Abstract
Human γ-herpesviruses include the closely related tumor viruses Epstein Barr virus (EBV) and Kaposi sarcoma-associated herpesvirus (KSHV). EBV is the most growth-transforming pathogen known and is linked to at least seven human malignancies. KSHV is also associated with three human cancers. Most EBV- and KSHV-infected individuals fortunately remain disease-free despite persistent infection and this is likely due to the robustness of the immune control that they mount against these tumor viruses. However, upon immune suppression EBV- and KSHV-associated malignancies emerge at increased frequencies. Moreover, primary immunodeficiencies with individual mutations that predispose to EBV or KSHV disease allow us to gain insights into a catalog of molecules that are required for the immune control of these tumor viruses. Curiously, there is little overlap between the mutation targets that predispose individuals to EBV versus KSHV disease, even so both viruses can infect the same host cell, human B cells. These differences will be discussed in this review. A better understanding of the crucial components in the near-perfect life-long immune control of EBV and KSHV should allow us to target malignancies that are associated with these viruses, but also induce similar immune responses against other tumors.
Keywords: Epstein Barr virus, Kaposi sarcoma-associated herpesvirus, primary effusion lymphoma, Kaposi sarcoma, Hodgkin's lymphoma, Burkitt's lymphoma, hemophagocytic lymphohistiocytosis
The comparison of molecular pathways and pathologies during immunodeficiencies that predispose for uncontrolled EBV and KSHV infection reveals similarities and differences in cell mediated immune control, and suggests that latent transforming EBV infection of epithelial cells does not play a significant role in healthy virus carriers.
INTRODUCTION
There are several human tumor viruses. These include Epstein Barr virus (EBV), Kaposi sarcoma-associated herpesvirus (KSHV), human papillomavirus, Merkel cell polyomavirus, hepatitis B virus, hepatitis C virus and human T-cell lymphotropic virus type 1 (Hopcraft and Damania 2017). Two of the seven human tumor viruses belong to the γ-herpesviruses, namely EBV or human herpesvirus 4, and KSHV or human herpesvirus 8 (Parkin 2006; Bouvard et al.2009). Each of them contributes 1–2% to the 20% of infectious disease-associated cancer burden among all malignancies in humans. EBV is mainly associated with lymphomas and carcinomas of B and epithelial cell origin, respectively (Cesarman 2014), including Burkitt's lymphoma, in which the virus was originally identified (Epstein et al.1965; Epstein, Achong and Barr 1964). However, epithelial cancers constitute the majority of the newly diagnosed 200 000 EBV-associated malignancies each year (Cohen et al.2011). In contrast, KSHV is mainly associated with endothelial and B cell-derived malignancies (Chang et al.1994; Cesarman 2014). The endothelial cell cancer Kaposi sarcoma (KS) is one of the acquired immune deficiency syndrome (AIDS)-defining illnesses in human immunodeficiency virus (HIV)-infected patients and KSHV was originally identified in this tumor (Chang et al.1994). Both EBV and KSHV have co-evolved with humans and are part of the primate associated γ1- and γ2-herpesviruses, respectively (McGeoch 2001; Ehlers et al.2010). Through this co-evolution they have both achieved a remarkable penetration of the human population, with EBV establishing persistence in more than 95% of the adult human population across the globe, while KSHV prevalence is more variable with over 50% seropositivity in equatorial Africa (Cesarman 2014). In light of this high prevalence, associated malignancies are still pretty rare and are likely to emerge from combinations of the viruses’ growth-transforming capacities and the failure of the immune system to control the virus. In this regard, studies on the gene expression programs in healthy EBV and KSHV carriers and associated diseases that emerge in patients with immunodeficiencies can inform us as to which viral gene expression programs continuously threaten healthy EBV and KSHV carriers with tumorigenesis and which associated malignancies can be prevented by an intact immune system during persistent infections with these γ-herpesviruses for life.
EBV is the more transforming of the two viruses and readily immortalizes human B cells upon infection in vitro (Miller and Lipman 1973a,b). Eight latent EBV proteins, two clusters of EBV-encoded microRNAs (miRNAs) and two small non-translated RNAs (EBERs) out of a total of around 90 open reading frames are expressed in the resulting lymphoblastoid cell lines and this latency III gene expression pattern can also be found in naïve B cells of healthy EBV carriers (Babcock, Hochberg and Thorley-Lawson 2000; Palser et al.2015). This latency gene expression program can also be found in EBV-associated large B cell lymphomas that primarily occur in immune-suppressed individuals, as in the case of post-transplant lymphoproliferative disease due to iatrogenic inhibition of the immune system to preserve a transplant or due to compromised immune reactivity upon HIV co-infection leading to immunoblastic lymphomas (Cesarman 2014). A much more restricted gene expression pattern with only one of the six nuclear proteins of EBV (EBNAs), the two latent membrane proteins and the non-translated RNAs expressed, can be found in germinal center B cells of healthy EBV carriers and in Hodgkin's lymphoma (Babcock and Thorley-Lawson 2000; Babcock, Hochberg and Thorley-Lawson 2000). A similar latency II gene expression pattern is also found in many of the EBV-associated epithelial cell cancers, like nasopharyngeal carcinoma (Kutok and Wang 2006). Finally, Burkitt's lymphoma, the tumor that EBV was discovered in, expresses only EBNA1 and the non-translated RNAs, but compensates for the loss of the pro-proliferative expression of the other latent EBV gene products with translocations of the cellular oncogene c-myc into the immunoglobulin loci (Cesarman 2014). This latency I gene expression pattern is also found in homeostatically proliferating memory B cells of healthy EBV carriers and in the 10% of EBV-positive gastric carcinomas (Hochberg et al.2004; Kutok and Wang 2006). EBV persists in quiescent memory B cell compartments with only non-translated RNA expression (Babcock et al.1998). This latency 0 in memory B cells might be reached upon B cell differentiation of latency III via latency II in a germinal center-dependent fashion or after early EBNA2-driven proliferation without the expression of EBNA3A or EBNA3C viral oncogenes (Murer et al.2018). It can reactivate from this persistence reservoir upon B cell receptor cross-linking by cognate antigen (Binne, Amon and Farrell 2002) and lytic EBV replication is found in the resulting plasma cells of healthy EBV carriers (Laichalk and Thorley-Lawson 2005). Early lytic EBV gene products of the around 80 open reading frames of EBV replication are thought to support tumor microenvironment changes that are beneficial for the establishment of EBV-associated lymphomas (Hong et al.2005; Ma et al.2011; Antsiferova et al.2014). Indeed, primary central nervous system lymphoma treatment benefitted from lytic EBV replication inhibition (Dugan et al.2018). Therefore, premalignant EBV gene expression programs are carried by B cells of healthy EBV carriers, but it remains unclear if epithelial cell infection and especially latent EBV gene expression in epithelial cells as in nasopharyngeal and gastric carcinoma contributes to persistent infection by this γ-herpesvirus in humans.
Similar to EBV, KSHV is thought to be transmitted by saliva exchange (Pauk et al.2000) and can be found in B cells of KSHV carriers (Ambroziak et al.1995). However, latent and lytic KSHV gene expression are much less segregated in the KSHV-associated malignancies KS, primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (Schulz and Cesarman 2015). Most of the tumor cells express the classical KSHV latency gene products [latency-associated nuclear antigen (LANA), viral D-type cyclin (vCyclin) and viral FLICE inhibitory protein (vFLIP), K15 and viral miRNAs] (Dittmer and Damania 2016; Abere et al.2017). However, a small percentage of the tumor cells do express lytic proteins including K1, K15, viral interleukin (IL)-6 and viral G protein-coupled receptor (Dittmer and Damania 2016). These lytic proteins are thought to contribute to tumor growth in a paracrine fashion by increasing angiogenesis and cell proliferation (Schulz and Cesarman 2015). Furthermore, inhibiting lytic KSHV replication might prevent the development of KS (Martin et al.1999), possibly eliminating the inflammatory tumor-nurturing microenvironment, similar to the contribution of early lytic EBV replication for virus-associated lymphomagenesis (Dugan et al.2018). Lytic EBV replication also seems to be important for PEL formation, a tumor entity that in up to 90% of cases contains both KSHV and EBV (McHugh et al.2017). This is also consistent with plasma cell differentiation being associated with lytic EBV replication (Laichalk and Thorley-Lawson 2005) and KSHV infection or the KSHV latent genes vFLIP and LANA driving immunoglobulin M λ light chain-expressing plasma cell accumulations (Ballon et al.2011; Hassman, Ellison and Kedes 2011; Sin and Dittmer 2013; Sin et al.2015), reminiscent of MCD (Du et al.2001). Thus, for both EBV and KSHV, B cells are proposed as the latency reservoir, and both lytic as well as latent gene products seem to contribute to tumorigenesis by these two human γ-herpesviruses. However, the role that epithelial and endothelial cell infection play for EBV and KSHV infection, respectively, remains unclear. We propose that immunodeficiencies caused by monogenic mutations or co-infections can at least document to what extent these non-hematopoietic infections by the two human γ-herpesviruses occur and by which means immune control of the different pre-malignant EBV and KSHV reservoirs is maintained.
IMMUNODEFICIENCIES THAT PREDISPOSE FOR EBV-ASSOCIATED DISEASES
Lymphomas and especially those associated with EBV are considered AIDS-defining diseases and constitute currently more than 50% of cancers that are associated with HIV co-infection (Simard and Engels 2010; Simard, Pfeiffer and Engels 2011). While non-Hodgkin's lymphomas, primarily latency III tumors, have significantly decreased in incidence due to combined anti-retroviral therapy (cART), EBV-positive Hodgkin's lymphomas have rather increased in frequency (Carbone et al.2014; Brugnaro et al.2015). In addition, EBV-associated smooth muscle tumors are increased during HIV co-infection (McClain et al.1995; Ehresman et al.2018). In contrast, EBV-positive epithelial cell-derived malignancies, such as nasopharyngeal carcinoma, are not significantly increased in HIV-infected individuals (Melbye et al.1996); even so, in most of these studies the prevalence of nasopharyngeal carcinoma was in general too low to draw firm conclusions (Shebl, Bhatia and Engels 2010; Zhang et al.2011; Grulich and Vajdic 2015). However, lytic EBV replication can progress uncontrolled in tongue epithelium after AIDS development and then causes oral hairy leukoplakia (Becker et al.1991). These observations during immune suppression caused by HIV-mediated CD4+ T cell elimination seem to suggest that defective immune control allows for the emergence of EBV-associated non-Hodgkin's lymphomas and more rarely EBV-positive smooth muscle tumors and oral hairy leukoplakia, but not EBV-associated epithelial cell cancers like nasopharyngeal carcinoma. Thus, HIV-mediated CD4+ T cell depletion does not seem to compromise immune control of a premalignant state of epithelial cell infection by EBV.
This picture is also mirrored by monogenic primary immunodeficiencies that predispose for EBV-associated pathologies, and affect primarily the development and function of cytotoxic lymphocytes (Cohen 2015; Tangye, Palendira and Edwards 2017; Münz 2017a) (Fig. 1). The affected patients present with a variety of EBV-associated diseases ranging from uncontrolled viremia, as in chronic active EBV infection (CAEBV), which often spreads from B cells to T and natural killer (NK) cells, EBV infection driven immunopathologies, like infectious mononucleosis and hemophagocytic lymphohistiocytosis (HLH), to EBV-associated malignancies, including lymphoproliferative diseases, non-Hodgkin's lymphomas, Hodgkin's lymphomas, smooth muscle tumors and EBV-associated Castleman's disease. Interestingly, again no increased incidence of EBV-associated epithelial cell-derived cancers, such as nasopharyngeal carcinoma, has so far been reported. Furthermore, different monogenic deficiencies predispose for different EBV-associated pathologies, which might pinpoint the protective role of individual immune pathways in the control of distinct virus-induced diseases. Along these lines, deficiencies in the effector machinery of cytotoxic lymphocytes, affecting NK and non-classical innate as well as classical T cell populations, result in EBV-driven HLH immunopathologies (Katano et al.2004; Rohr et al.2010; Cohen et al.2015). The respective loss of cytotoxicity compromises either perforin itself or the degranulation machinery for cytotoxic granules, like Munc13-4 and 18-2 (Table 1).
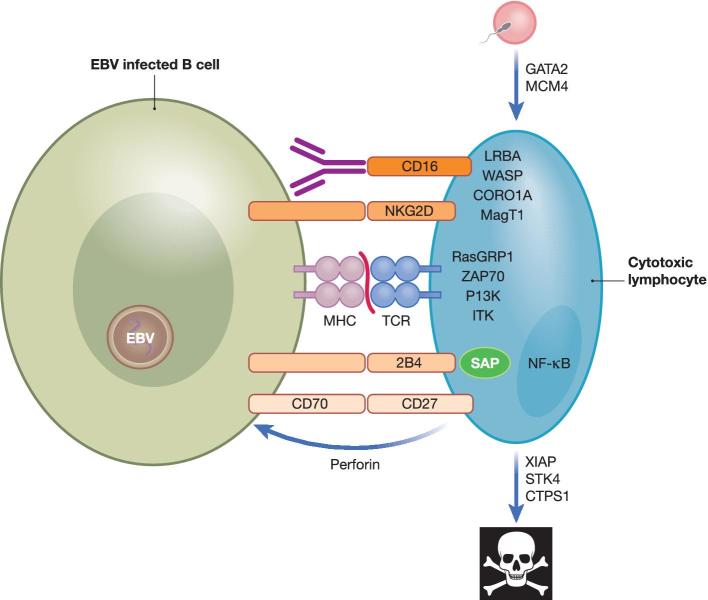
Figure 1.
Primary immunodeficiencies that compromise the function of cytotoxic lymphocytes and predispose for EBV-associated diseases. Immune control of EBV-infected B cells is compromised upon deficiencies in TCR signaling, co-stimulation, leucocyte development, lymphocyte cell death and cytotoxic effector functions. For TCR signaling, RasGRP1, ZAP70, PI3K and ITK are required during EBV-specific immune control. CORO1A and WASP deficiencies compromise actin cytoskeleton arrangements during EBV-specific immune control. GATA2 and MCM4 compromise the development of protective lymphocytes against EBV, and loss of XIAP, STK4 and CTPS1 accelerate their cell death. LRBA and MAGT1 influence the expression levels of co-receptors on cytotoxic lymphocytes, of which CD16, NKG2D, SLAM receptors like 2B4, which is compromised by SAP mutations, and CD27 are required for EBV-specific immune control. NF-κB is involved in the signaling of CD27 and NKG2D. Perforin-mediated cytotoxicity is crucial for EBV-specific immune control. TCR, T cell receptor.
Table 1.
Primary immunodeficiencies that predispose for EBV-associated diseases.
| Affected protein ‘name of syndrome’ | EBV-associated diseases | Innate immune system changes | Adaptive immune system changes | References |
|---|---|---|---|---|
| Cytotoxic machinery | ||||
| Perforin ‘FHL2’ | HLH, EBV VIR | Low neutrophils, compromised NK cell killing | Compromised T cell killing | Katano et al.2004 |
| Munc13-4 ‘FHL3’ | EBV VIR | Low neutrophils, compromised NK cell killing | Compromised T cell killing | Rohr et al.2010 |
| Munc18-2 ‘FHL5’ | EBV VIR, EBV NHL | Low neutrophils, compromised NK cell killing | Compromised T cell killing | Rohr et al.2010; Cohen et al.2015 |
| Leucocyte development | ||||
| GATA2 ‘MonoMac’ | IM, EBV SMT, EBV VIR, HLH | Low NK, DC and monocytes | CD4+ T cell lymphopenia | Biron, Byron and Sullivan 1989; Mace et al. 2013 |
| MCM4 | EBV NHL | Low NK | – | Eidenschenk et al.2006; Gineau et al.2012 |
| TCR signaling | ||||
| ITK | EBV HL, HLH | Loss of NKT | CD4+ T cell lymphopenia | Huck et al.2009; Linka et al.2012 |
| PI3K 110δ ‘PASLI, APDS’ | EBV NHL, EBV VIR | Compromised NK cell killing | CD4+ T cell lymphopenia | Angulo et al.2013, Kuehn et al.2013; Lucas et al.2014 |
| RasGRP1 | EBV NHL | Loss of NKT | CD4+ T cell lymphopenia | Salzer et al.2016; Winter et al.2018 |
| ZAP70 | EBV NHL | Loss of NKT | CD4+ T cell lymphopenia, compromised CD8+ T cell function | Hoshino et al.2018 |
| CORO1A | EBV NHL | Loss of NKT | CD4+ and CD8+ T cell lymphopenia | Moshous et al.2013 |
| Co-stimulation | ||||
| CD27 | HLH, EBV NHL | Loss of NKT, compromised NK cell function | Compromised T cell function | Salzer et al.2012; van Montfrans et al.2012 |
| CD70 | EBV HL | Loss of NKT | Compromised B cell recognition by T cells | Alkhairy et al.2015; Abolhassani et al.2017; Izawa et al.2017 |
| CD16 | EBV CD | Compromised NK cell function | – | de Vries et al.1996; Grier et al.2012 |
| CTLA-4 | EBV NHL | Low NK | Low T and B cells | Schwab et al.2018 |
| MagT1 ‘XMEN’ | EBV NHL | Compromised NKG2D expression on NK cells | CD4+ T cell lymphopenia, impaired B cell recognition by T cells | Li et al.2011; Chaigne-Delalande et al.2013; Dhalla et al.2015 |
| SAP ‘XLP1’ | EBV NHL, IM, HLH | Loss of NKT, compromised NK cell function | Compromised T cell function | Coffey et al.1998; Nichols et al. 1998; Sayos et al. 1998; Sumegi et al. 2000; Booth et al. 2011; Pachlopnik Schmid et al. 2011 |
| NF-κB1 | EBV VIR, EBV NHL | – | Compromised T cell function | Boztug et al.2016; Schipp et al.2016 |
| LRBA | EBV NHL, EBV VIR | – | – | Alangari et al.2012 |
| Cell death | ||||
| XIAP ‘XLP2’ | HLH, IM | Low NKT | Compromised T cell survival after activation | Rigaud et al.2006; Pachlopnik Schmid et al. 2011; Speckmann et al.2013 |
| STK4 | EBV NHL | Low neutrophils | CD4+ T cell lymphopenia | Abdollahpour et al.2012; Nehme et al.2012 |
| Cell proliferation | ||||
| CTPS1 | IM, EBV NHL | Loss of NKT | CD4+ T cell lymphopenia | Martin et al.2014 |
IM, infectious mononucleosis; EBV VIR, EBV viremia; EBV NHL, EBV-associated non-Hodgkin's lymphoma; EBV HL, EBV-positive Hodgkin's lymphoma; EBV CD, EBV-positive Castleman's disease; EBV SMT, EBV-associated smooth muscle tumor.
Of similar severity are GATA2 loss-of-function mutations that affect the development of multiple leucocyte populations, including NK and CD4+ T cells (Cohen 2017). These patients present with CAEBV, HLH and EBV-positive smooth muscle tumors. In contrast EBV-associated lymphoproliferations develop when just one cytotoxic lymphocyte population is compromised. This is for example the case for loss-of-function mutations in the minichromosome maintenance complex component 4 (MCM4), which compromises NK cell development and leads to absence of CD56bright NK cells (Eidenschenk et al.2006; Gineau et al.2012).
Similarly, compromised as well as hyperactive T cell receptor signaling leads primarily to EBV-associated lymphomas. The signaling components, which are affected by mutations that predispose for EBV-associated diseases, are IL-2 inducible T cell kinase (ITK), phosphoinositide 3-kinase (PI3K) 110δ (both loss- and gain-of-function mutations), the guanine nucleotide exchange factor RasGRP1, caspase recruitment domain-containing protein 11 (CARD11), phospholipase Cγ1, which is affected by deficient Mg2+ influx due to mutations in the magnesium transporter MAGT1, and ZAP70 (Huck et al.2009; Li et al.2011; Stepensky et al.2011; Linka et al.2012; Mansouri et al.2012; Snow et al.2012; Angulo et al.2013; Chaigne-Delalande et al.2013; Kuehn et al.2013; Ghosh et al.2014; Lucas et al.2014; Bienemann et al.2015; Cipe et al.2015; Dhalla et al.2015; Patiroglu et al.2015; Salzer et al.2016; Brigida et al.2017; Hoshino et al.2018; Latour and Winter 2018; Winter et al.2018). In addition, loss of the ability for cytoskeletal rearrangement by actin at immune synapses for efficient T cell receptor signaling due to Coronin actin binding protein 1A (CORO1A) deficiency also predisposes for EBV-associated lymphoproliferations (Moshous et al.2013).
NK and cytotoxic T cell function can also be compromised by deficient NK cell receptor or T cell co-receptor expression or signaling. Along these lines mutations in CD16, CTLA-4, CD27 and its ligand CD70, as well as in the adaptor molecules for the co-stimulatory SLAM family (SAP), including 2B4, and compromised NKG2D expression due to MAGT1 mutations have been found to predispose for EBV-associated lymphomas (de Vries et al.1996; Grier et al.2012; Salzer et al.2012; van Montfrans et al.2012; Chaigne-Delalande et al.2013; Alkhairy et al.2015; Abolhassani et al.2017; Izawa et al.2017; Schwab et al.2018). Interestingly, nuclear factor (NF)-κB1 deficiency, which is required for SAP and CD27 signaling, was also reported to be associated with EBV-driven lymphoproliferations (Boztug et al.2016; Schipp et al.2016). Moreover, lipopolysaccharide-responsive beige-like anchor (LRBA) protein regulates co-receptor internalization and its mutations have been found associated with EBV-induced lymphoproliferations (Alangari et al.2012). However, the pathological manifestations of EBV infection differ between these different primary immunodeficiencies that affect co-stimulation. In particular, CD16 deficiency leads to EBV-positive Castleman's disease and all patients with CD70 deficiency have so far presented with EBV-positive Hodgkin's lymphoma, while CD27 loss leads to more overt lymphoproliferations and sometimes even HLH.
A final category of primary immunodeficiencies that predispose for symptomatic EBV infection are mutations that affect survival and expansion of cytotoxic lymphocytes. These include deficiencies in serine/threonine kinase 4 (STK4), cytidine triphosphate synthase 1 (CTPS1) and X-linked inhibitor of apoptosis (XIAP) (Rigaud et al.2006; Abdollahpour et al.2012; Nehme et al.2012; Speckmann et al.2013; Martin et al.2014). CD27 and CD70 also play important roles in this cytotoxic lymphocyte expansion (Latour and Winter 2018). Depending on the role of these proteins in EBV-transformed B cell expansion, their deficiencies either preferentially cause immunopathologies such as HLH (for XIAP mutations) or, when preferentially T cell expansion is affected, EBV-associated lymphoproliferative disease (for STK4 mutations).
Of these primary immunodeficiencies some have a particularly high penetrance of EBV diseases, including deficiencies in SAP, CD27 and CD70 (Coffey et al.1998; Nichols et al.1998; Sayos et al.1998; Sumegi et al.2000; Booth et al.2011; Pachlopnik Schmid et al.2011; Salzer et al.2012; van Montfrans et al.2012; Alkhairy et al.2015; Abolhassani et al.2017; Izawa et al.2017).
For immune suppression by HIV co-infection and the primary immunodeficiencies in cytotoxic effector function, cytotoxic lymphocyte differentiation, T cell receptor signaling, lymphocyte co-stimulation, expansion and survival, however, affected patients have so far not been described to suffer from epithelial cell cancers. This suggests that latent EBV infection either does not occur in epithelial cells of healthy EBV carriers, or is not cell growth transforming, even in inflammatory settings like HIV co-infection and XIAP deficiency. Thus, the growth-transforming latency programs observed in nasopharyngeal carcinoma and the 10% of EBV-associated gastric carcinoma are most likely not a component of the EBV life cycle in healthy virus carriers.
IMMUNODEFICIENCIES THAT PREDISPOSE FOR KS
KS is the most common AIDS-associated cancer and in contrast to EBV, all of the KSHV-associated malignancies are considerably increased in the HIV-infected population, although these cancers can be seen in HIV-negative individuals as well (Bouvard et al.2009; Powles et al.2009a,b; Yarchoan and Uldrick 2018). Interestingly, as for EBV latency III tumors, KS incidence has declined and stabilized under cART (Krown et al.2008), while PEL and MCD development seem to be rather unaffected, similar to EBV-associated Hodgkin's lymphoma. KS also occurs during iatrogenic immune suppression after transplantation, especially under cyclosporine or tacrolimus (FK506) inhibition of calcineurin to prevent NF-AT activation downstream of T cell receptor signaling (Stallone et al.2005; Riva et al.2012; Jackson et al.2016), further suggesting that KSHV infection needs to be continuously immune-controlled during persistent infection.
Despite the increased frequency of PEL and MCD during immune suppression, KSHV-positive B cell malignancies have so far not been found associated with monogenic immunodeficiencies, although KSHV-positive MCD has been reported in a child born to consanguineous parents (Leroy et al.2012). Only KSHV-negative EBV-positive PELs have been reported as individual cases in combined and common variable immunodeficiencies (CVIDs) (Hisamoto et al.2003; Lam et al.2016), suggesting that EBV-associated plasmacytomas are very efficiently controlled by the immune system (Chatterjee et al.2017). In contrast, monogenic primary immunodeficiencies have been characterized that predispose either for isolated KS development or more generally for susceptibility to infectious diseases, including KS (Table 2). KS has been described in the context of CVID. CVID is a heterogeneous class of primary immune deficiencies associated with reduced level of serum antibodies, absent or impaired antibody production, and frequent infections. KS has been demonstrated to occur in the context of CVID (Wheat et al.2005; Gangemi, Allegra and Musolino 2015; Stenton et al.2016). Furthermore, mutations in both stromal interaction molecule 1 (STIM1) and tumor necrosis factor receptor superfamily member 4 (TNFRSF4, OX40 or CD134) predispose selectively for KS in children (Byun et al.2010; Byun et al.2013) (Fig. 2). STIM1 assists in the Ca2+ mobilization after T cell receptor signaling, while OX40 is a co-stimulatory receptor on non-classical innate and classical T cells as well as NK cells, and is expressed upon their activation (Saheki and De Camilli 2017; Buchan, Rogel and Al-Shamkhani 2018). OX40 ligand (OX40L) is expressed on many hematopoietic cells upon their activation, but can also be induced on endothelia and smooth muscle cells. This suggests that the OX40 interaction with OX40L is required for immune surveillance of KSHV-infected endothelial cells, which otherwise can give rise to KS. It is much less clear why STIM1 deficiency selectively predisposes for KS. In addition, KS has been found in children with mutations in interferon (IFN) γR1, STAT4, MAGT1 and Wiscott Aldrich syndrome protein (WASP) (Camcioglu et al.2004; Picard et al.2006; Aavikko et al.2015; Brigida et al.2017). While deficiencies in IFNγR1 and signaling for IFNγ production involving IL-12 are primarily known to confer susceptibility to mycobacterial infection (Jouanguy et al.1996; Newport et al.1996; Zhang et al.2008; Boisson-Dupuis et al.2015), IFNγR1 deficiency and diminished Th1 differentiation ability due to STAT4 mutations that compromise IL-12 signaling for IFNγ production seem to also predispose individuals towards developing KS (Camcioglu et al.2004; Aavikko et al.2015). This suggests an important role for IFNγ in KSHV-specific immune control. WASP deficiencies compromise actin stabilization of immunological synapse formation for T cell activation and have also been described to compromise EBV-specific immune control. Patients with WASP mutations have been reported to develop EBV-associated lymphomas (Du et al.2011). Furthermore, MAGT1 deficiencies have been proposed to compromise both NK- and T cell-mediated immune control of EBV (Chaigne-Delalande et al.2013), in addition to predisposing individuals to developing KS (Brigida et al.2017). Finally, KS has also been reported in a patient with Good's syndrome, which is a combined B- and T-cell immunodeficiency that occurs in association with a thymoma (Agarwal et al.2011). Thus, T cell-mediated immune control seems essential for maintaining asymptomatic KSHV infection. In contrast to EBV, however, both endothelial as well as B cell infection by this virus need to be immune controlled with cell-mediated immunity. In addition, IFNγ production by T cells might be more important for KSHV- than for EBV-specific immune control.
Table 2.
Primary immunodeficiencies that predispose for KSHV-associated diseases.
| Affected protein ‘name of syndrome’ | KSHV-associated disease | Innate immune system changes | Adaptive immune system changes | References |
|---|---|---|---|---|
| Th1 effector function | ||||
| IFNγR1 | KS | – | CD4+ T cell lymphopenia | Camcioglu et al.2004 |
| STAT4 | KS | – | Decreased Th1 differentiation | Aavikko et al.2015 |
| TCR signaling | ||||
| STIM1 | KS | – | Deficient T cell activation due to compromised Ca2+ influx | Byun et al.2010 |
| WASP ‘Wiskott Aldrich syndrome’ | KS | – | CD4+ T cell lymphopenia, deficiency in immunological synapse formation | Picard et al.2006 |
| Co-stimulation | ||||
| OX40 | KS | – | Impaired effector memory T cell populations | Byun et al.2013 |
| MagT1 ‘XMEN’ | KS | Decreased NK cell maturation and NKG2D expression | Compromised T cell receptor signaling | Brigida et al.2017 |
Figure 2.
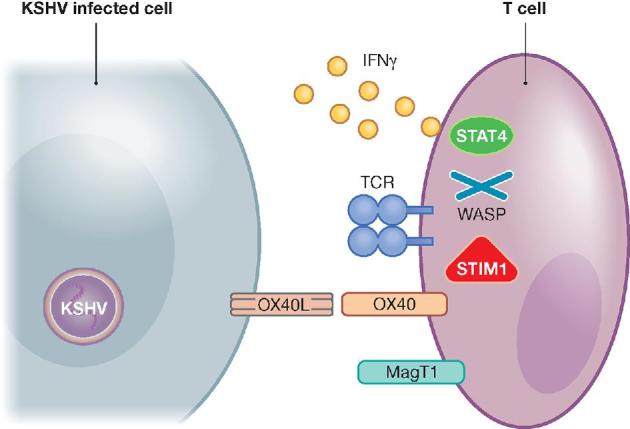
Primary immunodeficiencies that compromise the function of Th1-polarized lymphocytes and predispose for KSHV-associated diseases. Immune control of KSHV-infected endothelial cells is compromised upon deficiencies in TCR signaling, co-stimulation and Th1 cytokine functions. STIM1 is required for TCR signaling during KSHV-specific immune control. WASP deficiencies compromise actin cytoskeleton arrangements thereby hindering immune control of KSHV. OX40 is required to keep KSHV in check and MAGT1 allows co-receptor maintenance on lymphocytes. IFNγ plays an essential role in the immune surveillance of KSHV and STAT4 is required for its Th1-polarizing signaling. TCR, T cell receptor.
DIFFERENCES IN THE REQUIREMENT FOR IMMUNE SURVEILLANCE OF INFECTED NON-HEMATOPOIETIC COMPARTMENTS DURING PERSISTENT EBV AND KSHV INFECTION
The studies discussed above suggest an essential role for cytotoxic classical and innate non-classical T as well as NK cells in the immune control of EBV and KSHV. For EBV this was indeed functionally tested in preclinical in vivo models of mice with reconstituted or adoptively transferred human immune system compartments, and by therapeutic transfer of EBV-specific T cell populations into patients with EBV-associated malignancies (Münz 2017a,b).
During primary EBV infection in patients with infectious mononucleosis and mice with reconstituted human immune system components there is an expansion of NK cells (Williams et al.2005; Balfour et al.2013; Chijioke et al.2013; Azzi et al.2014; Dunmire et al.2015). In these mice, NK cell depletion during EBV infection leads to increased viral loads and tumorigenesis (Chijioke et al.2013; Landtwing et al.2016). The expanding NK cells primarily control lytic EBV infection in vivo and in vitro (Pappworth, Wang and Rowe 2007; Chijioke et al.2013; Azzi et al.2014). In addition to NK cells, Vγ9Vδ2 innate T cells expand in up to 50% of infectious mononucleosis patients (Djaoud et al.2017). Expansion or adoptive transfer for these Vγ9Vδ2 T cells in mice with reconstituted human immune system compartments reduces tumorigenesis after EBV-transformed B cell transfer or infection (Xiang et al.2014; Zumwalde et al.2017). Primarily, latency I Burkitt's lymphoma cells stimulate these Vγ9Vδ2 T cells by producing their mevalonate metabolite ligands and expressing the BTN 3A1 (CD277) restriction element (Djaoud et al.2017). As a third innate lymphocyte subset, NKT cells with the invariant Vα24-Jα18/Vβ11 T cell receptor can restrict EBV-associated lymphomas in mice with reconstituted human immune system components (Yuling et al.2009). They seem to primarily recognize latency II Hodgkin's lymphoma cells (Chung et al.2013).
Apart from these innate lymphocyte populations, CD4+ and CD8+ αβ T cells are thought to mediate EBV-specific immune control. Depletion of T cells or their CD4+ and CD8+ subpopulations individually increases EBV viral loads and associated lymphomagenesis in mice with reconstituted human immune system components (Strowig et al.2009; Yajima et al.2009; Chijioke et al.2015). Adoptive transfer of EBV-specific T cell lines was successfully used for the treatment of several EBV-associated lymphomas in patients (McLaughlin et al.2017). In addition, adoptive transfer of T cells against distinct EBV antigens provided clinical benefits in EBV infected mice with reconstituted human immune system components and patients (Icheva et al.2013; Antsiferova et al.2014). These studies corroborate the findings in immunodeficient patients that cytotoxic innate and adaptive lymphocytes mediate immune control of EBV, but so far no similar information on the function of cell-mediated immune control against KSHV infection in vivo is available, although immune restriction of KSHV infection by innate immune pathways has been demonstrated in cell culture studies (West and Damania 2008; West et al.2011; Ma et al.2015). This knowledge gap is mostly due to the absence of a preclinical in vivo model of persistent KSHV infection and associated pathologies, and to the lack of clinical trials of adoptive T cell transfer into patients with KSHV-associated malignancies.
Such an in vivo model to test these immunotherapeutic modalities might now be at hand. While previously transient infection was reported (Wang et al.2014), only co-infection with EBV allows for persistence of KSHV infection in mice with reconstituted human immune system components (McHugh et al.2017). Double-infection leads to PEL-like lymphoma formation with characteristic plasma cell differentiation. This in turn results in elevated lytic EBV replication and co-infection with a mutant EBV virus that cannot switch into lytic infection abolishes increased lymphomagenesis upon KSHV co-infection. This model should now allow us to dissect EBV- and KSHV-specific cell-mediated immune control in the same mice with reconstituted human immune system components to better understand the differences and similarities of cell-mediated immune control of these two oncogenic γ-herpesviruses, and how the genes affected by primary immunodeficiencies compromise it.
These models allow only lymphotropic infections by KSHV and EBV. Therefore, it is important to know if and how infection of non-hematopoietic cells contribute to the life cycle of these γ-herpesviruses. One criterion for the importance of a certain host cell is the adaptation of the virus to use specific entry receptors of the respective cell type. EBV and KSHV entry into B cells uses quite different receptors. Complement receptors 1 and 2 (CD35 and CD21) are used for EBV attachment and human major histocompatibility complex class II molecules are required as co-receptors for EBV entry into B cells (Fingeroth et al.1984; Li et al.1997; Ogembo et al.2013). In contrast, KSHV seems to use dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin for entry into B cells (Rappocciolo et al.2008). Curiously, both KSHV and EBV have been reported to use ephrin A2 receptor and the integrin αvβ5 for endothelial or epithelial cell infection, respectively (Akula et al.2002; Garrigues et al.2008; Chesnokova and Hutt-Fletcher 2011; Hahn et al.2012; Chen et al.2018; Zhang et al.2018). Therefore, the entry mechanisms of EBV and KSHV do not seem to be specific for epithelial or endothelial cells. Most likely, establishment of latent KSHV infection in endothelial cells, and possibly lytic EBV replication in epithelial cells after entry, rather than differences in entry mechanisms seem to determine the non-hematopoietic tropism of these two γ-herpesviruses. Moreover, EBV entry into epithelial cells seems to prefer infection from the basolateral side (Tugizov, Berline and Palefsky 2003), and EBV lytic reactivation was suggested to occur only efficiently from epigenetically silenced viral DNA, requiring about 2 weeks of DNA methylation (Woellmer, Arteaga-Salas and Hammerschmidt 2012). Therefore, lytic EBV replication might only play a role for viral shedding into saliva and further transmission. Similarly, endothelial cell infection by KSHV might occur secondary to submucosal B cell infection after salivary transmission of KSHV. Therefore, the above suggested cell-mediated immune control of EBV and KSHV infection in B cells should also be relevant for secondary epithelial and endothelial cell infections, which most likely only amplify the lymphotropic infections by the two viruses.
While increased incidence of KS in the context of immune deficiencies argues for transforming KSHV infection of endothelial cells being present also during asymptomatic KSHV infection, the lack of increased epithelial cell tumors argues against latent epithelial cell infection being part of the EBV life cycle in healthy virus carriers.
CONCLUSIONS AND OUTLOOK
The comparison of the influence of immunodeficiencies on persistent EBV and KSHV infections reveals interesting insights into the life cycle and immune control of these tumor viruses in healthy virus carriers. Firstly, both are controlled by cell-mediated immunity and cause AIDS-defining malignancies in HIV-infected individuals (AIDS-associated EBV lymphomas, PEL, MCD and KS). However, cytotoxicity might be primarily responsible for restricting EBV, while lymphocyte-derived IFNγ production seems to contribute to KSHV-specific immune control. Secondly, this cell-mediated immune control seems to depend on different T cell receptor signaling and co-stimulatory components for T cells to control persistent EBV and KSHV infection. These include ITK, PI3K 110δ, ZAP70 and RasGRP1 for T cell receptor signaling, or CD27, the SLAM receptor 2B4 and NKG2D for co-stimulation during EBV-specific immune control. For KSHV immune control, STIM1-assisted T cell receptor signaling and OX40-mediated co-stimulation seem to be more important. Thirdly, loss of these immune control components reveals in which cells the two oncogenic γ-herpesviruses need to be restricted to avoid EBV- and KSHV-associated pathologies. Surprisingly, transforming KSHV infection may be present in both B and endothelial cells from apparently healthy individuals, while EBV latent oncogene expression might be restricted to B cells.
These considerations suggest that transforming latent EBV infection that contributes to epithelial cell cancers like nasopharyngeal carcinoma and a subset of gastric carcinoma, constituting the majority of EBV-associated malignancies, does not readily occur in healthy virus carriers. Additional environmental factors and possible premalignant modifications of epithelial cells could render these susceptible to latent EBV infection. The characterization of these requirements and premalignant transformation should be important to understand and target EBV-associated epithelial cell cancers.
FUNDING
Research in our laboratories is supported by grants to CM from the Swiss National Science Foundation (310 030_162 560 and CRSII3_160 708), Cancer Research Switzerland (KFS-4091–02-2017), the Sobek Foundation, the Vontobel Foundation, the Baugarten Foundation, the Swiss MS Society, the Swiss Vaccine Research Institute and the clinical research priority programs on Multiple sclerosis (KFSPMS and KFSP-PrecisionMS) and human hemato-lymphatic diseases (KFSPHHLD) of the University of Zürich. B.D. is supported by NIH grants CA096500, DE028211, CA228172 and CA019014.
Conflict of interest. None declared.
REFERENCES
- Aavikko M, Kaasinen E, Nieminen JK et al.. Whole-genome sequencing identifies STAT4 as a putative susceptibility gene in classic Kaposi sarcoma. J Infect Dis 2015;211:1842–51. [DOI] [PubMed] [Google Scholar]
- Abdollahpour H, Appaswamy G, Kotlarz D et al.. The phenotype of human STK4 deficiency. Blood 2012;119:3450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abere B, Mamo TM, Hartmann S et al.. The Kaposi's sarcoma-associated herpesvirus (KSHV) non-structural membrane protein K15 is required for viral lytic replication and may represent a therapeutic target. PLoS Pathog 2017;13:e1006639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolhassani H, Edwards ES, Ikinciogullari A et al.. Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency. J Exp Med 2017;214:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Smereka P, Harpaz N et al.. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm Bowel Dis 2011;17:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula SM, Pramod NP, Wang FZ et al.. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 2002;108:407–19. [DOI] [PubMed] [Google Scholar]
- Alangari A, Alsultan A, Adly N et al.. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol 2012;130:481–8.e2. e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhairy OK, Perez-Becker R, Driessen GJ et al.. Novel mutations in TNFRSF7/CD27: Clinical, immunologic, and genetic characterization of human CD27 deficiency. J Allergy Clin Immunol 2015;136:703–12.e10. [DOI] [PubMed] [Google Scholar]
- Ambroziak JA, Blackbourn DJ, Herndier BG et al.. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 1995;268:582–3. [DOI] [PubMed] [Google Scholar]
- Angulo I, Vadas O, Garcon F et al.. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science 2013;342:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antsiferova O, Müller A, Rämer P et al.. Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog 2014;10:e1004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi T, Lunemann A, Murer A et al.. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood 2014;124:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc Natl Acad Sci USA 2000;97:12250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 2000;13:497–506. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Decker LL, Volk M et al.. EBV persistence in memory B cells in vivo. Immunity 1998;9:395–404. [DOI] [PubMed] [Google Scholar]
- Balfour HH Jr, Odumade OA, Schmeling DO et al.. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein–Barr virus infection in university students. J Infect Dis 2013;207:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon G, Chen K, Perez R et al.. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice. J Clin Invest 2011;121:1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Leser U, Marschall M et al.. Expression of proteins encoded by Epstein-Barr virus trans-activator genes depends on the differentiation of epithelial cells in oral hairy leukoplakia. Proc Natl Acad Sci USA 1991;88:8332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienemann K, Borkhardt A, Klapper W et al.. High incidence of Epstein-Barr virus (EBV)-positive Hodgkin lymphoma and Hodgkin lymphoma-like B-cell lymphoproliferations with EBV latency profile 2 in children with interleukin-2-inducible T-cell kinase deficiency. Histopathology 2015;67:607–16. [DOI] [PubMed] [Google Scholar]
- Binne UK, Amon W, Farrell PJ. Promoter sequences required for reactivation of Epstein-Barr virus from latency. J Virol 2002;76:10282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 1989;320:1731–5. [DOI] [PubMed] [Google Scholar]
- Boisson-Dupuis S, Bustamante J, El-Baghdadi J et al.. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev 2015;264:103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C, Gilmour KC, Veys P et al.. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood 2011;117:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K et al.. A review of human carcinogens—Part B: biological agents. Lancet Oncol 2009;10:321–2. [DOI] [PubMed] [Google Scholar]
- Boztug H, Hirschmugl T, Holter W et al.. NF-kappaB1 haploinsufficiency causing immunodeficiency and EBV-driven lymphoproliferation. J Clin Immunol 2016;36:533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigida I, Chiriaco M, Di Cesare S et al.. Large deletion of MAGT1 gene in a patient with classic Kaposi sarcoma, CD4 lymphopenia, and EBV infection. J Clin Immunol 2017;37:32–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnaro P, Morelli E, Cattelan F et al.. Non-AIDS definings malignancies among human immunodeficiency virus-positive subjects: Epidemiology and outcome after two decades of HAART era. World J Virol 2015;4:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan SL, Rogel A, Al-Shamkhani A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 2018;131:39–48. [DOI] [PubMed] [Google Scholar]
- Byun M, Abhyankar A, Lelarge V et al.. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med 2010;207:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun M, Ma CS, Akcay A et al.. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med 2013;210:1743–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camcioglu Y, Picard C, Lacoste V et al.. HHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiency. J Pediatr 2004;144:519–23. [DOI] [PubMed] [Google Scholar]
- Carbone A, Vaccher E, Gloghini A et al.. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol 2014;11:223–38. [DOI] [PubMed] [Google Scholar]
- Cesarman E. Gammaherpesviruses and lymphoproliferative disorders. Annu Rev Pathol Mech Dis 2014;9:349–72. [DOI] [PubMed] [Google Scholar]
- Chaigne-Delalande B, Li FY, O’Connor GM et al.. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 2013;341:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS et al.. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994;266:1865–9. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Sahli L, Chijioke O et al.. An immunocompetent patient with a recurrence-free Epstein-Barr virus positive plasmacytoma possesses robust Epstein-Barr virus specific T-cell responses. Haematologica 2017;102:e419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sathiyamoorthy K, Zhang X et al.. Ephrin receptor A2 is a functional entry receptor for Epstein–Barr virus. Nat Microbiol 2018;3:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by v 5 in addition to v 6 and v 8, and integrin binding triggers a conformational change in glycoproteins gHgL. J Virol 2011;85:13214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijioke O, Muller A, Feederle R et al.. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep 2013;5:1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijioke O, Marcenaro E, Moretta A et al.. The SAP-dependent 2B4 receptor mediates CD8+ T cell dependent immune control of Epstein Barr virus infection in mice with reconstituted human immune system components. J Infect Dis 2015;212:803–7. [DOI] [PubMed] [Google Scholar]
- Chung BK, Tsai K, Allan LL et al.. Innate immune control of EBV-infected B cells by invariant natural killer T cells. Blood 2013;122:2600–8. [DOI] [PubMed] [Google Scholar]
- Cipe FE, Aydogmus C, Serwas NK et al.. ITK deficiency: how can EBV be treated before lymphoma? Pediatr Blood Cancer 2015;62:2247–8. [DOI] [PubMed] [Google Scholar]
- Coffey AJ, Brooksbank RA, Brandau O et al.. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet 1998;20:129–35. [DOI] [PubMed] [Google Scholar]
- Cohen JI. Primary immunodeficiencies associated with EBV disease. Curr Top Microbiol Immunol 2015;390:241–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI. GATA2 deficiency and Epstein–Barr virus disease. Front Immunol 2017;8:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Fauci AS, Varmus H et al.. Epstein-Barr virus: An important vaccine target for cancer prevention. Sci Transl Med 2011;3:107fs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Niemela JE, Stoddard JL et al.. Late-onset severe chronic active EBV in a patient for five years with mutations in STXBP2 (MUNC18-2) and PRF1 (perforin 1). J Clin Immunol 2015;35:445–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E, Koene HR, Vossen JM et al.. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood 1996;88:3022–7. [PubMed] [Google Scholar]
- Dhalla F, Murray S, Sadler R et al.. Identification of a novel mutation in MAGT1 and progressive multifocal leucoencephalopathy in a 58-year-old man with XMEN disease. J Clin Immunol 2015;35:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Invest 2016;126:3165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaoud Z, Guethlein LA, Horowitz A et al.. Two alternate strategies for innate immunity to Epstein-Barr virus: One using NK cells and the other NK cells and gammadelta T cells. J Exp Med 2017;214:1827–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du MQ, Liu H, Diss TC et al.. Kaposi sarcoma-associated herpesvirus infects monotypic (IgMlambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 2001;97:2130–6. [DOI] [PubMed] [Google Scholar]
- Du S, Scuderi R, Malicki DM et al.. Hodgkin's and non-Hodgkin's lymphomas occurring in two brothers with Wiskott-Aldrich syndrome and review of the literature. Pediatr Dev Pathol 2011;14:64–70. [DOI] [PubMed] [Google Scholar]
- Dugan JP, Haverkos BM, Villagomez L et al.. Complete and durable responses in primary central nervous system posttransplant lymphoproliferative disorder with Zidovudine, Ganciclovir, Rituximab, and Dexamethasone. Clin Cancer Res 2018;24:3273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunmire SK, Grimm JM, Schmeling DO et al.. The incubation period of primary epstein-barr virus infection: Viral dynamics and immunologic events. PLoS Pathog 2015;11:e1005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers B, Spiess K, Leendertz F et al.. Lymphocryptovirus phylogeny and the origins of Epstein-Barr virus. J Gen Virol 2010;91:630–42. [DOI] [PubMed] [Google Scholar]
- Ehresman JS, Ahmed AK, Palsgrove DN et al.. Epstein-Barr virus-associated smooth muscle tumor involving the spine of an HIV-infected patient: Case report and review of the literature. J Clin Neurosci 2018;52:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidenschenk C, Dunne J, Jouanguy E et al.. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet 2006;78:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet North Am Ed 1964;1:702–3. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Henle G, Achong BG et al.. Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt's lymphoma. J Exp Med 1965;121:761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth JD, Weis JJ, Tedder TF et al.. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci USA 1984;81:4510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi S, Allegra A, Musolino C. Lymphoproliferative disease and cancer among patients with common variable immunodeficiency. Leuk Res 2015;39:389–96. [DOI] [PubMed] [Google Scholar]
- Garrigues HJ, Rubinchikova YE, Dipersio CM et al.. Integrin alphaVbeta3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J Virol 2008;82:1570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Bienemann K, Boztug K et al.. Interleukin-2-inducible T-cell kinase (ITK) deficiency - clinical and molecular aspects. J Clin Immunol 2014;34:892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gineau L, Cognet C, Kara N et al.. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest 2012;122:821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier JT, Forbes LR, Monaco-Shawver L et al.. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J Clin Invest 2012;122:3769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich AE, Vajdic CM. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol 2015;42:247–57. [DOI] [PubMed] [Google Scholar]
- Hahn AS, Kaufmann JK, Wies E et al.. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi's sarcoma-associated herpesvirus. Nat Med 2012;18:961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassman LM, Ellison TJ, Kedes DH. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J Clin Invest 2011;121:752–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto A, Yamane H, Hiraki A et al.. Human herpes virus-8-negative primary effusion lymphoma in a patient with common variable immunodeficiency. Leuk Lymphoma 2003;44:2019–22. [DOI] [PubMed] [Google Scholar]
- Hochberg D, Middeldorp JM, Catalina M et al.. Demonstration of the Burkitt's lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA 2004;101:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong GK, Gulley ML, Feng WH et al.. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J Virol 2005;79:13993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopcraft SE. and Damania B. Tumour viruses and innate immunity. Phil Trans R Soc Lond B Biol Sci 2017;372:20160267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Takashima T, Yoshida K et al.. Dysregulation of Epstein-Barr virus infection in hypomorphic ZAP70 mutation. J Infect Dis 2018;218:825–34. [DOI] [PubMed] [Google Scholar]
- Huck K, Feyen O, Niehues T et al.. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest 2009;119:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icheva V, Kayser S, Wolff D et al.. Adoptive transfer of Epstein-Barr virus (EBV) nuclear antigen 1-specific T cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol 2013;31:39–48. [DOI] [PubMed] [Google Scholar]
- Izawa K, Martin E, Soudais C et al.. Inherited CD70 deficiency in humans reveals a critical role for the CD70–CD27 pathway in immunity to Epstein-Barr virus infection. J Exp Med 2017;214:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CC, Dickson MA, Sadjadi M et al.. Kaposi sarcoma of childhood: inborn or acquired immunodeficiency to oncogenic HHV-8. Pediatr Blood Cancer 2016;63:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E, Altare F, Lamhamedi S et al.. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med 1996;335:1956–62. [DOI] [PubMed] [Google Scholar]
- Katano H, Ali MA, Patera AC et al.. Chronic active Epstein-Barr virus infection associated with mutations in perforin that impair its maturation. Blood 2004;103:1244–52. [DOI] [PubMed] [Google Scholar]
- Krown SE, Lee JY, Dittmer DP et al.. More on HIV-associated Kaposi's sarcoma. N Engl J Med 2008;358:535–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Niemela JE, Rangel-Santos A et al.. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood 2013;121:3117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol Mech Dis 2006;1:375–404. [DOI] [PubMed] [Google Scholar]
- Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol 2005;79:1296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam GK, Abdelhaleem M, Somers GR et al.. Primary effusion lymphoma (PEL)-like lymphoma in a child with congenital immunodeficiency. Pediatr Blood Cancer 2016;63:1674–6. [DOI] [PubMed] [Google Scholar]
- Landtwing V, Raykova A, Pezzino G et al.. Cognate HLA absence in trans diminishes human NK cell education. J Clin Invest 2016;126:3772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S, Winter S. Inherited immunodeficiencies with high predisposition to Epstein–Barr virus-driven lymphoproliferative diseases. Front Immunol 2018;9:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy S, Moshous D, Cassar O et al.. Multicentric Castleman disease in an HHV8-infected child born to consanguineous parents with systematic review. Pediatrics 2012;129:e199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FY, Chaigne-Delalande B, Kanellopoulou C et al.. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011;475:471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Spriggs MK, Kovats S et al.. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol 1997;71:4657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linka RM, Risse SL, Bienemann K et al.. Loss-of-function mutations within the IL-2 inducible kinase ITK in patients with EBV-associated lymphoproliferative diseases. Leukemia 2012;26:963–71. [DOI] [PubMed] [Google Scholar]
- Lucas CL, Kuehn HS, Zhao F et al.. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol 2014;15:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SD, Hegde S, Young KH et al.. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol 2011;85:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Jacobs SR, West JA et al.. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci USA 2015;112:E4306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace EM, Hsu AP, Monaco-Shawver L et al.. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood 2013;121:2669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri D, Mahdaviani SA, Khalilzadeh S et al.. IL-2-inducible T-cell kinase deficiency with pulmonary manifestations due to disseminated Epstein-Barr virus infection. Int Arch Allergy Immunol 2012;158:418–22. [DOI] [PubMed] [Google Scholar]
- Martin DF, Kuppermann BD, Wolitz RA et al.. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med 1999;340:1063–70. [DOI] [PubMed] [Google Scholar]
- Martin E, Palmic N, Sanquer S et al.. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature 2014;510:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain KL, Leach CT, Jenson HB et al.. Association of Epstein–Barr virus with leiomyosarcomas in young people with AIDS. N Engl J Med 1995;332:12–8. [DOI] [PubMed] [Google Scholar]
- McGeoch DJ. Molecular evolution of the gamma-Herpesvirinae. Philos Trans R Soc Lond B Biol Sci 2001;356:421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Caduff N, Barros MHM et al.. Persistent KSHV infection increases EBV-associated tumor formation in vivo via enhanced EBV lytic gene expression. Cell Host Microbe 2017;22:61–73.e7. [DOI] [PubMed] [Google Scholar]
- McLaughlin LP, Gottschalk S, Rooney CM et al.. EBV-directed T cell therapeutics for EBV-associated lymphomas. Methods Mol Biol 2017;1532:255–65. [DOI] [PubMed] [Google Scholar]
- Melbye M, Cote TR, West D et al.. Nasopharyngeal carcinoma: An EBV-associated tumour not significantly influenced by HIV-induced immunosuppression. The AIDS/Cancer Working Group. Br J Cancer 1996;73:995–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA 1973a;70:190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Lipman M. Comparison of the yield of infectious virus from clones of human and simian lymphoblastoid lines transformed by Epstein-Barr virus. J Exp Med 1973b;138:1398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshous D, Martin E, Carpentier W et al.. Whole-exome sequencing identifies Coronin-1A deficiency in 3 siblings with immunodeficiency and EBV-associated B-cell lymphoproliferation. J Allergy Clin Immunol 2013;131:1594–1603.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz C. Epstein–Barr virus-specific immune control by innate lymphocytes. Front Immunol 2017a;8:1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz C. Humanized mouse models for Epstein Barr virus infection. Current Opinion in Virology 2017b;25:113–8. [DOI] [PubMed] [Google Scholar]
- Murer A, McHugh D, Caduff N et al.. EBV persistence without its EBNA3A and 3C oncogenes in vivo. PLoS Pathog 2018;14:e1007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme NT, Schmid JP, Debeurme F et al.. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 2012;119:3458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport MJ, Huxley CM, Huston S et al.. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 1996;335:1941–9. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Harkin DP, Levitz S et al.. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci USA 1998;95:13765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogembo JG, Kannan L, Ghiran I et al.. Human complement receptor type 1/CD35 is an Epstein-Barr virus receptor. Cell Rep 2013;3:371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachlopnik Schmid J, Canioni D, Moshous D et al.. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood 2011;117:1522–9. [DOI] [PubMed] [Google Scholar]
- Palser AL, Grayson NE, White RE et al.. Genome diversity of Epstein-Barr virus from multiple tumor types and normal infection. J Virol 2015;89:5222–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappworth IY, Wang EC, Rowe M. The switch from latent to productive infection in Epstein-Barr virus-infected B cells is associated with sensitization to NK cell killing. J Virol 2007;81:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030–44. [DOI] [PubMed] [Google Scholar]
- Patiroglu T, Haluk Akar H, Gilmour K et al.. A case of XMEN syndrome presented with severe auto-immune disorders mimicking autoimmune lymphoproliferative disease. Clin Immunol 2015;159:58–62. [DOI] [PubMed] [Google Scholar]
- Pauk J, Huang ML, Brodie SJ et al.. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med 2000;343:1369–77. [DOI] [PubMed] [Google Scholar]
- Picard C, Mellouli F, Duprez R et al.. Kaposi's sarcoma in a child with Wiskott-Aldrich syndrome. Eur J Pediatr 2006;165:453–7. [DOI] [PubMed] [Google Scholar]
- Powles T, Stebbing J, Bazeos A et al.. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman's disease. Ann Oncol 2009a;20:775–9. [DOI] [PubMed] [Google Scholar]
- Powles T, Robinson D, Stebbing J et al.. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2009b;27:884–90. [DOI] [PubMed] [Google Scholar]
- Rappocciolo G, Hensler HR, Jais M et al.. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008;82:4793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud S, Fondaneche MC, Lambert N et al.. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 2006;444:110–4. [DOI] [PubMed] [Google Scholar]
- Riva G, Luppi M, Barozzi P et al.. How I treat HHV8/KSHV-related diseases in posttransplant patients. Blood 2012;120:4150–9. [DOI] [PubMed] [Google Scholar]
- Rohr J, Beutel K, Maul-Pavicic A et al.. Atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2 overlaps with primary immunodeficiency diseases. Haematologica 2010;95:2080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P. Endoplasmic reticulum–plasma membrane contact sites. Annu Rev Biochem 2017;86:659–84. [DOI] [PubMed] [Google Scholar]
- Salzer E, Daschkey S, Choo S et al.. Combined immunodeficiency with life-threatening EBV-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica 2012;98:473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer E, Cagdas D, Hons M et al.. RASGRP1 deficiency causes immunodeficiency with impaired cytoskeletal dynamics. Nat Immunol 2016;17:1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayos J, Wu C, Morra M et al.. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM [see comments]. Nature 1998;395:462–9. [DOI] [PubMed] [Google Scholar]
- Schipp C, Nabhani S, Bienemann K et al.. Specific antibody deficiency and autoinflammatory disease extend the clinical and immunological spectrum of heterozygous NFKB1 loss-of-function mutations in humans. Haematologica 2016;101:e392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TF, Cesarman E. Kaposi sarcoma-associated herpesvirus: mechanisms of oncogenesis. Curr Opin Virol 2015;14:116–28. [DOI] [PubMed] [Google Scholar]
- Schwab C, Gabrysch A, Olbrich P et al.. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol 2018;142:1932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shebl FM, Bhatia K, Engels EA. Salivary gland and nasopharyngeal cancers in individuals with acquired immunodeficiency syndrome in United States. Int J Cancer 2010;126:2503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis 2010;51:957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer 2011;117:1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin SH, Dittmer DP. Viral latency locus augments B-cell response in vivo to induce chronic marginal zone enlargement, plasma cell hyperplasia, and lymphoma. Blood 2013;121:2952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin SH, Kim Y, Eason A et al.. KSHV latency locus cooperates with myc to drive lymphoma in mice. PLoS Pathog 2015;11:e1005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow AL, Xiao W, Stinson JR et al.. Congenital B cell lymphocytosis explained by novel germline CARD11 mutations. J Exp Med 2012;209:2247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckmann C, Lehmberg K, Albert MH et al.. X-linked inhibitor of apoptosis (XIAP) deficiency: The spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol 2013;149:133–41. [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B et al.. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 2005;352:1317–23. [DOI] [PubMed] [Google Scholar]
- Stenton S, Fernando M, Currie Z et al.. Metachronous diffuse large B-cell lymphoma and Kaposi sarcoma of the right eyelid and lacrimal gland in a patient with granulomatous common variable immunodeficiency. Ocul Oncol Pathol 2016;2:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepensky P, Weintraub M, Yanir A et al.. IL-2-inducible T-cell kinase deficiency: Clinical presentation and therapeutic approach. Haematologica 2011;96:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Gurer C, Ploss A et al.. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med 2009;206:1423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumegi J, Huang D, Lanyi A et al.. Correlation of mutations of the SH2D1A gene and epstein-barr virus infection with clinical phenotype and outcome in X-linked lymphoproliferative disease. Blood 2000;96:3118–25. [PubMed] [Google Scholar]
- Tangye SG, Palendira U, Edwards ES. Human immunity against EBV-lessons from the clinic. J Exp Med 2017;214:269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Erratum: Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med 2003;9:307–14. [DOI] [PubMed] [Google Scholar]
- van Montfrans JM, Hoepelman AI, Otto S et al.. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol 2012;129:787–793.e6. e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Kang G, Kumar P et al.. Humanized-BLT mouse model of Kaposi's sarcoma-associated herpesvirus infection. Proc Natl Acad Sci USA 2014;111:3146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Damania B. Upregulation of the TLR3 Pathway by Kaposi's sarcoma-associated herpesvirus during primary infection. J Virol 2008;82:5440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JA, Gregory SM, Sivaraman V et al.. Activation of plasmacytoid dendritic cells by Kaposi's sarcoma-associated Herpesvirus. J Virol 2011;85:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat WH, Cool CD, Morimoto Y et al.. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med 2005;202:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, McAulay K, Macsween KF et al.. The immune response to primary EBV infection: A role for natural killer cells. Br J Haematol 2005;129:266–74. [DOI] [PubMed] [Google Scholar]
- Winter S, Martin E, Boutboul D et al.. Loss of RASGRP1 in humans impairs T-cell expansion leading to Epstein-Barr virus susceptibility. EMBO Mol Med 2018;10:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woellmer A, Arteaga-Salas JM, Hammerschmidt W. BZLF1 governs CpG-methylated chromatin of Epstein-Barr virus reversing epigenetic repression. PLoS Pathog 2012;8:e1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Liu Y, Zheng J et al.. Targeted activation of human Vgamma9Vdelta2-T cells controls Epstein-Barr virus-induced B cell lymphoproliferative disease. Cancer Cell 2014;26:565–76. [DOI] [PubMed] [Google Scholar]
- Yajima M, Imadome K, Nakagawa A et al.. T cell-mediated control of Epstein-Barr virus infection in humanized mice. J Infect Dis 2009;200:1611–5. [DOI] [PubMed] [Google Scholar]
- Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med 2018;378:1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuling H, Ruijing X, Li L et al.. EBV-induced human CD8+ NKT cells suppress tumorigenesis by EBV-associated malignancies. Cancer Res 2009;69:7935–44. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Y, Wang HB et al.. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat Microbiol 2018;3:1–8. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Boisson-Dupuis S, Chapgier A et al.. Inborn errors of interferon (IFN)-mediated immunity in humans: Insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev 2008;226:29–40. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Gui XE, Zhong YH et al.. Cancer in cohort of HIV-infected population: Prevalence and clinical characteristics. J Cancer Res Clin Oncol 2011;137:609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumwalde NA, Sharma A, Xu X. et al Adoptively transferred Vgamma9Vdelta2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight 2017;2:93179. [DOI] [PMC free article] [PubMed] [Google Scholar]