Abstract
World Health Organization recommends hepatitis B virus (HBV) immunization at 0, 1, and 6 months. However, studies have suggested that shortening the interval between the first and last HBV immunization can improve completion rates. Less clear is whether accelerated immunization is as immunogenic as standard immunization. Thus, the present study aimed to compare the short-term immunogenicity of yeast-derived hepatitis B vaccine in healthy adults immunized on an accelerated or standard schedule. Between June 2013 and March 2014, individuals from Jinfeng and Longmen, China were randomly assigned to receive the vaccine on an accelerated schedule (at 0, 1, and 2 months; n=201) or a standard schedule (at 0, 1, and 6 months; n=206). Subjects filled out a questionnaire asking about demographic and other health data, and they underwent physical examination. Blood was assayed for HBV surface antigen and HBV surface antibody (HBsAb) at 1–2 months after the three-dose schedule. Multivariate binary logistic regression was used to determine whether the rate of anti-HBs seroconversion differed with immunization schedule. Covariance analysis was used to compare geometric mean HBsAb concentration between the two schedules. The anti-HBs seroconversion rate was 84.6% in the accelerated group and 90.3% in the standard group. After controlling for several potential confounders, the accelerated schedule was associated with significantly lower anti-HBs seroconversion rate (OR: 0.560, 95% CI: 0.318–0.988). Similarly, the accelerated schedule was associated with significantly lower geometric mean HBsAb concentration. These results suggest that the standard schedule is more likely to lead to anti-HBs seroconversion and higher HBsAb levels in adults.
Keywords: accelerated schedule, Hepatitis B virus, hepatitis B vaccine, immunization program, standard schedule
Introduction
Incidence of hepatitis B virus (HBV) in China among children younger than 15 years fell significantly after 1992, when the Ministry of Health included HBV vaccination in the national immunization program [1]. However, HBV incidence among adults remains substantial. A national survey in China showed that 8.57% of adults aged 15–59 years are positive for HBV surface antigen (HBsAg), and 47.38% of adults are positive for anti-HBV surface antibodies (HBsAb) [2].
HBV vaccination is considered the safest, most effective way to prevent HBV infection [3–5]. The World Health Organization and US Centers for Disease Control and Prevention, as well as Chinese National Guidelines on chronic hepatitis B prevention and treatment (2015) recommend HBV immunization at 0, 1, and 6 months. This long duration translates to low completion rates [6] and has contributed to the fact that in the US only 24.5% of adults aged 19 and older were vaccinated against HBV in 2014 [7]; in China, the adult vaccination rate is below 10%, according to a 2006 national HBV seroprevalence survey [8].
Studies have suggested that shortening the interval between the first and last HBV immunization can improve completion rates and even stimulate earlier and faster HBsAb production [9,10]. For example, an accelerated immunization schedule can encourage injected drug users to complete HBV vaccination [11], and it can increase the acceptability of vaccination among the general population [12].
Less clear, however, is whether accelerated immunization is as immunogenic as standard immunization. One study, for example, showed that although 93% of individuals vaccinated on an accelerated schedule achieved the desired HBsAb titer within 1 month after the third injection, the rate of individual’s positive for such antibodies as well as the titer of such antibodies tended to decrease after 12 months [13]. Given the attractiveness of accelerated HBV immunization, whether it is as effective at eliciting antibody production as the standard immunization needs to be further studied.
Therefore, the present epidemiological field study was undertaken to compare the immunogenicity of an accelerated HBV vaccination schedule (at 0, 1, and 2 months) with the standard schedule (at 0, 1, and 6 months).
Methods
Setting
The towns of Jinfeng and Longmen were randomly selected from among the 21 towns and subdistricts in Mianyang, China covered by the National Science & Technology Pillar Program of the 12th Five-Year Plan. Eligible subjects in the towns were enrolled and randomly assigned to receive accelerated HBV vaccination (at 0, 1, and 2 months) or standard vaccination (at 0, 1, and 6 months). In the end, subjects in Jinfeng received the accelerated schedule, while subjects in Longmen received the standard schedule. The same vaccine and dose were used in all cases.
Eligibility criteria
Study subjects were recruited from the cohort of individuals enrolled in the National Science & Technology Pillar Program during the 12th Five-Year Plan. This cohort contained individuals from Mianyang City aged 15–59 years who were negative for HBsAg and HBsAb at the time of enrollment.
The present study was carried out from 1 June 2013 to 1 March 2014.
Individuals were eligible to participate in the study if they (1) were negative for HBsAg and HBsAb, (2) were 15–59 years old, (3) had lived in Mianyang City longer than 6 months by the time of enrollment, and (4) voluntarily consented to participate in the study. The study was approved by the Ethics Committee of West China Hospital, Sichuan University. All subjects gave written informed consent to receive HBV vaccination and to participate in follow-up.
Subjects were excluded from the study if they (1) were positive for HBsAg or HBsAb, (2) had a history of serious vaccine reaction, (3) were known to have immune dysfunction or were considered susceptible to it, (4) were currently on immunosuppressive therapy, (5) had experienced fever (>38°C) during 3 days prior to enrollment, or (6) had been vaccinated with live attenuated virus during the preceding month, such as measles (including MMR, and leprosy), Japanese encephalitis virus, hepatitis A virus, or varicella zoster virus.
Vaccination and data collection
Research staff received appropriate training from the lead project investigators, and then collected data at examination centers in local health stations and community clinics in Jinfeng and Longmen. Staff conducted standardized, questionnaire-based face-to-face interviews after obtaining written consent from participants. The questionnaire collected information about demographics (including gender, age, height, and weight), health-related behaviors (smoking and drinking), family history of hepatitis B (parents infected by HBV or not), and other data. Each questionnaire was assigned a unique identification number.
Subjects who, upon screening, showed no serological evidence of past HBV infection or immunization were offered vaccination through the regular service of township hospitals, where trained medical stuff performed vaccinations by intramuscular injection. The vaccine (Hualan Biological Vaccine Company, Chengdu, China) contained 10 μg recombinant HBsAg per dose.
All subjects were tested before and after vaccination for the presence of HBsAg, anti-HBs, and anti-HBc. Testing was performed at an external laboratory using commercially available kits (Sichuan Kingmed Center for Clinical Laboratory, Chengdu, China). At 1–2 months after the third vaccine injection, subjects’ blood was sampled (5 ml) and assayed for anti-HBs seroconversion.
Blood sample tests
The chemiluminescence microparticle immunoassay was used to detect HBV serum markers (ARCHITET i2000, Abbott, U.S.A.). Seroprotection was defined as HBsAg ≥ 0.05 IU/ml, an anti-HBs titer ≥10 IU/l, and anti-HBc (s/co) ≥1 at 1–2 months after the third vaccination. The immune response of Hep B was classified based on anti-HBs concentration as none (<10 IU/l) [13], low (10–100 IU/l) [14], normal (100–1000 IU/l), or high (≥1000 IU/l).
Statistical analysis
SPSS 22.0 (IBM, Chicago, IL, U.S.A.) was used for all statistical analyses, with significance defined as P<0.05. Subjects on accelerated or standard vaccination schedules were compared in terms of demographic characteristics, health-related behaviors, family history of hepatitis B, and anti-HBs seroconversion rate. Differences were assessed for significance using the Pearson χ2 and Fisher’s exact tests. Multivariate binary logistic regression was used to compare anti-HBs seroconversion rates between the two vaccination schedules after controlling for age, gender, body mass index (BMI), smoking, drinking, anti-HBc, and family history of hepatitis B.
Anti-HBs concentrations were log-transformed to give them a normal distribution, and then used to calculate anti-HBs geometric mean concentration (GMC), which was compared between subjects on accelerated and standard vaccination schedules using the t test. GMC was also compared between subgroups of subjects stratified by different characteristics, and differences were assessed for significance using the t test, ANOVA, and Scheffe test. Finally, covariance analysis was used to compare anti-HBs GMC between the accelerated and standard schedules after controlling for various potential confounders.
Ethical approval
The present study was approved by the Ethics Committee of West China Hospital, Sichuan University, and it conformed to the provisions of the Declaration of Helsinki. Each participant signed an informed consent form before enrollment.
Results
Study population
Between 1 June 2013 and 1 March 2014, 407 individuals underwent blood testing and were allocated to undergo vaccination on the accelerated schedule (201, 49.39%) or standard schedule (206, 50.61%). The remaining eligible individuals did not consent to participate in the study.
Subjects on the accelerated schedule (36.3% men) had an average age of 38.1 ± 12.8 years and average BMI of 23.0 ± 3.6 kg/m2. Subjects on the standard schedule (38.8% men) had an average age of 39.7 ± 11.8 years and average BMI of 23.0 ± 3.5 kg/m2. The two groups were similar in age, gender, BMI, smoking, drinking, anti-HBc, and family history of hepatitis B (P>0.05, Table 1).
Table 1. Characteristics of the study population.
| Variable | Accelerated schedule (n=201) | Standard schedule (n=206) | χ2/u | P | |
|---|---|---|---|---|---|
| Age, year | 15–29 | 60 (29.9) | 52 (25.3) | 2.891 | 0.236 |
| 30–49 | 89 (44.3) | 107 (51.9) | |||
| 50–59 | 52 (25.9) | 47 (22.8) | |||
| Sex | Male | 73 (36.3) | 80 (38.8) | 0.327 | 0.568 |
| Female | 128 (63.7) | 126 (61.2) | |||
| BMI, kg/m2 | 23.0 ± 3.6 | 23.0 ± 3.5 | 0.022 | 0.983 | |
| Smoking | Yes | 33 (16.4) | 44 (21.4) | 1.819 | 0.177 |
| No | 168 (83.6) | 162 (78.6) | |||
| Drinking, g/day | No | 149 (74.2) | 149 (72.3) | 1.588 | 0.452 |
| <20 | 25 (12.4) | 33 (16.0) | |||
| ≥20 | 27 (13.4) | 24 (11.7) | |||
| Family history of hepatitis B | Yes | 3 (1.5) | 2 (1.0) | 0.230 | 0.631 |
| No | 198 (98.5) | 204 (99.0) | |||
| Anti-HBc | Negative | 110 (54.7) | 112 (54.4) | 0.005 | 0.941 |
| Positive | 91 (45.3) | 94 (45.6) | |||
Values are n ± S.D. or n (%), unless otherwise noted.
Rate of anti-HBs seroconversion
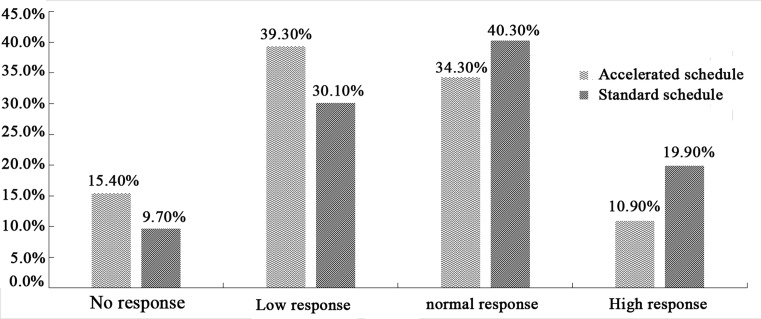
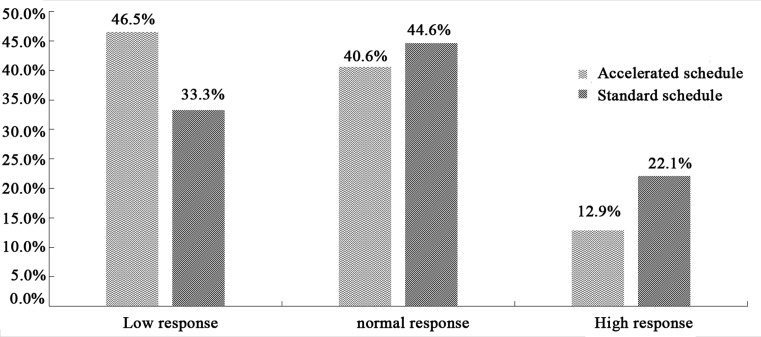
The rate of anti-HBs seroconversion was 84.6% in the accelerated group and 90.3% in the standard group. The proportion of subjects showing low response to HBV vaccination was higher in the accelerated group (39.3%) than in the standard group (30.1%). Conversely, lower proportions of subjects in the accelerated group showed normal response (34.3% vs 40.3%) or high response (10.9% vs 19.9%; Figure 1). Among subjects on the accelerated schedule who produced anti-HBs, 46.5% showed low response, 40.6% normal response, and 12.9% high response. The corresponding proportions among subjects on the standard schedule who produced anti-HBs were 33.3%, 44.6%, and 22.1% (Figure 2).
Figure 1. Anti-HBs seroconversion rates on the two vaccination schedules.
The left vertical axis represents the rate of anti-HBs seroconversion. Wave represents the anti-HBs seroconversion rates on accelerated schedule in each group, and slash represents the anti-HBs seroconversion rates on standard schedule in each group. The horizontal axis represents the reaction type.
Figure 2. Anti-HBs seroconversion rates on the two vaccination schedules among subjects who produced anti-HBs.
The left vertical axis represents the constituent ratio. Wave represents the constituent ratio on accelerated schedule in each group, and slash represents the constituent ratio on standard schedule in each group. The horizontal axis represents the reaction type among subjects who produced anti-HBs.
Univariate analyses revealed a significantly lower rate of anti-HBs seroconversion among men on the accelerated schedule than among men on the standard schedule (χ2 = 4.520, P<0.05; Table 2). Subjects with BMI between 24 and 28 showed a significantly lower anti-HBs seroconversion rate on the accelerated schedule than on the standard one (χ2 = 5.289, P<0.05). Similarly, the accelerated schedule was associated with significantly lower anti-HBs seroconversion rate among subjects without a family history of hepatitis B (χ2 = 4.069, P<0.05). The following factors were negatively associated with anti-HBs seroconversion rate: BMI below 18.5, between 18.5 and 24, or above 28; age; female gender; smoking; drinking; anti-HBc; and family history of hepatitis B (Table 2).
Table 2. Univariate analysis to identify variables associated with anti-HBs seroconversion rate on the two vaccination schedules.
| Variable | Accelerated schedule | Standard schedule | χ2 | P | |
|---|---|---|---|---|---|
| Seroconversion % (n/N) | Seroconversion % (n/N) | ||||
| Age, year | 15–29 | 93.3 (56/60) | 94.2 (49/52) | 1.000† | |
| 30–49 | 85.4 (76/89) | 90.7 (97/107) | 1.737 | 0.187 | |
| 50–59 | 73.1 (38/52) | 85.1 (40/47) | 2.617 | 0.106 | |
| χ2 | 8.620 | 3.747 | |||
| P | 0.003* | 0.053* | |||
| Sex | Male | 79.5 (58/73) | 90.0 (72/80) | 4.520 | 0.034 |
| Female | 87.5 (112/128) | 90.5 (114/126) | 0.384 | 0.759 | |
| χ2 | 2.308 | 0.002 | |||
| P | 0.129 | 0.960 | |||
| BMI | <18.5 | 100 (20/20) | 95.0 (19/20) | 1.000† | |
| 18.5–23.9 | 89.2 (91/102) | 92.8 (103/111) | 1.203 | 0.273 | |
| 24–27.9 | 75.0 (45/60) | 89.8 (53/59) | 5.289 | 0.021 | |
| ≥28 | 73.7 (14/19) | 68.8 (11/16) | 0.043 | 0.836 | |
| χ2 | 10.206 | 9.866 | |||
| P | 0.001* | 0.002* | |||
| Smoking | Yes | 78.8 (26/33) | 86.4 (38/44) | 3.833 | 0.052 |
| No | 85.7 (144/168) | 92.1 (116/126) | 0.748 | 0.387 | |
| χ2 | 1.014 | 2.177 | |||
| P | 0.314 | 0.140 | |||
| Drinking | |||||
| (g/day) | No | 86.6 (129/149) | 91.3 (136/149) | 2.354 | 0.125 |
| <20 | 80.0 (20/25) | 87.9 (29/33) | 0.494† | ||
| ≥20 | 77.8 (21/27) | 87.5 (21/24) | 0.315† | ||
| P | 0.335† | 0.551† | |||
| Anti-HBc | Negative | 90.0 (99/110) | 93.8 (105/112) | 1.312 | 0.252 |
| Positive | 78.0 (71/91) | 86.2 (81/94) | 2.904 | 0.088 | |
| χ2 | 5.478 | 4.349 | |||
| P | 0.019 | 0.037 | |||
| Family history of hepatitis B | Yes | 100 (3/3) | 100 (2/2) | ||
| No | 84.3 (167/198) | 90.2 (184/204) | 4.069 | 0.044 | |
| P | 1.000† | 1.000 | |||
Cochran–Armitage trend test.
Fisher’s exact test.
Multivariate analyses showed that, after controlling for age, gender, BMI, smoking, drinking, anti-HBc, and family history of hepatitis B, the anti-HBs seroconversion rate was significantly lower for the accelerated schedule than for the standard one (odds ratio [OR]: 0.560, 95% confidence interval [CI]: 0.318–0.988).
Anti-HBs GMC
Anti-HBs GMC was 73.197 mIU/ml (95% CI: 57.320–95.302) in the accelerated group and 140.134 mIU/ml (95% CI: 115.193–170.425) in the standard group.
Univariate analysis indicated that for subjects aged 15–29 or 30–49 years, the accelerated schedule was associated with significantly lower anti-HBs GMC than the standard schedule (Table 3). The accelerated schedule was also associated with significantly lower anti-HBs GMC than the standard schedule for the following subgroups: men, women, non-smokers, subjects with BMI between 18.5 and 24, or between 24 and 28, subjects who drink <20 g/day, subjects positive for anti-HBc, and subjects without a family history of hepatitis B.
Table 3. Univariate analysis to identify variables associated with anti-HBs GMC on the two vaccination schedules.
| Variable | Anti-HBs GMC (mIU/ml) | t | P | ||
|---|---|---|---|---|---|
| Accelerated schedule | Standard schedule | ||||
| Age, year | 15–29 | 96.107 | 287.268 | 4.101 | <0.001 |
| 30–49 | 78.686 | 137.155 | 2.401 | 0.017 | |
| 50–59 | 47.023 | 65.406 | 1.037 | 0.302 | |
| F | 2.483 | 14.411 | |||
| P | 0.086 | <0.001 | |||
| Sex | Male | 70.996 | 129.432 | 2.243 | 0.026 |
| Female | 74.472 | 147.383 | 3.489 | 0.001 | |
| t | 0.185 | 0.636 | |||
| P | 0.853 | 0.525 | |||
| BMI | <18.5 | 160.405 | 288.402 | 1.266 | 0.212 |
| 18.5–23.9 | 76.325 | 165.687 | 3.828 | <0.001 | |
| 24–27.9 | 48.420 | 114.950 | 2.969 | 0.004 | |
| ≥28 | 92.842 | 39.210 | 1.275 | 0.209 | |
| F | 2.663 | 7.308 | |||
| P | 0.049 | <0.001 | |||
| Smoking | Yes | 52.288 | 93.874 | 1.565 | 0.121 |
| No | 77.799 | 156.088 | 4.003 | <0.001 | |
| t | 1.343 | 2.097 | |||
| P | 0.181 | 0.037 | |||
| Drinking | |||||
| (g/day) | No | 78.910 | 147.727 | 3.463 | 0.001 |
| <20 | 60.734 | 147.383 | 2.023 | 0.047 | |
| ≥20 | 57.306 | 96.494 | 1.022 | 0.311 | |
| F | 1.012 | 0.539 | |||
| P | 0.365 | 0.584 | |||
| Anti-HBc | Negative | 86.044 | 220.485 | 4.740 | <0.001 |
| Positive | 60.130 | 81.311 | 1.249 | 0.213 | |
| t | 1.448 | 5.203 | |||
| P | 0.149 | <0.001 | |||
| Family history of hepatitis B | Yes | 246.776 | 345.776 | 0.475 | 0.660 |
| No | 71.841 | 138.864 | 4.133 | <0.001 | |
| t | 1.228 | 0.923 | |||
| P | 0.221 | 0.357 | |||
Covariance analysis showed that, after controlling for age, gender, BMI, smoking, drinking, anti-HBc, and family history of hepatitis B, the calibrated anti-HBs GMC was 72.473 mIU/ml (95% CI: 57.429–91.325) in the accelerated group and 141.219 mIU/ml (95% CI: 116.850–170.187) in the standard group; the calibrated value was significantly lower in the accelerated group (F = 19.287, P<0.001; Table 4).
Table 4. Covariance analysis to compare anti-HBs GMC (mIU/ml) on the two vaccination schedules.
| Schedule | Raw GMC | Calibrated GMC | Calibrated 95% CI | F | P |
|---|---|---|---|---|---|
| Accelerated (n=201) | 73.197 | 72.473 | 57.429–91.325 | 19.287 | <0.001 |
| Standard (n=296) | 140.134 | 141.219 | 116.850–170.187 |
Discussion
The present study shows that, after controlling for age, sex, BMI, smoking, drinking, anti-HBc, and family history of hepatitis B, an accelerated HBV immunization schedule was associated with significantly lower anti-HBs seroconversion rate than the standard immunization schedule (OR: 0.560, 95% CI: 0.318–0.988). These results are consistent with previous work in a different region of China [15]. Similarly, we found by covariance analysis that the accelerated schedule was associated with significantly lower anti-HBs GMC (F = 19.287, P<0.001). Our results are consistent with several studies reporting lower immunogenicity of accelerated vaccination schedules [16–21]. This may be explained by the shorter interval between the second and third dose in the accelerated schedule [21].
The higher frequency of low vaccination response in our accelerated group than in the standard group suggests that the standard schedule stimulates an immune response more easily [22]. Studies suggest that individuals usually possess long-term immunity to HBV if anti-HBs concentration is at least 100 mIU/ml. Our results suggest that the standard schedule is better at ensuring minimum immune response levels for sustained protective effects.
Consistent with this, we found that only 84.6% of subjects on the accelerated schedule developed anti-HBs titer ≥10 mIU/ml, which is considered the minimum needed to withstand HBV infection [23]. In contrast, 90.3% of subjects on the standard schedule achieved this minimum. A higher percentage of subjects on an accelerated schedule (93.6%) achieved this minimum in a study in South Korea [24], which may reflect the fact that the vaccine in that study was delivered subcutaneously and contained 0.15 ml recombinant HBsAg per dose. Administering a higher dose subcutaneously may enhance immune responses to HBV [25,26].
Despite the apparent superiority of the standard schedule, both the standard and accelerated schedules achieved anti-HBs seroconversion rates above 80%, which compares well with the rates reported for healthy adults on a standard schedule [27,28]. Our results add to the evidence base showing that HBV vaccination can elicit protective immune responses in healthy adults.
One potential benefit of an accelerated vaccination schedule is earlier protection even before the series of injections is complete [29–31]. In fact, one study examined anti-HBV immune responses at 2 years after vaccination on an accelerated or standard schedule and found lower HBV infection prevalence among those immunized on an accelerated schedule [32]. One possible explanation is that subjects on the standard schedule are at greater risk of suffering infection before they complete the vaccination course. This implies that individuals who urgently require protection from HBV may benefit from the accelerated schedule. It may also benefit individuals who, for various reasons, are at higher risk of failing to complete the lengthy standard schedule [33].
The results of the present study should be interpreted with caution in light of several limitations. First, we convenience-sampled residents of only two towns in one region of China, which increases the risk of bias in our study. We collected data only on anti-HBs response at 1–2 months after the third injection, preventing us from comparing the long-term immune effects of the two vaccination schedules.
Conclusion
More than 80% of subjects vaccinated on a standard or accelerated schedule presented anti-HBs concentrations above 10 mIU/ml at 1–2 months after the last vaccination. This highlights the usefulness of vaccinating adults against HBV. The accelerated schedule was associated with lower anti-HBs seroconversion rate and lower anti-HBs GMC than the standard schedule. Therefore, the standard schedule may be more effective for eliciting anti-HBV immune protection.
Acknowledgments
We thank the workers at the Center for Disease Control and Prevention of Mianyang City, Jiangyou County and Fucheng District for their assistance in carrying out the present study. We also thank the physicians and nurses at the community health service centers and township hospitals that helped us perform this work.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- GMC
geometric mean concentration
- HBsAb
HBV surface antibody
- HBsAg
HBV surface antigen
- HBV
hepatitis B virus
- OR
odds ratio
Funding
This study was supported by the National Science & Technology Pillar Program during the 12th Five-year Plan Period: Integrated Demonstration of Major Infectious Disease Prevention in Mianyang [grant number 2012ZX10004-901].
Author contribution
P.Y. conceived and designed the study. X.Z. and J.W. performed the study. X.C., M.Y., S.Y., and Y.S. were responsible for literature research and statistical analysis; J.D. and H.S. collected the data. X.Z. analyzed the data and drafted the paper. X.Z., J.W., X.C., M.Y., S.Y., and Y.S. revised the manuscript accordingly. P.Y. guided the whole study. All authors have read and agreed with the final version of this manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Pingge Yuan. (2015) China has ranged from the ‘high endemic areas’ to the ‘middle endemic areas’ by the hepatitis B virus infection. Gan Bing Lun Tan 5, 28–29 (In Chinese) [Google Scholar]
- 2.Xiaoqiu Qi, Yu Wang, Jingjin Yu et al. (2011) National Population Survey of Viral Hepatitis B Serological Investigation Report, pp. 49–54, People’s Health Publishing House, Beijing: (In Chinese) [Google Scholar]
- 3.Centers for Disease Control and Prevention (1991) Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the immunization practices advisory committee(ACIP). Morb. Mortal. Wkly Rep. 40, 1–19 [PubMed] [Google Scholar]
- 4.Van Damme P., Kane M. and Meheus A. (1997) Integration of hepatitis B vaccination into national immunization programmes. BMJ 314, 1033–1037 10.1136/bmj.314.7086.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao J.H. and Chen D.S. (2002) Global control of hepatitis B virus infection. Lancet Infect. Dis. 2, 395–403 10.1016/S1473-3099(02)00315-8 [DOI] [PubMed] [Google Scholar]
- 6.Dawei Zhu. (2013) Study on Utilization, Demand and Provider Choice for Hepatitis B Vaccination among Adults with User Fees in Rural China, Shandong University, Shandong: (In Chinese) [Google Scholar]
- 7.Williams W.W., Lu P.J., O’Halloran A. et al. (2016) Surveillance of vaccination coverage among adult populations - United States, 2014. MMWR Surveill. Summ. 65, 1–36 10.15585/mmwr.ss6501a1 [DOI] [PubMed] [Google Scholar]
- 8.Guomin Zhang, jin Sun Xiao, Fuzhen Wang et al. (2013) Analysis of epidemiological characteristics of hepatitis B among the population of 18-59 year old and Hep B immunization strategies. Chin. J. Vaccines Immun. 19, 266–270 (In Chinese) [Google Scholar]
- 9.Van Herck K., Leuridan E. and Van Damme P. (2007) Schedules for hepatitis B vaccination of risk groups: balancing immunogenicity and compliance. Sex. Transm. Infect. 83, 426–432 10.1136/sti.2006.022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wouters K., Leuridan E., Van Herck K. et al. (2007) Compliance and immunogenicity of two hepatitis B vaccination schedules in sex workers in Belgium. Vaccine 25, 1893–1900 10.1016/j.vaccine.2006.09.073 [DOI] [PubMed] [Google Scholar]
- 11.Bowman S., Grau L.E., Singer M. et al. (2014) Factors associated with hepatitis B vaccine series completion in a randomized trial for injection drug users reached through syringe exchange programs in three US cities. BMC Public Health 14, 820 10.1186/1471-2458-14-820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang L.Y., Grimes C.Z., Tran T.Q. et al. (2010) Accelerated hepatitis B vaccination schedule among drug users: a randomized controlled trial. J. Infect. Dis. 202, 1500–1509 10.1086/656776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irungu E., Mugo N., Ngure K. et al. (2013) Immune response to hepatitis B virus vaccination among HIV-1 infected and uninfected adults in Kenya. J. Infect. Dis. 207, 402–410 10.1093/infdis/jis695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jianbo Zhang, Wenbo Luo, Shunying Huang, Weimin Huang and Fengjuan Chen (2015) Related factors of no or weak immune response after inoculation of hepatitis B vaccine. Int. J. Lab. Me. 36, 2172–2174 (In Chinese) [Google Scholar]
- 15.Yin-zhong Chen, Ren-jie Jiang and Jin-jin Shen (2005) Study on the immunization schedule and dose of genetic recombinant hepatitis B vaccine wed for adults. Zhong Guo. Ji Hua Mian Yi 11, 100–105 (In Chinese) [Google Scholar]
- 16.Belloni C., Pistorio A., Tinelli C. et al. (2000) Early immunisation with hepatitis B vaccine: a five-year study. Vaccine 18, 1307–1311 10.1016/S0264-410X(99)00414-4 [DOI] [PubMed] [Google Scholar]
- 17.Bosnak M., Dikiei B., Bosnak V. et al. (2000) Accelerated hepatitis B vaccination schedule in childhood. Pediatr. Int. 44, 663–665 10.1046/j.1442-200X.2002.01621.x [DOI] [PubMed] [Google Scholar]
- 18.Zuckemau J. (2003) The place of accelerated schedules for hepatitis A and B vaccinations. Drugs 63, 1779–1784 10.2165/00003495-200363170-00001 [DOI] [PubMed] [Google Scholar]
- 19.Shengyu Chen, Xuecai Wang, Xiaolian Dong, Haitao Xu, Fadi Wang, Zhifeng Tang et al. (2013) Effects of two immunization schedules of recombinant yeast-derived hepatitis B vaccine for adults. Chin. Prev. Med. 14, 96–98 (In Chinese) [Google Scholar]
- 20.Minjian Guo, Jun Yao and Jing Li (2015) Observation on the immune effect of hepatitis B vaccination by different immunization schedules among adults. Zhejiang Pre. Med. 27, 757–760 (In Chinese) [Google Scholar]
- 21.Jilg W., Schmidt M. and Deinhardt F. (1989) Vaccination against Hepatitis B: comparison of three different vaccination schedules. J. Infect. Dis. 160, 766–769 10.1093/infdis/160.5.766 [DOI] [PubMed] [Google Scholar]
- 22.McMahon B.J., Bruden D.L., Petersen K.M. et al. (2005) Protection after hepatitis B vaccination: results of a 15-year follow-up. Ann. Intern. Med. 142, 333–341 10.7326/0003-4819-142-5-200503010-00008 [DOI] [PubMed] [Google Scholar]
- 23.Mast E.E., Weinbaum C.M., Fiore A.E. et al. (2006) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm. Rep. 55, 1–33 [PubMed] [Google Scholar]
- 24.Kyi K.P., Oo K.M., Htun M.M. et al. (2002) Clinical trial of the intradermal administration of hepatitis B vaccine produced at the Department of Medical Research, Myanmar. Vaccine 20, 1649–1652 10.1016/S0264-410X(01)00468-6 [DOI] [PubMed] [Google Scholar]
- 25.Fabrizi F., Dixit V., Magnini M et al. (2006) Meta-analysis: intradermal vs. intramuscular vaccination against hepatitis B virus in patients with chronic kidney disease. Aliment. Pharmacol. Ther. 24, 497–506 10.1111/j.1365-2036.2006.03002.x [DOI] [PubMed] [Google Scholar]
- 26.Pettit N.N., DePestel D.D., Malani P.N. et al. (2010) Factors associated with seroconversion after standard dose hepatitis B vaccination and high-dose revaccination among HIV-infected patients. HIV Clin. Trials 11, 332–339, 10.1310/hct1105-332 [DOI] [PubMed] [Google Scholar]
- 27.Kai Cu, Jianfang Xu, Jianguo Zhang and Yuemei Hu (2015) Analysis on immune-effects of recombinant yeset hepatitis B vaccine on adults. Jiangsu Yu Fang Yi Xue 26, 22–24 (In Chinese) [Google Scholar]
- 28.André F.E. (1990) Overview of a five year clinical experience with a yeast derived hepatitis B vaccine. Vaccine 8, S74–S78, discussion S79-80 10.1016/0264-410X(90)90222-8 [DOI] [PubMed] [Google Scholar]
- 29.Lum P.J., Ochoa K.C., Hahn J.A. et al. (2003) Hepatitis B virus immunization among young injection drug users in San Francisco, Calif: the UFO Study. Am. J. Public Health 93, 919–923 10.2105/AJPH.93.6.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchou B., Excler J.L., Bourderioux C. et al. (1995) A 3-week hepatitis B vaccination schedule provides rapid and persistent protective immunity: a multicenter, randomized trial comparing accelerated and classic vaccination schedules. J. Infect. Dis. 172, 258–260 10.1093/infdis/172.1.258 [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury A., Santra A., Habibullah C.M. et al. (2005) Immune response to an indigenously developed r-hepatitis B vaccine in mixed population: study of an accelerated vaccination schedule. World J. Gastroenterol. 11, 1037–1039 10.3748/wjg.v11.i7.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah D.P., Grimes C.Z., Nguyen A.T. et al. (2015) Long-term effectiveness of accelerated hepatitis B vaccination schedule in drug users. Am. J. Public Health 105, e36–e43 10.2105/AJPH.2014.302487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froehlich H. and West D.J. (2001) Compliance with hepatitis B virus vaccination in a high-risk population. Ethn. Dis. 11, 548–553 [PubMed] [Google Scholar]