Abstract
We aimed at identifying the predictive role of endothelial function assessed by the RH-PAT index (RHI) for future major cardiovascular events (MACEs) in acute coronary syndrome (ACS) patients treated with percutaneous coronary intervention (PCI). We measured RHI in 308 subjects with ACS, and they were divided into the normal endothelial function (NEF) group and the endothelial dysfunction (DEF) group according to the RHI. The subjects were followed up for a mean of 16 months (interquartile range [IQR]: 14–20 months) after PCI treatment, and their MACEs were also recorded. Cumulative incidence curves were constructed for time-to-event variables with Kaplan–Meier estimates and compared using the log-rank test. The overall incidence of MACEs was 25.39% in the DEF group and 15.96% in the NEF group (P<0.05). Kaplan–Meier analysis also demonstrated a significantly higher probability of MACEs in the DEF group than in the NEF group (log-rank test: P<0.05). Multivariate Cox hazard analysis identified RHI (Model 2, adjusted by blood pressure, hazard ratio [HR]: 0.425; 95% confidence interval [CI]: 0.198–0.914; P=0.029) and SYNTAX score (HR: 1.043; 95% CI: 1.019–1.067; P<0.001) as independent predictors of future MACEs after PCI treatment in ACS patients. Endothelial function measured by reactive hyperemia-peripheral arterial tonometry (RH-PAT) is impaired in ACS subjects treated with PCI. The RHI was an independent predictor of MACEs, suggesting that RHI may be useful as a candidate biomarker in the risk stratification of patients with ACS after PCI treatment.
Keywords: acute coronary syndrome, endothelial function, major cardiovascular events, RH-PAT index
Introduction
Endothelial dysfunction (DEF), including coronary DEF and peripheral DEF, has been demonstrated to be an essential step in the initiation and progression of atherosclerosis [1,2]. It has also been considered to be a key process in atherogenesis and to contribute to the development of clinical cardiovascular diseases, including acute coronary syndrome (ACS) [3–6]. Although endothelial function studies in the past few decades have mainly focused on the coronary circulation, DEF in the peripheral arteries has also attracted considerable attention [7,8]. It has been widely indicated that DEF of peripheral arteries is strongly associated with coronary artery atherosclerosis and is an independent prognostic predictor of coronary artery disease (CAD), heart failure, and cerebrovascular diseases [2,7,9,10].
To date, various non-invasive assessments of peripheral artery endothelial function have been used extensively in clinic research and practice [2]. The high-resolution ultrasonographic measurement of brachial artery flow-mediated dilatation (FMD) was initially applied frequently to evaluate endothelial function and has been indicated to be correlated with coronary DEF and cardiovascular diseases. FMD measured in the forearm provides information that predicts the extent and severity of coronary atherosclerosis and is correlated with coronary endothelial function [11–14]. However, though this method provides information on the ‘recruitability’ of endothelial function, it does not take into account resting endothelial activity [15].
Subsequently, a novel non-invasive, automated, quantitative, and reproducible clinical examination for the evaluation of peripheral endothelial function, known as reactive hyperemia-peripheral arterial tonometry (RH-PAT), has been proposed [16–18]. It has identified invasively proven coronary DEF and ischemic heart disease while also predicting future cardiovascular events. Furthermore, the reactive hyperemia index (RHI) measured by RH-PAT serves as an excellent marker for cardiovascular events in patients in different stages of the cardiovascular disease continuum [19–22].
The impairment of reactive hyperemia (reduced RHI) in CAD patients has been observed previously [7,20]. Previous studies have also revealed associations between RHI and cardiovascular diseases, including the specific and emergent type, ACS, which requires early and emergent treatment. Despite advances in pharmacological therapy and percutaneous coronary intervention (PCI), recurrent major adverse cardiac events (MACEs) still occur in patients with ACS [23].
Although several studies have indicated the practical applications of RH-PAT, the role of RHI in MACEs among ACS patients treated with PCI has not been confirmed. In addition, the Framingham Heart Study revealed no statistically significant relationship between RHI and FMD, indicating that RHI and FMD represent distinct aspects of or risk factors for endothelial function and cardiovascular diseases [24]. RH-PAT may measure components of vasodilation that are not reflected in FMD, providing at least a theoretical basis for a more comprehensive assessment of vascular function [24]. Thus, we postulate that endothelial function measured by RH-PAT is impaired in ACS patients who are treated with PCI and may be a predictor of MACEs in this population.
Methods
Study design and population
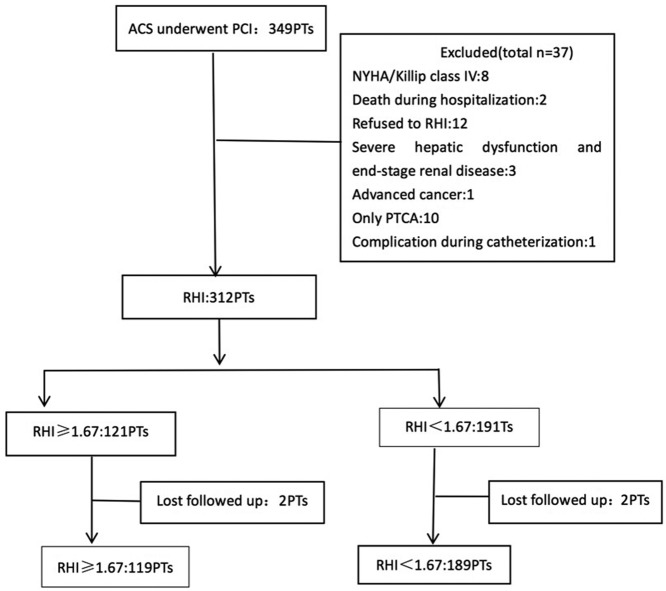
The present study is a prospective, observational, single-center study of all consecutive patients with ACS treated with PCI at Xinqiao Hospital, a tertiary hospital located in the Shapingba district in Chongqing City, from 1 December 2015 to 30 September 2017. The inclusion criterion was ACS patients who were treated with PCI. The exclusion criteria were as follows: NYHA/Killip class IV (n=8), balloon angioplasty only without stent implantation (n=10), death during hospitalization (n=2), severe hepatic dysfunction and end-stage renal disease (n=3), advanced cancer (n=1), refused to RHI (n=12), and complication during catheterization (n=1), as shown in Figure 1. The study complied with the Declaration of Helsinki with respect to human investigations was approved by an institutional review committee, and was conducted in accordance with the guidelines of the ethics committee of Xinqiao Hospital. All the subjects provided written informed consent.
Figure 1. Study profile.
The flow chart of the present study showed our procedure in the performing this research.
Measurement of RHI
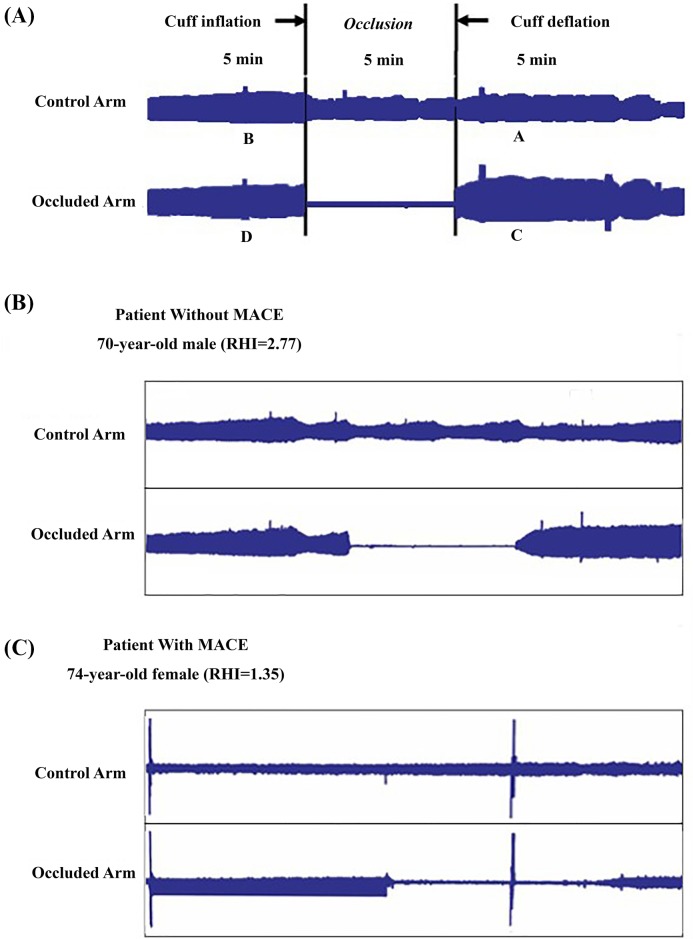
RHI was measured using the RH-PAT system (EndoPAT 2000; Itamar Medical, Caesarea, Israel) with the following procedures 48–72 h after PCI treatment. All measurements were performed in the early morning in a dedicated laboratory after fasting for at least 8 h. Medications containing long-lasting nitroglycerin, anti-ischemic, and anti-hypertensive medications for 24 h, and short-lasting nitroglycerin were withheld 1 h before the examination. The patients also had to refrain from caffeine consumption, smoking, and vasoactive medications. Before any measurements, the patients had an acclimatization period of 20 min in a quiet room, lying in a hospital bed at an ambient temperature of 21–24°C. The RH-PAT measurement protocol has previously been reported in detail [7]. Briefly, measurements were performed by the use of probes on the index fingers of both the study and control arms. A blood pressure cuff was placed on one upper arm with the contralateral arm serving as a control. The finger pulse wave amplitude was assessed with the EndoPAT-2000 sensing device and finger plethysmographic probes at baseline and during ischemia-induced hyperemia. Baseline measurements were recorded for 5 min prior to ischemia induction by inflating a blood pressure cuff on the upper arm of the study arm for 5 min to suprasystolic pressures. All subjects underwent RHI evaluation, and a representative figure is shown in Figure 2.
Figure 2. A representative for the measurement of RHI.
(A) RH-PAT ratio calculated: RH-PAT ratio = (C/D)/(A/B). Representative results of RH-PAT of subjects without cardiovascular events (B) and (C) those with cardiovascular events.
In the present study, RHI was calculated as the ratio of the mean hyperemic pulse wave analysis results over a period of 60 s, beginning at 90 s after cuff deflation, divided by the baseline pulse wave analysis results (mean baseline measurements for 2.5 min), and it was normalized to the concurrent measurements of the control arm. Participants were divided into two groups according to RHI: normal group (RHI ≥ 1.67, normal endothelial function [NEF] group) and abnormal RHI group (RHI < 1.67, DEF group) [22].
Echocardiogram examination
Each subject received an echocardiogram examination (ultrasonography system, CX50, Philips, U.S.A.) in a quiet room in the supine position. The end-diastolic internal diameters of the left atrium (LA), left ventricle (LV), right atrium (RA), right ventricle (RV), and pulmonary artery (PA), in addition to the stroke volume (SV) and left ventricle ejection fraction (LVEF), were measured and recorded.
Biomarker variables determination
Venous blood samples were obtained from subjects in the morning after an overnight fast (at least 12 h) within 24 h after admission. Plasma triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) concentrations were assessed from venous blood samples using commercially available ELISA kits (Roche Diagnostics GmbH, Mannheim, Germany). The concentration of plasma creatinine (Cr) was measured by an enzymatic assay (Roche Diagnostics GmbH). All of the biochemical variables were measured from blood specimens in the Clinical Laboratory Department, Xinqiao Hospital.
Coronary angiography
Coronary angiography was performed by a standard technique using 4–7 French right and left catheters through a femoral or radial artery approach. Clinically significant CAD was defined by the presence of a coronary lesion resulting in luminal stenosis ≥70% (≥50% at the left main coronary artery) or fractional flow reserve ≤0.80 in one or more major coronary arteries or their major branches. The PCI treatments were performed according to the guidelines on treatments of ST-segment elevation myocardial infarction (STEMI) [25] and NSTE-ACS recommended by Chinese Society of Cardiology [26].
Clinical follow-up
After PCI treatment, the subjects were prospectively followed monthly at the outpatient clinic of Xinqiao Hospital until 30 September 2017 or until an endpoint event occurred. The MACE endpoints included cardiovascular death, acute myocardial infarction (AMI), target vessel revascularization (TVR), non-fatal ischemic stroke, and cardiac hospitalization. MACEs in subjects were evaluated and confirmed according to their medical records. Moreover, the first MACE for a subject was considered in the analysis if he/she suffered from more than two types of MACEs.
Statistical analysis
Continuous variables with a normal distribution were expressed as the means ± S.D. and were compared using unpaired Student’s t test or one-way ANOVA, whereas the remaining variables with a non-normal distribution were compared using the Wilcoxon rank sum test. Dichotomous variables were assessed by the Chi-square test or Fisher’s exact test. Univariate and multivariate Cox regression analyses were performed to assess the effect of DEF on MACEs and to identify independent predictors of MACEs. Variables with P values less than 0.1 in the analyses, including differences between MACEs and non-MACEs groups and univariate Cox regression, were included into the multivariate Cox regression analysis. The variables with closely associations were analyzed in the regression independently (Model 1: the hypertension cases as a dichotomy variable selected into the regression; Model 2: included SBP and DBP into the analysis instead of hypertension). Hazard ratios (HRs) were calculated with 95% confidence intervals (95% CIs). Cumulative incidence curves were constructed for time-to-event variables with Kaplan–Meier estimates and compared using the log-rank test. The statistical analysis was performed with the statistical software ‘EZR’ (Easy R), which is based on R and R commander, and SPSS 20.0 for windows.
Results
A total of 312 of the 349 ACS patients who were treated with PCI underwent the endothelial function assessment by EndoPAT. Finally, outpatient follow-up was completed in 308 patients, whereas four patients were lost to follow-up during the median follow-up period of 16 months (interquartile range [IQR]: 14–20 months).
Baseline clinical characteristics were compared between the DEF group (n=191) and NEF group (n=121, Table 1). There were no significant differences in age, gender, BMI, incidence of diabetes mellitus, or percentages of smoking and alcohol consumption between the two above-mentioned groups. The LVEF of the subjects was significantly lower in the DEF group than the NEF group (P<0.05). A calcium channel blocker (CCB) was used in 12.04% of the patients in the DEF group compared with 22.31% in the NEF group (P<0.05). Other medication use, including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), β-blockers, antiplatelets, statins, and ezetimibe, showed no significant differences between the NEF and DEF groups (P>0.05). Moreover, the brain-type natriuretic peptide (BNP) level in the DEF group was significantly higher than that in the NEF group (P=0.035), while the systolic blood pressure was lower in the DEF group (P=0.013). In addition, the number of patients with STEMI was also higher in the DEF group than in the NEF group (P=0.003).
Table 1. Baseline characteristics of the subjects.
| Variables | NEF group (n=121) | DEF group (n=191) | P value | Power of test |
|---|---|---|---|---|
| Age, mean (SD), years | 59.44 ± 9.55 | 60.63 ± 9.94 | 0.291 | 0.183 |
| Male gender, n (%) | 89 (73.55%) | 151 (79.06%) | 0.273 | |
| BMI, mean (SD), kg/m2 | 24.90 ± 3.05 | 24.32 ± 2.82 | 0.084 | 0.389 |
| LV mean (SD), mm | 46.47 ± 5.02 | 47.64 ± 5.98 | 0.135 | 0.458 |
| LVEF, mean (SD), % | 62.66 ± 6.00 | 59.67 ± 9.39 | 0.002† | 0.928 |
| BNP, Median (IQR), pg/ml | 37.40 (139.20) | 68.30 (188.00) | 0.035* | |
| Peripheral vascular disease, n (%) | 77 (63.63%) | 120 (62.83%) | 0.905 | |
| Pre-PCI, n (%) | 18 (14.88%) | 15 (7.85%) | 0.059 | |
| Pre-CABG, n (%) | 1 (0.83%) | 0 (0.00%) | 0.388 | |
| Current smoking, n (%) | 56 (46.28%) | 107 (56.02%) | 0.104 | |
| Creatinine mean (SD), μmol/l | 75.45 ± 17.33 | 77.36 ± 19.06 | 0.386 | 0.149 |
| ALT, mean (SD), IU/l | 30.60 ± 20.30 | 35.48 ± 28.11 | 0.095 | 0.425 |
| AST, mean (SD), IU/l | 25.89 ± 14.62 | 28.86 ± 21.45 | 0.177 | 0.305 |
| Hypertension, n (%) | 76 (62.81%) | 110 (57.59%) | 0.408 | |
| Systolic BP, mean (SD), mm Hg | 133.35 ± 18.96 | 127.98 ± 18.66 | 0.013* | 0.686 |
| Diastolic BP, mean (SD), mm Hg | 79.45 ± 11.23 | 77.05 ± 12.03 | 0.075 | 0.430 |
| TC, mean (SD), mmol/l | 4.02 ± 1.11 | 3.98 ± 1.14 | 0.774 | 0.061 |
| TG, mean (SD), mmol/l | 1.55 ± 0.89 | 1.67 ± 1.01 | 0.263 | 0.195 |
| LDL-C, mean (SD), mmol/l | 2.61 ± 0.93 | 2.60 ± 0.83 | 0.760 | 0.051 |
| HDL-C, mean (SD), mmol/l | 1.00 ± 0.21 | 1.01 ± 0.25 | 0.973 | 0.067 |
| Diabetes mellitus, n (%) | 30 (24.79%) | 60 (31.41%) | 0.202 | |
| HbA1c, mean (SD), % | 6.57 ± 1.55 | 6.74 ± 1.51 | 0.433 | 0.158 |
| Diagnosis | 0.014* | |||
| STEMI, n (%) | 15 (12.39%) | 50 (26.18%) | 0.003* | |
| NSTEMI, n (%) | 8 (6.61%) | 12 (6.28%) | 0.136 | |
| UA, n (%) | 98 (81.00%) | 129 (67.54%) | 0.784 | |
| Medication | ||||
| ACEI/ARB, n (%) | 88 (72.72%) | 127 (66.49%) | 0.261 | |
| CCB, n (%) | 27 (22.31%) | 23 (12.04%) | 0.018 | |
| β-Blockers, n (%) | 92 (76.03%) | 147 (76.96%) | 0.891 | |
| Antiplatelet | ||||
| Aspirin + Clopidogrel, n (%) | 45 (37.19%) | 68 (35.60%) | 0.810 | |
| Aspirin + Ticagrelor, n (%) | 76 (62.81%) | 123 (64.40%) | 0.810 | |
| Trimetazidine, n (%) | 70 (57.85%) | 102 (53.40%) | 0.559 | |
| Statins, n (%) | 121 (100.00%) | 191 (100.00%) | 1.000 |
Abbreviations: ALT, aspartate aminotransferase; AST, aspartate transaminase; BMI, body mass index; CABG, cardiac artery bypass graft; GRACE, Score, Global Registry of Acute Coronary Events Risk Score; LV, left ventricular end diastolic dimension; NSTEMI, non ST-elevation myocardial infarction; PCI, percutaneous coronary interventional treatment; TC, total cholesterol; UA, unstable angina pectoris.
*P≤0.05; †P≤0.01.
Table 2 showed the differences in the coronary artery lesion characteristics of subjects between the DEF and NEF groups. There were no significant differences in SYNTAX score, the percent of thrombus lesions, number of diseased coronary vessels, and other parameters between the NEF and DEF groups (all P values > 0.05).
Table 2. Patient coronary artery lesion characteristics.
| NEF group (n=121) | DEF group (n=191) | P value | Power for test | |
|---|---|---|---|---|
| Number of diseased coronary vessels | 0.052 | |||
| One, n (%) | 51 (42.15%) | 55 (28.80%) | ||
| Two, n (%) | 30 (24.79%) | 57 (29.84%) | ||
| Three, n (%) | 40 (33.06%) | 79 (41.36%) | ||
| SYNTAX score (SD) | 16.21 ± 10.20 | 18.30 ± 9.64 | 0.069 | 0.434 |
| Thrombus lesions, n (%) | 8 (6.61%) | 22 (11.52%) | 0.172 | |
| Chronic total occlusion, n (%) | 16 (13.22%) | 32 (16.75%) | 0.426 | |
| Curve lesions, n (%) | 4 (3.30%) | 10 (5.24%) | 0.577 | |
| Calcificaton lesions, n (%) | 14 (11.57%) | 33 (17.28%) | 0.129 | |
| LM lesions, n (%) | 20 (16.52%) | 25 (13.09%) | 0.412 | |
| Number of stents, n | 1.83 ± 0.97 | 1.93 ± 0.98 | 0.391 | |
| Medina, 1,1,1, n (%) | 32 (26.45%) | 55 (28.80%) | 0.699 | |
| T/TAP technique, n (%) | 3 (2.48%) | 2 (1.05%) | 0.380 | |
| Crush/mini crush technique, n (%) | 2 (1.65%) | 6 (3.14%) | 0.491 | |
| BT/ BSKT technique, n (%) | 11 (9.90%) | 9 (4.71%) | 0.155 |
Abbreviations: BSKT, balloon stent kissing technique; JBT, jailed-balloon technique; LM, left main coronary artery; SYNTAX score, synergy between PCI with taxus and cardiac surgery score; T/TAP, T stenting and small protrusion technique.
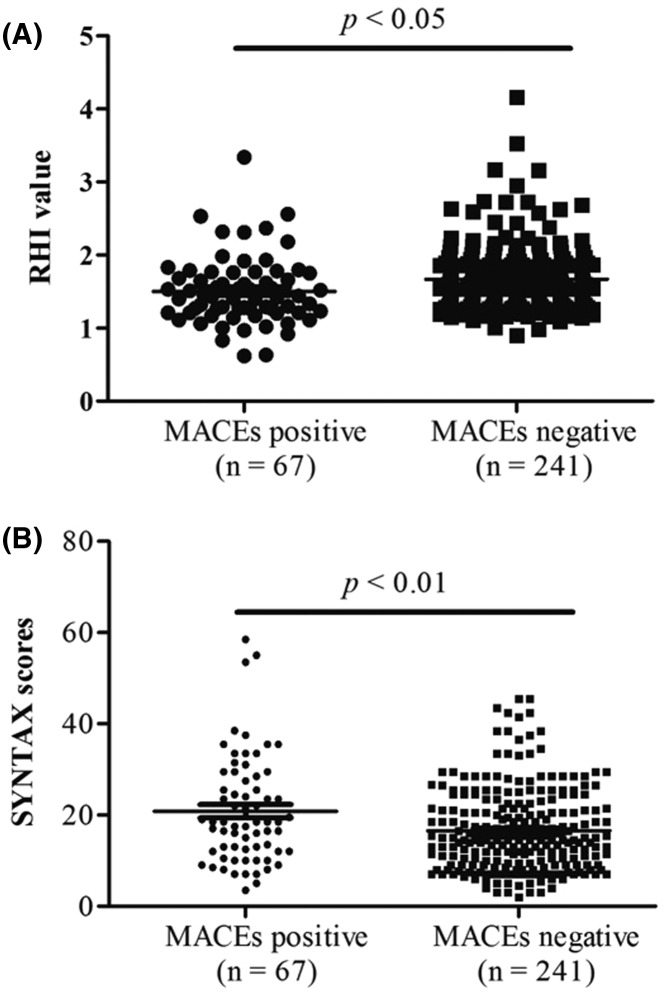
Patients were followed up for a median period of 16 months, during which 67 (21.75%) patients suffered from a MACE. The incidence of cardiovascular events in the DEF group (n=48, 25.39%) was significantly higher than that in the NEF group (n=19, 15.96%, P<0.05), as shown in Table 3. Specifically, non-fatal ischemic stroke occurred in seven patients (3.70%) in the DEF group, while no patients suffered from stroke in the NEF group (P<0.05). However, the incidences of other types of MACEs, including AMI, TVR, cardiac death, and cardiac hospitalization, showed no difference between the DEF group and NEF group (P>0.05). Additionally, the RHI in patients with MACEs was obviously lower than that in patients without MACEs (P<0.05, Figure 3A).
Table 3. Estimated incidence of major adverse cardiac events in patients undergoing PCIs.
| Overall (n=308) | NEF group (n=119) | DEF group (n=189) | HR (95% CIs) | P value | |
|---|---|---|---|---|---|
| AMI, n (%) | 13 (4.22%) | 4 (5.04%) | 9 (4.76%) | 1.015 (0.969–1.063) | 0.389 |
| TVR, n (%) | 4 (1.30%) | 1 (0.84%) | 3 (1.59%) | 1.016 (0.998–1.034) | 0.285 |
| Cardiac death, n (%) | 8 (2.60%) | 1 (0.84%) | 7 (3.70%) | 1.029 (0.997–1.063) | 0.157 |
| Non-fatal ischemic stroke, n (%) | 7 (2.27%) | 0 (0.00%) | 7 (3.70%) | ∞ (1.010 -∞) | 0.046* |
| Cardiac hospitalization, n (%) | 35 (11.36%) | 13 (10.92%) | 22 (11.64%) | 1.008 (0.929–1.094) | 0.501 |
| MACEs, n (%) | 67 (21.75%) | 19 (15.96%) | 48 (25.39%) | 1.142 (1.019–1.280) | 0.036* |
*P≤0.05.
Figure 3. Differences of RHI and SYNTAX score between MACEs and non-MACEs groups.
RHI (A) value was lower (B) was significantly higher in MACEs subjects than non-MACE ones.
The overall SYNTAX score was higher in subjects suffering from MACEs than in the non-MACEs subjects (P<0.05, Figure 3B). Furthermore, the LVEF and systolic BP were also significantly lower in the MACEs group than that in the non-MACEs group, while the diastolic BP was higher in the MACEs group (P<0.05, Supplementary Table S1). However, the coronary artery lesion characteristics showed no significant differences between the MACEs positive and MACEs negative groups (all P values <0.05, Supplementary Table S2).
Cox proportional hazards analyses were also performed to identify the associations of endothelial function, cardiovascular risk factors, and heart function with future MACEs after PCI treatment. Unadjusted Cox proportional hazards regression models showed that RHI (HR: 0.335, 95% CI: 0.167–0.675; P<0.05), LVEF (HR: 0.973, 95% CI : 0.948–0.998; P<0.05), and SYNTAX score (HR: 1.052, 95% CI: 1.029–1.075; P<0.01) were predictive of MACEs after PCI treatment (Table 4). However, multivariate regression (stepwise backward algorithm multivariate Cox proportional hazard analysis) revealed that RHI (Model 2 adjusted by blood pressure, HR: 0.425, 95% CI: 0.198–0.914; P=0.029) was an independent predictor of future MACEs after PCI treatment (Table 5). Furthermore, we have also identified that the SYNTAX score is also an independent predictor for MACEs (HR: 1.043; 95% CI: 1.019–1.067; P<0.001, Table 5).
Table 4. Cox proportional comparison hazards for future MACEs in patients after PCI.
| Variables | Univariate regression | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| RHI | 0.335 | 0.167–0.675 | 0.002† |
| Age (years old) | 1.002 | 0.978–1.026 | 0.900 |
| Male (yes) | 0.745 | 0.431–1.288 | 0.292 |
| Current smoker (yes) | 0.859 | 0.529–1.395 | 0.539 |
| Diabetes mellitus (yes) | 1.397 | 0.836–2.337 | 0.202 |
| Hypertension (yes) | 1.661 | 0.982–2.811 | 0.059 |
| Systolic BP (mm Hg) | 1.002 | 0.989–1.015 | 0.798 |
| Diastolic BP (mm Hg) | 0.990 | 0.968–1.013 | 0.383 |
| Heart rate (per beats/min) | 0.997 | 0.977–1.017 | 0.753 |
| LVEF (%) | 0.973 | 0.948–0.998 | 0.036* |
| TG (mmol/l) | 1.106 | 0.884–1.385 | 0.378 |
| HDL (mmol/l) | 1.838 | 0.696–4.851 | 0.219 |
| LDL (mmol/l) | 1.147 | 0.931–1.607 | 0.147 |
| SYNTAX score | 1.052 | 1.029–1.075 | <0.001† |
| TC (mmol/l) | 1.128 | 0.915–1.390 | 0.259 |
| BNP (pg/ml) | 1.001 | 1.000–1.001 | 0.098 |
| Cr (μmol/l) | 0.998 | 0.985–1.012 | 0.811 |
| ALT (IU/l) | 0.998 | 0.988–1.008 | 0.639 |
| AST (IU/l) | 0.999 | 0.988–1.009 | 0.814 |
| Pre-PCI | 0.473 | 0.171–1.304 | 0.148 |
| Peripheral vascular disease | 1.139 | 0.691–1.878 | 0.609 |
*P≤0.05; †P≤0.01.
Table 5. Cox proportional comparison hazards for future MACEs in patients after PCI (multivariate regression).
| Variables | Multivariate regression Model 1 | Multivariate regression Model 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| RHI | 0.415 | 0.195–0.884 | 0.023* | 0.425 | 0.198–0.914 | 0.029* |
| Hypertension (yes) | 1.153 | 0.686–1.939 | 0.591 | – | – | – |
| Systolic BP (mm Hg) | – | – | – | 1.011 | 0.994–1.029 | 0.202 |
| Diastolic BP (mm Hg) | – | – | – | 1.010 | 0.982–1.010 | 0.210 |
| LVEF (%) | 1.000 | 0.998–1.001 | 0.153 | 1.004 | 0.952–1.004 | 0.095 |
| SYNTAX score | 1.043 | 1.019–1.067 | <0.001† | 1.043 | 1.019–1.067 | <0.001† |
| BNP (pg/ml) | 1.000 | 0.998–1.001 | 0.593 | 1.000 | 0.998–1.001 | 0.527 |
| BMI (kg/m2) | 1.042 | 0.958–1.134 | 0.335 | 1.043 | 0.958–1.135 | 0.333 |
| Number of diseased coronary vessels | 0.897 | 0.673–1.197 | 0.461 | 0.897 | 0.675–1.196 | 0.465 |
| Pre-PCI history | 1.103 | 0.537–2.266 | 0.790 | 1.057 | 0.510–2.191 | 0.881 |
| Creatinine, (μmol/l) | 0.997 | 0.983–1.010 | 0.638 | 0.998 | 0.984–1.011 | 0.722 |
Model 1: The hypertension cases as a dichotomy variable selected into the regression; Model 2: Included SBP and DBP into the analysis instead of hypertension.
*P≤0.05; †P≤0.01.
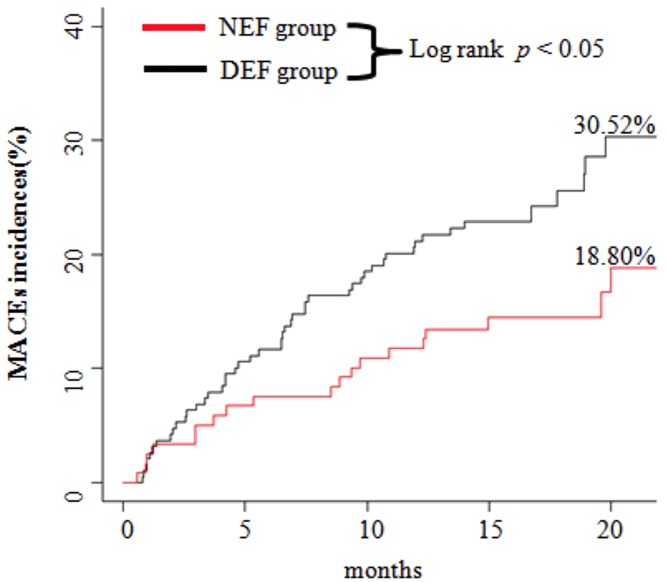
Additionally, Kaplan–Meier survival curves illustrated the relationship between RHI and MACEs (Figure 4). Compared with the RHI ≥ 1.67 group (NEF group) as a reference, subjects in the RHI < 1.67 group (DEF group) were shown to have a higher risk of MACEs after PCI treatment (P=0.008).
Figure 4. Kaplan–Meier curves of cumulative incidences of MACEs.
Subjects in the RHI < 1.67 group (DEF group) were shown to have a higher risk of MACEs after PCI treatment (P=0.008).
Discussion
In the present study, we focused on the association between peripheral DEF and MACEs in a specific type of CAD patients; namely, patients with ACS after they received PCI treatment, in whom this association has not been previously reported. We found that the incidence of MACEs was significantly higher in the DEF group (RHI < 1.67) than in the NEF group among ACS patients who were treated with PCI during the follow-up period (median: 16 months). Furthermore, we also observed that the patients in the DEF group were characterized by a lower LVEF. Finally, we demonstrated that in addition to the traditional predictors, RHI was an independent predictor of future MACEs after PCI treatment in ACS patients.
As mentioned above, peripheral DEF plays a pivotal role in the development of clinical cardiovascular diseases [1,4,7]. Recently, a novel peripheral endothelial function assessment technique, RH-PAT, has been widely applied in clinical research and practice. Although both FMD and RHI were associated with CAD and were also predictors of cardiac events, the relationship between FMD and RHI has been controversial [2,27]. RH-PAT also has clear advantages over the established methods of coronary angiography and ultrasound used to measure endothelial function [2]. Thus, it is important to identify the roles of RHI in cardiovascular diseases. The associations between RHI and coronary blood flow, coronary DEF, coronary microvascular function, heart failure, and their future events have been reported. The results above were consistent with those of previous reports, in which RHI was shown to be a risk factor in addition to the traditional risk factors [22,28], which may provide guidance for ACS risk stratification.
In addition, we found that LVEF was much lower in the DEF group than in the NEF group, suggesting that cardiac function was also affected to some degree. The lower LVEF in the DEF group may be also a reason for the lower systolic BP in this group. Also of note is that BNP levels were significantly higher in the DEF group than in the NEF group, which supports the previously reported association between cardiac function and peripheral DEF [9]. This association may also be a mechanism underlying the predictive roles of RHI in future cardiovascular disease events. However, in the adjusted model, LVEF could not predict future MACEs after PCI treatment following RHI adjustment.
Interestingly, we found that in the coronary artery lesion characteristic analysis, there were no significant differences in the number of diseased coronary vessels, lesion types in coronary arteries (including the left main artery), number of implanted stents, calcification, occlusion cases, or other characteristics. Although ACS patients had the same baseline and major coronary artery lesion characteristics at the time of PCI treatment, patients in the DEF group had a poor prognosis and a higher incidence of MACEs in the future, implying that DEF may be a prognostic marker in ACS patients. This predictive role may be attributed to the higher incidence of STEMI in populations with peripheral DEF. Further analyses revealed that in the MACE subtype analysis, non-fatal ischemic stroke had a higher incidence in the DEF group, indicating DEF in the peripheral arteries as well as cerebral arterioles. In addition, in the populations with MACEs, RHI was significantly lower than that in the non-MACEs subjects, indicating associations between DEF and MACEs. The higher incidence of non-fatal ischemic stroke in the DEF group suggested that DEF may be a risk factor for cardiovascular diseases as well as cerebral artery atherosclerosis, microvascular diseases and thromboembolism or, consequently, stroke. This may provide insight into predicting or assessing cerebral artery atherosclerosis or DEF, which needs further methodological and basic mechanisms studies. Furthermore, the RHI assessment may also be part of the risk management, stratification of ischemic stroke and prediction of stroke.
Finally, we performed Cox regression analyses to identify predictors of MACEs in ACS patients after PCI treatment. In the univariate regression, RHI, SYNTAX score, and lower LVEF were predictors of MACEs. However, there were many differences in clinical characteristics, blood pressure, and medication between patients with normal and abnormal endothelial function, thus it is also possible that these factors influenced the endothelial function in the MACEs occurrence. Furthermore, in the adjusted regression, other variables were adjusted by the RHI and SYNTAX score, meaning that RHI and SYNTAX score were still independent predictors of MACEs during the 16-month (median) follow-up period in the PCI-treated ACS patients. Combined with the Kaplan–Meier survival analysis findings (patients in the DEF group had a higher risk of MACEs), RHI impairment could provide a role in peripheral DEF as an independent predictor of prognosis (MACEs) in PCI-treated ACS patients.
Limitations
First, this was a single-center study with a limited sample size and number of events. Further large, multicenter studies with various ethnic groups are required.
Second, patients with hemodynamic instability or who died as a consequence of ACS before the RHI was measured were not included in the present study, which may lead to a reduced ability of the SYNTAX score to predict MACEs by excluding patients with serious CAD.
Third, our study excluded ACS patients with an unstable condition despite maximal therapy (NYHA class IV) due to difficulty in performing RHI measurements.
Fourth, we knew the RHI of patients during to follow for MACE, which makes the trial non-blind. Moreover, though this is a 5-year study, the current result can indicate that RHI can predict the MACE in these patients. Further prospective clinical trials are needed, as an observational study cannot determine causality between RHI and MACEs in the selected populations.
Conclusion
Endothelial function measured by RH-PAT is impaired in ACS subjects treated with PCI. The founding that RHI was an independent predictor of MACEs, suggesting that RHI may be useful as a candidate biomarker in the risk stratification of patients with ACS after PCI treatment.
Supporting information
Table S1. Baseline Characteristics of patients between MACEs and non-MACEs groups.
Table S2. The coronary artery lesion characteristics of patients divided by MACEs.
Acknowledgments
We thank to all study participants for their participation in the study. We thank colleagues at the Department of Laboratory Medicine, Xinqiao Hospital for their help with biochemical measurements.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- ACS
acute coronary syndrome
- AMI
acute myocardial infarction
- BNP
brain-type natriuretic peptide
- BP
Blood pressure
- CAD
coronary artery disease
- CCB
calcium channel blocker
- CI
confidence interval
- Cr
creatinine
- DEF
endothelial dysfunction
- FMD
flow-mediated dilatation
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- IQR
interquartile range
- LV
left ventricle
- LVEF
left ventricle ejection fraction
- LDL-C
low-density lipoprotein cholesterol
- MACE
major cardiovascular event
- NEF
normal endothelial function
- NSTE-ACS
non ST-elevation acute coronary syndrome
- NSTEMI
non ST-elevation myocardial infarction
- PCI
percutaneous coronary intervention
- RHI
reactive hyperemia index
- RH-PAT
reactive hyperemia-peripheral arterial tonometry
- STEMI
ST-segment elevation myocardial infarction
- SYNTAX
Synergy Between PCI with Taxus and Cardiac Surgery
- TG
triglyceride
- TVR
target vessel revascularization
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
X.C. and J.J. designed the research and wrote the paper. Y.H. and H.F. performed the research. X.C. and T.L. analyzed the data. W.P. and K.W. guided and supervised the experiment process.
Funding
National Key Research and Invention Program of the Thirteenth [grant number 2016YFC1301304 to Jun Jin]; Clinical Scientific Research Project of Xinqiao Hospital [grant number 2015YLC07 to Jun Jin]; Military special grant [grant number 16BJZ37 to Jun Jin].
References
- 1.Celermajer D.S., Sorensen K.E., Bull C., Robinson J. and Deanfield J.E. (1994) Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J. Am. Coll. Cardiol. 24, 1468–1474 10.1016/0735-1097(94)90141-4 [DOI] [PubMed] [Google Scholar]
- 2.Lian B.Q. and Keaney J.F. Jr (2010) Predicting ischemic heart disease in women: the value of endothelial function. J. Am. Coll. Cardiol. 55, 1697–1699 10.1016/j.jacc.2009.10.074 [DOI] [PubMed] [Google Scholar]
- 3.Gori T., Muxel S., Damaske A., Radmacher M.C., Fasola F., Schaefer S. et al. (2012) Endothelial function assessment: flow-mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. Eur. Heart J. 33, 363–371 10.1093/eurheartj/ehr361 [DOI] [PubMed] [Google Scholar]
- 4.Hays A.G., Hirsch G.A., Kelle S., Gerstenblith G., Weiss R.G. and Stuber M. (2010) Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J. Am. Coll. Cardiol. 56, 1657–1665 10.1016/j.jacc.2010.06.036 [DOI] [PubMed] [Google Scholar]
- 5.Sherwood A., Hinderliter A.L., Watkins L.L., Waugh R.A. and Blumenthal J.A. (2005) Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J. Am. Coll. Cardiol. 46, 656–659 10.1016/j.jacc.2005.05.041 [DOI] [PubMed] [Google Scholar]
- 6.Karatzis E.N., Ikonomidis I., Vamvakou G.D., Papaioannou T.G., Protogerou A.D., Andreadou I. et al. (2006) Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am. J. Cardiol. 98, 1424–1428 10.1016/j.amjcard.2006.06.043 [DOI] [PubMed] [Google Scholar]
- 7.Bonetti P.O., Pumper G.M., Higano S.T., Holmes D.R. Jr, Kuvin J.T. and Lerman A. (2004) Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 44, 2137–2141 10.1016/j.jacc.2004.08.062 [DOI] [PubMed] [Google Scholar]
- 8.Ras R.T., Streppel M.T., Draijer R. and Zock P.L. (2013) Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int. J. Cardiol. 168, 344–351 10.1016/j.ijcard.2012.09.047 [DOI] [PubMed] [Google Scholar]
- 9.Akiyama E., Sugiyama S., Matsuzawa Y., Konishi M., Suzuki H., Nozaki T. et al. (2012) Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J. Am. Coll. Cardiol. 60, 1778–1786 10.1016/j.jacc.2012.07.036 [DOI] [PubMed] [Google Scholar]
- 10.Matsuzawa Y., Li J., Aoki T., Guddeti R.R., Kwon T.G., Cilluffo R. et al. (2015) Predictive value of endothelial function by noninvasive peripheral arterial tonometry for coronary artery disease. Coron. Artery Dis. 26, 231–238 10.1097/MCA.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadhav U.M., Sivaramakrishnan A. and Kadam N.N. (2003) Noninvasive assessment of endothelial dysfunction by brachial artery flow-mediated dilatation in prediction of coronary artery disease in Indian subjects. Indian Heart J. 55, 44–48 [PubMed] [Google Scholar]
- 12.Kanahara M., Harada H., Katoh A. and Ikeda H. (2014) New methodological approach to improve reproducibility of brachial artery flow-mediated dilatation. Echocardiography 31, 197–202 10.1111/echo.12307 [DOI] [PubMed] [Google Scholar]
- 13.Lee C.R., Bass A., Ellis K., Tran B., Steele S., Caughey M. et al. (2012) Relation between digital peripheral arterial tonometry and brachial artery ultrasound measures of vascular function in patients with coronary artery disease and in healthy volunteers. Am. J. Cardiol. 109, 651–657 10.1016/j.amjcard.2011.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanivadekar A. (2003) Brachial artery flow-mediated dilatation in prediction of coronary artery disease in Indian subjects. Indian Heart J. 55, 283, author reply 283-285 [PubMed] [Google Scholar]
- 15.Sejda T., Pit’ha J., Svandova E. and Poledne R. (2005) Limitations of non-invasive endothelial function assessment by brachial artery flow-mediated dilatation. Clin. Physiol. Funct. Imaging 25, 58–61 10.1111/j.1475-097X.2004.00590.x [DOI] [PubMed] [Google Scholar]
- 16.Igari K., Kudo T., Toyofuku T. and Inoue Y. (2016) Endothelial dysfunction of patients with peripheral arterial disease measured by peripheral arterial tonometry. Int. J. Vasc. Med. 2016, 3805380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzawa Y., Sugiyama S., Sugamura K., Nozaki T., Ohba K., Konishi M. et al. (2010) Digital assessment of endothelial function and ischemic heart disease in women. J. Am. Coll. Cardiol. 55, 1688–1696 10.1016/j.jacc.2009.10.073 [DOI] [PubMed] [Google Scholar]
- 18.Nil M., Schafer D., Radtke T., Saner H., Wilhelm M. and Eser P. (2014) Reproducibility of peripheral arterial tonometry measurements in male cardiovascular patients. Eur. J. Clin. Invest. 44, 1065–1071 10.1111/eci.12341 [DOI] [PubMed] [Google Scholar]
- 19.Hamburg N.M. and Benjamin E.J. (2009) Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc. Med. 19, 6–11 10.1016/j.tcm.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heffernan K.S., Karas R.H., Patvardhan E.A., Jafri H. and Kuvin J.T. (2010) Peripheral arterial tonometry for risk stratification in men with coronary artery disease. Clin. Cardiol. 33, 94–98 10.1002/clc.20705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komura N., Tsujita K., Yamanaga K., Sakamoto K., Kaikita K., Hokimoto S. et al. (2016) Impaired peripheral endothelial function assessed by digital reactive hyperemia peripheral arterial tonometry and risk of in-stent restenosis. J. Am. Heart Assoc. 5, e003202 10.1161/JAHA.116.003202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michelsen M.M., Mygind N.D., Pena A., Aziz A., Frestad D., Host N. et al. (2016) Peripheral reactive hyperemia index and coronary microvascular function in women with no obstructive CAD: the iPOWER study. JACC Cardiovasc. Imaging 9, 411–417 10.1016/j.jcmg.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 23.Schoenenberger A.W., Radovanovic D., Windecker S., Iglesias J.F., Pedrazzini G., Stuck A.E. et al. (2016) Temporal trends in the treatment and outcomes of elderly patients with acute coronary syndrome. Eur. Heart J. 37, 1304–1311 10.1093/eurheartj/ehv698 [DOI] [PubMed] [Google Scholar]
- 24.Woo J.S., Jang W.S., Kim H.S., Lee J.H., Choi E.Y., Kim J.B. et al. (2014) Comparison of peripheral arterial tonometry and flow-mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron. Artery Dis. 25, 421–426 10.1097/MCA.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 25.Chinese Journal of Cardiology (2015) 2015 Chinese Guidelines for diagnosis and treatment on ST-segment elevation myocardial infarction (STEMI). Chin. J. Cardiol. 43, 12 [Google Scholar]
- 26.Chinese Journal of Cardiology (2012) 2012 Guidelines for diagnosis and treatment on non-ST-segment elevation acute coronary syndrome (NSTE-ACS). Chin. J. Cardiol. 40, 14 [Google Scholar]
- 27.Rubinshtein R., Kuvin J.T., Soffler M., Lennon R.J., Lavi S., Nelson R.E. et al. (2010) Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 31, 1142–1148 10.1093/eurheartj/ehq010 [DOI] [PubMed] [Google Scholar]
- 28.Suessenbacher A., Dorler J., Wanitschek M., Alber H.F., Pachinger O. and Frick M. (2014) Prognostic value of peripheral arterial tonometry in patients with coronary artery disease and a high cardiovascular risk profile. J. Atheroscler. Thromb. 21, 230–238 10.5551/jat.18986 [DOI] [PubMed] [Google Scholar]