Abstract
Preoperative serum albumin has been considered to be closely correlated with the prognosis of various cancers, including urothelial carcinoma (UC). However, to date, this conclusion remains controversial. The aim of this meta-analysis is to investigate the prognostic significance of preoperative serum albumin in UC. A literature search was performed in PubMed, Web of Science, Embase, and Cochrane Library up to 4 July 2017. Herein, a total of 15506 patients from 23 studies were enrolled in our meta-analysis. Decreased preoperative serum albumin level predicted poor overall survival (OS) (HR = 1.88, 95% CI: 1.44–2.45, P<0.0001), cancer-specific survival (CSS) (HR = 2.03, 95% CI: 1.42–2.90, P=0.0001), recurrence-free survival (HR = 1.85, 95% CI: 1.15–2.97, P=0.01), 30-day complications (30dCs) after surgery (odds ratio (OR) = 1.93, 95% CI: 1.16–3.20, P=0.01), and 90-day mortality after surgery (OR = 4.24, 95% CI: 2.20–8.16, P<0.001). The subgroup analyses indicated that low preoperative serum albumin level is still positively associated with a worse prognosis of UC based on ethnicity, cut-off value, tumor type, analyses type, and sample size. Our meta-analysis indicated that reduced preoperative serum albumin level was a predictor of poor prognosis of UC.
Keywords: Albumin, meta-analysis, prognosis, urothelial carcinoma
Introduction
Urothelial carcinoma (UC), the major histologic type of bladder cancer (BC), is one of the most common and fatal types of genitourinary tract malignancies [1], while upper tract UC (UTUC) makes up only 5–10% of UC with a poor prognosis [2–4]. The standard treatments for UC are radical resection including radical cystectomy (RC) with pelvic lymph node (LN) dissection for BC and radical nephroureterectomy (RNU) coupled with excision of a bladder cuff for UTUC [2,5]. However, radical surgery correlates with a high incidence of early postoperative complications [6,7] and mortality [6,8]. Even worse, tumor recurrence occurs in more than 20% of patients within 10 years of the operation [7,9,10]. Therefore, it is imperative to establish an effective prognostic model to stratify patients and then make a plan for an optimal preoperative management. Presently, postoperative TNM stage and grade are the factors that are most widely used to stratify UC patients, but their accuracy may be unsatisfactory. Additional predictive factors should be explored to solve the intractable clinical problem.
Serum albumin, the main serum protein [11], can be tested to estimate visceral protein function. The normal level of serum albumin for an adult varies from 3.5 to 5.0 g/dl, and the definition of hypoalbuminemia is <3.5 g/dl [12,13]. It has been demonstrated that albumin synthesis can be inhibited by malnutrition and inflammation during the later stages of disease [14,15]. Moreover, inflammation is a key step in the development and progression of cancers [16,17]. In recent years, many studies have indicated that preoperative serum albumin level can serve as an indicator of inflammation [18,19] and is closely related to the prognosis of various types of cancers [20–22]. In particular, the link between the preoperative serum albumin level and the prognosis of UC patients has been widely investigated in many studies [23–27]. Nevertheless, the prognostic significance of preoperative serum albumin level in UC patient remains controversial. For instance, some studies reported that preoperative serum albumin can act as a predictor of the prognosis of UC patients [24,28,29], but the conclusion was different in the other studies [27,30,31]. Hence, we performed a systematic review and meta-analysis to assess the prognostic significance of preoperative serum albumin in UC patients.
Materials and methods
Publication search strategy
A comprehensive literature search was conducted in PubMed, Embase, Web of Science, and Cochrane library up to 4 July 2017. The following terms were used to perform the search: ‘urothelial carcinoma or urothelial cancer or bladder cancer or upper tract urothelial carcinoma or radical cystectomy or radical nephroureterectomy’ and ‘albumin or serum albumin or hypoalbuminemia’ and ‘prognosis or survival or outcome or prognostic’. The search was limited to articles published in English.
Inclusion criteria
The enrolled studies were required to meet the following criteria: (i) the diagnosis of UC patients was histopathologically validated; (ii) preoperative serum albumin was measured and the correlation with prognosis of UC patients was analyzed; and (iii) the full text of publications should be available in order to access the data. The exclusion criteria were as follows: (i) reviews, case reports, animal research, letters, and meeting abstracts; and (ii) the same institution or authors between articles, as this may result in duplicate data.
Data extraction
The eligible relevant data were extracted from the included articles by two independent investigators. Any disagreements encountered during data extraction were resolved through a consensus. The extracted data included the author, year, number of cases, sex, the use of neoadjuvant chemotherapy, follow-up duration, cut-off value, and the end points. We were interested in the overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS), 30-day complication (30dC), and 90-day mortality (90dM).
If data from multivariable and univariate analyses were both available in the publications, the former was chosen. Survival data were extracted by applying Engauge Digitizer (version 4.1) if studies only included Kaplan–Meier curves. The HRs and their 95% CIs for prognosis were estimated according to the Tierney et al. [32] methods.
Quality assessment
The quality of included articles was evaluated independently by two investigators using the Newcastle–Ottawa Scale (NOS), in which the scores ranged from 0 to 9 [33]. In present meta-analysis, a study was regarded as high quality if it obtained 6 or more points.
Statistical analysis
This meta-analysis was carried out using Review sManager 5.0 (Cochrane Collaboration, Oxford, U.K.) and Stata SE12.0 (StataCorp, College Station, TX). The association between preoperative serum albumin and survival outcomes of UC patients was described by HRs with 95% CIs. In addition, odds ratios (ORs) with 95% CIs were used for the description of the relationship between early postoperative outcomes and preoperative serum albumin. Chi-square-based Q and I2 tests were used to estimate the heterogeneity amongst the studies. In the present study, I2 > 50% and P<0.05 indicate that a significant statistical heterogeneity exists. A random effects model was applied to pool the data if heterogeneity was significant. On the contrary, a fixed effects model was used when there is no significant heterogeneity.
Subgroup analysis, meta-regression, and sensitivity analysis were conducted to explore the possible source of heterogeneity, while also determining whether our pooled analyses were robust. The subgroup analysis and meta-regression were performed according to tumor type, ethnicity, analysis type, cut-off value, and sample size, and the sensitivity analysis was performed by omitting a single study in each step.
Publication bias
Begg’s and the Egger’s tests [34] were used to test the publication bias for the outcomes mentioned in the least included studies. Significant publication bias was considered to exist if the funnel plot was asymmetric and the P-values in Egger’s or Begg’s test are less than 0.05.
Results
Search results and characteristics of eligible articles
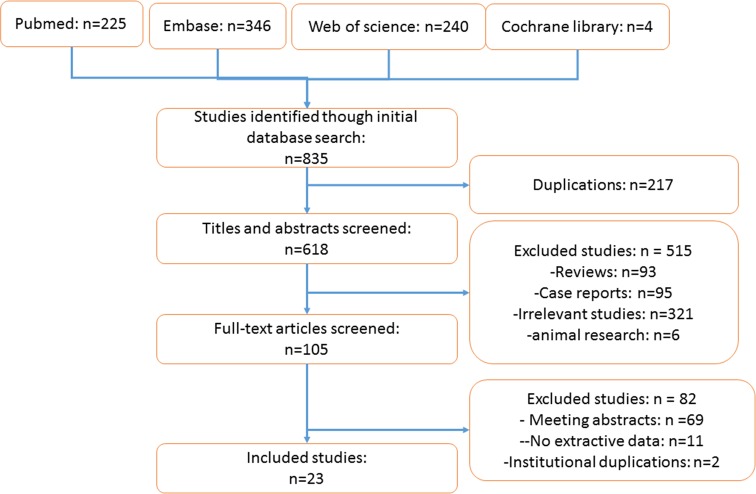
The publication search found 835 potentially relevant articles, but only 23 eligible studies with 15506 patients were finally included in this meta-analysis. The study selection process is shown in Figure 1.
Figure 1. The study flow of study selection process.
The detailed information on the eligible articles is presented in Table 1. The main characteristics that we were interested in included country, tumor type, case number, age, sex, the use of neoadjuvant chemotherapy, follow-up duration, and cut-off value. The 23 studies used for the meta-analysis included 4 from Japan, 4 from China, 2 from Korea, 9 from the U.S.A., 1 from Britain, 1 from France, 1 from Turkey, 1 from Egypt, and 1 from Canada. Amongst the studies, 18 analyzed the prognostic value of preoperative serum albumin in participants with BC, 5 with UTUC, and 1 with both BC and UTUC. Meanwhile, the relationship between preoperative serum albumin and OS was assessed in 12 studies; CSS was analyzed in 9 studies; RFS was reported in 6 studies; 30dCs after surgery were evaluated in 7 studies; and 90dM after surgery was reported in 6 studies (Table 2). HRs were directly presented in 15 studies, and there were 2 articles only providing Kaplan–Meier curves, which could be used to estimate HRs. In addition, the quality of the included studies was assessed according to the NOS, and the scores ranged from 5 to 7, with a mean of 6.5, indicating that the included studies had moderate to high quality (Table 3).
Table 1. Main characteristics of the included studies.
| Study | Country | Tumor type | Case number (LSA/HSA) | Age (years) | Sex (M/F) | Neoadjuvant chemotherapy (LSA/HSA) | Surgical treatment | Follow-up (months) | Survival analysis | Cut-off value |
|---|---|---|---|---|---|---|---|---|---|---|
| Caras et al. (2017) [23] | U.S.A. | BC and UTUC | 1292/4282 | 65 | 4228/1340 | NA | RC or TURBT | 1 | NA | 3.5 g/dl |
| Chan et al. (2013) [35] | China | BC | 62/55 | 68 ± 10 | 99/18 | NA | RC | 31 ± 29 | CSS, OCS, OS | 3.9 g/l |
| Cho et al. (2014) [30] | Korea | UTUC | 35/112 | 70 | 40/106 | NA | RNU | 33 | RFS | 3.5 g/dl |
| Cui et al. (2017) [24] | China | UTUC | 54/36 | 65.66 | 107/62 | NA | RNU | 53.7 | OS, CSS | 4.37 g/l |
| Djaladat et al. (2014) [28] | U.S.A. | BC | 197/1274 | 67 | 1154/317 | 15/92 | RC | 148.8 | OS, RFS | 3.5 g/dl |
| Fujita et al. (2015) [36] | Japan | UTUC | NA | 70 | 221/85 | NA | RNU | 41 | RFS, CSS | NA |
| Garg et al. (2014) [37] | U.S.A. | BC | 150/947 | 68 | 831/266 | 19/109 | RC | 25.2 | NA | 4.0 g/dl |
| Hinata et al. (2015) [31] | Japan | BC | NA | 68.6 | 575/155 | NA | RC | 52 | OS, RFS | 3.5 g/l |
| Huang et al. (2017) [25] | China | UTUC | 17/408 | 67 | 279/146 | NA | RUN | 38.5 | OS, CSS | 3.5 g/l |
| Johnson et al. (2015) [38] | U.S.A. | BC | 102/587 | 73 | 530/159 | NA | RC | 1 | NA | 3.5 g/dl |
| Kluth et al. (2014) [39] | U.S.A. | UTUC | NA | 70 | 175/67 | NA | RNU | 9 | CSS | 3.7 g/dl |
| Krane et al. (2013) [29] | U.S.A. | BC | NA | 67.4 | 55/13 | NA | RC | 25 | OS, CSS | 3.5 g/dl |
| Ku et al. (2015) [40] | Korea | BC | NA | 65.1 | 362 /57 | NA | RC | 37.7 | OS, CSS | 3.5 g/dl |
| Lambert et al. (2013) [41] | Britain | BC | 31/156 | 67.4 | 153/34 | 29/6 | RC | 26.2 | OS, CSS | 3.5 g/dl |
| Laurent et al. (2017) [26] | France | BC | 95/98 | 75.2 | 164/29 | NA | RC | 9.1 | OS | 3.5 g/dl |
| Lavallee et al. (2014) [42] | Canada | BC | 341/1090 | 70 | 1819/484 | NA | RC | NA | NA | 3.7 g/dl |
| Liu et al. (2016) [43] | China | BC | 129/167 | 61.71 | 250/45 | NA | RC | 72 | RFS, CSS | 4.0 g/dl |
| Morgan et al. (2011) [44] | U.S.A. | BC | 30/139 | 78.8 | 122/47 | NA | RC | 3 | NA | 3.7 g/dl |
| Mursi et al. (2013) [45] | Egypt | BC | 24/7 | 58.4 | 22/9 | NA | RC | 3 | NA | 3.5 g/dl |
| Nakagawa et al. (2017) [27] | Japan | BC | NA | 69 | 248/58 | NA | RC | 6.8 | OS | 3.5 g/dl |
| Sharma et al. (2016) [46] | U.S.A. | BC | NA | 70.1 | 209/65 | NA | RC | NA | NA | 4.0 g/dl |
| Sheth et al. (2016) [47] | U.S.A. | UTUC | NA | 71 | 77/24 | NA | RNU or partial ureterectomy | 18.5 | RFS, OS | 4.0 g/dl |
| Taguchi et al. (2016) [48] | Japan | UC | NA | 68 | 160/40 | NA | RNU and RC | 12 | OS | 3.5 g/dl |
Abbreviations: HSA, high serum albumin; LSA, low serum albumin; NA, not available; TURBT, transurethral resection of bladder tumor.
Table 2. The interest outcomes extracted from included studies.
| Study | HR | HR | HR | OR | OR |
|---|---|---|---|---|---|
| (95% CI) for OS | (95% CI) for CSS | (95% CI) for RFS | (95% CI) for 30dC | (95% CI) for 90dM | |
| Caras et al. (2017) | NA | NA | NA | 3.14 (2.86, 3.45) (overall morbidity)* | 7.66 (5.80, 10.12) (overall morbidity)* |
| 1.85 (1.41, 2.44) (RC)* | 1.71 (0.85, 3.45) (RC)* | ||||
| 4.32 (3.47, 5.39) (TURBT)* | 9.89 (6.05, 16.16)(TURBT)* | ||||
| Chan et al. (2013) | NA | 1.79 (0.78, 4.08)* | NA | NA | NA |
| Cho et al. (2014) | 0.60 (0.26–1.39) | NA | 2.88 (1.80–4.62) | NA | NA |
| Cui et al. (2017) | 5.509 (2.144–14.158) | 5.521 (2.074–14.697) | NA | NA | NA |
| Djaladat et al. (2014) | 1.93 (1.43–2.63) | NA | 1.68 (1.16–2.43) | 1.41 (0.98–2.02) | 2.42 (1.31, 4.45)* |
| Fujita et al. (2015) | 2.63 (1.149, 6.02) | 2.63 (5.882, 1.149) | |||
| Garg et al. (2014) | NA | 1.68 (1.17, 2.41) | 3.03 (7.143, 1.33) | ||
| Hinata et al. (2015) | 1.062 (0.643–1.703) | NA | 1.077 (0.654–1.718) | NA | NA |
| Huang et al. (2017) | 1.96 (0.96–4.01) | 2.51 (1.22–5.18) | NA | NA | NA |
| Johnson et al. (2015) | NA | NA | NA | 1.79 (1.06, 3.03) | NA |
| Kluth et al. (2014) | NA | 1.754 (1.2987, 2.326) | NA | NA | NA |
| Krane et al. (2013) | 4.96 (2.18–11.67) | 8.10 (2.63–27.59) | NA | NA | NA |
| Ku et al. (2015) | 1.670 (1.007–2.767) | 1.794 (1.010–3.187) | NA | NA | NA |
| Lambert et al. (2013) | 1.76 (1.03, 2.12)* | 1.57 (1.24, 1.90)* | NA | 3.9 (1.3–12.2) | 22.96 (2.47, 213.36)* |
| Laurent et al. (2017) | 3.06 (1.81–5.17) | NA | NA | NA | NA |
| Lavallee et al. (2014) | NA | NA | NA | 1.16 (1.06–1.26) | NA |
| Liu et al. (2016) | NA | 0.979 (0.880–1.089) | 0.998 (0.908–1.096) | NA | NA |
| Morgan et al. (2011) | NA | NA | NA | NA | 2.50 (1.40–4.45) |
| Mursi et al. (2013) | NA | NA | NA | NA | 9.20 (0.69, 122.38)* |
| Nakagawa et al. (2017) | 1.51 (0.95–2.41) | NA | NA | NA | NA |
| Sharma et al. (2016) | NA | NA | NA | 2.27 (5.56, 0.94) | NA |
| Sheth et al. (2016) | 3.37 (1.43–7.92) | NA | 4.4 (2.04–9.30) | NA | NA |
| Taguchi et al. (2016) | 1.345 (0.969–1.855) | NA | NA | NA | NA |
Abbreviation: NA, not available; TURBT, transurethral resection of bladder tumor.
*Data extracted indirectly.
Table 3. The NOS quality assessment of the included studies.
| Study ID | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of the study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | ||
| Caras et al. (2017) | ★ | ★ | ★ | ★ | ★☆ | ☆ | ★ | ★ | 7 |
| Chan et al. (2013) | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 6 |
| Cho et al. (2014) | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 7 |
| Cui et al. (2017) | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 7 |
| Djaladat et al. (2014) | ★ | ★ | ☆ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Fujita et al. (2015) | ★ | ★ | ☆ | ★ | ★☆ | ★ | ★ | ☆ | 6 |
| Garg et al. (2014) | ★ | ★ | ☆ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Hinata et al. (2015) | ★ | ☆ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Huang et al. (2017) | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Johnson et al. (2015) | ★ | ☆ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Kluth et al. (2014) | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 7 |
| Krane et al. (2013) | ☆ | ☆ | ★ | ★ | ★☆ | ★ | ★ | ★ | 6 |
| Ku et al. (2015) | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 7 |
| Lambert et al. (2013) | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Laurent et al. (2017) | ★ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ★ | 7 |
| Lavallee et al. (2014) | ★ | ★ | ☆ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Liu et al. (2016) | ☆ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ★ | 6 |
| Morgan et al. (2011) | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Mursi et al. (2013) | ☆ | ★ | ☆ | ★ | ★☆ | ★ | ☆ | ★ | 5 |
| Nakagawa et al. (2017) | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 7 |
| Sharma et al. (2016) | ☆ | ★ | ☆ | ★ | ★☆ | ★ | ★ | ★ | 6 |
| Sheth et al. (2016) | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Taguchi et al. (2016) | ★ | ★ | ☆ | ★ | ★☆ | ★ | ★ | ★ | 7 |
★ indicates that a score (1) was assigned; ☆ indicates a score of zero.
Preoperative serum albumin level and the survival of UC patients
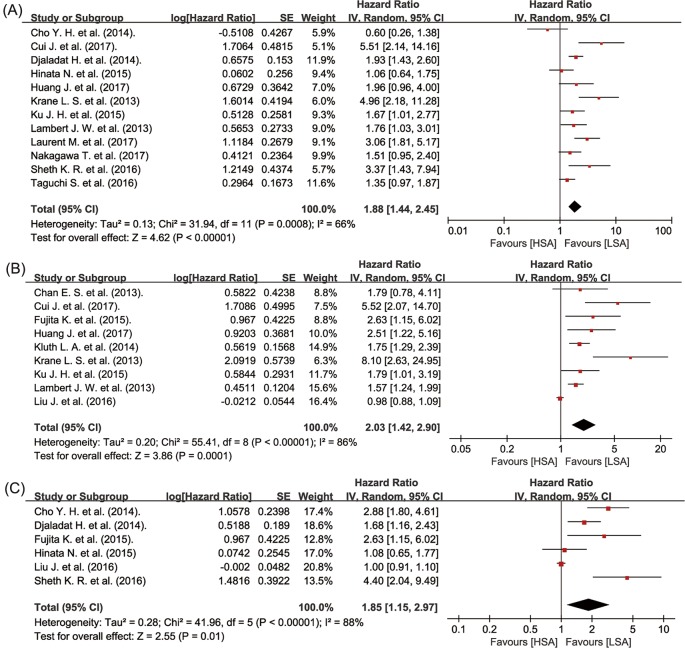
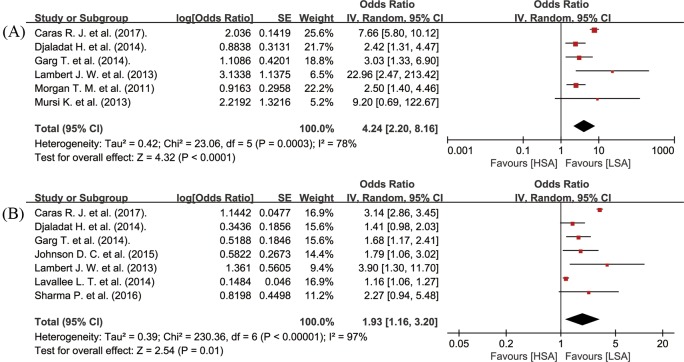
The UC patients with decreased preoperative serum albumin level suffered from significantly worse OS (random-effects model; HR = 1.88, 95% CI: 1.44–2.45, P<0.00001; I2 = 66%, P=0.0008) (Figure 2A), CSS (random-effects model; HR = 2.03, 95% CI: 1.42–2.90, P=0.0001; I2 = 86%, P<0.00001) (Figure 2B), and RFS (random-effects model; HR = 1.85, 95% CI: 1.15–2.97, P=0.01; I2 = 88%, P<0.00001) (Figure 2C). With respect to short-term outcomes after surgery, low preoperative serum albumin level was significantly related to a lower 90dM after surgery (random-effects model; OR = 4.24, 95% CI: 2.20–8.16, P<0.0001; I2 = 78%, P=0.0003) (Figure 3A), and a lower rate of 30dCs after surgery (random-effects model; OR = 1.93, 95% CI: 1.16–3.20, P=0.01; I2 = 97%, PP<0.00001) (Figure 3B).
Figure 2. Meta-analysis of preoperative serum albumin level and OS (A), CSS (B), and RFS (C) in UC patients.
Abbreviations: LSA, low level of preoperative serum albumin; HSA, high level of preoperative serum albumin.
Figure 3. Meta-analysis of preoperative serum albumin level at 30dC (A) and 90dM (B) in UC patients.
The analysis of potential sources of heterogeneity
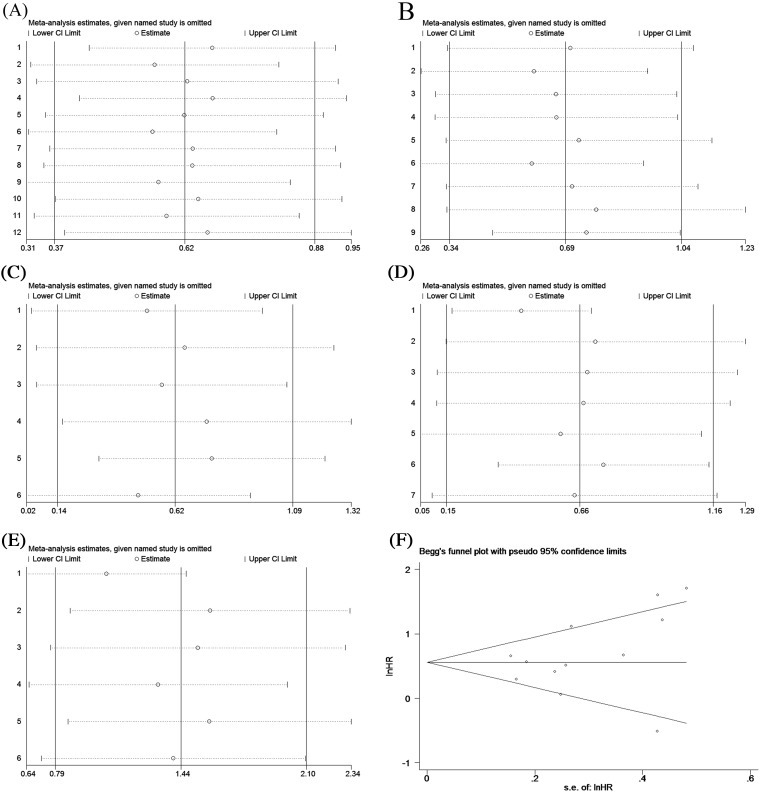
To explore the potential sources of heterogeneity amongst the studies, the subgroup analysis, sensitivity analysis, and meta-regression analysis were performed in our meta-analysis. The subgroup analyses were conducted to evaluate the prognostic values of preoperative serum albumin according to ethnicity, cut-off value, tumor type, analysis type, and sample size. Our results showed that the significant heterogeneity still existed in all the subgroups, indicating that these factors might not be the sources of heterogeneity in our meta-analysis. In addition, except for RFS, the pooled results of the other outcomes in subgroup analyses did not fluctuate significantly, demonstrating the robustness of our pooled analyses. The detailed results of all the subgroup analyses are presented in Table 4. The sensitivity analyses were conducted by omitting a single study step by step and the results showed that no single study exerted significant influence on the pooled results of the outcomes, also indicating that our findings were stable (Figure 4). Next, we performed the meta-regression analysis to further explore the potential sources of heterogeneity based on five covariates, including tumor type (UTUC compared with BC), ethnicity (Asian compared with non-Asian), analysis type (univariate compared with multivariate), cut-off value (3.5 g/dl compared with others), and sample size (>600 compared with ≤600). The results indicated that tumor type accounted for most heterogeneity of the pooled HR or OR of RFS (Coef. = 0.27, 95% CI: 0.18–0.79, P=0.02), 30dC (Coef. = 0.48, 95% CI: 0.25–0.92, P=0.03), and 90dM (Coef. = 0.36, 95% CI: 0.18–0.74, P=0.02), and explained 84.61, 69.5, and 100% between-study variance in RFS, 30dC, and 90dM, respectively. The other covariates could not clarify the heterogeneity, and the details of meta-regression were presented in Table 5.
Table 4. Subgroup analysis of preoperative serum albumin and the prognosis of UC patients.
| Variables | Outcome | Studies | Patients | HR (95% CI) | P-value | Model | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | |||||||
| Tumor type | ||||||||
| UTUC | OS | 4 | 841 | 2.03 (1.34, 3.07) | <0.01 | Random | 78 | <0.01 |
| CSS | 6 | 900 | 1.69 (1.16, 2.46) | <0.01 | Random | 87 | <0.01 | |
| RFS | 3 | 553 | 3.11 (2.17, 4.46) | <0.01 | Fixed | 0 | 0.59 | |
| 30dC | 1 | 5735 | 3.14 (2.86, 3.45) | - | - | - | - | |
| 90dM | 1 | 2669 | 7.66 (5.80, 10.12) | - | - | - | - | |
| BC | OS | 8 | 3288 | 1.80 (1.38, 2.33) | <0.01 | Random | 60 | 0.01 |
| CSS | 6 | 1328 | 1.69 (1.16, 2.46) | <0.01 | Random | 87 | <0.01 | |
| RFS | 3 | 2210 | 1.19 (0.85, 1.68) | 0.32 | Random | 72 | 0.03 | |
| 30dC | 6 | 5568 | 1.55 (1.19, 2.02) | <0.01 | Random | 62 | 0.02 | |
| 90dM | 5 | 5568 | 2.87 (1.89, 4.36) | <0.01 | Fixed | 14 | 0.33 | |
| Ethnicity | ||||||||
| Asian | OS | 6 | 1665 | 1.59 (1.09, 2.32) | 0.02 | Random | 62 | 0.02 |
| CSS | 6 | 1731 | 2.03 (1.17, 3.54) | 0.01 | Random | 82 | <0.01 | |
| RFS | 4 | 1477 | 1.60 (0.90, 2.86) | 0.11 | Random | 87 | <0.01 | |
| Non-Asian | OS | 6 | 2271 | 2.20 (1.50, 3.23) | <0.01 | Random | 67 | 0.01 |
| CSS | 3 | 497 | 2.02 (1.28, 3.19) | <0.01 | Random | 75 | 0.02 | |
| RFS | 2 | 1286 | 2.56 (1.00, 6.52) | 0.05 | Random | 80 | 0.03 | |
| Analysis type | ||||||||
| Univariate | OS | 10 | 3859 | 1.70 (1.31, 2.20) | <0.01 | Random | 62 | <0.01 |
| CSS | 4 | 953 | 2.25 (1.46, 3.47) | <0.01 | Fixed | 43 | 0.15 | |
| RFS | 2 | 247 | 1.64 (0.23, 11.53) | 0.62 | Random | 92 | <0.01 | |
| 30dC | 2 | 6939 | 2.35 (1.43, 3.87) | <0.01 | Random | 82 | <0.01 | |
| 90dM | 5 | 7140 | 4.64 (2.15, 10.03) | <0.01 | Random | 82 | <0.01 | |
| Multivariate | OS | 2 | 270 | 4.21 (2.23, 7.94) | <0.01 | Fixed | 0 | 0.45 |
| CSS | 5 | 1275 | 1.81 (1.14, 2.87) | 0.01 | Random | 88 | <0.01 | |
| RFS | 4 | 2516 | 1.33 (0.91, 1.94) | 0.13 | Random | 75 | <0.01 | |
| 30dC | 5 | 4364 | 1.46 (1.06, 2.00) | 0.02 | Random | 62 | 0.05 | |
| 90dM | 1 | 1097 | 3.03 (1.33, 6.90) | - | - | - | - | |
| Cut-off value | ||||||||
| =3.5 g/dl | OS | 10 | 3859 | 1.70 (1.31, 2.20) | <0.01 | Random | 62 | <0.01 |
| CSS | 5 | 1216 | 2.11 (1.40, 3.17) | <0.01 | Random | 55 | 0.06 | |
| RFS | 3 | 2061 | 1.74 (1.04, 2.91) | 0.03 | Random | 75 | 0.02 | |
| 30dC | 5 | 8726 | 2.09 (1.35, 3.25) | <0.01 | Random | 87 | <0.01 | |
| 90dM | 5 | 8068 | 4.92 (2.35, 10.31) | <0.01 | Random | 74 | <0.01 | |
| Others | OS | 2 | 270 | 4.21 (2.23, 7.94) | <0.01 | Fixed | 0 | 0.45 |
| CSS | 4 | 1012 | 1.92 (1.06, 3.47) | 0.03 | Random | 89 | <0.01 | |
| RFS | 3 | 702 | 2.14 (0.77, 5.97) | 0.15 | Random | 90 | <0.01 | |
| 30dC | 2 | 2577 | 1.40 (0.78, 2.52) | 0.27 | Random | 55 | 0.14 | |
| 90dM | 1 | 169 | 2.50 (1.40, 4.46) | - | - | - | - | |
| Sample size | ||||||||
| n>600 | OS | 2 | 1915 | 1.48 (0.83, 2.65) | 0.18 | Random | 75 | 0.05 |
| RFS | 4 | 1915 | 2.30 (1.02, 5.18) | 0.13 | Random | 49 | 0.16 | |
| 30dC | 5 | 10842 | 1.73 (0.98, 3.08) | 0.06 | Random | 98 | <0.01 | |
| 90dM | 3 | 387 | 4.01 (1.72, 9.32) | <0.01 | Random | 86 | <0.01 | |
| n<600 | OS | 10 | 2214 | 2.02 (1.45, 2.81) | <0.01 | Random | 67 | <0.01 |
| RFS | 2 | 848 | 1.39 (0.90, 2.14) | 0.05 | Random | 92 | <0.01 | |
| 30dC | 2 | 461 | 2.81 (1.41, 5.58) | <0.01 | Fixed | 0 | 0.45 | |
| 90dM | 3 | 7850 | 5.68 (1.32, 24.41) | 0.02 | Random | 53 | 0.12 | |
Figure 4. Sensitivity analyses for OS (A), CSS (B), RFS (C), 30dC (D), 90dM (E), and the Begg’s and Egger’s test results for UC patients’ OS (F).
Table 5. Assessment of potential sources of heterogeneity amongst studies by meta-regression.
| Covariates | Outcomes | P-value | Regression coefficient (95% CI) |
|---|---|---|---|
| Tumor type (UTUC compared with BC) | |||
| OS | 0.82 | 0.92 (0.36–2.37) | |
| CSS | 0.15 | 0.52 (0.20–1.35) | |
| RFS | 0.02 | 0.27 (0.18–0.79) | |
| 30dC | 0.03 | 0.48 (0.25–0.92) | |
| 90dM | 0.02 | 0.36 (0.18–0.74) | |
| Ethnicity (Asian compared with non-Asian) | |||
| OS | 0.10 | 1.68 (0.90–3.14) | |
| CSS | 0.81 | 1.11 (0.39–3.16) | |
| RFS | 0.43 | 1.58 (0.37–6.80) | |
| Analysis type (univariate compared with multivariate) | |||
| OS | 0.74 | 0.88 (0.36–2.10) | |
| CSS | 0.54 | 0.77 (0.29–2.04) | |
| RFS | 0.05 | 0.39 (0.19–1.02) | |
| 30dC | 0.16 | 0.62 (0.30–1.30) | |
| 90dM | 0.66 | 0.66 (0.06–7.13) | |
| Cut-off value (3.5 g/dl compared with others) | |||
| OS | 0.08 | 2.50 (0.89–7.01) | |
| CSS | 0.72 | 0.86 (0.32–2.30) | |
| RFS | 0.78 | 1.18 (0.27–5.22) | |
| 30dC | 0.32 | 0.64 (0.21–1.92) | |
| 90dM | 0.42 | 0.52 (0.07–4.04) | |
| Sample size (>600 compared with ≤600) | |||
| OS | 0.46 | 1.38 (0.54–3.49) | |
| RFS | 0.40 | 1.63 (0.38–6.97) | |
| 30dC | 0.35 | 1.64 (0.47–5.72) | |
| 90dM | 0.82 | 1.20 (0.16–9.20) |
Publication bias
As Figure 4F shows, the funnel plots for OS were almost symmetrical, and the P-values of Begg’s and Egger’s tests were 0.170 and 0.266, respectively, which suggested that there was no significant publication bias in our meta-analysis.
Discussion
Numerous articles have reported that pretreatment serum albumin is correlated with the prognosis of UC patients, but the conclusions amongst studies remain inconsistent [23–31,35–48]. Therefore, we combined 23 studies with 15506 patients to perform this meta-analysis to evaluate the prognostic role of preoperative serum albumin in UC patients [23–31,35–48]. Our results demonstrated that low levels of preoperative serum albumin are significantly associated with worse OS, CSS, RFS, complications, and early mortality. In spite of significant heterogeneity, subgroup analyses conducted according to ethnicity, tumor type, analysis type, cut-off value, and sample size showed that our pooled results did not alter significantly, which indicated the robustness of the pooled results. Generally, all these findings suggested that preoperative serum albumin level played an important prognostic role in UC patients.
Although it has been demonstrated that preoperative serum albumin is closely correlated with the prognosis of UC and other cancers [20–22,24,29], the latent mechanisms remain complex and unclear. However, it is widely recognized that malnutrition and inflammation may be partly responsible for the mechanisms [49,50]. Malnutrition, partly mirrored by hypoalbuminemia, is a severe problem in cancer patients, due to a variety of mechanisms, which involve anticancer therapies and the host response to the tumor [51]. Furthermore, many unfavorable clinical consequences are related to malnutrition, including a deteriorated quality of life, an enhanced risk of chemotherapy-induced toxicity, and poor long-term survival [52]. In addition, inflammation, which is a critical step in cancer initiation and progression [16,53], can alter the levels of serum albumin [15]. Under inflammatory conditions, immune cells and tumor cells release various inflammatory mediators, including interleukin-1β, interleukin-6, and tumor necrosis factor (TNF), which can suppress albumin synthesis in liver cells [53–55]. Moreover, albumin might directly be lost from the circulatory system, since TNF can increase the permeability of capillaries [55]. In addition, previous studies have also demonstrated that serum albumin is associated with several anticancer mechanisms, including its antioxidant function [56]. Therefore, preoperative serum albumin could serve as a good predictor of the prognosis of cancers.
Serum albumin is a low cost and easily accessible predictor for UC patients, but it still does have some limitations for clinical implications. For instance, under overhydrated conditions or other disease processes, hypoalbuminemia may not indicate malnutrition [57,58], so its prognostic value in UC patients will be reduced. In addition, diet and other non-tumor-related factors can also affect the levels of serum albumin. To some degree, those factors mentioned above may explain the significant heterogeneities in our meta-analysis. Actually, to predict the prognosis of UC patients more precisely, several predictive factors involving serum albumin have been studied for clinical practice. For instance, Liu et al. [43] indicated that the albumin/globulin ratio calculated from preoperative serum albumin and globulin levels can act as an independent predictor of long-term RFS and CSS in bladder UC. Additionally, Cui et al. [24] recently reported that a predictive model based on preoperative plasma fibrinogen and serum albumin level, also known as an FA score, can be used to predict OS and CSS in UTUC. Regardless, our meta-analysis demonstrated that preoperative serum albumin plays a significant prognostic role in UC patients.
To the best of our knowledge, the present study is the first to systematically analyze the predictive value of preoperative serum albumin for prognosis of UC patients. However, the results of our meta-analysis may be challenged by some limitations. First, most of the included studies were retrospective and thus may have bias in patient selection and data analysis. Second, the cutoffs of low preoperative serum albumin were not consistent amongst the included studies. Other inconsistencies amongst the various studies included the follow-up period and the base characteristics of patients, which may cause significant heterogeneities and thus affect the robustness of the pooled analysis. In summary, heterogeneity may be the biggest limitation of our meta-analysis. Therefore, the value of preoperative serum albumin as a prognostic predictor in UC patients still requires further investigation in the future.
Conclusion
In conclusion, this meta-analysis indicated that preoperative serum albumin is a useful predictor for the prognosis of patients with UC. The patients with decreased preoperative serum albumin have more unfavorable long-term survival and short-term outcomes. Considering the limitations in present meta-analyses, further homogeneous prospective studies are needed to confirm our findings.
Acknowledgments
We thank American Journal Experts (AJE) to help us edit our manuscript.
Abbreviations
- BC
bladder cancer
- CI
confidence interval
- CSS
cancer-specific survival
- HR
hazard ratio
- NOS
Newcastle–Ottawa scale
- OR
odds ratio
- OS
overall survival
- RC
radical cystectomy
- RFS
recurrence-free survival
- RNU
radical nephroureterectomy
- TNF
tumor necrosis factor
- UC
urothelial carcinoma
- UTUC
upper tract UC
- 30dC
30-day complication
- 90dM
90-day mortality
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Youth science and technology foundation of Gansu Province (1606RJYA286),the National Natural Science Foundation (31360508), the Health industry Research Project of Gansu Province (GSWSKY2018-04), and the Health industry Research Project of Gansu Province (GSWSKY2017-16).
Author contribution
Jing Liu and Fang Wang extracted data and writed this paper. Shaohong Li and Wenhui Huang searched for the eligible studies. Yanjuan Jia performed the statistical analysis. Chaojun Wei designed this work.
References
- 1.Van Batavia J., et al. (2014) Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 16, 982–991, 1-5 10.1038/ncb3038 [DOI] [PubMed] [Google Scholar]
- 2.Roupret M., et al. (2015) European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur. Urol. 68, 868–879 10.1016/j.eururo.2015.06.044 [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L. and Miller K.D. Jemal A. (2016) Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 4.Lughezzani G., et al. (2012) Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur. Urol. 62, 100–114 10.1016/j.eururo.2012.02.030 [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M., et al. (2017) EAU Guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur. Urol. 71, 447–461 10.1016/j.eururo.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 6.Shabsigh A., et al. (2009) Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur. Urol. 55, 164–174 10.1016/j.eururo.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 7.Stein J.P., et al. (2001) Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 10.1200/JCO.2001.19.3.666 [DOI] [PubMed] [Google Scholar]
- 8.Chahal R., et al. (2003) A study of the morbidity, mortality and long-term survival following radical cystectomy and radical radiotherapy in the treatment of invasive bladder cancer in Yorkshire. Eur. Urol. 43, 246–257 10.1016/S0302-2838(02)00581-X [DOI] [PubMed] [Google Scholar]
- 9.Yin M., et al. (2016) Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist 21, 708–715 10.1634/theoncologist.2015-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shariat S.F., et al. (2010) Statistical consideration for clinical biomarker research in bladder cancer. Urol. Oncol. 28, 389–400 10.1016/j.urolonc.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margarson M.P. and Soni N. (1998) Serum albumin: touchstone or totem? Anaesthesia 53, 789–803 10.1046/j.1365-2044.1998.00438.x [DOI] [PubMed] [Google Scholar]
- 12.Di Fiore F., et al. (2007) Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am. J. Gastroenterol. 102, 2557–2563 10.1111/j.1572-0241.2007.01437.x [DOI] [PubMed] [Google Scholar]
- 13.Ishizuka M., et al. (2007) Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann. Surg. 246, 1047–1051 10.1097/SLA.0b013e3181454171 [DOI] [PubMed] [Google Scholar]
- 14.Yeun J.Y. and Kaysen G.A. (1998) Factors influencing serum albumin in dialysis patients. Am. J. Kidney Dis. 32 (6 Suppl. 4), S118–S125 10.1016/S0272-6386(98)70174-X [DOI] [PubMed] [Google Scholar]
- 15.Ballmer P.E. and Ochsenbein A.F. Schutz-Hofmann S. (1994) Transcapillary escape rate of albumin positively correlates with plasma albumin concentration in acute but not in chronic inflammatory disease. Metabolism 43, 697–705 10.1016/0026-0495(94)90117-1 [DOI] [PubMed] [Google Scholar]
- 16.Coussens L.M. and Werb Z. (2002) Inflammation and cancer. Nature 420, 860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Prete A., et al. (2011) Molecular pathways in cancer-related inflammation. Biochem. Med. (Zagreb) 21, 264–275 10.11613/BM.2011.036 [DOI] [PubMed] [Google Scholar]
- 18.Gabay C. and Kushner I. (1999) Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 19.McMillan D.C. (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc. Nutr. Soc. 67, 257–262 10.1017/S0029665108007131 [DOI] [PubMed] [Google Scholar]
- 20.Lai C.C., et al. (2011) Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int. J. Colorectal Dis. 26, 473–481 10.1007/s00384-010-1113-4 [DOI] [PubMed] [Google Scholar]
- 21.Seebacher V., et al. (2013) Does thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trial. Br. J. Cancer 109, 215–218 10.1038/bjc.2013.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanriverdi O., et al. (2015) Pretreatment serum albumin level is an independent prognostic factor in patients with stage IIIB non-small cell lung cancer: a study of the Turkish descriptive oncological researches group. Asian Pac. J. Cancer Prev. 16, 5971–5976 10.7314/APJCP.2015.16.14.5971 [DOI] [PubMed] [Google Scholar]
- 23.Caras R.J., et al. (2017) Preoperative albumin is predictive of early postoperative morbidity and mortality in common urologic oncologic surgeries. Clin. Genitourin. Cancer 15, e255–e262 10.1016/j.clgc.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Cui J., et al. (2017) Prognostic scores based on the preoperative plasma fibrinogen and serum albumin level as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Oncotarget 8, 68964–68973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J., et al. (2017) Preoperative serum pre-albumin as an independent prognostic indicator in patients with localized upper tract urothelial carcinoma after radical nephroureterectomy. Oncotarget 8, 36772–36779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent M., et al. (2017) Early chemotherapy discontinuation and mortality in older patients with metastatic bladder cancer: the AGEVIM multicenter cohort study. Urol. Oncol. 35, 34e9–34e16 10.1016/j.urolonc.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T., et al. (2017) Nomogram for predicting survival of postcystectomy recurrent urothelial carcinoma of the bladder. Urol. Oncol. 35, 457.e15–457.e21 10.1016/j.urolonc.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 28.Djaladat H., et al. (2014) The association of preoperative serum albumin level and American Society of Anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int. 113, 887–893 10.1111/bju.12240 [DOI] [PubMed] [Google Scholar]
- 29.Krane L.S., et al. (2013) Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J. Endourol. 27, 1046–1050 10.1089/end.2012.0606 [DOI] [PubMed] [Google Scholar]
- 30.Cho Y.H., et al. (2014) Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: an inflammation-based prognostic score. Korean J. Urol. 55, 453–459 10.4111/kju.2014.55.7.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinata N., et al. (2015) Performance status as a significant prognostic predictor in patients with urothelial carcinoma of the bladder who underwent radical cystectomy. Int. J. Urol. 22, 742–746 10.1111/iju.12804 [DOI] [PubMed] [Google Scholar]
- 32.Tierney J.F., et al. (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 34.Egger M., et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan E.S., et al. (2013) Age, tumour stage, and preoperative serum albumin level are independent predictors of mortality after radical cystectomy for treatment of bladder cancer in Hong Kong Chinese. Hong Kong Med. J. 19, 400–406 [DOI] [PubMed] [Google Scholar]
- 36.Fujita K., et al. (2015) Preoperative risk stratification for cancer-specific survival of patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int. J. Clin. Oncol. 20, 156–163 10.1007/s10147-014-0695-1 [DOI] [PubMed] [Google Scholar]
- 37.Garg T., et al. (2014) Preoperative serum albumin is associated with mortality and complications after radical cystectomy. BJU Int. 113, 918–923 10.1111/bju.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson D.C., et al. (2015) Nutritional predictors of complications following radical cystectomy. World J. Urol. 33, 1129–1137 10.1007/s00345-014-1409-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kluth L.A., et al. (2014) Predictors of survival in patients with disease recurrence after radical nephroureterectomy. BJU Int. 113, 911–917 10.1111/bju.12369 [DOI] [PubMed] [Google Scholar]
- 40.Ku J.H., et al. (2015) The prognostic value of pretreatment of systemic inflammatory responses in patients with urothelial carcinoma undergoing radical cystectomy. Br. J. Cancer 112, 461–467 10.1038/bjc.2014.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert J.W., et al. (2013) Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology 81, 587–592 10.1016/j.urology.2012.10.055 [DOI] [PubMed] [Google Scholar]
- 42.Lavallee L.T., et al. (2014) Peri-operative morbidity associated with radical cystectomy in a multicenter database of community and academic hospitals. PLoS ONE 9, e111281 10.1371/journal.pone.0111281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J., et al. (2016) The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol. Oncol. 34, 484.e1–484.e8 10.1016/j.urolonc.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 44.Morgan T.M., et al. (2011) Predicting the probability of 90-day survival of elderly patients with bladder cancer treated with radical cystectomy. J. Urol. 186, 829–834 10.1016/j.juro.2011.04.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mursi K., et al. (2013) The effect of preoperative clinical variables on the 30- and 90-day morbidity and mortality after radical cystectomy: a single-centre study. Arab J. Urol. 11, 152–158 10.1016/j.aju.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma P., et al. (2016) Preoperative patient reported mental health is associated with high grade complications after radical cystectomy. J. Urol. 195, 47–52 10.1016/j.juro.2015.07.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheth K.R., et al. (2016) Prognostic serum markers in patients with high-grade upper tract urothelial carcinoma. Urol. Oncol. 34, 418.e9–418.e16 10.1016/j.urolonc.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 48.Taguchi S., et al. (2016) Validation of major prognostic models for metastatic urothelial carcinoma using a multi-institutional cohort of the real world. World J. Urol. 34, 163–171 10.1007/s00345-015-1631-3 [DOI] [PubMed] [Google Scholar]
- 49.Lien Y.C., et al. (2004) Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J. Gastrointest. Surg. 8, 1041–1048 10.1016/j.gassur.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 50.Wu N., et al. (2015) Low pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomas. Nutr. Cancer 67, 481–485 10.1080/01635581.2015.1004726 [DOI] [PubMed] [Google Scholar]
- 51.von Meyenfeldt M. (2005) Cancer-associated malnutrition: an introduction. Eur. J. Oncol. Nurs. 9, S35–S38 10.1016/j.ejon.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 52.Dewys W.D., et al. (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 69, 491–497 10.1016/S0149-2918(05)80001-3 [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A., et al. (2008) Cancer-related inflammation. Nature 454, 436–444 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 54.Elinav E., et al. (2013) Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 55.Gupta D. and Lis C.G. (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 9, 69 10.1186/1475-2891-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Namiesnik J., et al. (2014) In vitro studies on the relationship between the antioxidant activities of some berry extracts and their binding properties to serum albumin. Appl. Biochem. Biotechnol. 172, 2849–2865 10.1007/s12010-013-0712-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Detsky A.S., et al. (1984) Evaluating the accuracy of nutritional assessment techniques applied to hospitalized patients: methodology and comparisons. JPEN J. Parenter. Enteral Nutr. 8, 153–159 10.1177/0148607184008002153 [DOI] [PubMed] [Google Scholar]
- 58.Mahdavi A.M. and Ostadrahimi A. Safaiyan A. (2010) Subjective global assessment of nutritional status in children. Matern. Child Nutr. 6, 374–381 10.1111/j.1740-8709.2009.00214.x [DOI] [PMC free article] [PubMed] [Google Scholar]