Abstract
Intracellular occupancy of the respiratory epithelium is a useful pathogenic strategy facilitating microbial replication and evasion of professional phagocytes or circulating antimicrobial drugs. A less appreciated but growing body of evidence indicates that the airway epithelium also plays a crucial role in host defence against inhaled pathogens, by promoting ingestion and quelling of microorganisms, processes that become subverted to favour pathogen activities and promote respiratory disease. To achieve a deeper understanding of beneficial and deleterious activities of respiratory epithelia during antimicrobial defence, we have comprehensively surveyed all current knowledge on airway epithelial uptake of bacterial and fungal pathogens. We find that microbial uptake by airway epithelial cells (AECs) is a common feature of respiratory host–microbe interactions whose stepwise execution, and impacts upon the host, vary by pathogen. Amidst the diversity of underlying mechanisms and disease outcomes, we identify four key infection scenarios and use best-characterised host–pathogen interactions as prototypical examples of each. The emergent view is one in which effi-ciency of AEC-mediated pathogen clearance correlates directly with severity of disease outcome, therefore highlighting an important unmet need to broaden our understanding of the antimicrobial properties of respiratory epithelia and associated drivers of pathogen entry and intracellular fate.
Keywords: respiratory epithelium, airway epithelial cells (AECs), microbial uptake, epithelial responses, pathogenesis, microbicidal activities
With a view to better understanding the role of the respiratory epithelium in host defence against inhaled pathogens, this review explores the mechanistic basis of microbial uptake by airway epithelia for a diverse range of bacterial and fungal pathogens, with an emphasis on microbial intracellular trafficking and fate, and correlations with pathogen clearance or host disease.
INTRODUCTION
A broad range of inhaled pathogens including bacterial and fungal species, as well as viruses and some protozoa, cause diseases of the lower respiratory tract including bronchitis, pneumonia, flu, bronchiolitis and tuberculosis. The respiratory epithelium is a common point of initial host contact for this myriad of microbes that provoke highly varied and multifaceted host responses. Entry into host mucosae has been conventionally recognised as a pathogenic strategy exploited by microbes to access a nutrient-rich niche within which to replicate and promote invasion of substrata, at the same time avoiding immune detection or antimicrobial drugs. However, an increasing body of evidence indicates that the airway epithelium also plays a crucial role in host defence against inhaled pathogens by opsonising, ingesting and quelling microorganisms. In most instances, the relevance of microbial uptake to pathogenesis or resolution of respiratory disease remains to be directly addressed, thereby obscuring potentially useful commonalities amongst pathogenic or host behaviours and associated disease outcomes. With a view to identifying common regulatory paradigms, we here define a substantial cohort of bacterial and fungal pathogens of the respiratory tract for which airway epithelial uptake has been demonstrated (Table S1, Supporting Information), and explore the relationship between pathogen processing in human respiratory epithelia and disease pathology.
For ease of readability, we focus on several key examples in the field, and refer the reader to Table S1 (Supporting Information) for a more thorough tableau of lesser-studied species and diseases. Although virus entry into airway epithelial cells (AECs) is sometimes achieved via similar mechanisms to those exploited by bacterial and fungal species, and leads to similar downstream host activation and intracellular trafficking, we consider the differences between uptake of living, metabolically active microbes versus lifeless particles to lie beyond the scope of this review. Similarly, since inhalation is rarely a route of transmission for protozoa causing respiratory disease, protozoa will not be analysed in this review.
MICROBIAL UPTAKE BY AECs IS A STEPWISE PROCESS RESULTING IN SPECIES-SPECIFIC OUTCOMES
While the canonical classification of professional phagocytes is limited to polymorphonuclear granulocytes, monocytes and macrophages, AECs can also neutralise microbes via phagocytic means. However, phagocytic indices, microbicidal activity and associated host responses are muted compared to that of their professional counterparts (Aderem and Underhill 1999; DeMali and Burridge 2003; Niedergang and Chavrier 2004). Broadly speaking, internalisation by AECs occurs in a stepwise manner (Fig. 1) whereby receptor- or effector-mediated uptake, also referred to as the ‘zipper’ or ‘trigger’ mechanisms, respectively (Swanson and Baer 1995), leads to cytoskeletal remodelling and signalling activation of the host cell. The trigger mechanism is induced by pathogen-derived soluble effectors that, once delivered into the host cell by microbial contact or proximity, induce the actin-mediated formation of transient membrane projections to engulf the pathogen. In the case of zipper-mediated uptake, molecular coupling of specific pathogen ligand(s) and host cell receptor(s) leads, with minimal changes in the cell surface, to pathogen engulfment by the host cell membrane.
Figure 1.
Microbial uptake by AECs occurs in a step wise manner and leads to species-specific outcomes for both host and pathogen. Receptor- or effector-mediated uptake of microbes by AECs leads to the activation of host cellular signalling pathways and remodelling of the host cytoskeleton at the site of pathogen entry. A successful antimicrobial defence results from trafficking of the internalised pathogen, through a series of intracellular compartments culminating in phagolysosome-mediated killing. In some cases however, intracellular pathogens evade intracellular killing either by thriving in the extremely hostile environment of the AEC phagolysosome or by preventing the fusion of the microbe-containing vacuole with lysosomes. By residing latently in the intracellular niche or replicating, such pathogens successfully exploit internalisation by AECs to cause host damage and trigger inflammatory responses, ultimately leading to disseminated infection.
Following uptake, microbes are trafficked via various membrane-bound vacuoles to a series of intracellular compartments for killing (Fig. 1). Some intracellular pathogens are able to evade intracellular killing, therefore exploiting internalisation by AECs as a means to cause host damage and/or to disseminate. On a species-specific basis, this capability might promote disease in fully immunocompetent or immunodeficient hosts or both. Various intracellular replicative niches have been identified including (i) lysosome-like vacuoles, which have an acidic pH and contain hydrolytic enzymes; (ii) non-acidic vacuoles, whose fusion to lysosomes has been subverted by the pathogen; (iii) the autophagosome, a double-membraned compartment, normally delivered to lysosomal compartments for degradation of the enclosed material; and (iv) in the cytosol of the host cell, after escape of the pathogen from endocytic compartments (Mostowy and Cossart 2012; Gomes and Dikic 2014).
Some respiratory pathogens trigger apoptosis or cell death of AECs to exit their replicative niche and/or breach the epithelial barrier to enter underlying tissues (Fig. 1). Alternatively, induction of epithelial cell death or apoptosis can in some instances promote host defence by (i) preventing pathogen access to nutrients, (ii) downregulating inflammation, (iii) eliminating diseased cells and (iv) delivering the intracellular pathogen in apoptotic bodies to competent professional phagocytes. Accordingly, some respiratory pathogens have evolved strategies to safeguard their replicative niche by exhibiting anti-apoptotic activities to restrain host apoptosis or block epithelial cell death.
In professional phagocytes, such as macrophages, the phagocytosis of pathogens is coupled with the production of inflammatory cytokines via the cooperation of two classes of innate immune receptors, namely the phagocytic receptors and the Toll-like receptors, which respectively signal particle internalisation and trigger appropriate inflammatory responses (Underhill et al.1999). The connection between microbial uptake and the activation of an AEC-mediated inflammatory response is also well documented for several respiratory pathogens. Amongst the plethora of human AEC responses to microbial challenge, IL-8 expression predominates as a potent chemoattractant and activator of neutrophils, monocytes and T lymphocytes, especially in association with airway inflammatory diseases (Sagel, Chmiel and Konstan 2007).
MICROBIAL UPTAKE LEADING TO EFFICIENT PATHOGEN CLEARANCE
There are many examples of efficient AEC-mediated defence against respiratory pathogens including, but not limited to, Pseudomonas aeruginosa, Aspergillus fumigatus (the major mould pathogen of human lungs) and species of the Burkholderia cepacia complex (Bcc). All of these microbes cause disease predominantly amongst patients with impaired immunity or pre-existing chronic lung disease, thereby indicating the critical importance of a healthy respiratory niche in delivering efficient defence against infectious disease. In health, it is likely that efficiency of antimicrobial activity is achieved in collaboration with professional phagocytes, whilst in disease the paucity of innate defences is likely compounded by deficient AEC-mediated clearance. The manner in which microbes are cleared by AECs varies in a species-specific manner, in some instances being mediated by the directly microbicidal activities of AECs (Fig. 2A and B), and in others by despatching infected AECs (including their intracellular pathogenic cargo) from the airway epithelium (Fig. 3). Sometimes, naturally occurring genetic variants, such as unencapsulated isolates of the Gram-negative bacterium Klebsiella pneumoniae (Fig. 2C), serve to illustrate the immense potency with which microbial attributes (such as capsular polysaccharide) can undermine otherwise highly efficacious AEC-mediated antimicrobial defence.
Figure 2.
Microbial uptake leading to direct neutralisation of pathogen. (A) Burkholderia cepacia complex: once internalised by wild-type AECs, species of the B. cepacia complex (Bcc) are trafficked to cathepsin D-positive endocytic vesicles and killed. Uptake by AECs occurs in a CFTR-dependent manner and via an uncharacterised glycolipid receptor and requires Bcc lipases, the flagellum, cable pilin and the 22-kDa adhesin protein, which binds to the host surface protein cytokeratin 13 (CK13). Exogenous addition of IL-8 enhances intracellular bacterial growth. (B) Aspergillus fumigatus spores: following uptake by AECs, the majority of internalised A. fumigatus spores are killed. Aspergillus fumigatus uptake is mediated by E-cadherin and CFTR, by Dectin-1 via binding of fungal β-glucan and α5β1 integrin via binding of A. fumigatus CalA. The gliotoxin immunotoxin also facilitates spore internalisation by AECs. (C) Capsule-deficient K. pneumoniae variants: upon uptake by AECs, capsule-deficient K. pneumoniae are efficiently killed. Klebsiella pneumoniae is internalised by AECs in a process that involves a GlcNAc-binding surface component and an N-glycosylated receptor on the host cell surface. Also, AEC-mediated C3 opsonisation enhances dramatically CD46-mediated microbial uptake. Klebsiella pneumoniae uptake increases surface expression of ICAM-1 and secretion of IL-8 by AECs in an NF-kB-dependent manner. Pathogen-derived effectors of uptake are indicated in bold black font, putative or proven host receptors, opsonins or bridging factors driving uptake are indicated in bold red font.
Figure 3.
Microbial clearance facilitated by cellular desquamation and apoptosis of infected AECs: P. aeruginosa. (A) In healthy AECs, internalisation of P. aeruginosa leads to initiation of NF-κB nuclear translocation, cellular desquamation and eventual apoptosis and shedding of the infected cells. Intracellular P. aeruginosa viability is not reduced within the host cell and P. aeruginosa replicates in plasma membrane blebs (PMBs) via products secreted by a bacterial type III secretion system. Pseudomonas aeruginosa uptake is dependent on the bacterial lipopolysaccharide (LPS)–core oligosaccharide and CFTR and, in a strain-dependent manner, on αvβ5 and α5β1integrins via vitronectin (Vn) and fibronectin (Fn) bridging, respectively. The interaction of the two major bacterial adhesion factors, namely type IV (Tfp) and flagella, with the N-glycoproteins and heparate sulfate proteoglycans (HSPG), respectively, is also required for microbial uptake, as well as the effector proteins of the secretion systems H2-T6SS and H3-T6SS. Internalisation-mediated apoptosis limits the release of cytokines, such as IL-1β. (B) Following uptake of P. aeruginosa, CF epithelial cells undergo significantly delayed apoptosis compared with wild-type AECs allowing higher rates of intracellular replication and sustained host damage. Pathogen-derived effectors of uptake are indicated in bold black font, putative or proven host receptors, opsonins or bridging factors driving uptake are indicated in bold red font.
Microbial uptake leading to direct neutralisation of pathogen: B. cepacia species complex and A. fumigatus
Bcc species are the major cause of morbidity and mortality (30%) in cystic fibrosis (CF) patients (Razvi et al.2009; Folescu et al.2015). In vitro uptake of Bcc has been demonstrated using several types of AECs (Burns et al.1996; Martin and Mohr 2000; Cieri et al.2002; Duff et al.2006; Moura et al.2008; Mullen, Callaghan and McClean 2010), where the intracellular bacteria become enclosed within membrane-bound vacuoles (Fig. 2A), an observation corroborated as also being relevant in vivo via electron microscopy of murine respiratory epithelial cells (Burns et al.1996; Chiu, Ostry and Speert 2001). Comparative analysis of microbial uptake of 31 environmental and clinical Bcc isolates via gentamycin protection assay showed mean percent uptake rates of 0.003%–3.613% and indicated a statistically significant correlation between the ability to invade A549 cells and to cause infection in an in vivo mouse agar bead model (Cieri et al.2002). Importantly, the highly virulent and transmissible clinical isolate J2315 is able to enter, survive and replicate efficiently within A549 cells, whereas uptake of the environmental isolate J2540 is shown to be six times less efficient and this isolate is unable to survive and replicate within AECs (Martin and Mohr 2000). By 24 h post-infection, epithelial monolayers infected with J2540 appear intact; however, monolayers infected with J2315 exhibit physical disruption, mitochondrial rounding and vacuolisation (Martin and Mohr 2000).
Once internalised by healthy AECs, most Bcc genomovars are contained in endocytic vesicles and killed, but in CF cell lines aberrant maturation of endocytic vesicles promotes bacterial viability and replication, leading to extensive host damage (Fig. 2A) (Martin and Mohr 2000; Sajjan, Keshavjee and Forstner 2004; Sajjan et al.2006, 2008; Kaza, McClean and Callaghan 2011). In CF airway cells, less than 10% of Bcc-containing vacuoles acquire cathepsin D, promoting bacterial avoidance of intracellular killing and intracellular replication (Sajjan et al.2008), and intracellular survival and replication requires at least one of the two type IV secretion systems (T4SS) encoded by Bcc genomes. Accordingly, insertional inactivation of the Ptw secretion system leads to altered intracellular trafficking whereby ∼30% of ptwD4 mutant cells become localised to cathepsin D-positive vacuoles, becoming thereby targeted for lysosomal degradation (Sajjan et al.2008).
The ability of Bcc complex to preferentially invade CF airway cells and to avoid killing once internalised has been hypothesised to contribute to their persistence in the lungs of infected CF patients (Sajjan, Ackerley and Forstner 2002). In agreement with this hypothesis, lung sections of infected CF patients reveal an abundance of intra-AEC bacteria and bacterial colonisation of the intercellular junctions of the airway epithelium (Sajjan et al.2001). Furthermore, bacteria can be found in CF lungs despite treatment with antibiotics having demonstrated activity against Bcc (Gold et al.1983), although it should be also noted that serum and airway surface concentrations of systemically administered antibiotics are likely to differ.
While uptake of Bcc genomovars has been widely demonstrated in various types of AECs, the mechanistic basis of Bcc uptake remains poorly characterised (Fig. 2A). Bcc lipases facilitate AEC uptake, since pre-treatment of AEC monolayers with Bcc lipase or with the lipase active-site inhibitor tetrahydrolipstatin (Orlistat) respectively increases or decreases Bcc uptake by up to 2-fold, depending on the genomovar used for infection (Mullen, Callaghan and McClean 2010). Pre-treatment of AECs with α- or β-galactosidase or inhibitors of host glycolipid biosynthesis also significantly reduces Bcc uptake compared to untreated epithelial monolayers, supporting the involvement of one or more still unidentified glycosphingolipid host receptors in driving bacterial uptake (Mullen, Callaghan and McClean 2010). Confocal and transmission electron microscopy of a highly transmissible Bcc strain expressing the cable pilin and the 22-kDa adhesin proteins demonstrated the requirement for both surface components for fully efficient Bcc uptake by primary bronchial epithelial cultures differentiated into squamous epithelia (Sajjan, Ackerley and Forstner 2002). Importantly, the 22-kDa adhesin protein has been found to bind to the host surface protein cytokeratin 13 (CK13), which is not only abundantly expressed by differentiated cultured squamous epithelia in vitro, but also in the airway of CF patients (Sajjan et al.2000). Furthermore, non-motile Bcc mutants lacking flagellar genes show a 50% reduction in uptake by A549 cells relative to their parental isolates in an in vitro infection (Tomich et al.2002).
Relative to uninfected cultures, infection of healthy AECs with Bcc isolates leads to significant early secretion of tumour necrosis factor α (TNF-α), IL-6, IL-1β and IL-8 (Gillette et al.2013). The exogenous addition of IL-8 to in vitro infection assays enhances intracellular replication of Bcc (Fig. 2A) (Kaza, McClean and Callaghan 2011), suggesting that strains eliciting more IL-8 secretion may have a higher propensity to survive intracellularly and accordingly, compared to other Bcc isolates, the epidemic strain LMG16656 showed greater levels of IL-8 induction and intracellular growth upon uptake by AECs (Kaza, McClean and Callaghan 2011). Despite higher basal levels in CFTR (cystic fibrosis transmembrane conductance regulator)-negative cells, internalisation of Bcc stimulates secretion of IL-8 by both CFTR-positive and -negative cell lines (Kaza, McClean and Callaghan 2011). At high IL-8 concentrations, growth of bacteria is significantly stimulated in both CFTR-positive and -negative cells; thus, B. cepacia strains eliciting more IL-8 secretion are able to survive better within epithelial cells (Kaza, McClean and Callaghan 2011).
The environmental mould A. fumigatus causes a broad spectrum of diseases, the differential pathologies of which are dependent upon host immune response and underlying disease. Invasive aspergillosis is associated with an estimated 200 000 deaths per annum, while allergic bronchopulmonary aspergillosis affects more than 4 million asthmatic and CF sufferers and chronic pulmonary aspergillosis exacerbates pre-existing structural and immunological lung defects in more than 3 million people worldwide (Brown et al.2012). In vitro and ex vivo infection models have determined spore internalisation to be one of the earliest events following spore adhesion to AECs, occurring in both ciliated and non-ciliated cells (Amitani and Kawanami 2009). Bronchial and type II alveolar epithelia are able to internalise approximately 30%–50% of adherent A. fumigatus conidia in a concentration- and time-dependent manner (Fig. 2B) (Wasylnka and Moore 2002, 2003; Han et al.2011; Xu et al.2012; Bertuzzi et al.2014; Bao et al.2015), while cultured human tracheobronchial explants have demonstrated present, but scarce, uptake by ciliated epithelial cells from the upper airways (Paris et al.1997).
Epithelial uptake of A. fumigatus spores can result in either fungal killing or intraphagosomal occupancy (Osherov 2012; Sheppard and Filler 2014; Croft et al.2016; Bertuzzi et al.2018), the latter occurring at a low frequency in vitro. Internalised conidia quickly acquire late endosomal/lysosomal markers such as the lysosome-associated membrane protein 1 (LAMP-1), and CD63 and cathepsin D (Fig. 2B). Following rapid intracellular trafficking through the endosomal system to the phagolysosomes (Wasylnka and Moore 2003), phagolysosomal acidification leads to killing of most internalised conidia; however, ∼3% of the internalised conidia remain viable and 34% of these can eventually germinate by 36 h, without lysis of the host cells (Wasylnka and Moore 2003). Aspergillus fumigatus is able to inhibit A549 apoptosis induced by TNF-α, staurosporine and cycloheximide (CHX) (Berkova et al.2006; Amin et al.2014), suggesting that pathogen-mediated viability of the hosting cell might facilitate longevity within the intracellular niche.
The notion that suboptimal quenching of internalised A. fumigatus spores might promote subsequent invasive disease is further supported by the recent discovery of a role for mammalian α5β1 integrin-mediated engagement of an A. fumigatus thaumatin-like protein, called CalA, in spore uptake and pulmonary infection of mice (Fig. 2B) (Liu et al.2016). In in vitro infection assays, both anti-β1 or -α5 antibody- and siRNA-mediated inhibition of integrin functionality reduce internalisation of A. fumigatus by ∼50% (Liu et al.2016) as does administration of anti-CalA antibody. Moreover, intraperitoneal injection of an anti-CalA antibody prior to A. fumigatus infection in cortisone-acetate-treated mice increased survival of the infected mice by 20% (Liu et al.2016).
The uptake of A. fumigatus by cultured alveolar epithelial cells has also been found to depend on the C-type lectin type receptor Dectin-1 (Fig. 2B) (Han et al.2011; Bertuzzi et al.2014) that drives spore uptake, in a host phospholipase (PLD1)-dependent manner, via fungal cell wall β-1,3-glucan exposed on the surface of swollen and germinated fungal spores (Han et al.2011). Antibody-mediated Dectin-1 depletion inhibits both PLD activity (Han et al.2011) and A. fumigatus internalisation into A549 cells by as much as 50% (Han et al.2011); however, neither siRNA-mediated Dectin-1 knockdown nor antibody-mediated blocking of receptor functionality completely abrogates uptake of A. fumigatus suggesting the existence of other uptake mechanisms (Han et al.2011; Bertuzzi et al.2014). Concordantly, knockdown of E-cadherin expression via blocking antibody or siRNA also results in reduced, but not absent, cell–conidial associations and conidial internalisation (Xu et al.2012, 2016). Dectin-1 has been described primarily as a pattern recognition receptor of myeloid-derived phagocytes; however, immunohistochemical staining of human lung sections reveals that Dectin-1 is expressed mainly apically on both bronchial and alveolar human epithelium (Heyl et al.2014) and flow cytometric analyses of normal human bronchial epithelial (NHBE) cells cultured in vitro from the same donors, as well as commercially available primary small AECs, similarly confirm Dectin-1 expression (Heyl et al.2014).
Aspergillus fumigatus secretes several secondary metabolites, the most potent of which is gliotoxin, an epipolythiodioxopiperazine immunotoxin described as a facilitator of spore internalisation by A549 cells (Jia et al.2014). Mutants lacking the gene gliP, which regulates the production of gliotoxin in A. fumigatus, show a 40% reduction in internalisation by A549 cells compared to wild-type isolates, a defect that is rescued by endogenous supplementation of gliotoxin (Jia et al.2014).
In conclusion, the potency of AEC-mediated fungicidality supports a role for AECs in clearance of inhaled A. fumigatus spores, a role that might become dysfunctional in settings of respiratory disease or immunosuppression. Concordantly, Chaudhary et al. (2012) reported that AECs lacking the CFTR or expressing a non-functional CFTR (ΔF508) are deficient for internalisation and intracellular killing of ingested A. fumigatus spores compared to bronchial cell lines and AECs derived from murine tracheas and also undergo more conidial-induced apoptosis in response to spore uptake.
Naturally occurring pathogen variants that heighten efficiency of AEC-mediated microbial clearance: K. pneumoniae
Klebsiella pneumoniae causes hospital-acquired bacterial pneumonia, with associated mortality of 25%–60% (Sahly and Podschun 1997). The efficiency of K. pneumoniae uptake by AECs (Fig. 2C) is highly dependent upon bacterial capsule polysaccharide whereby poorly encapsulated or capsule-deficient mutants of K. pneumoniae are internalised by A549 cells significantly more efficiently than their encapsulated wild-type counterparts (Huang et al.2013). Although the basal rate of uptake into AECs is quite low (only ∼0.01% of the total bacterial inoculum is internalised after 24 h of infection), AECs incubated with poorly encapsulated or capsule-deficient strains of K. pneumoniae kill more than 90% of the internalised bacteria, indicating a striking ability of AECs to quell intracellular K. pneumoniae (Fig. 2C) (Cortes et al.2002; de Astorza et al.2004). In stark contrast, infection of AECs with encapsulated K. pneumoniae isolates, which are significantly less efficiently internalised by AECs, triggers extensive AEC detachment and cytotoxicity (Cano et al.2009). In vivo, transmission electron microscopy of lung sections obtained from mice infected with a poorly encapsulated, avirulent strain of K. pneumoniae, showed that the bacterium reaches the alveolus, becoming adhered to, or localised within, the epithelial cells in vacuole-like structures (de Astorza et al.2004). In contrast, the less efficiently internalised wild-type strain produces pneumonia and systemic infection, indicating that uptake by AECs represents a curative mechanism against K. pneumoniae infection (Cortes et al.2002; de Astorza et al.2004; Cano et al.2009).
In vitro infection experiments in the presence of various glycan-binding competitors or inhibitors of eukaryotic glycosylation demonstrate that K. pneumoniae uptake by A549 cells involves the coupling of glycan moieties and N-glycosylated receptor(s) respectively on the bacterial and host cell surface (Fig. 2C) (Fumagalli et al.1997). Furthermore, AEC-mediated C3 opsonisation enhanced dramatically (by 8-fold) CD46-mediated microbial uptake (de Astorza et al.2004).
A K. pneumoniae capsule-deficient mutant caused increased surface expression of ICAM-1 and secretion of IL-8 by primary airway cells (NHBE) and A549 cells, in comparison with a wild-type strain (Fig. 2C) (Regueiro et al.2006). This inflammatory response was dependent on NF-kB activation, mediated by TLR2 and TLR4 and was abrogated in the presence of cytochalasin D, indicating that bacterial internalisation by AECs triggers a signal for the activation of the innate immune system of the airway (Regueiro et al.2006). Furthermore, IL-8 enhanced the fungicidality of neutrophils supporting a crucial facilitative role for AECs in the activation of pulmonary innate immune responses following uptake. In contrast, poorly internalised wild-type K. pneumoniae does not elicit an inflammatory response in AECs (Regueiro et al.2006; Moranta et al.2010), instead attenuating IL-8 secretion via inhibition of NF-kB and MAPK signalling in an NOD1-dependent manner (Regueiro et al.2011). Similarly, in the whole animal, infection with a wild-type encapsulated K. pneumoniae results in a dampened secretion of inflammatory mediators and reduced infiltration of inflammatory cells (Yoshida et al.2000).
Microbial clearance facilitated by cellular desquamation and apoptosis of infected AECs: P. aeruginosa
The Gram-negative bacterium P. aeruginosa causes persistent infections in patients with chronic respiratory diseases such as CF and Chronic obstructive pulmonary disease (COPD). Microscopy and culture tests on sputum from CF patients indicate that at least 40% of CF patients are infected by P. aeruginosa (Lambiase et al.2006; Razvi et al.2009) and chronic P. aeruginosa infection accounts for disease-related morbidity and mortality in 60%–80% of CF patients (Moore and Mastoridis 2017). Pseudomonas aeruginosa is internalised by various type of AECs such as A549 (Chi et al.1991; Gagniere and Di Martino 2004; Leroy-Dudal et al.2004) and 16HBE14 (Darling, Dewar and Evans 2004) cells via a process that is dependent on the bacterial lipopolysaccharide (LPS)–core oligosaccharide (Fig. 3A) (Pier et al.1996).
CFTR is crucial for P. aeruginosa uptake and cultured human AECs expressing the non-functional ΔF508 allele are impaired in the uptake of P. aeruginosa compared to non-mutated counterparts, supporting a direct link between defective uptake by AECs and hypersusceptibility of CF patients to lung infections (Pier et al.1996). CFTR-mediated uptake of P. aeruginosa into epithelial cells leads to NF-κB nuclear translocation, cellular desquamation and eventual apoptosis of the infected cells, ultimately driving bacterial clearance and quelling of the infection (Fig. 3A) (Pier et al.1996; Pier, Grout and Zaidi 1997; Grassme et al.2000; Schroeder et al.2001, 2002). AECs expressing ΔF508 undergo significantly delayed apoptosis compared with cells expressing wild-type CFTR (Fig. 3B) and lungs from CF mice show no apoptosis after infection with P. aeruginosa compared with their wild-type counterparts (Cannon et al.2003). Following infection with P. aeruginosa, lipid rafts isolated from wild-type CFTR-expressing AECs showed high abundance of the major vault protein (MVP) in comparison with those isolated from infected CF airway cells (Kowalski et al.2007) and moreover, AECs isolated from MVP−/− mice internalised 55% less bacteria than their wild-type counterparts, a finding that correlated with a > 3-fold increase in bacterial burden and mortality amongst infected MVP−/− mice compared to wild-type mice (Kowalski et al.2007).
Within AECs, P. aeruginosa viability is not reduced, instead intracellular replication occurs in plasma membrane blebs (Jolly et al.2015), protrusions of the plasma membrane associated with multiple types of cell death (Fig. 3A). Survival and replication of P. aeruginosa in membrane blebs depends on the bacterial type III secretion system (T3SS), since mutants lacking the three T3SS effectors ExoS, ExoT or ExoY are instead trafficked to acidic vacuoles where they are unable to replicate (Jolly et al.2015). CFTR is also implicated as controlling survival and replication of bacteria after internalisation by AECs (Fig 3A and B) as P. aeruginosa has an enhanced survival rate in CFTR-deficient cells (Jolly et al.2015). While not directly reducing bacterial viability, P. aeruginosa internalisation by AECs leads to shedding of infected cells, therefore providing a direct means of reducing bacterial burden (Fig. 3A). Thus, uptake by AECs likely has a beneficial role for the healthy host in defence against P. aeruginosa infection. In support of this view, a decreased ability of lung cells from transgenic CF mice, relative to wild-type mice, to ingest LPS-smooth P. aeruginosa results in a significant impairment in bacterial clearance and greater bacterial lung burden (Schroeder et al.2001).
In addition to CFTR, the efficiency of P. aeruginosa uptake by AECs is also mediated by engagement of the integrin family of αβ heterodimeric transmembrane cell adhesion receptors (Hynes and Zhao 2000), whereby integrin engagement prompts the engulfment of bound pathogen via F-actin filaments (Fig. 3A) (Brakebusch and Fassler 2003; DeMali, Wennerberg and Burridge 2003; Goodwin and Yap 2004; Hauck and Ohlsen 2006). Pseudomonas aeruginosa binding to members of the mammalian integrin family occurs indirectly via the bridging activity of the extracellular matrix components vitronectin (Vn) and fibronectin (Fn) in a strain-specific manner. While Vn bridges αvβ5 integrin-mediated uptake of the PAK reference strain by A549 cells (Leroy-Dudal et al.2004), the clinical isolate ER97314 adheres to α5β1 integrins on A549 epithelial cells via an Fn-mediated interaction (Gagniere and Di Martino 2004). In vitro, a rapid increase of α5, αv, β1 and β4 integrin expression is stimulated by infection with P. aeruginosa (Gravelle et al.2010).
In addition to integrins, P. aeruginosa also enters AECs via interaction with other apical and basolateral receptors, the expression of which is affected by cell polarisation in in vitro infection systems and/or prompted during wound-repairing processes (Fig. 3A). For example, in vitro infection experiments with isogenic sets of P. aeruginosa mutants and pharmacological inhibitors show that binding, invasion and cytotoxicity of polarised Calu-3 monolayers are due to the interaction of the two major bacterial adhesion factors, type IV (Tfp) and flagella, with the N-glycoproteins and heparate sulfate proteoglycans (HSPG) respectively at the apical and basolateral host surfaces (Bucior, Mostov and Engel 2010; Bucior, Pielage and Engel 2012). In contrast, in incompletely polarised Calu-3 monolayers, HSPG abundance is increased at the apical surface resulting in a 2-fold higher AEC susceptibility to P. aeruginosa internalisation and damage (Bucior, Mostov and Engel 2010). Concordantly, in both in vitro and in vivo experimentation, manipulation of cell polarity was found to enhance bacterial internalisation and subsequent cytotoxicity, hence explaining the striking ability of P. aeruginosa to attach to and infect wounded, as opposed to healthy, tissues (Fleiszig et al.1997; Roger et al.1999; Bucior, Mostov and Engel 2010; Engel and Eran 2011; Plotkowski et al.1999).
Pseudomonas aeruginosa is able to regulate its internalisation into epithelial cells by producing and delivering effector proteins to the host cell via type III (T3SS) and type VI (T6SS) secretion systems, whose effectors facilitate host cell entry and bacterial toxicity (Fig. 3A). Among these, H2-T6SS is included, which interacts with the AEC microtubule network, promoting bacterial uptake into HeLa and polarised human airway epithelial Calu-3 cells (Sana et al.2012) and PldB, a H3-T6SS phospholipase D effector of P. aeruginosa, which facilitates uptake by HeLa and A549 cells (Jiang et al.2014).
Bronchoalveolar lavages of CF patients show elevated levels of IL-6 and IL-8 (Bonfield et al.1995), and pre-treatment of CF AECs with IL-6 or IL-8 increases P. aeruginosa internalisation, suggesting that appropriate inflammatory responsiveness might hamper the establishment of chronic colonisation in CF patients (Hussain et al.2015). Despite controversy, glucocorticoids are used as anti-inflammatory agents; however, it has been shown that concomitant treatment of CF airway cells with glucocorticoids and IL-6 decreases bacterial internalisation, in turn reducing bacterial clearance and possibly increasing the risk of pulmonary infection in CF patients (Hussain et al.2015). Heightened release of IL-1β is also central to the lethal effect of acute P. aeruginosa infection and mice deficient for the IL-1 receptor show improved host defence against infection in the lung (Schultz et al.2002, 2004). Following uptake of P. aeruginosa, cytokine release by AECs seems to be negatively regulated by internalisation, whereby uptake inhibition causes an 8-fold increase in release and a 100-fold increase in mRNA of IL-1β (Grassme et al.2003). Internalisation-mediated apoptosis might therefore limit cytokine release in the whole animal to a level that is beneficial to the host (Grassme et al.2003).
EPITHELIAL ENTRY AS A PATHOGENIC STRATEGY
Some respiratory pathogens, including, but not limited to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Mycobacterium tuberculosis and Chlamydia species, are able to resist the antimicrobial properties of healthy AECs leading to pathogen persistence and establishment of chronic or acute infection. The ability to reside within intracellular niches can be a transient component of a more complex journey through the respiratory epithelium that occurs with minimal in situ host damage but promotes disseminated disease, or an opportunity for the pathogen to replicate, leading to host cell apoptosis, fibrosis and inflammation.
Microbial uptake with incomplete killing leading to intracellular replication and host damage: S. aureus
Staphylococcus aureus is a common coloniser of the nasopharynx that causes lower respiratory tract infections, particularly in the hospital setting (Diekema et al.2001; Jones 2010) and in CF patients (Kahl 2010; Wong, Ranganathan and Hart 2013). While skin and soft tissue infections by S. aureus are extremely common, lower respiratory infections are much less frequent but are associated with much poorer outcomes (Klevens et al.2007), with staphylococcal pneumonia being associated with up to 37% mortality in the USA (Haque et al.2012). Staphylococcus aureus is internalised by—and survives within AECs—a process that has been postulated as leading to bacterial persistence and establishment of chronic infections (Alexander and Hudson 2001). In support of this theory, in patients with recurrent rhinosinusitis where intranasal biopsies were examined over a period of more than three consecutive years, intracellular bacteria were found located inside the nasal epithelium (Clement et al.2005). Molecular typing demonstrated patient-specific clonality of the staphylococci, suggesting that intracellular staphylococcal reservoirs contribute to recurrent infection (Clement et al.2005). Accordingly, naturally occurring S. aureus small colony variants, which have been linked to chronic, recurrent and antibiotic-resistant infections (Proctor et al.1995), show enhanced uptake and survival within A549 cells (Tuchscherr et al.2011) and more importantly invade significantly more CF-like AECs than normal AECs (Mitchell et al.2011).
Staphylococcus aureus is able to undergo intracellular replication within cultured wild-type AECs (Li et al.2009; Korea et al.2014) and in cultured respiratory epithelial cells (CFT-1) derived from a CF patient (Kahl et al.2000), leading to host cell apoptosis (Fig. 4A). It has been hypothesised that recurrent infections in CF patients are due to defective bacterial clearance by AECs but no difference in internalisation rate or timing has been observed between CFT-1 cells and LCFSN cells, their functionally reconstituted counterparts (Kahl et al.2000). However, clear differences in intracellular fate of S. aureus are demonstrable in CFT-1 and LCFSN cells, whereby LCFSN cells are more efficiently able to contain intracellular replication of the bacteria than their mutated counterpart (Jarry and Cheung 2006). In LCFSN, most of the internalised bacteria remain contained, at 4 h after invasion (Jarry and Cheung 2006), in a non-replicative state in vesicles that have acquired vacuolar-ATPase, lysosomal markers LAMP-1 and -2, and the lysomotrophic dye LysoTracker (Fig. 4A). In CFT-1 cells, S. aureus-containing vesicles cells rapidly lose their association with lysosomal markers and transmission electron microscopy analysis shows that the majority of bacteria become free in the cytosol at 4 h after invasion (Jarry and Cheung 2006).
Figure 4.
Microbial uptake leading to incomplete killing, host damage and disseminated infection. (A) Staphylococcus aureus: upon uptake by AECs, S. aureus is able to undergo intracellular replication within, and cause apoptosis of, cultured AECs to further disseminate and cause disease. EsxA and EsxB, members of the ESAT-6-like secretion system, contribute to bacterial release from host cells. Staphylococcus aureus uptake by AECs occurs via the interaction of FnBPs, FN-binding proteins, and the host integrin α5β1. The extracellular adherence protein Eap also binds to Fn, while FnBPs bind the heat shock protein 60 (Hsp60). (B) Streptococcus pneumoniae: upon uptake by AECs, internalised S. pneumoniae co-localises with several endosomal markers such as LAMP-1, Rab5, Rab4 and Rab7, indicating bacterial intracellular trafficking to recycling endosomes for transcytosis and/or killing in phagolysosomes. However, complete killing is not achieved and remaining pneumococci may persist, without multiplying, leading to transcytosis. Streptococcus pneumoniae αvβ3 integrin internalisation by AECs is mediated by Vn, the plasminogen and Fn-binding protein PepO and the choline-binding protein CbpA/PspC, which binds to the complement regulator factor H and the human polymeric immunoglobulin receptor (pIgR). AECs infected with S. pneumoniae release IL-8 in an internalisation-dependent manner. (C) Haemophilus influenzae: NTHI variants can become internalised by AECs to subsequently reside intracellularly and trigger apoptosis of the infected cells. Integrins, Dectin-1, CEACAMs, PAFr and Vn are essential for H. influenzae uptake by AECs. Internalisation via PAFr occurs via the interaction with ChoP of the lipooligosaccharide (LOS), while surface fibrils on NTHIs allow the interaction with Vn. Pathogen-derived effectors of uptake are indicated in bold black font, putative or proven host receptors, opsonins or bridging factors driving uptake are indicated in bold red font.
Staphylococcus aureus causes apoptosis of AECs (Fig. 4A), and infection of A549 cells with an isogenic panel of S. aureus deletion strains indicates that secretion of EsxA, a substrate of the ESAT-6-like secretion system (Ess) together with EsxB, interferes with this process (Korea et al.2014). Epithelial cells infected with the ΔesxAB mutant show an increased presence of intracellular bacteria over those infected with the wild type suggesting that EsxA and EsxB together contribute to bacterial release from host cells by modulating host apoptosis. Indeed, transcriptional profiling of HEp-2 cell monolayers infected with S. aureus indicates that intracellular S. aureus causes differential expression of genes involved in cellular stress responses and signal transduction, inflammation, apoptosis, fibrosis and cholesterol biosynthesis (Li et al.2009). A transient apoptotic process is then followed by necrosis and after 24 h of infection with 108 CFU mL−1 bacteria, most airway cells exhibit a necrotic phenotype (da Silva et al.2004).
Staphylococcus aureus is internalised by A549 cells via the host integrin α5β1 (Wang et al.2013), but studies on several other non-AEC human cells (for example, the nasopharyngeal cell line Detroit 562 and the embryonic kidney cell line 293T) suggest that the interaction between S. aureus and AECs occurs indirectly via Fn bridging activity (Fig. 4A) (Sinha et al.1999; Massey et al.2001; Jett and Gilmore 2002). Indeed, S. aureus mutants lacking the fibronectin-binding proteins (FnBPs) serve as excellent examples of redundancy in uptake mechanisms. Such mutants, despite being unable to engage the host α5β1 integrin via Fn bridging, are still internalised although at low efficiencies (Sinha et al.1999). Furthermore, antibody-mediated blocking of host α5β1 integrin achieves only 60% reduction relative to untreated monolayers (Sinha et al.1999; Kintarak et al.2004). Besides FnBPs, the extracellular adherence protein Eap has also been shown to bind Fn and supplementation with exogenous Eap1 in in vitro infections enhances S. aureus uptake by epithelial cells (Haggar et al.2003). Staphylococcus aureus FnBPs were also found to bind to another cellular partner beyond integrins, namely the heat shock protein 60 (Hsp60) from membrane preparations of the bronchial cell line HEp-2, which are deficient in Fn production (Dziewanowska et al.2000).
Staphylococcus aureus infection of A549 cells has been found to induce a strong inflammatory response with peak expression of genes encoding CCL5, CXCL11 and IL-6 in S. aureus-infected cells at 8 to 24 h post-infection (Strobel et al.2016). In S. aureus-infected fibroblasts, IL-6 expression is prompted by binding of the cytoskeletal focal adhesion protein vinculin to the Rab small GTPase Rab5 to induce bacterial uptake and phosphorylation of host MAP kinases, including p38, ERK and JNK (Hagiwara et al.2014). Similarly, in the mouse lung, siRNA-mediated knockdown of vinculin or Rab5 heightens p38, ERK and JNK phosphorylation and IL-6 expression leading to reduced infection by S. aureus (Hagiwara et al.2014) and highlighting the potential of vinculin as a target for therapy against S. aureus pneumonia.
Microbial uptake leading to incomplete killing and disseminated infection: S. pneumoniae and H. influenzae
Despite being common colonisers of the human respiratory tract, S. pneumoniae and H. influenzae are the most frequent causes of lower respiratory tract infection in patients with impaired immunity or pre-existing chronic lung disease. Streptococcus pneumoniae, a common Gram-positive coloniser of the nasopharynx, is the most frequent causative agent of community-acquired pneumonia (CAP), especially in children and the elderly (O’Brien et al.2009; Naucler et al.2013; Heron 2015), while H. influenzae, a Gram-negative commensal of the human respiratory tract, is one of the most prevalent pathogens identified during COPD exacerbations (Garcha et al.2012). Importantly, both pathogens are thought to exploit uptake by AECs as a way of accessing areas underlying the respiratory epithelium, hence leading to the dissemination of the infection beyond the epithelial barrier. Supporting a role for intracellular localisation of unencapsulated (non-typeable) H. influenzae (NTHI) in exacerbations of chronic bronchitis, in situ hybridisation and immunofluorescence microscopy performed on human bronchial biopsies revealed intracellular bacteria in 21 of 39 patients having chronic or acute bronchitis versus none of seven healthy individuals (Bandi et al.2001). Furthermore, intermittent NTHI exacerbations caused by the same strain suggest that, in COPD, AECs might provide a protected reservoir of infectious agents (Murphy et al.2004).
Primarily an extracellular pathogen, S. pneumoniae is internalised at low rates by AECs, a process (Fig. 4B) that occurs via multiple redundant entry mechanisms (Bergmann et al.2009; Asmat et al.2011, 2014; Agarwal et al.2013a,b). In vitro infection studies show that internalised pneumococci co-localise with early, recycling and late endosomal markers such as LAMP-1, Rab5, Rab4 and Rab7, indicating bacterial intracellular trafficking to the recycling endosomes for transcytosis and/or killing in phagolysosomes (Asmat et al.2014). Antibiotic protection assays demonstrate, in both Calu-3 and A549 cells (Asmat et al.2011), that more than 95% of intracellular pneumococci are killed after 7 h of co-incubation. However, complete killing is not achieved (Asmat et al.2011, 2014; Agarwal et al.2013a,b) and remaining pneumococci may persist, without multiplying, in lysosomes for 4 h and longer to subsequently exit the phagosome and undergo transcytosis across in vitro cultured epithelia, which remain largely undamaged (Asmat et al.2014).
An important body of work has identified that transcytosis occurs via a receptor-dependent vacuole-assisted mechanism requiring engagement of the platelet-activating factor (PAF) receptor (PAFr), a G-protein coupled receptor (GPCR) for the circulating lipid chemokine PAF (Fig. 4B) (Cundell et al.1995; Le Gouill et al.1997; Rijneveld et al.2004; Radin et al.2005). PAFr is now well recognised as a portal for invasion for many respiratory pathogens displaying phosphorylcholine on their surface (Swords et al.2000, 2001; Fillon et al.2006) and is also required for S. pneumoniae uptake by AECs (Cundell et al.1995; Radin et al.2005). Cell wall phosphorycholine on the bacterial surface mimics the natural ligand of PAFr, and this interaction initiates bacterial uptake of virulent S. pneumoniae in a β-arrestin-dependent way (Radin et al.2005). Following S. pneumonia binding to PAFr, the receptor is internalised and recycled, transporting the bacterium to the basolateral side of the epithelium, ultimately leading to systemic dissemination. Whole animal imaging studies using PAFr−/– mice have demonstrated that PAFr deficiency leads to lessened dissemination of intratracheally administered S. pneumoniae providing robust evidence that transcytosis facilitates disseminated disease (Cundell et al.1995; Rijneveld et al.2004).
Maximal uptake of S. pneumoniae by AECs and other host cells is facilitated by exploitation of several host receptors that has diversified host tropism to include a broad range of cell types (Fig. 4B). Heparin competitive binding experiments indicate that internalisation of S. pneumoniae by A549 cells is also mediated by αvβ3 integrin via Vn bridging (Bergmann et al.2009). In addition to the integrin/Vn- and the PAFr-mediated mechanisms (Cundell et al.1995; Radin et al.2005; Bergmann et al.2009), S. pneumoniae also employs at least one further cell surface component, namely the plasminogen and Fn-binding protein PepO, for achieving epithelial entry (Agarwal et al.2013b, 2014).
Streptococcus pneumonia expresses the choline-binding protein CbpA/PspC (Fig. 4B), which interacts, via distinct epitopes, with the complement regulator factor H and the human polymeric immunoglobulin receptor (pIgR), mediating pneumococcal internalisation by AECs (Zhang et al.2000; Hammerschmidt et al.2007; Agarwal and Hammerschmidt 2009; Agarwal et al.2010). Accordingly, PspC-deficient strains are 100-fold less efficient colonisers of the nasopharynx than the respective wild-type isolates (Rosenow et al.1997). pIgR mediates the transport of polymeric IgA or IgM from the basolateral to the apical surface of polarised respiratory epithelial cells to then be recycled and transported in a retrograde manner (Rojas and Apodaca 2002). Several lines of evidence suggest that S. pneumoniae exploits the pIgR-transcytosis machinery for uptake and further dissemination beyond the mucosal barrier (Zhang et al.2000; Agarwal and Hammerschmidt 2009; Asmat et al.2014). The level of pIgR expression in various epithelial cell lines correlates with the degree of bacterial adherence and invasion, whereby the highest pIgR-expressing cells, namely the nasopharyngeal epithelial Detroit-562, were found to take up 10–50 times more S. pneumonia than other cell lines (Zhang et al.2000). While no pIgR expression has been demonstrated for the alveolar and bronchial models, such as A549 and BEAS-2B cells (Zhang et al.2000; Agarwal et al.2010; Iovino, Molema and Bijlsma 2014), Calu-3 bronchial epithelial cells do express pIgR (Agarwal and Hammerschmidt 2009), indicating that different ligand-receptor pairs are employed by different cell types and possibly in different host niches.
A549 cells infected with group B streptococci release IL-8 and this process occurs in an internalisation-dependent manner, as suggested by comparative analysis of a wild-type isolate and a poorly internalised cylE mutant, lacking a beta-haemolysin/cytolysin (Doran et al.2002). Importantly, following 4 h of infection of A549 cells, the cylE mutant shows attenuated cytolytic activity in comparison with the respective wild-type isolates, hence substantiating a damaging role for pneumococcal uptake by AECs (Doran et al.2002). Accodingly, Interferon (IFN)β treatment of AEC monolayers leads to the downregulation of PAFr and subsequently of pneumococcal uptake in vitro and this corresponds to a reduced development of bacteraemia following intranasal infection with S. pneumoniae in mice treated with IFNβ or IFN-I-inducing synthetic double-stranded RNA (LeMessurier et al.2013).
Several studies have demonstrated that NTHI variants can become internalised by AECs to subsequently reside within intracellular membrane-bound vacuoles (Fig. 4C) (Ketterer et al.1999; Swords et al.2000, 2001; Ahren et al.2001; Morey et al.2011; Lopez-Gomez et al.2012; Clementi, Hakansson and Murphy 2014; Heyl et al.2014; Singh et al.2014). Co-localisation with endosomal markers including early endosomal antigen 1 (EEA1), LAMP-1, LAMP-2, CD63 and Rab7 indicates that the internalised bacteria are trafficked via the endosomal pathway (Morey et al.2011; Clementi, Hakansson and Murphy 2014). Some of the bacilli undergo lysosomal killing after variable durations of persistence (Clementi, Hakansson and Murphy 2014); however, incomplete co-localisation of the internalised bacilli with cathepsin D, a protease that accumulates in lysosomes, indicates that the majority of intracellular bacteria do not reach mature lysosomes but remain in alternative acidic intracellular compartments in a metabolically active but non-proliferative state (Morey et al.2011; Goyal et al.2015). The bacterial protease IgaB, which belongs to the class of IgA1 proteases directed against the predominant secretory immunoglobulin of human mucosal surfaces IgA1, is required for optimal intracellular survival and persistence of NTHI in AECs by cleaving LAMP-1 at pHs characteristic of endolysosomal trafficking (Clementi, Hakansson and Murphy 2014).
Flow cytometric analysis of apoptotic markers in A549 cells infected with NTHI in the presence or absence of cytochalasin D indicates that bacterial internalisation causes the apoptosis of type II AECs (Goyal et al.2015); however, apoptosis only occurs at later stages of infection thereby promoting the deepest possible tissue penetration and bacterial access to the host's lymphatic and circulatory systems and affording protection of the pathogen from antibiotics and bactericidal antibody (van Schilfgaarde et al.1999).
NTHIs bind to a multitude (Fig. 4C) of adhesion molecules and receptors (Kubiet et al.2000; Swords et al.2000; Hill et al.2001; Avadhanula et al.2006; Lopez-Gomez et al.2012; Singh et al.2014), amongst which only a minority have been experimentally recognised as being essential for NTHI uptake by AECs, including Dectin-1, the carcinoembryonic antigen-related cell adhesion molecules (CEACAMs), PAFr and Vn (Swords et al.2001; Heyl et al.2014; Singh et al.2014; Tchoupa et al.2015). Dectin-1 mediates the internalisation of NTHI since the soluble β-glucan, laminarin, has an inhibitory effect on the uptake of NTHI by A549 cells (Ahren et al.2001) and the expression of a non-functional mutated Dectin-1 receptor in these cells significantly decreases NTHI uptake compared to that of wild-type counterparts (Heyl et al.2014).
Screening of a panel of NTHI mutant interactions with A549 cells has also recently revealed that host CEACAMs mediate NTHI uptake by AECs by binding to the bacterial outer membrane protein (OMP) P1 (Fig. 4C) (Tchoupa et al.2015). Importantly, while heterologous expression of OMP P1 in Escherichia coli is sufficient to promote NTHI-like uptake of E. coli by AECs, OMP P1 recognition of CEACAMs is exclusively limited to the human, not murine, homologues of the host receptor (Tchoupa et al.2015). Immunological analysis of human lung sections revealed that CEACAM1, CEACAM5 and CEACAM6 are the most highly expressed members of the family in bronchial and alveolar epithelia and in vitro infection of NHBEs with NTHI significantly increased CEACAM1 mRNA and protein levels (Klaile et al.2013), suggesting NTHI-driven upregulation of CEACAM1 expression is exploited by the microbe as a pathogenic strategy to invade the host.
Epithelial cells also internalise NTHI via PAFr engagement with the bacterial phosphorylcholine moiety (ChoP) of the LOS lipooligosaccharide (Fig. 4C) (Swords et al.2000, 2001). ChoP+ bacilli co-localise with PAFr, while mutations causing the premature truncation of LOS and subsequent loss of ChoP, or pre-treatment of ChoP+ bacilli with a PAFr inhibitor significantly decreases uptake of NHTI by AECs (Swords et al.2000). Importantly, PAFr expression is elevated in chronically inflamed airways, such as those of patients with chronic bronchitis and asthma (Hinojosa, Boyd and Orihuela 2009), substantiating a damaging role for ChoP-PAFr-mediated bacterial uptake by AECs in the settings of NTHI exacerbations.
Vn binding to NTHI via the Haemophilus surface fibrils (Hsf), a major trimeric autotransporter adhesion (Fig. 4C), increases bacterial adherence to, and internalisation by Vn-expressing cells, such as the embryonic kidney cell line 293T (Singh et al.2014). While no host receptor has been so far identified for Vn-bound NHTI, a separate study has demonstrated that NHTI uptake by A549 cells requires the members of the integrin family α5 and β1, known host receptors for other Vn-bound respiratory pathogens (Lopez-Gomez et al.2012).
Challenge of epithelial monolayers with NTHI causes the secretion of significant quantities of IL-8 and increased expression of ICAM-1 mRNA and protein relative to unchallenged monolayers (Fig. 4C) (Frick et al.2000). Both IL-8 and ICAM-1 play a crucial role in AEC-mediated leukocyte recruitment via interaction with leukocyte CD18/β2 integrin-containing receptors. Accordingly, during NTHI infection in the whole animal, increased epithelial ICAM-1 expression coincides with increased chemokine secretion and neutrophil recruitment (Frick et al.2000). It is thus far unclear whether the inflammatory response observed in response to NTHI challenge of monolayers is specifically due to NTHI uptake by AECs. In primary NHBE cells and in an A549 cell line stably transfected with Dectin-1, cytokine release upon challenge with NTHI involves IL-8 and IL-6 (Heyl et al.2014). However, in vitro infection experiments comparing AECs expressing a non-functional mutated Dectin-1 receptor and a wild-type Dectin-1 show that the decrease of IL-8 and IL-6 in AECs lacking a functional Dectin-1 is much more pronounced than the decrease in NTHI uptake (Heyl et al.2014). Thus, Dectin-1-mediated immune responses to NTHI infection are likely to occur independently from NTHI uptake by AECs.
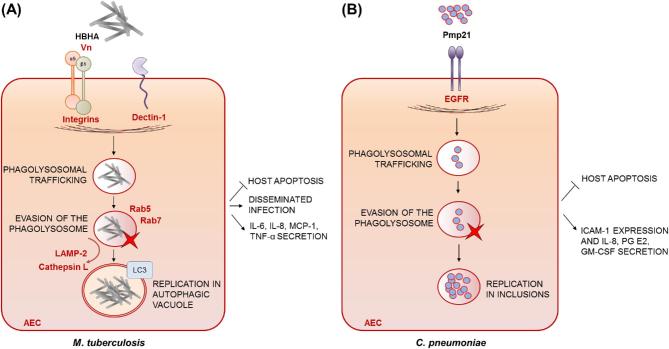
Microbial uptake leading to intracellular survival of pathogen and AEC apoptosis: M. tuberculosis and Chlamydia species
Mycobacterium tuberculosis and the Gram-negative bacteria C. pneumoniae and C. psittaci are obligate intracellular pathogens able to cause pneumonia in humans. In 2015, infection with M. tuberculosis still accounted for 1.8 million deaths from tuberculosis (TB), maintaining TB as a top 10 cause of mortality worldwide (World Health Organization, http://www.who.int). While C. psittaci is mostly a pathogen of birds, but can occasionally infect humans, C. pneumoniae causes mild pneumonia or bronchitis in young adults, but can lead to more severe disease and repeated infections in older adults. Often a primary pathogen (300 000 cases a year in the USA only), C. pneumoniae is also a major cause of infection in CAP patients, with an incidence of 1%–20% among adults, but up to 50% in children (Ewig and Torres 2003; Blasi, Tarsia and Aliberti 2009; Cillóniz et al.2011; Choroszy-Krol et al.2013; Dumke et al.2015).
Mycobacterium tuberculosis is well known as an intracellular pathogen of alveolar macrophages but also invades and multiplies within cultured AECs (Bermudez and Goodman 1996; Reddy and Hayworth 2002; Garcia-Perez et al.2003; Lee et al.2009; Ashiru, Pillay and Sturm 2010), and mycobacterial DNA has been recovered from several types of primary human lung cells, including type-II pneumocytes derived from deceased but undiseased persons, perhaps indicating that AECs constitute a genuine mycobacterial reservoir (Hernandez-Pando et al.2000).
Once internalised by AECs (Fig. 5A), M. tuberculosis is trafficked to late endosomes, as indicated by positive staining with Rab5- and Rab7-specific antibodies, but lysosomal fusion appears to be inhibited, as bacterial compartments fail to stain with LAMP-2 and cathepsin L-specific antibodies (Fine et al.2012). Mycobacterium tuberculosis is able to proliferate intracellularly within AECs (Bermudez and Goodman 1996; Garcia-Perez et al.2003) in double-membraned compartments positive for the LC3 autophagy marker (Fine et al.2012). Diversion of intracellular M. tuberculosis via the autophagy pathway promotes evasion of intracellular killing, a finding further substantiated by the impact of 3-methyladenine, an inhibitor of autophagy, in blocking (i) intracellular bacterial replication and (ii) epithelial damage, which is reduced by 15%–25% (Fine et al.2012).
Figure 5.
Microbial uptake leading to intracellular survival of pathogen and AEC apoptosis. (A) Mycobacterium tuberculosis: once internalised by AECs, M. tuberculosis is trafficked to late endosomes (Rab5 and Rab7 positive) but no co-localisation with the lysosomal markers LAMP-2 and cathepsin L is observed. Instead, M. tuberculosis is diverted to the autophagy pathway, providing the means to evade intracellular killing. Mycobacterium tuberculosis is able to proliferate intracellularly within AECs and safeguard its replicative niche by suppressing epithelial apoptosis. β1 and αν integrin act synergistically in mediating uptake by AECs as do an unidentified Vn receptor and Dectin-1. The heparin-binding haemagglutinin (HBHA) is the main M. tuberculosis surface component interacting with AECs, but its cognate receptor remains unidentified. Mycobacterium tuberculosis uptake stimulates the release of IL-8, IL-6, monocyte chemotactic protein-1 (MCP-1) and tumour necrosis factor α (TNF-α) in a process dependent on intracellular bacterial growth and Dectin-1. (B) Chlamydia pneumoniae: following uptake by AECs, C. pneumoniae occupies membrane-bound vacuoles called inclusions, and inhibits apoptosis of infected cells. The bacterial invasin Pmp21 drives internalisation into AECs via binding to the human epithermal growth factor receptor (EGFR). Chlamydia pneumonia uptake by AECs induces the release of IL-8, prostaglandin E2 and granulocyte macrophage colony-stimulating factor (GM-CSF), as well as an increase in the expression of ICAM-1. Pathogen-derived effectors of uptake are indicated in bold black font, putative or proven host receptors, opsonins or bridging factors driving uptake are indicated in bold red font.
Mycobacterium tuberculosis is known to safeguard its replicative niche by suppressing staurosporine-mediated epithelial apoptosis (Fig. 5A) (Danelishvili et al.2003). After 5 days, infection with M. tuberculosis results in only 14% of apoptotic type II alveolar epithelial cells versus 59% of necrotic ones (Danelishvili et al.2003). Indeed, internalisation of M. tuberculosis by A549 cells leads to fragmentation of host mitochondria (Fine-Coulson et al.2015) and is highly cytotoxic (Bermudez and Goodman 1996). In stark contrast, infected macrophages undergo significant apoptosis following infection with both virulent and avirulent M. tuberculosis strains (Danelishvili et al.2003). At 48 h post-infection of alveolar epithelial cells, pathway-specific cDNA microarray analysis reveals a 2.5-fold upregulation of the inhibitors of apoptosis, bcl-2 and Rb, and a downregulation of the proapoptotic genes, bad, bax, and caspases -1, -3 and -10 (Danelishvili et al.2003).
Multiple receptors mediate M. tuberculosis uptake by AECs (Fig. 5A) including β1 integrin and Vn which act synergistically to support up to 79% of observed uptake (Bermudez and Goodman 1996). To date, however, no direct or indirect mycobacterial ligand for these receptors has been identified. Dectin-1 has also been found to mediate the uptake of M. tuberculosis by cultured alveolar epithelial cells, as silencing of Dectin-1 expression leads to partial inhibition of M. tuberculosis uptake by A549 cells (Lee et al.2009). Pre-treatment of A549 cells with laminarin or anti-Dectin-1 siRNA significantly enhances survival and intracellular growth of M. tuberculosis over a period of 3 days suggesting that Dectin-1 is necessary for AECs to control M. tuberculosis infection (Lee et al.2009).
Several effector molecules on the M. tuberculosis cell surface (Fig. 5A) have been reported to induce uptake by AECs (Chitale et al.2001; Pethe et al.2001; Reddy and Hayworth 2002). Whilst cognate receptors remain thus far unidentified, probing of mycobacterial sonicates with biotinylated host extracts from in vitro cultured AECs revealed that heparin-binding haemagglutinin (HBHA) is the main M. tuberculosis surface component involved in the interaction with HEp-2 cells (Reddy and Hayworth 2002). Accordingly, HBHA deletion markedly affects mycobacterial adhesion and uptake by A549 cells, but not by J774.1 macrophage-like cells (Pethe et al.2001). Most importantly, upon nasal murine infection, a mutant strain lacking the hbhA gene showed impaired splenic, but not pulmonary colonisation, indicating that HBHA-mediated uptake by AECs has a detrimental effect for the host during mycobacterial pathogenesis, as it facilitates extrapulmonary dissemination of M. tuberculosis infection to the spleen (Pethe et al.2001).
The intracellular pathogen M. tuberculosis stimulates the release of chemokines, in particular IL-8, monocyte chemotactic protein-1 (MCP-1) and TNF-α, from alveolar epithelial cells in a process dependent on intracellular bacterial growth (Zhang et al.1997; Lin et al.1998; Sato et al.2001). Mycobacterium tuberculosis-mediated stimulation of TNF-α, IL-6 and IL-8 in A549 cells has been shown to be Dectin-1 dependent using siRNA-mediated inhibition of this receptor (Lee et al.2009).
Chlamydia pneumoniae and C. psittaci can reside and replicate within epithelial cells, inducing a profound proinflammatory response (Korhonen et al.2012; Molleken, Becker and Hegemann 2013). During the developmental cycle, Chlamydia spp. are contained in membrane-bound vacuoles, named inclusions (Fig. 5B), which do not accumulate the pH-sensitive probes acridine orange or LysoTracker (Al-Younes, Rudel and Meyer 1999). Thus, Chlamydia spp. avoid fusion with lysosomes and multiply in the inclusions, with little apparent damage to the infected epithelial cells (Al-Younes, Rudel and Meyer 1999). The stealth nature of pathogenesis explains, in part, why Chlamydia infections are mostly chronic and persistent (Wyrick 2000). A key pathogenicity trait of Chlamydia spp. is the ability to induce or actively block host-cell apoptosis under specific circumstances (Byrne and Ojcius 2004). Chlamydia psittaci triggers apoptosis of human epithelioid cells as demonstrated using the TUNEL method, but apoptosis is induced through a caspase-3-independent pathway (Ojcius et al.1998). In contrast to C. psittaci, C. pneumoniae infection protects human epithelial cells against apoptosis, as indicated by the significant inhibition of processing of caspase-3 and caspase-9, and redistribution of cytochrome C in infected cells (Fischer et al.2001).
Internalisation of C. pneumoniae and C. psittaci into AECs is driven by the binding of the bacterial invasin Pmp21 (Fig. 5B) to the human epidermal growth factor receptor (EGFR), a ubiquitous cell-surface receptor tyrosine kinase (Molleken, Becker and Hegemann 2013). Accordingly, 30% of Pmp21-coated latex beads are taken up by HEp-2 cells (Molleken, Becker and Hegemann 2013). Pmp21 is the first discovered pathogen-derived EGFR ligand, activation of which leads to recruitment of the adaptors Grb2 and c-Cbl, activation of ERK1/2 signalling and, eventually, bacterial internalisation (Molleken, Becker and Hegemann 2013). Indirect immunofluorescence microscopy indicates that activated EGFR receptor remains tightly associated in ring-like structures around the internalised pathogen (Molleken, Becker and Hegemann 2013). In accordance with the obligate intracellular lifestyle of C. pneumoniae, a direct dependence between the level of EGFR on the cell surface and susceptibility to infection is demonstrated by the finding that siRNA-mediated depletion, antibody-mediated blocking or chemical inhibition of the receptor in Hep-2 cells drastically reduces C. pneumoniae infectivity (Molleken, Becker and Hegemann 2013). Remarkably, heterologous expression of EGFR in otherwise EGFR-negative hamster cells leads to a 365% increase in microbial uptake and massively heightened susceptibility to C. pneumoniae infection (Molleken, Becker and Hegemann 2013).
In various types of AEC, in vitro internalisation of C. pneumonia induces a time-dependent release of IL-8, prostaglandin E2 and granulocyte macrophage colony-stimulating factor, as well as an increase in the expression of ICAM-1 (Jahn et al.2000; Yang et al.2003; Krull et al.2006).
CONCLUSIONS AND PERSPECTIVES
Relative to the antimicrobial properties of so-called professional phagocytes, the involvement of the respiratory epithelium in preventing or advancing pathogen invasion is poorly understood. The physiological relevance of the intracellular AEC niche in humans is well substantiated by clinical evidence that suggests the widespread uptake of microbes by human respiratory epithelia. In many instances, however, the pathological significance of such findings, for many diseases, has remained in doubt.
The high prevalence of certain pathogens in settings of airway epithelial dysfunction indicates an important role for healthy AECs in controlling everyday microbial exposures. For example, in CF, where AECs harbouring CFTR mutations are deficient for internalisation and intracellular killing of ingested fungal spores (Chaudhary et al.2012) and are less able than healthy AECs to contain intracellular replication of S. aureus bacteria (Jarry and Cheung 2006). It is currently unknown how other pulmonary abnormalities, such as chronic respiratory disease and/or the impact of immunosuppressive conditioning regimens, affect antimicrobial defences; however, there is good cause to assume that such factors will affect antimicrobial potency of the respiratory epithelium as, for example, epithelial injury upregulates α5β1 integrin expression, thereby greatly enhancing P. aeruginosa attachment (Roger et al.1999).
Clearly, given the broad spectrum of pathogens capable of infecting the respiratory niche, there is a collective urgency to understand more, and more precisely, how microbes enter the host via the respiratory tract and evade the curative potential of AEC activities to colonise and undermine the integrity of the respiratory epithelial niche.
Our analysis of the literature has identified four predominating disease scenarios whereby efficiency of AEC-mediated pathogen clearance (irrespectively of underlying mechanistic basis) correlates directly with severity of disease, and highlights an important but unmet need to distinguish curative uptake activities from those where respiratory pathogens exploit AECs as a means to cause severe infection. If the power of novel immunotherapeutic and biologic therapies can be appropriately harnessed, potentiation of airway defences at the epithelial interface might fall within grasp. In order to harness or refute the value of such approaches, it will be necessary to develop infection models which can (i) prove a causal relationship between microbial uptake and pathogen clearance and (ii) promote the testing of novel interventions.
Supplementary Material
FUNDING
This work was supported in part by grants to EMB from the Medical Research Council (G0501164, MR/L000822/1 and MR/M02010X/1), the Biotechnology and Biological Sciences Research Council (BB/G009619/1), the Wellcome Trust (WT093596MA) and the Chelsea & Westminster Healthcare National Health Service Trust Charity, to MB from Imperial College London (Division of Investigative Sciences PhD Studentships) and to EMB and MB from a University of Manchester Medical Research Council Discovery Award (MC-PC_15072).
Conflict of interest. None declared.
REFERENCES
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 1999;17:593–623. [DOI] [PubMed] [Google Scholar]
- Agarwal V, Ahl J, Riesbeck K et al.. An alternative role of C1q in bacterial infections: facilitating Streptococcus pneumoniae adherence and invasion of host cells. J Immunol 2013a;191:4235–45. [DOI] [PubMed] [Google Scholar]
- Agarwal V, Asmat TM, Luo S et al.. Complement regulator Factor H mediates a two-step uptake of Streptococcus pneumoniae by human cells. J Biol Chem 2010;285:23486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal V, Hammerschmidt S. Cdc42 and the phosphatidylinositol 3-kinase-Akt pathway are essential for PspC-mediated internalization of pneumococci by respiratory epithelial cells. J Biol Chem 2009;284:19427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal V, Kuchipudi A, Fulde M et al.. Streptococcus pneumoniae endopeptidase O (PepO) is a multifunctional plasminogen- and fibronectin-binding protein, facilitating evasion of innate immunity and invasion of host cells. J Biol Chem 2013b;288:6849–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal V, Sroka M, Fulde M et al.. Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J Biol Chem 2014;289:15833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren IL, Williams DL, Rice PJ et al.. The importance of a b-glucan receptor in the nonopsonic entry of nontypeable Haemophilus influenzae into human monocytic and epithelial cells. J Infect Dis 2001;184:150–8. [DOI] [PubMed] [Google Scholar]
- Al-Younes HM, Rudel T, Meyer TF. Characterization and intracellular trafficking pattern of vacuoles containing Chlamydia pneumoniae in human epithelial cells. Cell Microbiol 1999;1:237–47. [DOI] [PubMed] [Google Scholar]
- Alexander E, Hudson M. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl Microbiol Biot 2001;56:361–6. [DOI] [PubMed] [Google Scholar]
- Amin S, Thywissen A, Heinekamp T et al.. Melanin dependent survival of Aspergillus fumigatus conidia in lung epithelial cells. Int J Med Microbiol 2014;304:626–36. [DOI] [PubMed] [Google Scholar]
- Amitani R, Kawanami R. Interaction of Aspergillus with human respiratory mucosa: a study with organ culture model. Med Mycol 2009;47:S127–31. [DOI] [PubMed] [Google Scholar]
- Ashiru OT, Pillay M, Sturm AW. Adhesion to and invasion of pulmonary epithelial cells by the F15/LAM4/KZN and Beijing strains of Mycobacterium tuberculosis. J Med Microbiol 2010;59:528–33. [DOI] [PubMed] [Google Scholar]
- Asmat TM, Agarwal V, Rath S et al.. Streptococcus pneumoniae infection of host epithelial cells via polymeric immunoglobulin receptor transiently induces calcium release from intracellular stores. J Biol Chem 2011;286:17861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmat TM, Agarwal V, Saleh M et al.. Endocytosis of Streptococcus pneumoniae via the polymeric immunoglobulin receptor of epithelial cells relies on clathrin and caveolin dependent mechanisms. Int J Med Microbiol 2014;304:1233–46. [DOI] [PubMed] [Google Scholar]
- Avadhanula V, Rodriguez CA, Ulett GC et al.. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect Immun 2006;74:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandi V, Apicella MA, Mason E et al.. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Resp Crit Care 2001;164:2114–9. [DOI] [PubMed] [Google Scholar]
- Bao Z, Han X, Chen F et al.. Evidence for the involvement of cofilin in Aspergillus fumigatus internalization into type II alveolar epithelial cells. BMC Microbiol 2015;15:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S, Lang A, Rohde M et al.. Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. J Cell Sci 2009;122:256–67. [DOI] [PubMed] [Google Scholar]
- Berkova N, Lair-Fulleringer S, Femenia F et al.. Aspergillus fumigatus conidia inhibit tumour necrosis factor- or staurosporine-induced apoptosis in epithelial cells. Int Immunol 2006;18:139–50. [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun 1996;64:1400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi M, Hayes GE, Icheoku UJ et al.. Anti-Aspergillus activities of the respiratory epithelium in health and disease. J Fungi (Basel) 2018;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi M, Schrettl M, Alcazar-Fuoli L et al.. The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog 2014;10:e1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F, Tarsia P, Aliberti S. Chlamydophila pneumoniae. Clin Microbiol Infect 2009;15:29–35. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Panuska JR, Konstan MW et al.. Inflammatory cytokines in cystic fibrosis lungs. Am J Resp Crit Care 1995;152:2111–8. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R. New Embo Member's Review: the integrin-actin connection, an eternal love affair. EMBO J 2003;22:2324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA et al.. Hidden killers: human fungal infections. Sci Transl Med 2012;4:165rv13. [DOI] [PubMed] [Google Scholar]
- Bucior I, Mostov K, Engel JN. Pseudomonas aeruginosa-mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infect Immun 2010;78:939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucior I, Pielage JF, Engel JN. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog 2012;8:e1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JL, Jonas M, Chi EY et al.. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect Immun 1996;64:4054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne GI, Ojcius DM. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol 2004;2:802–8. [DOI] [PubMed] [Google Scholar]
- Cannon CL, Kowalski MP, Stopak KS et al.. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am J Resp Cell Mol 2003;29:188–97. [DOI] [PubMed] [Google Scholar]
- Cano V, Moranta D, Llobet-Brossa E et al.. Klebsiella pneumoniae triggers a cytotoxic effect on airway epithelial cells. BMC Microbiol 2009;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Datta K, Askin FB et al.. Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to Aspergillus and resultant pulmonary inflammation. Am J Resp Crit Care 2012;185:301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E, Mehl T, Nunn D et al.. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun 1991;59:822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitale S, Ehrt S, Kawamura I et al.. Recombinant Mycobacterium tuberculosis protein associated with mammalian cell entry. Cell Microbiol 2001;3:247–54. [DOI] [PubMed] [Google Scholar]
- Chiu CH, Ostry A, Speert DP. Invasion of murine respiratory epithelial cells in vivo by Burkholderia cepacia. J Med Microbiol 2001;50:594–601. [DOI] [PubMed] [Google Scholar]
- Choroszy-Krol I, Frej-Madrzak M, Jama-Kmiecik A et al.. Incidence of Chlamydophila pneumoniae infection in children during 2007–2010. In: Pokorski M (ed). Neurobiology of Respiration. Netherlands, Dordrecht: Springer, 2013, 83–7. [DOI] [PubMed] [Google Scholar]
- Cieri MV, Mayer-Hamblett N, Griffith A et al.. Correlation between an in vitro invasion assay and a murine model of Burkholderia cepacia lung Infection. Infect Immun 2002;70:1081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillóniz C, Ewig S, Polverino E et al.. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 2011;66:340–6. [DOI] [PubMed] [Google Scholar]
- Clement S, Vaudaux P, Francois P et al.. Evidence of an intracellular reservoir in the nasal mucosa of patients with recurrent Staphylococcus aureus rhinosinusitis. J Infect Dis 2005;192:1023–8. [DOI] [PubMed] [Google Scholar]
- Clementi CF, Hakansson AP, Murphy TF. Internalization and trafficking of nontypeable Haemophilus influenzae in human respiratory epithelial cells and roles of IgA1 proteases for optimal invasion and persistence. Infect Immun 2014;82:433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes G, Alvarez D, Saus C et al.. Role of lung epithelial cells in defense against Klebsiella pneumoniae pneumonia. Infect Immun 2002;70:1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft CA, Culibrk L, Moore MM et al.. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: a critical review. Front Microbiol 2016;7:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell DR, Gerard NP, Gerard C et al.. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 1995;377:435–8. [DOI] [PubMed] [Google Scholar]
- da Silva MCA, Zahm J-M, Gras D et al.. Dynamic interaction between airway epithelial cells and Staphylococcus aureus. Am J Physiol - Lung C 2004;287:L543–51. [DOI] [PubMed] [Google Scholar]
- Danelishvili L, McGarvey J, Li YJ et al.. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol 2003;5:649–60. [DOI] [PubMed] [Google Scholar]
- Darling KEA, Dewar A, Evans TJ. Role of the cystic fibrosis transmembrane conductance regulator in internalization of Pseudomonas aeruginosa by polarized respiratory epithelial cells. Cell Microbiol 2004;6:521–33. [DOI] [PubMed] [Google Scholar]
- de Astorza B, Cortes G, Crespi C et al.. C3 promotes clearance of Klebsiella pneumoniae by A549 epithelial cells. Infect Immun 2004;72:1767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Burridge K. Coupling membrane protrusion and cell adhesion. J Cell Sci 2003;116:2389–97. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol 2003;15:572–82. [DOI] [PubMed] [Google Scholar]
- Diekema DJ, Pfaller MA, Schmitz FJ et al.. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis 2001;32:S114–32. [DOI] [PubMed] [Google Scholar]
- Doran KS, Chang JC, Benoit VM et al.. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis 2002;185:196–203. [DOI] [PubMed] [Google Scholar]
- Duff C, Murphy PG, Callaghan M et al.. Differences in invasion and translocation of Burkholderia cepacia complex species in polarised lung epithelial cells in vitro. Microb Pathog 2006;41:183–92. [DOI] [PubMed] [Google Scholar]
- Dumke R, Schnee C, Pletz MW et al.. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg Infect Dis 2015;21:426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewanowska K, Carson AR, Patti JM et al.. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect Immun 2000;68:6321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Eran Y. Subversion of mucosal barrier polarity by Pseudomonas aeruginosa. Front Microbiol 2011;2:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewig S, Torres A. Is Chlamydia pneumoniae an important pathogen in patients with community-acquired pneumonia? Eur Resp J 2003;21:741–2. [DOI] [PubMed] [Google Scholar]
- Fillon S, Soulis K, Rajasekaran S et al.. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol 2006;177:6182–91. [DOI] [PubMed] [Google Scholar]
- Fine KL, Metcalfe MG, White E et al.. Involvement of the autophagy pathway in trafficking of Mycobacterium tuberculosis bacilli through cultured human type II epithelial cells. Cell Microbiol 2012;14:1402–14. [DOI] [PubMed] [Google Scholar]
- Fine-Coulson K, Giguere S, Quinn FD et al.. Infection of A549 human type II epithelial cells with Mycobacterium tuberculosis induces changes in mitochondrial morphology, distribution and mass that are dependent on the early secreted antigen, ESAT-6. Microbes Infect 2015;17:689–97. [DOI] [PubMed] [Google Scholar]
- Fischer SF, Schwarz C, Vier J et al.. Characterization of antiapoptotic activities of Chlamydia pneumoniae in human cells. Infect Immun 2001;69:7121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Evans DJ, Do N et al.. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun 1997;65:2861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folescu TW, da Costa CH, Cohen RW et al.. Burkholderia cepacia complex: clinical course in cystic fibrosis patients. BMC Pulm Med 2015;15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick AG, Joseph TD, Pang L et al.. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J Immunol 2000;164:4185–96. [DOI] [PubMed] [Google Scholar]
- Fumagalli O, Tall BD, Schipper C et al.. N-glycosylated proteins are involved in efficient internalization of Klebsiella pneumoniae by cultured human epithelial cells. Infect Immun 1997;65:4445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagniere H, Di Martino P. #945;5β1 integrins and fibronectin are involved in adherence of the Pseudomonas aeruginosa ER97314 clinical strain to A549 cells. Folia Microbiol (Praha) 2004;49:757–62. [DOI] [PubMed] [Google Scholar]
- Garcha DS, Thurston SJ, Patel AR et al.. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 2012;67:1075–80. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez BE, Mondragon-Flores R, Luna-Herrera J. Internalization of Mycobacterium tuberculosis by macropinocytosis in non-phagocytic cells. Microb Pathog 2003;35:49–55. [DOI] [PubMed] [Google Scholar]
- Gillette DD, Shah PA, Cremer T et al.. Analysis of human bronchial epithelial cell proinflammatory response to Burkholderia cenocepacia infection: inability to secrete IL-1b. J Biol Chem 2013;288:3691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, Jin E, Levison H et al.. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J Antimicrob Chemoth 1983;12(suppl A):331–6. [DOI] [PubMed] [Google Scholar]
- Gomes LC, Dikic I. Autophagy in antimicrobial immunity. Mol Cell 2014;54:224–33. [DOI] [PubMed] [Google Scholar]
- Goodwin M, Yap AS. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Hist 2004;35:839–44. [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh M, Ray P et al.. Cellular interaction of nontypeable Haemophilus influenzae triggers cytotoxicity of infected type II alveolar cells via apoptosis. Pathog Dis 2015;73:1–12. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Riehle A et al.. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med 2003;9:322–30. [DOI] [PubMed] [Google Scholar]
- Grassme H, Kirschnek S, Riethmueller J et al.. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 2000;290:527–30. [DOI] [PubMed] [Google Scholar]
- Gravelle S, Barnes R, Hawdon N et al.. Up-regulation of integrin expression in lung adenocarcinoma cells caused by bacterial infection: in vitro study. Innate Immun 2010;16:14–26. [DOI] [PubMed] [Google Scholar]
- Haggar A, Hussain M, Lonnies H et al.. Extracellular adherence protein from Staphylococcus aureus enhances internalization into eukaryotic cells. Infect Immun 2003;71:2310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Kokubu E, Sugiura S et al.. Vinculin and Rab5 complex is required for uptake of Staphylococcus aureus and interleukin-6 expression. PLoS One 2014;9:e87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt S, Agarwal V, Kunert A et al.. The host immune regulator factor H interacts via two contact sites with the PspC protein of Streptococcus pneumoniae and mediates adhesion to host epithelial cells. J Immunol 2007;178:5848–58. [DOI] [PubMed] [Google Scholar]
- Han X, Yu R, Zhen D et al.. β-1,3-glucan-induced host phospholipase D activation is involved in Aspergillus fumigatus internalization into type II human pneumocyte A549 cells. PLoS One 2011;6:e21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque NZ, Arshad S, Peyrani P et al.. Analysis of pathogen and host factors related to clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2012;50:1640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck CR, Ohlsen K. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr Opin Microbiol 2006;9:5–11. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R, Jeyanathan M, Mengistu G et al.. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet North Am Ed 2000;356:2133–8. [DOI] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2012. Natl Vital Stat Rep 2015;64:1–93. [PubMed] [Google Scholar]
- Heyl KA, Klassert TE, Heinrich A et al.. Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. mBio 2014;5:e01492–01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DJ, Toleman MA, Evans DJ et al.. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol Microbiol 2001;39:850–62. [DOI] [PubMed] [Google Scholar]
- Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and Toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis 2009;200:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weaver A, Wu E et al.. Lipid-based signaling modulates DNA repair response and survival against Klebsiella pneumoniae infection in host cells and in mice. Am J Resp Cell Mol 2013;49:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R, Shahror R, Karpati F et al.. Glucocorticoids can affect Pseudomonas aeruginosa (ATCC 27853) internalization and intracellular calcium concentration in cystic fibrosis bronchial epithelial cells. Exp Lung Res 2015;41:383–92. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Zhao Q. The evolution of cell adhesion. J Cell Biol 2000;150:F89–96. [DOI] [PubMed] [Google Scholar]
- Iovino F, Molema G, Bijlsma JJ. Streptococcus pneumoniae Interacts with pIgR expressed by the brain microvascular endothelium but does not co-localize with PAF receptor. PLoS One 2014;9:e97914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn HU, Krull M, Wuppermann FN et al.. Infection and activation of airway epithelial cells by Chlamydia pneumoniae. J Infect Dis 2000;182:1678–87. [DOI] [PubMed] [Google Scholar]
- Jarry TM, Cheung AL. Staphylococcus aureus escapes more efficiently from the phagosome of a cystic fibrosis bronchial epithelial cell line than from its normal counterpart. Infect Immun 2006;74:2568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett BD, Gilmore MS. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect Immun 2002;70:4697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Chen F, Pan W et al.. Gliotoxin promotes Aspergillus fumigatus internalization into type II human pneumocyte A549 cells by inducing host phospholipase D activation. Microbes Infect 2014;16:491–501. [DOI] [PubMed] [Google Scholar]
- Jiang F, Waterfield Nicholas R, Yang J et al.. A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 2014;15:600–10. [DOI] [PubMed] [Google Scholar]
- Jolly AL, Takawira D, Oke OO et al.. Pseudomonas aeruginosa-induced bleb-niche formation in epithelial cells is independent of actinomyosin contraction and enhanced by loss of cystic fibrosis transmembrane-conductance regulator osmoregulatory function. mBio 2015;6:e02533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010;51 (S1):S81–7. [DOI] [PubMed] [Google Scholar]
- Kahl BC. Impact of Staphylococcus aureus on the pathogenesis of chronic cystic fibrosis lung disease. Int J Med Microbiol 2010;300:514–9. [DOI] [PubMed] [Google Scholar]
- Kahl BC, Goulian M, van Wamel W et al.. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun 2000;68:5385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaza SK, McClean S, Callaghan M. IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth and intracellular survival of bacteria. Int J Med Microbiol 2011;301:26–33. [DOI] [PubMed] [Google Scholar]
- Ketterer MR, Shao JQ, Hornick DB et al.. Infection of primary human bronchial epithelial cells by Haemophilus influenzae: macropinocytosis as a mechanism of airway epithelial cell entry. Infect Immun 1999;67:4161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintarak S, Whawell SA, Speight PM et al.. Internalization of Staphylococcus aureus by human keratinocytes. Infect Immun 2004;72:5668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaile E, Klassert TE, Scheffrahn I et al.. Carcinoembryonic antigen (CEA)-related cell adhesion molecules are co-expressed in the human lung and their expression can be modulated in bronchial epithelial cells by non-typable Haemophilus influenzae, Moraxella catarrhalis, TLR3, and type I and II interferons. Respir Res 2013;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens R, Morrison MA, Nadle J et al.. Invasive methicillin-resistant Staphylococcus aureus infections in the united states. JAMA 2007;298:1763–71. [DOI] [PubMed] [Google Scholar]
- Korea CG, Balsamo G, Pezzicoli A et al.. Staphylococcal Esx proteins modulate apoptosis and release of intracellular Staphylococcus aureus during infection in epithelial cells. Infect Immun 2014;82:4144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen JT, Puolakkainen M, Haveri A et al.. Chlamydia pneumoniae entry into epithelial cells by clathrin-independent endocytosis. Microb Pathog 2012;52:157–64. [DOI] [PubMed] [Google Scholar]
- Kowalski MP, Dubouix-Bourandy A, Bajmoczi M et al.. Host resistance to lung infection mediated by major vault protein in epithelial cells. Science 2007;317:130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull M, Bockstaller P, Wuppermann FN et al.. Mechanisms of Chlamydophila pneumoniae-mediated GM-CSF release in human bronchial epithelial cells. Am J Resp Cell Mol 2006;34:375–82. [DOI] [PubMed] [Google Scholar]
- Kubiet M, Ramphal R, Weber A et al.. Pilus-mediated adherence of Haemophilus influenzae to human respiratory mucins. Infect Immun 2000;68:3362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Raia V, Del Pezzo M et al.. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gouill C, Parent J-L, Rola-Pleszczynski M et al.. Structural and functional requirements for agonist-induced internalization of the human platelet-activating factor receptor. J Biol Chem 1997;272:21289–95. [DOI] [PubMed] [Google Scholar]
- Lee HM, Yuk JM, Shin DM et al.. Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol 2009;29:795–805. [DOI] [PubMed] [Google Scholar]
- LeMessurier KS, Hacker H, Chi L et al.. Type I interferon protects against pneumococcal invasive disease by inhibiting bacterial transmigration across the lung. PLoS Pathog 2013;9:e1003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy-Dudal J, Gagniere H, Cossard E et al.. Role of alphavbeta5 integrins and vitronectin in Pseudomonas aeruginosa PAK interaction with A549 respiratory cells. Microbes Infect 2004;6:875–81. [DOI] [PubMed] [Google Scholar]
- Li X, Fusco WG, Seo KS et al.. Epithelial cell gene expression induced by intracellular Staphylococcus aureus. Int J Microbiol 2009;2009:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang M, Barnes PF. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun 1998;66:1121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Lee MJ, Solis NV. et al. Aspergillus fumigatus CalA binds to integrin α5β1 and mediates host cell invasion. Nat Microbiol 2017;2:16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gomez A, Cano V, Moranta D et al.. Host cell kinases, α5 and β1 integrins, and Rac1 signalling on the microtubule cytoskeleton are important for non-typable Haemophilus influenzae invasion of respiratory epithelial cells. Microbiology 2012;158:2384–98. [DOI] [PubMed] [Google Scholar]
- Martin DW, Mohr CD. Invasion and intracellular survival of Burkholderia cepacia. Infect Immun 2000;68:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey RC, Kantzanou MN, Fowler T et al.. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell Microbiol 2001;3:839–51. [DOI] [PubMed] [Google Scholar]
- Mitchell G, Grondin G, Bilodeau G et al.. Infection of polarized airway epithelial cells by normal and small-colony variant strains of Staphylococcus aureus is increased in cells with abnormal CFTR function and is influenced by NF-κB. Infect Immun 2011;79:3541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleken K, Becker E, Hegemann JH. The Chlamydia pneumoniae invasin protein Pmp21 recruits the EGF receptor for host cell entry. PLoS Pathog 2013;9:e1003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JE, Mastoridis P. Clinical implications of Pseudomonas aeruginosa location in the lungs of patients with cystic fibrosis. J Clin Pharm Ther 2017;42:259–67. [DOI] [PubMed] [Google Scholar]
- Moranta D, Regueiro V, March C et al.. Klebsiella pneumoniae capsule polysaccharide impedes the expression of β-defensins by airway epithelial cells. Infect Immun 2010;78:1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey P, Cano V, Marti-Lliteras P et al.. Evidence for a non-replicative intracellular stage of nontypable Haemophilus influenzae in epithelial cells. Microbiology 2011;157:234–50. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Cossart P. Bacterial autophagy: restriction or promotion of bacterial replication? Trends Cell Biol 2012;22:283–91. [DOI] [PubMed] [Google Scholar]
- Moura JA, Cristina de Assis M, Ventura GC et al.. Differential interaction of bacterial species from the Burkholderia cepacia complex with human airway epithelial cells. Microbes Infect 2008;10:52–9. [DOI] [PubMed] [Google Scholar]
- Mullen T, Callaghan M, McClean S. Invasion of Burkholderia cepacia complex isolates into lung epithelial cells involves glycolipid receptors. Microb Pathog 2010;49:381–7. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Brauer AL, Schiffmacher AT et al.. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Resp Crit Care 2004;170:266–72. [DOI] [PubMed] [Google Scholar]
- Naucler P, Darenberg J, Morfeldt E et al.. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax 2013;68:571–9. [DOI] [PubMed] [Google Scholar]
- Niedergang F, Chavrier P. Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr Opin Cell Biol 2004;16:422–8. [DOI] [PubMed] [Google Scholar]
- O’Brien KL, Wolfson LJ, Watt JP et al.. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet North Am Ed 2009;374:893–902. [DOI] [PubMed] [Google Scholar]
- Ojcius DM, Souque P, Perfettini JL et al.. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol 1998;161:4220–6. [PubMed] [Google Scholar]
- Osherov N. Interaction of the pathogenic mold Aspergillus fumigatus with lung epithelial cells. Front Microbiol 2012;3:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, Boisvieux-Ulrich E, Crestani B et al.. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect Immun 1997;65:1510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethe K, Alonso S, Biet F et al.. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 2001;412:190–4. [DOI] [PubMed] [Google Scholar]
- Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA 1997;94:12088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Grout M, Zaidi TS et al.. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 1996;271:64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkowski MC, de Bentzmann S, Pereira SH et al.. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am J Resp Cell Mol 1999;20:880–90. [DOI] [PubMed] [Google Scholar]
- Proctor RA, van Langevelde P, Kristjansson M et al.. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis 1995;20:95–102. [DOI] [PubMed] [Google Scholar]
- Radin JN, Orihuela CJ, Murti G et al. β-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun 2005;73:7827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razvi S, Quittell L, Sewall A et al.. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 2009;136:1554–60. [DOI] [PubMed] [Google Scholar]
- Reddy VM, Hayworth DA. Interaction of Mycobacterium tuberculosis with human respiratory epithelial cells (HEp-2). Tuberculosis 2002;82:31–6. [DOI] [PubMed] [Google Scholar]
- Regueiro V, Campos MA, Pons J et al.. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology 2006;152:555–66. [DOI] [PubMed] [Google Scholar]
- Regueiro V, Moranta D, Frank CG et al.. Klebsiella pneumoniae subverts the activation of inflammatory responses in a NOD1-dependent manner. Cell Microbiol 2011;13:135–53. [DOI] [PubMed] [Google Scholar]
- Rijneveld AW, Weijer S, Florquin S et al.. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J Infect Dis 2004;189:711–6. [DOI] [PubMed] [Google Scholar]
- Roger P, Puchelle E, Bajolet-Laudinat O et al.. Fibronectin and α5β1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur Respir J 1999;13:1301–9. [PubMed] [Google Scholar]
- Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Bio 2002;3:944–56. [DOI] [PubMed] [Google Scholar]
- Rosenow C, Ryan P, Weiser JN et al.. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol 1997;25:819–29. [DOI] [PubMed] [Google Scholar]
- Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc 2007;4:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly H, Podschun R. Clinical, bacteriological, and serological aspects of Klebsiella infections and their spondylarthropathic sequelae. Clin Diagn Lab Immunol 1997;4:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U, Ackerley C, Forstner J. Interaction of cblA/adhesin-positive Burkholderia cepacia with squamous epithelium. Cell Microbiol 2002;4:73–86. [DOI] [PubMed] [Google Scholar]
- Sajjan U, Corey M, Humar A et al.. Immunolocalisation of Burkholderia cepacia in the lungs of cystic fibrosis patients. J Med Microbiol 2001;50:535–46. [DOI] [PubMed] [Google Scholar]
- Sajjan U, Keshavjee S, Forstner J. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infect Immun 2004;72:4188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U, Wu Y, Kent G et al.. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J Med Microbiol 2000;49:875–85. [DOI] [PubMed] [Google Scholar]
- Sajjan US, Hershenson MB, Forstner JF et al.. Burkholderia cenocepacia ET12 strain activates TNFR1 signalling in cystic fibrosis airway epithelial cells. Cell Microbiol 2008;10:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan US, Yang JH, Hershenson MB et al.. Intracellular trafficking and replication of Burkholderia cenocepacia in human cystic fibrosis airway epithelial cells. Cell Microbiol 2006;8:1456–66. [DOI] [PubMed] [Google Scholar]
- Sana TG, Hachani A, Bucior I et al.. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J Biol Chem 2012;287:27095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaki T, Shimizu T et al.. Invasion and intracellular growth of Mycobacterium tuberculosis and Mycobacterium avium complex adapted to intramacrophagic environment within macrophages and type II alveolar epithelial cells. Kekkaku 2001;76:53–7. [PubMed] [Google Scholar]
- Schroeder TH, Lee MM, Yacono PW et al.. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF- B translocation. Proc Natl Acad Sci USA 2002;99:6907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TH, Reiniger N, Meluleni G et al.. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol 2001;166:7410–8. [DOI] [PubMed] [Google Scholar]
- Schultz J, Schwarz A, Neidhold S et al.. Role of interleukin-1 in prion disease-associated astrocyte activation. Am J Pathol 2004;165:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MJ, Rijneveld AW, Florquin S et al.. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung C 2002;282:L285–290. [DOI] [PubMed] [Google Scholar]
- Sheppard DC, Filler SG. Host cell invasion by medically important fungi. Cold Spring Harbor Perspect Med 2015;5:a019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Su YC, Al-Jubair T et al.. A fine-tuned interaction between trimeric autotransporter haemophilus surface fibrils and vitronectin leads to serum resistance and adherence to respiratory epithelial cells. Infect Immun 2014;82:2378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Francois PP, Nusse O et al.. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell Microbiol 1999;1:101–17. [DOI] [PubMed] [Google Scholar]
- Strobel M, Pfortner H, Tuchscherr L et al.. Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain. Clin Microbiol Infect 2016;22:799–809. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol 1995;5:89–93. [DOI] [PubMed] [Google Scholar]
- Swords WE, Buscher BA, Ver Steeg Ii K et al.. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol 2000;37:13–27. [DOI] [PubMed] [Google Scholar]
- Swords WE, Ketterer MR, Shao J et al.. Binding of the non-typeable Haemophilus influenzae lipooligosaccharide to the PAF receptor initiates host cell signalling. Cell Microbiol 2001;3:525–36. [DOI] [PubMed] [Google Scholar]
- Tchoupa AK, Lichtenegger S, Reidl J et al.. Outer membrane protein P1 is the CEACAM-binding adhesin of Haemophilus influenzae. Mol Microbiol 2015;98:440–55. [DOI] [PubMed] [Google Scholar]
- Tomich M, Herfst CA, Golden JW et al.. Role of flagella in host cell invasion by Burkholderia cepacia. Infect Immun 2002;70:1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherr L, Medina E, Hussain M et al.. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 2011;3:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM et al.. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 1999;401:811–5. [DOI] [PubMed] [Google Scholar]
- van Schilfgaarde M, Eijk P, Regelink A et al.. Haemophilus influenzae localized in epithelial cell layers is shielded from antibiotics and antibody-mediated bactericidal activity. Microb Pathog 1999;26:249–62. [DOI] [PubMed] [Google Scholar]
- Wang J-H, Zhang K, Wang N et al.. Involvement of phosphatidylinositol 3-Kinase/Akt signaling pathway in β1 integrin-mediated internalization of Staphylococcus aureus by alveolar epithelial cells. J Microbiol. 2013;51:644–50. [DOI] [PubMed] [Google Scholar]
- Wasylnka JA, Moore MM. Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect Immun 2002;70:3156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylnka JA, Moore MM. Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J Cell Sci 2003;116:1579–87. [DOI] [PubMed] [Google Scholar]
- Wong JK, Ranganathan SC, Hart E, Australian Respiratory Early Surveillance Team for Cystic Fibrosis. Staphylococcus aureus in early cystic fibrosis lung disease. Pediatr Pulmonol 2013;48:1151–9. [DOI] [PubMed] [Google Scholar]
- Wyrick PB. Intracellular survival by Chlamydia . Microreview. Cell Microbiol 2000;2:275–82. [DOI] [PubMed] [Google Scholar]
- Xu XY, Chen F, Sun H et al.. Important factors mediates the adhesion of Aspergillus fumigatus to alveolar epithelial cells with E-cadherin. Am J Transl Res 2016;8:2419–25. [PMC free article] [PubMed] [Google Scholar]
- Xu XY, Shi Y, Zhang PP et al.. E-cadherin mediates adhesion and endocytosis of Aspergillus fumigatus blastospores in human epithelial cells. Chin Med J (Engl) 2012;125:617–21. [PubMed] [Google Scholar]
- Yang J, Hooper WC, Phillips DJ et al.. Induction of proinflammatory cytokines in human lung epithelial cells during Chlamydia pneumoniae infection. Infect Immun 2003;71:614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Matsumoto T, Tateda K et al.. Role of bacterial capsule in local and systemic inflammatory responses of mice during pulmonary infection with Klebsiella pneumoniae. J Med Microbiol 2000;49:1003–10. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Mostov KE, Lamm ME et al.. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 2000;102:827–37. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kim KJ, Iyer D et al.. Effects of Mycobacterium tuberculosis on the bioelectric properties of the alveolar epithelium. Infect Immun 1997;65:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.