in-person 24-h dietary recall (Supplemental Table 3).
Analyses of data from the most recent decade (2005–2014) indicated significant variability in LCS consumption across sociodemographic characteristics (Table 4). Although comparisons for LCS foods and LCS packets were limited because of the small sample size and large relative SEs, prevalence of any LCSs and LCS beverage consumption were highest among non-Hispanic white women (P < 0.0001), whereas LCS beverage consumption increased with higher education (P = 0.003) and income (P = 0.01) and was higher among married than among not-married women (P = 0.02). Prevalence of LCS consumption also increased with maternal age (P = 0.02). No differences were observed across prepregnancy weight status or pregnancy trimester (Table 4).
TABLE 4.
Prevalence (%) of LCS consumption among US pregnant women, NHANES 2005–20141
| Percentage of consumers (95% CI) | |||||
|---|---|---|---|---|---|
| N | Any LCSs | LCS beverages | LCS foods | LCS packets | |
| All women | 539 | 29.4 (23.6, 35.2) | 21.6 (16.5, 26.6) | 7.7 (4.2, 11.2) | 6.5 (3.0, 10.0) |
| Age at screening, y | |||||
| 20–24 | 182 | 25.5 (15.7, 35.2) | 17.5 (9.8, 25.1) | 3.3 (0.6, 5.9)2 | 5.4 (0.5, 10.2)2 |
| 25–29 | 184 | 23.0 (12.9, 33.0) | 17.7 (8.1, 27.4) | 6.9 (1.9, 12.0)2 | 5.3 (0.0, 11.6)2 |
| 30–34 | 119 | 30.1 (18.8, 41.4) | 18.6 (8.1, 29.1) | 9.6 (2.2, 17.0)2 | 6.9 (0.0, 14.8)2 |
| 35–39 | 54 | 48.7 (32.8, 64.6) | 41.1 (25.4, 56.7) | 14.6 (0.0, 29.3)2 | 10.4 (0.8, 20.1)2 |
| P value | 0.0264* | 0.0221* | 0.28 | 0.79 | |
| Race/ethnicity | |||||
| NH white | 210 | 39.3 (31.2, 47.5) | 31.7 (24.1, 39.2) | 9.4 (3.3, 15.5)2 | 9.5 (3.2, 15.8)2 |
| Mexican American | 145 | 18.5 (10.5, 26.6) | 11.4 (3.9, 18.9)2 | 5.1 (2.3, 7.8) | 3.4 (0.0, 6.9)2 |
| NH black | 102 | 15.5 (8.0, 23.0) | 12.1 (6.4, 17.8) | 4.5 (0.0, 8.9)2 | 0 |
| Other | 82 | 20.4 (10.2, 30.7) | 6.8 (2.4, 11.1)2 | 7.6, (0.28, 15.0)2 | 6.0 (0.0, 12.3)2 |
| P value | <0.0001* | <0.0001* | 0.47 | – | |
| Family income3 | |||||
| PIR <130% | 181 | 18.9 (11.5, 26.3) | 11.8 (5.9, 17.8) | 5.3 (1.4, 9.3)2 | 2.8 (0.0, 6.2)2 |
| PIR 130–350% | 178 | 34.7 (24.2, 45.3) | 21.9 (13.0, 30.9) | 8.8 (2.8, 14.7)2 | 11.0 (2.6, 19.5)2 |
| PIR >350% | 143 | 35.0 (24.5, 45.4) | 30.5 (20.9, 40.1) | 8.6 (1.9, 15.3)2 | 6.2 (0.1, 12.4)2 |
| P value | 0.0427* | 0.0097* | 0.66 | 0.26 | |
| Education | |||||
| Less than high school | 142 | 23.6 (13.4, 33.8) | 18.1 (8.4, 27.7) | 1.7 (0.0, 3.5)2 | 4.2 (0.0, 9.0)2 |
| HS diploma or GED | 116 | 14.1 (6.1, 22.1) | 11.6 (3.9, 19.2)2 | 3.6, (1.1, 6.0)2 | 0.4 (0.0, 1.2)2 |
| Some college | 167 | 31.4 (19.6, 43.2) | 16.3 (8.3, 24.2) | 8.7, (3.3, 14.1)2 | 7.6 (0.0, 15.4)2 |
| College degree or above | 114 | 40.0 (28.4, 51.6) | 35.1 (23.3, 46.9) | 12.6 (3.3, 21.8)2 | 10.3 (1.7, 18.9)2 |
| P value | 0.0113* | 0.0030* | 0.0484* | 0.24 | |
| Marital status4 | |||||
| Married | 330 | 33.6 (26.2, 41.0) | 24.6 (17.4, 31.8) | 10.2 (4.9, 15.5) | 6.8 (2.2, 11.4)2 |
| Not married | 208 | 21.7 (14.0, 29.3) | 15.9 (9.2, 22.6) | 3.0 (0.6, 5.4)2 | 5.9 (0.1, 11.8)2 |
| P value | 0.0236* | 0.10 | 0.0214* | 0.82 | |
| Trimester5 | |||||
| 1st (1–3 mo) | 120 | 40.0 (25.1, 54.9) | 30.3 (19.3, 41.3) | 8.1 (0.0, 16.4)2 | 13.8 (1.7, 25.8)2 |
| 2nd (4–6 mo) | 157 | 31.0 (20.2, 41.8) | 19.0 (8.2, 29.8) | 8.2 (2.3, 14.0)2 | 5.8 (2.4, 9.1)2 |
| 3rd (7–10 mo) | 165 | 29.3 (17.9, 40.7) | 23.3 (12.1, 34.6) | 11.3 (3.5, 19.1)2 | 3.5 (0.0, 7.5)2 |
| P value | 0.47 | 0.38 | 0.36 | 0.17 | |
| Prepregnancy BMI6 | |||||
| Underweight | 23 | 12.1 (0.0, 28.5)2 | 12.1 (0.0, 28.5)2 | 0 | 0 |
| Normal weight | 271 | 26.7 (18.4, 35.0) | 18.8 (11.9, 25.7) | 8.8 (3.4, 14.2) | 5.0 (1.6, 8.3)2 |
| Overweight | 137 | 37.2 (25.7, 48.6) | 31 (19.5, 42.7) | 6.4 (0.6, 12.3)2 | 8.6 (0.3, 16.9)2 |
| Obese | 96 | 30.0 (17.6, 42.4) | 18.3 (6.8, 29.9)2 | 8.7 (1.8, 15.6)2 | 8.7 (0.0, 19.1)2 |
| P value | 0.21 | 0.14 | – | – | |
*Statistically significant, P < 0.05. HS, high school; LCS, low-calorie sweetener; NH, non-Hispanic; PIR, poverty to income ratio.
Relative SE >30%.
37 participants missing data for family income.
1 participant missing marital status.
97 participants missing data on pregnancy trimester.
12 participants missing data on prepregnancy BMI, underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obese (≥30 kg/m2). Survey-weighted percentages; Rao–Scott modified chi-square tests within LCS product types (omnibus P values shown).
Discussion
Our findings demonstrate that the prevalence of LCS consumption has increased among US pregnant women over the past 2 decades, which is consistent with reports in nonpregnant women of similar age and in the general US population (1, 2, 17, 24), albeit of a smaller magnitude. In NHANES 2007–2014, approximately 24% of US pregnant women reported LCS use during at least 1 of their 2 dietary recalls, similar to recent estimates reported among pregnant women in the Canadian Healthy Infant Longitudinal Development birth cohort (8). As has been demonstrated in nonpregnant individuals, an increase in LCS beverage consumption, rather than LCS-containing foods or packets, appears to be driving this trend (2, 25) with particularly marked increases observed in 2005–2006. Although the reason for this disproportionate increase in LCS consumption in 2005–2006 is not clear, considerable public health emphasis was placed on reducing consumption of added sugar during this time frame. For example, in 2005, the Center for Science in the Public Interest published its report on “Liquid Candy” (26). Furthermore, several LCS-containing food codes were reported in 2005–2006 that were not present in earlier cycles, although the appearance of these food codes does not fully explain the increases observed.
Despite rising LCS consumption during pregnancy, it is currently unclear whether LCS exposure in utero impacts fetal or infant weight and health (27). Although potential effects of prenatal LCS exposure have not been well studied in humans (19), 2 recent observational analyses have reported associations between maternal LCS consumption and child weight gain (8, 9), although a third analysis reported null findings (11). Epidemiologic associations between maternal LCS intake and preterm delivery have also been reported (28, 29), yet possible biological mechanisms explaining the observational link between LCSs and premature birth have not been elucidated.
In contrast with the observational literature, randomized controlled trials in nonpregnant individuals demonstrate neutral (3) or beneficial (5, 30) effects of LCS use on weight management. It is therefore possible that LCSs may serve as a useful tool for preventing excessive gestational weight gain and related pregnancy complications in pregnant women, if used carefully for the purpose of weight loss (31) and in parallel with more comprehensive lifestyle changes (32). As with in the general population, however, it is not clear whether LCSs are used in this manner among the majority of pregnant women, especially as many individuals consume LCSs inadvertently (33, 34), as opposed to intentionally for the purpose of restricting energy intake. It is also possible that in utero LCS exposure may predispose the fetus to metabolic complications, independently of weight (35) or postnatal diet (36). Experimental human data linking in utero LCS exposure with offspring weight and health are lacking to date (37), and more research is required to fully understand the implications, if any, of LCS use during pregnancy and to develop evidence-based guidelines for or against their use among expectant mothers (27).
Strengths of our study include evaluation of a large sample of pregnant women using nationally representative data collected in NHANES, enabling comparison across sociodemographic characteristics, prepregnancy weight status, and pregnancy trimester, and between product categories. As prevalence of LCS consumption during pregnancy has not, to our knowledge, been previously documented in the United States, our results complement prior studies reporting consumption estimates in the general, nonpregnant US population (1, 24, 38).
In addition to previously described limitations of assessing LCS consumption using NHANES (1, 2), including inability to assess specific types of LCSs, which have different properties and may exert different physiological effects, or to calculate absolute quantities of LCS consumed, our analysis was further limited by the small sample size, resulting in large relative SEs for sociodemographic comparisons for LCS foods and LCS packets. The relatively small number of pregnant women surveyed, particularly in the most recent 4 cycles of NHANES (2007–2014), also restricted our ability to assess temporal trends in overall LCS consumption within population subgroups. Despite these limitations, however, our estimates provide confirmation that recently documented increases in LCS use in the United States (1, 2) also apply to expectant mothers, with similar sociodemographic correlates as reported in nonpregnant adults. Our findings underscore the need for careful investigation as to whether use of LCSs by pregnant women reduces excessive gestational weight gain and if in utero LCS exposure influences infant and child taste preferences, weight trajectory, and long-term metabolic health.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—ACS, MIG, KIR, and JAW: designed the study; JF: performed the statistical analyses; ACS: wrote the first draft of the manuscript; and all authors: read and approved the final manuscript.
Notes
The study was funded in part by the Department of Exercise and Nutrition Sciences at the George Washington University and the Sumner M. Redstone Global Center for Prevention and Wellness Pilot Studies Program (PI: Sylvetsky).
None of the authors report a conflict of interest related to research presented in this article.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
References
- 1. Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet 2017;117(3):441–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr 2012;96:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiander M et al.. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017;189:E929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rother KI, Conway EM, Sylvetsky AC. How non-nutritive sweeteners influence hormones and health. Trends Endocrinol Metab 2018;29:455–67. [DOI] [PubMed] [Google Scholar]
- 5. Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR, Penfold C, Perry R, Putz P, Yeomans MR et al.. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes (Lond) 2016;40(3):381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitch C, Keim KS, Academy of N, Dietetics. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112:739–58. [DOI] [PubMed] [Google Scholar]
- 7. Sylvetsky A, Rother KI, Brown R. Artificial sweetener use among children: Epidemiology, recommendations, metabolic outcomes, and future directions. Pediatr Clin North Am 2011;58:1467–80, xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azad MB, Sharma AK, de Souza RJ, Dolinsky VW, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Lefebvre DL, Sears MR et al.. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr 2016;170:662–70. [DOI] [PubMed] [Google Scholar]
- 9. Zhu Y, Olsen SF, Mendola P, Halldorsson TI, Rawal S, Hinkle SN, Yeung EH, Chavarro JE, Grunnet LG, Granstrom C et al.. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. Int J Epidemiol 2017;46(5):1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maslova E, Strom M, Olsen SF, Halldorsson TI. Consumption of artificially-sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS One 2013;8:e57261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillman MW, Rifas-Shiman SL, Fernandez-Barres S, Kleinman K, Taveras EM, Oken E. Beverage intake during pregnancy and childhood adiposity. Pediatrics 2017;140(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phelan S, Hart C, Phipps M, Abrams B, Schaffner A, Adams A, Wing R. Maternal behaviors during pregnancy impact offspring obesity risk. Exp Diabetes Res 2011;2011:985139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mennella JA, Griffin CE, Beauchamp GK. Flavor programming during infancy. Pediatrics 2004;113:840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beauchamp GK, Mennella JA. Early flavor learning and its impact on later feeding behavior. J Pediatr Gastroenterol Nutr 2009;48 Suppl 1:S25–30. [DOI] [PubMed] [Google Scholar]
- 15. Mennella JA, Bobowski NK, Reed DR. The development of sweet taste: From biology to hedonics. Rev Endocr Metab Disord 2016;17:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sylvetsky AC, Walter PJ, Garraffo HM, Robien K, Rother KI. Widespread sucralose exposure in a randomized clinical trial in healthy young adults. Am J Clin Nutr 2017;105(4):820–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes 2013;8:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sylvetsky AC, Conway EM, Malhotra S, Rother KI. Development of sweet taste perception: Implications for artificial sweetener use. Endocr Dev 2017;32:87–99. [DOI] [PubMed] [Google Scholar]
- 19. Archibald AJ, Dolinsky VW, Azad MB. Early-life exposure to non-nutritive sweeteners and the developmental origins of childhood obesity: Global evidence from human and rodent studies. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. [Internet]. 2015[cited February 10, 2015]; Available from: http://www.cdc.gov/nchs/nhanes.htm
- 21. Cioffi CE, Figueroa J, Welsh JA. Added Sugar Intake among Pregnant Women in the United States: National Health and Nutrition Examination Survey 2003–2012. J Acad Nutr Diet 2018;118:886–95e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. USDA ARS. USDA National Nutrient Database for Standard Reference, Release 27. [Internet]. 2014[cited February 11, 2015]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl
- 23. Centers for Disease Control and Prevention. Healthy weight: About adult BMI. [Internet]. 2015. Available from: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/.
- 24. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr 2015;69:1035–41. [DOI] [PubMed] [Google Scholar]
- 25. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav 2016;164(Pt B):446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobson M. Liquid candy: How soft drinks are harming Americans’ health. Washington, DC: Center for Science in the Public Interest; 2005. [Google Scholar]
- 27. Pope E, Koren G, Bozzo P. Sugar substitutes during pregnancy. Can Fam Phys 2014;60:1003–5. [PMC free article] [PubMed] [Google Scholar]
- 28. Halldorsson TI, Strom M, Petersen SB, Olsen SF. Intake of artificially sweetened soft drinks and risk of preterm delivery: A prospective cohort study in 59,334 Danish pregnant women. Am J Clin Nutr 2010;92:626–33. [DOI] [PubMed] [Google Scholar]
- 29. Englund-Ogge L, Brantsaeter AL, Haugen M, Sengpiel V, Khatibi A, Myhre R, Myking S, Meltzer HM, Kacerovsky M, Nilsen RM et al.. Association between intake of artificially sweetened and sugar-sweetened beverages and preterm delivery: A large prospective cohort study. Am J Clin Nutr 2012;96:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: A meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014;100:765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters JC, Wyatt HR, Foster GD, Pan Z, Wojtanowski AC, Vander Veur SS, Herring SJ, Brill C, Hill JO. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring) 2014;22:1415–21. [DOI] [PubMed] [Google Scholar]
- 32. Sylvetsky AC, Rother KI. Nonnutritive Sweeteners in weight management and chronic disease: A review. Obesity (Silver Spring) 2018;26:635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sylvetsky AC, Dietz WH. Nutrient-content claims—guidance or cause for confusion? N Engl J Med 2014;371:195–8. [DOI] [PubMed] [Google Scholar]
- 34. Sylvetsky AC, Greenberg M, Zhao X, Rother KI. What parents think about giving nonnutritive sweeteners to their children: A pilot study. Int J Pediatr 2014;2014:819872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blackmore HL, Niu Y, Fernandez-Twinn DS, Tarry-Adkins JL, Giussani DA, Ozanne SE. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 2014;155:3970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 2009;587:905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fidler Mis N, Braegger C, Bronsky J, Campoy C, Domellof M, Embleton ND, Hojsak I, Hulst J, Indrio F, Lapillonne A et al.. Sugar in infants, children and adolescents: A position paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 2017;65:681–96. [DOI] [PubMed] [Google Scholar]
- 38. Fakhouri TH, Kit BK, Ogden CL. Consumption of diet drinks in the United States, 2009–2010. NCHS Data Brief 2012;109:1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.