Abstract
Infectious disease is recognized as an important complication among patients with end-stage renal disease, contributing to excess morbidity and health care costs. However, recent epidemiological studies have revealed that even mild to moderate stages of chronic kidney disease (CKD) substantially increase risk of infection. Regarding underlying mechanisms, evidence suggests various aspects of altered immune response in patients with CKD including impaired function of T cells, B cells and neutrophil. Multiple conditions surrounding CKD, such as older age, diabetes, and cardiovascular disease are important contributors in the increased susceptibility to infection in this population. In addition, several mechanisms impairing immune function have been hypothesized including accumulated uremic toxins, increased oxidative stress, endothelial dysfunction, low-grade inflammation, and mineral and bone disorders. In terms of prevention strategies, influenza and pneumococcal vaccines are most feasible and important. Nevertheless, the extent of vaccine utilization in CKD has not been well documented. In addition, antibody response to vaccination may be reduced in CKD patients, and thus a vaccine delivery strategy (e.g., dose and frequency) may need to be optimized among patients with CKD. Through this review, we demonstrate that infection is a major but underrecognized complication of CKD. As CKD is recognized as a serious public health issue, dedicated research is needed to better characterize the burden of infectious disease associated with CKD, understand the pathophysiology of infection in patients with CKD, and develop effective strategies to prevent infection and its sequela in this high risk population.
Keywords: Chronic kidney disease, Infections, Pneumonia, Bloodstream infections, Renal failure, Influenza vaccination, Pneumococcal vaccination
Introduction
Chronic kidney disease (CKD) is a serious public health issue, affecting 8–16% of adult population worldwide [1]. Although historically cardiovascular disease has been considered as one of the most important CKD complications [2], an accumulating body of evidence has revealed that CKD is also an important risk factor for non-cardiovascular outcomes (e.g., cognitive decline [3], fracture [4], bleeding [5]). In this context, infection is probably the most important non-cardiovascular outcome since it poses the second leading cause of hospitalization after cardiovascular disease [2]. While it is well-recognized that infection risk is extremely high among patients with end-stage renal disease (ESRD), a few recent studies suggest that even less severe CKD substantially increases the risk of infection. Nevertheless, data on the epidemiology of infectious disease are still sparse in the entire CKD population including its mild to moderate stages. Such a knowledge gap is critical since the vast majority of CKD patients are at mild to moderate stages [2]. In this review, we will discuss the current evidence regarding the epidemiology of infectious disease in CKD. In the first section, we will discuss the incidence of infectious disease associated with CKD in inpatient and outpatient settings. In the second section, we will discuss possible mechanisms and contributing factors to the increased susceptibility to infection in CKD. In the third section, we will discuss infection prevention strategies primarily focusing on vaccination programs. We will also list some potential future directions in this context.
Incidence of infectious disease associated with CKD
According to a report from the 2017 United States Renal Data System [6], the incidence of hospitalization was 614 per 1000 person-years in individuals aged 65 years or older with any stage of CKD, which was nearly 3 times higher as compared to the incidence of 214 per 1000 person-years in those without CKD. Regarding cause of hospitalization, cardiovascular disease was the leading cause of hospitalization, accounting for 23% of all-cause hospitalization. Infection was the second major cause, accounting for 21% of all hospitalizations—a burden almost identical to cardiovascular disease [6]. Thus, it is important to recognize infection as a leading cause of hospitalization among individuals with CKD.
Table 1 summarizes the characteristics of representative cohort studies investigating the association between eGFR and risk of infection. Regarding the risk of hospitalization with infection, previous studies consistently showed an association between lower eGFR and risk of hospitalization with infection [7–10]. The risk is substantially increased even at mildly to moderately reduced eGFR: as compared to those with eGFR ≥ 60 ml/min/1.73 m2, individuals with eGFR 30–59 ml/min/1.73 m2 had an approximately 50% higher risk of hospitalization with infection. This pattern was observed for all-cause infection, as well as type-specific infections. Although the low prevalence of eGFR < 30 ml/min/1.73 m2 in the general population tends to limit the statistical power, the risk is exponentially increased in eGFR < 30 ml/min/1.73 m2, with a 2–3 times higher risk compared to eGFR ≥ 60 ml/min/1.73 m2. The association between low eGFR and risk of infection tended to be stronger among younger adults than older adults [8]. This could be explained by the lower incidence rate of infection in younger adults, resulting in a substantial increase in the relative risk even with a modest increase in the absolute risk. In addition, younger adults with reduced eGFR might be likely to have a unique etiology of kidney disease such as glomerulonephritis, polycystic kidney disease, or severe diabetes (e.g., type 1 diabetes), posing a particularly high risk of infection.
Table 1.
Characteristics of representative cohort studies assessing risk of infection across eGFR
| eGFR | Year | Setting | High risk population* | Sample size | Mean age, years | Infection type | Crude IR per 1000 p-years | Relative risk (95%CI) by eGFR category (ml/min/1.73 m2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 90 | 60–89 | 45–59 | 30–44 | 15–29 | < 15 | ||||||||
| Hospitalization with infection | |||||||||||||
| James | 2008 | Canada | Yes | 25,675 | 75 | Bloodstream infections | 10.4 | Ref | 1.24 (1.01–1.52) | 1.59 (1.24–2.04) | 3.54 (2.69–4.69) | ||
| James | 2009 | Canada | No | 252,516 | range, 18–54 | Pneumonia | 1.7 | Ref | 3.23 (2.40–4.36) | 9.67 (6.36–14.69) | 15.04 (9.64–23.47) | ||
| James | 2009 | Canada | Yes | 252,516 | range, ≥ 75 | Pneumonia | 31.7 | Ref | 0.95 (0.85–1.05) | 1.03 (0.92–1.16) | 1.79 (1.55–2.06) | ||
| Dalrymple | 2012 | US | Yes | 5,142 | 72 | All-cause | 34.7 | Ref | 1.16 (1.02–1.32) | 1.37 (1.14–1.66) | 1.64 (1.28–2.12) | Excluded | |
| Ishigami | 2016 | US | No | 9,697 | 63 | All-cause | 23.6 | Ref | 1.07 (0.98–1.16) | 1.48 (1.28–1.71) | 2.55 (1.43–4.55) | Excluded | |
| Outpatient and inpatient infections | |||||||||||||
| McDonald | 2014 | UK | Yes | 191,709 | 71 | LRTI | 155.8 | Ref | 1.03 (1.01–1.04) | 1.08 (1.05–1.10) | 1.17 (1.13–1.22) | 1.47 (1.34–1.62) | |
| Xu | 2017 | Sweden | No | 1,139,470 | 52 | All-cause | 95.0 | Ref | 1.08 (1.01–1.14) | 1.53 (1.39–1.69) | |||
| Infection-related death | |||||||||||||
| James | 2009 | Canada | No | 252,516 | range, 18–64 | Pneumonia | 0.3 | Ref | 2.54 (1.40–4.60) | 13.15 (7.04-424.56) | 23.35 (11.52–47.32) | ||
| James | 2009 | Canada | Yes | 252,516 | range, ≥ 75 | Pneumonia | 4.9 | Ref | 1.22 (1.01–1.49) | 2.03 (1.64–2.50) | 4.94 (3.94–6.19) | ||
| Wang | 2011 | US | No | 7,400 | 61 | All-cause | 1.9 | Ref | 1.36 (0.81–2.30) | 2.36 (1.04–5.38) | Excluded | ||
| Ishigami | 2016 | US | No | 9,697 | 63 | All-cause | 4.1 | Ref | 0.99 (0.80–1.21) | 1.62 (1.20–2.19) | 3.76 (1.48–9.58) | Excluded | |
*The study investigated population at high risk of infection (e.g., older adults, diabetes)
IR incidence rate, eGFR estimated glomerular filtration rate, LRTI lower respiratory tract infections
Increased risk of infection associated with reduced eGFR was also observed in ambulatory settings (Table 1) [11, 12]. Of note, although the association (i.e., relative risk) for outpatient infections seems weaker compared to inpatient infections, outpatient infections are much more common than inpatient infections. The incidence rates including outpatient infections ranged 100–150 cases per 1000 person-years, which was 3–5 times more frequent as compared to the incidence of hospitalization with infection [7–10]. Thus, although outpatient infections should have less prognostic impact than inpatient infections, they still pose a significant burden on CKD patients in terms of excess clinic visits and frequent antibiotic prescriptions, which reduce patients’ quality of life, impact health care costs, and induce multidrug resistant microorganisms [13, 14].
Previous studies also reported an increased risk of infection-related death associated with reduced eGFR (Table 1) [8, 15, 16]. However, we should interpret those results carefully since definitions of infection-related death varied across studies. For example, in a study of 38,520 individuals with eGFR < 60 ml/min/1.73 m2 using data from the electronic medical record-base registry in Ohio, the leading causes of death were cardiovascular disease (34.7%) and malignant neoplasms (31.8%), and deaths due to infections only accounted for 1.7% (influenza and pneumonia), and 1.4% (septicemia), respectively [17]. However, in a secondary analysis of the Trial to Reduce Cardiovascular Events With Aranesp Therapy [18], cause of death was centrally adjudicated, and infection was the second leading cause of death after cardiovascular death accounting for ~ 35% of all-cause mortality.
As compared to eGFR, fewer studies have examined albuminuria (Table 2). Among patients aged 65 years or older with diabetes, persons with positive dipstick proteinuria had nearly 10% higher risk for lower respiratory tract infections and nearly 30% higher risk for pneumonia or sepsis compared to those without [11]. In the Atherosclerosis Risk in Communities study, we observed a strong dose–response association between urinary albumin-to-creatinine ratio (ACR) and risk of hospitalization with infection, and this association was independent of eGFR (Table 2) [15]. Indeed, when assessed in the context of CKD risk stage according to the Kidney Disease Improving Global Outcomes (KDIGO) [19], there were multiplicative contributions of low eGFR and high ACR to the risk of hospitalization with infection (Fig. 1): within each eGFR category, risk of hospitalization with infection was higher with higher ACR in a graded fashion. Importantly, those with preserved kidney function (i.e., eGFR ≥ 60 ml/min/1.73 m2), but ACR ≥ 300 mg/g had an equivalent or even greater infection risk compared to those with moderately to severely reduced kidney function, but without albuminuria. Thus, in addition to reduced eGFR, health care providers should recognize albuminuria as an important risk factor of infection.
Table 2.
Characteristics of representative cohort studies assessing risk of infection ACR
| ACR | Year | Setting | High risk population* | Sample size | Mean age, years | Infection type | Crude IR per 1000 p-years | Relative risk (95%CI) by ACR category (mg/dL) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 10 | 10–29 | 30–299 | 300+ | ||||||||
| Hospitalization with infection | |||||||||||
| Ishigami | 2016 | US | No | 9,697 | 63 | All-cause | 23.6 | Ref | 1.34 (1.20–1.50) | 1.56 (1.36–1.78) | 2.30 (1.81–2.91) |
| Outpatient and inpatient infections | |||||||||||
| McDonald | 2014 | UK | Yes | 191,709 | 71 | LRTI | 155.8 | Ref (Dipstick negative) | 1.07 (95% CI, 1.05–1.09) (Dipstick positive) | ||
*The study investigated population at high risk of infection (e.g., older adults, diabetes)
IR incidence rate, eGFR estimated glomerular filtration rate, LRTI lower respiratory tract infections
Fig. 1.
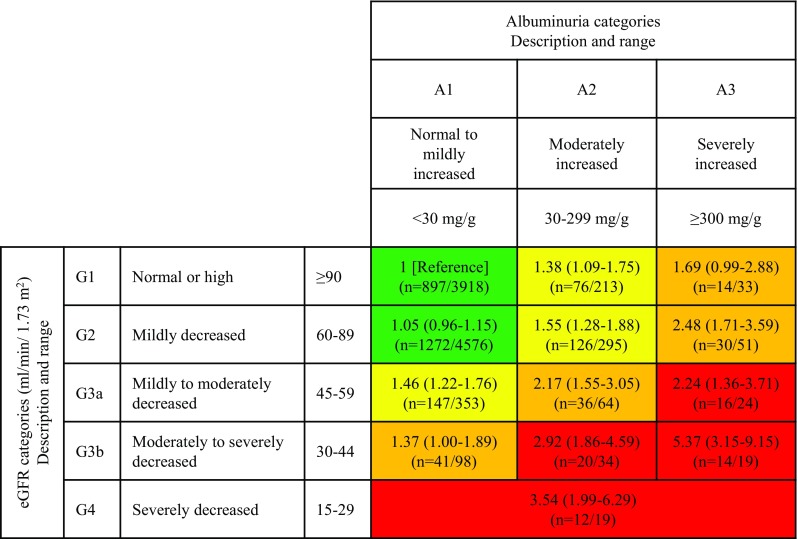
Adjusted hazard ratio of hospitalization with infection by eGFR and ACR categories. GFR glomerular filtration rate, ACR albumin-to-creatinine ratio. Green: low risk; yellow: moderately increased risk; orange: high risk; red, very high risk. For each category, hazard ratio and its 95% confidence interval were presented in the first row, and n = denotes number of events and number of individuals in the second row. The model was adjusted for age, race, sex, body mass index, smoking status, alcohol consumption, education level, use of antineoplastic agents and steroids, hypertension, diabetes, history of cancer, chronic obstructive pulmonary disease, prior heart failure, prior coronary disease, and prior stroke.
Reprinted from reference 15 with permission
Pathophysiological mechanisms increasing infection risk in CKD
Impaired immune system in CKD
Impaired immune system has been recognized in CKD patients. For example, in patients with reduced kidney function, the number of lymphocytes, primarily the B lymphocyte and CD4+ T lymphocyte subset, is decreased [20]. In addition, T-cell response to antigen stimulus is impaired in persons with CKD [21]. CKD patients are also unknown to have the impaired function of neutrophil. In contrast to the decreased count in lymphocytes, the number of neutrophils remain unchanged in ESRD patients [22]. However, as compared to healthy subjects, patients with ESRD seem to have a lower capacity of phagocytosis and greater rate of apoptosis [23, 24].
Potential mechanisms impairing immune system in CKD
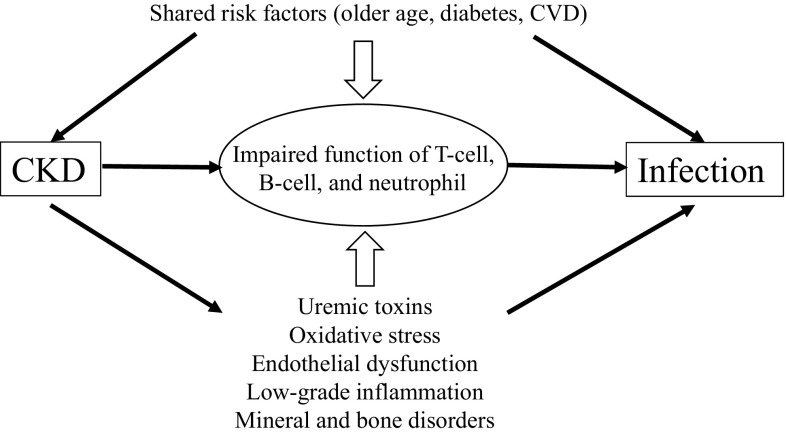
Underlying mechanisms of impaired immune system in CKD are considered multifactorial (Fig. 2). First, shared risk factors of CKD and infection are likely to play an important role. For example, CKD primarily affects older adults, a population at high risk of infection. Immunological changes similar to patients with CKD are also observed in older adults, including reduced lymphocyte production, impaired leukocyte, and neutrophil functions [25, 26]. In addition, diabetes and cardiovascular disease are prevalent among CKD patients, and are known to increase the risk of infection in this population [27–29].
Fig. 2.
Potential mechanisms increasing infection in chronic kidney disease. CKD chronic kidney disease, CVD cardiovascular disease
Also, several uremic toxins may contribute to the impaired immune system in CKD. For example, indoxyl sulfate and p-cresyl sulfate are metabolites of tryptophan and tyrosine [30], and previous in vitro studies suggest that these metabolites could impair the leucocyte and endothelial function [30–33]. A couple of small studies of hemodialysis patients suggested the positive association of p-cresol sulfate levels with risk of infection [34, 35]. Another potential metabolite would be trimethylamine-N-oxide (TMAO). TMAO is an oxidative product of trimethylamine [36], and some studies have shown pro-inflammatory aspects of TMAO through the activation of macrophage in patients with CKD, which may ultimately interfere with the immune system [37, 38]. Nonetheless, future studies are still needed to better understand the involvement of these uremic toxins in the impaired immune system in CKD.
Reactive oxygen species (ROS) are important components of the immune response such as activating inflammatory signals and eliminating damaged cells [39]. However, due to their cytotoxicity, excess levels of ROS can actually impair immune function [40]. Among CKD patients, oxidative stress is increased and antioxidant capacity is decreased, and several small studies suggested the link between oxidative stress and impaired immune response [41–43]. However, it is yet to be determined to what extent increased levels of ROS actually contribute to increased risk of infection in CKD patients.
Endothelial cells play an important role in the immune regulation such as cell migration, neutrophil adhesion, and permeability to circulating leukocytes. Several studies have reported the potential link between endothelial dysfunction and impaired immune function [44–46]. Patients with CKD have higher levels of markers for endothelial dysfunction (e.g., soluble P-selectin) compared to healthy controls [47]. Another study reported the association between decreased layer of endothelial surface, known as glycocalyx, and the incidence of albuminuria [48]. Thus, endothelial dysfunction may be another contributing factor for impaired immune response in CKD, and may be relevant to the underlying pathophysiology for elevated infection risk seen in individuals with albuminuria [49]. However, future studies are needed to specifically evaluate whether a measure of endothelial dysfunction, such as flow-mediated dilation [50, 51], is related to the increased risk of infection.
Previous cross-sectional studies showed increased levels of inflammatory cytokines among patients with CKD [52, 53]. For example, in the Chronic Renal Insufficiency Cohort study (eGFR 20–70 ml/min/1.73 m2), there was an inverse relationship between plasma levels of inflammatory markers (interleukin-1b, interluekin-1RA, interleukin-6 [IL-6], tumor necrosis factor-alpha [TNF-α], and C-reactive protein [CRP]) and eGFR [53]. Several prospective studies have also shown that an elevation of inflammatory markers such as CRP, IL-6, TNF-α, was associated with increased risk of infection [54–56]. These findings suggest a potential contribution of inflammation to infection, although causality has not yet be determined.
Some evidence suggests that dysregulation of bone and mineral metabolism contributes to the increased risk of infection. Animal studies have suggested that elevated serum level of fibroblast growth factor 23 (FGF23) disrupts the leukocyte and innate immune function [57, 58]. Among patients on dialysis in the Hemodialysis (HEMO) Study, patients in the highest quartile for 25-hydroxyvitamin D had a 33% lower risk of infectious events compared to the lowest quartile; whereas those in the highest quartile FGF23 had a 57% higher risk compared to the lowest quartile [59]. Similar results were also observed for elderly adults [60], as well as in the general population [61]. However, whether mineral and bone disorders can be targeted for an intervention to reduce infection risk is unknown, although a recent meta-analysis reported the protective effects of vitamin D supplementation on reducing respiratory infection in the general population [62].
Prevention strategies
Some types of infection are preventable through vaccinations such as the influenza and pneumococcal vaccine. Thus, adherence to vaccine recommendations should be the central strategy for reducing risk of vaccine-preventable infections [63, 64]. In addition, there are several non-vaccine prevention measures (e.g., standard preventative measures for hospital-acquired infection), which are also applicable to individuals with CKD.
Vaccination
Influenza vaccination is probably the most feasible and effective strategy to reduce influenza-related diseases [63, 64]. Although influenza vaccination is beneficial to all age groups, it is particularly important for those at high risk (e.g., older adults, individuals with chronic conditions). In the 2013 KDIGO guideline, annual vaccination with influenza vaccine is recommended to all adults with CKD unless contraindicated [19].
Previous studies in the general population have consistently shown protective effects of influenza vaccination in reducing risk of influenza-related complications by 20–40% [65–67]. However, the effectiveness is less clear among patients with CKD. In ESRD populations in the US, influenza vaccination was non-significantly associated with 10–15% lower risks of hospitalization with influenza/pneumonia [68, 69]. Similarly, a Taiwanese study of hemodialysis patients reported that the receipt of influenza vaccination was associated with ~ 20% lower risks of hospitalization with pneumonia/influenza [70]. These findings suggest that influenza vaccine may be less effective in patients with advanced CKD compared to the general population.
Reduced effectiveness of influenza vaccine in advanced CKD may be due to poorer antibody response to influenza vaccination compared to non-CKD [71–76]. Chang et al. studied antibody response to a single dose H1N1/09 vaccine among 110 hemodialysis patients and 173 healthy controls, and found that the seroconversion rate was 24.5% among hemodialysis patients compared to 86.7% among healthy controls [71]. However, some studies reported less evident difference between dialysis patients and control groups [76]. Further studies are needed to assess antibody response to influenza vaccination in advanced CKD.
Recent studies showed higher effectiveness of a high-dose or adjuvanted influenza vaccine compared to regular vaccine [77, 78]. However, whether these vaccines could benefit CKD patients is not fully clear. A few studies reported a higher vaccine antibody response with an adjuvanted trivalent influenza vaccine among hemodialysis patients and renal transplant recipients [79, 80]. In contrast, a study assessing one booster influenza vaccination among hemodialysis patients showed no differences in the seroconversion rate between a single dose group and one booster dose group [81]. Taken together, although newer influenza vaccines could theoretically induce a stronger vaccine response, additional studies are needed to assess whether CKD patients would benefit from such vaccines.
Pneumococcal vaccination is also an effective strategy to prevent diseases caused by Streptococcus Pneumoniae. Currently, two vaccine types, pneumococcal conjugate vaccine (PCV) and pneumococcal polysaccharide vaccine (PPSV), are available. In the 2013 KDIGO guideline [19], pneumococcal vaccination is recommended to all adults with eGFR < 30 ml/min/1.73 m2 and those at high risk of pneumococcal infection, such as individuals with nephrotic syndrome, diabetes, or those on immunosuppressive drugs. In addition, revaccination is recommended for adults with CKD within 5 years after receiving pneumococcal vaccination [19].
In a landmark trial of the Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA), PCV13 reduced the risk of community-acquired pneumonia due to vaccine-type strains by 46% in community-dwelling adults aged 65 years or older [82]. PCV13 could induce a stronger vaccine response than PPSV23, but the effectiveness is considered comparable [83]. Whether these data may be generalizable to individuals with CKD is unknown, but some observational studies suggested an improved survival among dialysis patients with pneumococcal vaccination compared to those without [84, 85].
To maintain the adequate immunogenicity, some experts suggest a booster dose of pneumococcal vaccination for patients with CKD [86], since individuals with CKD may have a faster decline in the antibody titers post-vaccination [87–91]. However, its benefits have been controversial. Tobudic et al. reported that the prime-boost strategy did not result in the increased antibody response among transplant patients [92]. Other previous studies have been limited by small number of study subjects and only investigating patients with ESRD. Thus, future studies are needed to assess the effectiveness of pneumococcal vaccination in a broader range of CKD and determine the optimal dose and vaccine delivery strategy.
Other strategies
Besides vaccine programs, there are several general approaches for preventing infection, which are also applicable to individuals with CKD. Patients with CKD have a high risk of all-cause hospitalization [93], and thus prevention of hospital acquired infections is crucial [94]. Medical devices such as ventilator, central venous catheter, and urinary catheter are frequently used for CKD patients, and are important sources of infection. Standard preventative measures such as good hand hygiene, maximal barrier precautions during the procedure, and prompt removal of devices are critical to minimize the chance of device associated infections [95]. In addition, some active interventions such as quality-improvement interventions [96] and clinical decision support systems (e.g., reminders for preventive care) [97] are shown to be effective but can be expensive. From the perspective of policymaking, these active interventions may be cost-effective when targeted to CKD patients given their high vulnerability to infection. Finally, antibiotic prophylaxis before some invasive procedures such as major surgeries (e.g., cardiac and abdominal surgery) [98] and dental procedures [99] are also generally encouraged to all patients including those with CKD. However, CKD patients may have a high prevalence of multidrug-resistant organism colonization [100], which may complicate the clinical management concerning antibiotic prophylaxis in this clinical population.
Future research directions
Despite the advancement in the management of CKD, there remains a substantial knowledge gap in the epidemiology of infectious disease in CKD. Future studies should characterize the incidence of overall and cause-specific infection across the spectrum of CKD, particularly including albuminuria stages. Additionally, whether these infections affect the subsequent outcomes of CKD, and if so, to what extent, should be assessed. In addition, we should better understand mechanisms elevating the risk of infection in CKD patients, which would have implications on preventive and therapeutic strategies for infection in CKD. Finally, effective strategies to maximize the benefits of vaccination programs should be developed to prevent vaccine-preventable diseases and improve the outcomes among patients with CKD.
Conclusions
A body of evidence demonstrated a high risk of infection even at mild to moderate stages of CKD. Nonetheless, infection has been underrecognized and understudied as a complication of CKD. Although several recent studies reported the increased risk of infection among individuals with reduced GFR, definitions of infection varied across studies, and statistical powers were limited in persons with eGFR < 30 ml/min/1.73 m2 not requiring renal replacement therapy. Thus, the actual burden of overall and type-specific infection across CKD stages is yet to be determined. Additionally, more studies are needed to quantify the burden of infection associated with albuminuria. Incomplete understanding of underlying mechanisms may preclude us from considering and planning effective preventive strategies for infection in CKD patients. As true in the entire population, vaccination is a major prevention approach for some types of infection in CKD populations, but whether its uptake is optimal is unknown and a few studies raise a question regarding the effectiveness of regular vaccinations in CKD patients. As the number of individuals with CKD is growing globally, it is time to focus on infectious disease as a complication of CKD and advance our understandings to reduce the burden of infection in CKD.
Acknowledgements
J.I. was supported by National Heart, Lung, and Blood Institute grant T32HL007024.
Conflict of interest
The author reports no conflicts of interest in this work.
Human and animal rights statement
This work does not include any analysis involving human or animal subjects.
Informed consent
There is no involvement of human subjects in this work.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Change history
3/2/2019
The article “Clinical epidemiology of infectious disease among patients with chronic kidney disease”, written by Junichi Ishigami and Kunihiro Matsushita, was originally published electronically on the publisher’s internet portal (currently SpringerLink) on 3rd September 2018 without open access.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System . 2015 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 3.Barzilay JI, Fitzpatrick AL, Luchsinger J, Yasar S, Bernick C, Jenny NS, et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis. 2008;52(2):216–226. doi: 10.1053/j.ajkd.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daya NR, Voskertchian A, Schneider AL, Ballew S, McAdams DeMarco M, Coresh J, et al. Kidney function and fracture risk: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2016;67(2):218–226. doi: 10.1053/j.ajkd.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishigami J, Grams ME, Naik RP, Coresh J, Matsushita K. Chronic kidney disease and risk for gastrointestinal bleeding in the community: the Atherosclerosis Risk in Communities (ARIC) Study. Clin J Am Soc Nephrol. 2016;11(10):1735–1743. doi: 10.2215/CJN.02170216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saran R, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, US Renal Data System 2016 Annual Data Report et al. Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3S1):A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MT, Laupland KB, Tonelli M, Manns BJ, Culleton BF, Hemmelgarn BR, et al. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med. 2008;168(21):2333–2339. doi: 10.1001/archinte.168.21.2333. [DOI] [PubMed] [Google Scholar]
- 8.James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis. 2009;54(1):24–32. doi: 10.1053/j.ajkd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K. CKD and risk for hospitalization with infection: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalrymple LS, Katz R, Kestenbaum B, de Boer IH, Fried L, Sarnak MJ, et al. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis. 2012;59(3):356–363. doi: 10.1053/j.ajkd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald HI, Thomas SL, Millett ER, Nitsch D. CKD and the risk of acute, community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using electronic health records. Am J Kidney Dis. 2015;66(1):60–68. doi: 10.1053/j.ajkd.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Gasparini A, Ishigami J, Mzayen K, Su G, Barany P, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017;12(9):1399–1408. doi: 10.2215/CJN.00250117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yates C, May K, Hale T, Allard B, Rowlings N, Freeman A, et al. Wound chronicity, inpatient care, and chronic kidney disease predispose to MRSA infection in diabetic foot ulcers. Diabetes Care. 2009;32(10):1907–1909. doi: 10.2337/dc09-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berns JS. Infection with antimicrobial-resistant microorganisms in dialysis patients. Semin Dial. 2003;16(1):30–37. doi: 10.1046/j.1525-139x.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- 15.Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K. CKD and risk for hospitalization with infection: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2017;69(6):752–761. doi: 10.1053/j.ajkd.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HE, Gamboa C, Warnock DG, Muntner P. Chronic kidney disease and risk of death from infection. Am J Nephrol. 2011;34(4):330–336. doi: 10.1159/000330673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Nally JV., Jr Cause-specific deaths in non-dialysis-dependent CKD. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charytan DM, Lewis EF, Desai AS, Weinrauch LA, Ivanovich P, Toto RD, et al. Cause of death in patients with diabetic CKD enrolled in the trial to reduce cardiovascular events with aranesp therapy (TREAT) Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.02.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 20.Litjens NH, van Druningen CJ, Betjes MG. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol. 2006;118(1):83–91. doi: 10.1016/j.clim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13(1):204–212. doi: 10.1681/ASN.V131204. [DOI] [PubMed] [Google Scholar]
- 22.Anding K, Gross P, Rost JM, Allgaier D, Jacobs E. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol Dial Transplant. 2003;18(10):2067–2073. doi: 10.1093/ndt/gfg330. [DOI] [PubMed] [Google Scholar]
- 23.Gollapudi P, Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Leukocyte toll-like receptor expression in end-stage kidney disease. Am J Nephrol. 2010;31(3):247–254. doi: 10.1159/000276764. [DOI] [PubMed] [Google Scholar]
- 24.Sela S, Shurtz-Swirski R, Cohen-Mazor M, Mazor R, Chezar J, Shapiro G, et al. Primed peripheral polymorphonuclear leukocyte: a culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol. 2005;16(8):2431–2438. doi: 10.1681/ASN.2004110929. [DOI] [PubMed] [Google Scholar]
- 25.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9(1):57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 27.Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–1065. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheikh Hassan HI, Tang M, Djurdjev O, Langsford D, Sood MM, Levin A. Infection in advanced chronic kidney disease leads to increased risk of cardiovascular events, end-stage kidney disease and mortality. Kidney Int. 2016;90(4):897–904. doi: 10.1016/j.kint.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol. 2003;14(7):1863–1870. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 30.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25(9):1897–1907. doi: 10.1681/ASN.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant. 2007;22(2):592–596. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 32.Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J, et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD) Sci Rep. 2017;7(1):3057. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pletinck A, Glorieux G, Schepers E, Cohen G, Gondouin B, Van Landschoot M, et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol. 2013;24(12):1981–1994. doi: 10.1681/ASN.2012030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CJ, Wu CJ, Pan CF, Chen YC, Sun FJ, Chen HH. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol Dial Transplant. 2010;25(11):3693–3700. doi: 10.1093/ndt/gfq251. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee T, Meyer TW, Shafi T, Hostetter TH, Melamed M, Zhu Y, et al. Free and total p-cresol sulfate levels and infectious hospitalizations in hemodialysis patients in CHOICE and HEMO. Medicine. 2017;96(6):e5799. doi: 10.1097/MD.0000000000005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeisel SH, Warrier M. Trimethylamine N-Oxide, the microbiome, and heart and kidney disease. Ann Rev Nutr. 2017;37:157–181. doi: 10.1146/annurev-nutr-071816-064732. [DOI] [PubMed] [Google Scholar]
- 39.Sack MN, Fyhrquist FY, Saijonmaa OJ, Fuster V, Kovacic JC. Basic biology of oxidative stress and the cardiovascular system: Part 1 of a 3-Part series. J Am Coll Cardiol. 2017;70(2):196–211. doi: 10.1016/j.jacc.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24(5):469–473. doi: 10.1016/j.semnephrol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22(6):636–643. doi: 10.1111/j.1525-139X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 42.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65(3):1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 2007;71(2):167–172. doi: 10.1038/sj.ki.5002019. [DOI] [PubMed] [Google Scholar]
- 44.Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, et al. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review. J Autoimmun. 2015;57:1–13. doi: 10.1016/j.jaut.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 47.Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43(2):244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 48.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23(8):1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seliger SL, Salimi S, Pierre V, Giffuni J, Katzel L, Parsa A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016;17(1):82. doi: 10.1186/s12882-016-0303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papaioannou GI, Seip RL, Grey NJ, Katten D, Taylor A, Inzucchi SE, et al. Brachial artery reactivity in asymptomatic patients with type 2 diabetes mellitus and microalbuminuria (from the Detection of Ischemia in Asymptomatic Diabetics-brachial artery reactivity study) Am J Cardiol. 2004;94(3):294–299. doi: 10.1016/j.amjcard.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41(6):1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 53.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7(12):1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang HE, Shapiro NI, Safford MM, Griffin R, Judd S, Rodgers JB, et al. High-sensitivity C-reactive protein and risk of sepsis. PloS One. 2013;8(7):e69232. doi: 10.1371/journal.pone.0069232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Inflammatory and endothelial activation biomarkers and risk of sepsis: a nested case-control study. J Crit Care. 2013;28(5):549–555. doi: 10.1016/j.jcrc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yende S, Tuomanen EI, Wunderink R, Kanaya A, Newman AB, Harris T, et al. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005;172(11):1440–1446. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naive and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70(2):371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 58.Bacchetta J, Salusky IB, Hewison M. Beyond mineral metabolism, is there an interplay between FGF23 and vitamin D in innate immunity? Pediatr Nephrol. 2013;28(4):577–582. doi: 10.1007/s00467-012-2336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol. 2016;27(1):227–237. doi: 10.1681/ASN.2014101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nowak KL, Bartz TM, Dalrymple L, de Boer IH, Kestenbaum B, Shlipak MG, et al. Fibroblast growth factor 23 and the risk of infection-related hospitalization in older adults. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishigami J, Jaar BG, Rebholz CM, Grams ME, Michos ED, Wolf M, et al. Biomarkers of mineral and bone metabolism and 20-year risk for hospitalization with infection: the ARIC study. J Clin Endocrinol Metab. 2017 doi: 10.1210/jc.2017-01868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.European Centre for Disease Prevention and Control. Seasonal influenza vaccination in Europe. https://ecdc.europa.eu/en/publications-data/seasonal-influenza-vaccination-europe. Accessed 30 Jan 2018.
- 64.World Health Organization. Influenza vaccine use. http://www.who.int/influenza/vaccines/use/en/. Accessed 30 Jan 2018.
- 65.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 66.Grijalva CG, Zhu Y, Williams DJ, Self WH, Ampofo K, Pavia AT, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA. 2015;314(14):1488–1497. doi: 10.1001/jama.2015.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hak E, Nordin J, Wei F, Mullooly J, Poblete S, Strikas R, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis. 2002;35(4):370–377. doi: 10.1086/341403. [DOI] [PubMed] [Google Scholar]
- 68.Gilbertson DT, Unruh M, McBean AM, Kausz AT, Snyder JJ, Collins AJ. Influenza vaccine delivery and effectiveness in end-stage renal disease. Kidney Int. 2003;63(2):738–743. doi: 10.1046/j.1523-1755.2003.00787.x. [DOI] [PubMed] [Google Scholar]
- 69.McGrath LJ, Kshirsagar AV, Cole SR, Wang L, Weber DJ, Sturmer T, et al. Influenza vaccine effectiveness in patients on hemodialysis: an analysis of a natural experiment. Arch Intern Med. 2012;172(7):548–554. doi: 10.1001/archinternmed.2011.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang IK, Lin CL, Lin PC, Liang CC, Liu YL, Chang CT, et al. Effectiveness of influenza vaccination in patients with end-stage renal disease receiving hemodialysis: a population-based study. PloS One. 2013;8(3):e58317. doi: 10.1371/journal.pone.0058317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang YT, Guo CY, Tsai MS, Cheng YY, Lin MT, Chen CH, et al. Poor immune response to a standard single dose non-adjuvanted vaccination against 2009 pandemic H1N1 influenza virus A in the adult and elder hemodialysis patients. Vaccine. 2012;30(33):5009–5018. doi: 10.1016/j.vaccine.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Lertdumrongluk P, Changsirikulchai S, Limkunakul C, Prachukthum P, Punpiput P, Buppanharun R, et al. Safety and immunogenicity of a 2009 influenza A (H1N1) vaccine in hemodialysis patients. Vaccine. 2012;30(6):1108–1114. doi: 10.1016/j.vaccine.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 73.Birdwell KA, Ikizler MR, Sannella EC, Wang L, Byrne DW, Ikizler TA, et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54(1):112–121. doi: 10.1053/j.ajkd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crespo M, Collado S, Mir M, Cao H, Barbosa F, Serra C, et al. Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients. Clin J Am Soc Nephrol. 2011;6(9):2208–2214. doi: 10.2215/CJN.02160311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scharpe J, Peetermans WE, Vanwalleghem J, Maes B, Bammens B, Claes K, et al. Immunogenicity of a standard trivalent influenza vaccine in patients on long-term hemodialysis: an open-label trial. Am J Kidney Dis. 2009;54(1):77–85. doi: 10.1053/j.ajkd.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 76.Ortbals DW, Marks ES, Liebhaber H. Influenza immunization in patients with chronic renal disease. JAMA. 1978;239(24):2562–2565. doi: 10.1001/jama.239.24.2562. [DOI] [PubMed] [Google Scholar]
- 77.Izurieta HS, Thadani N, Shay DK, Lu Y, Maurer A, Foppa IM, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015;15(3):293–300. doi: 10.1016/S1473-3099(14)71087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 79.Noh JY, Song JY, Choi WS, Lee J, Seo YB, Kwon YJ, et al. Immunogenicity of trivalent influenza vaccines in patients with chronic kidney disease undergoing hemodialysis: MF59-adjuvanted versus non-adjuvanted vaccines. Hum Vaccin Immunother. 2016;12(11):2902–2908. doi: 10.1080/21645515.2016.1191717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Broeders NE, Hombrouck A, Lemy A, Wissing KM, Racape J, Gastaldello K, et al. Influenza A/H1N1 vaccine in patients treated by kidney transplant or dialysis: a cohort study. Clin J Am Soc Nephrol. 2011;6(11):2573–2578. doi: 10.2215/CJN.04670511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang YT, Wang JR, Lin MT, Wu CJ, Tsai MS, Wen-Chi CL, et al. Changes of immunogenic profiles between a single dose and one booster influenza vaccination in hemodialysis patients—an 18-week, open-label trial. Sci Rep. 2016;6:20725. doi: 10.1038/srep20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 83.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. The Cochrane Database Syst Rev. 2013;1:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bond TC, Spaulding AC, Krisher J, McClellan W. Mortality of dialysis patients according to influenza and pneumococcal vaccination status. Am J Kidney Dis. 2012;60(6):959–965. doi: 10.1053/j.ajkd.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Gilbertson DT, Guo H, Arneson TJ, Collins AJ. The association of pneumococcal vaccination with hospitalization and mortality in hemodialysis patients. Nephrol Dial Transpl. 2011;26(9):2934–2939. doi: 10.1093/ndt/gfq853. [DOI] [PubMed] [Google Scholar]
- 86.Linnemann CC, Jr, First MR, Schiffman G. Revaccination of renal transplant and hemodialysis recipients with pneumococcal vaccine. Arch Intern Med. 1986;146(8):1554–1556. [PubMed] [Google Scholar]
- 87.Fuchshuber A, Kuhnemund O, Keuth B, Lutticken R, Michalk D, Querfeld U. Pneumococcal vaccine in children and young adults with chronic renal disease. Nephrol Dial Transpl. 1996;11(3):468–473. [PubMed] [Google Scholar]
- 88.Nikoskelainen J, Koskela M, Forsstrom J, Kasanen A, Leinonen M. Persistence of antibodies to pneumococcal vaccine in patients with chronic renal failure. Kidney Int. 1985;28(4):672–677. doi: 10.1038/ki.1985.182. [DOI] [PubMed] [Google Scholar]
- 89.Mitra S, Stein GE, Bhupalam S, Havlichek DH. Immunogenicity of 13-valent conjugate pneumococcal vaccine in patients 50 years and older with end-stage renal disease and on dialysis. Clin Vaccine Immunol. 2016;23(11):884–887. doi: 10.1128/CVI.00153-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis. 2003;187(10):1639–1645. doi: 10.1086/374784. [DOI] [PubMed] [Google Scholar]
- 91.Kumar D, Welsh B, Siegal D, Chen MH, Humar A. Immunogenicity of pneumococcal vaccine in renal transplant recipients–three year follow-up of a randomized trial. Am J Transplant. 2007;7(3):633–638. doi: 10.1111/j.1600-6143.2007.01668.x. [DOI] [PubMed] [Google Scholar]
- 92.Tobudic S, Plunger V, Sunder-Plassmann G, Riegersperger M, Burgmann H. Randomized, single blind, controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in renal transplant recipients. PloS One. 2012;7(9):e46133. doi: 10.1371/journal.pone.0046133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 94.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization. Department of Communicable Disease, Surveillance and Response. Prevention ofhospital-acquiredinfections. http://apps.who.int/medicinedocs/documents/s16355e/s16355e.pdf. Accessed 05 May 2018.
- 96.Flodgren G, Conterno LO, Mayhew A, Omar O, Pereira CR, Shepperd S. Interventions to improve professional adherence to guidelines for prevention of device-related infections. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD006559.pub2. [DOI] [PubMed] [Google Scholar]
- 97.Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. J Am Med Assoc. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 98.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst Pharm. 2013;70(3):195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 99.American Dental Association. Antibiotic Prophylaxis Prior to Dental Procedures. Available: https://www.ada.org/en/member-center/oral-health-topics/antibiotic-prophylaxis. Accessed 05 May 2018.
- 100.Calfee DP. Multidrug-resistant organisms in dialysis patients. Semin Dial. 2013;26(4):447–456. doi: 10.1111/sdi.12094. [DOI] [PubMed] [Google Scholar]