Abstract
During systemic infection of susceptible hosts, Salmonella enterica colonizes the gall bladder, which contains lethal concentrations of bile salts. Recovery of Salmonella cells from the gall bladder of infected mice yields two types of isolates: (i) bile-resistant mutants; (ii) isolates that survive lethal selection without mutation. Bile-resistant mutants are recovered at frequencies high enough to suggest that increased mutation rates may occur in the gall bladder, thus providing a tentative example of stress-induced mutation in a natural environment. However, most bile-resistant mutants characterized in this study show defects in traits that are relevant for Salmonella colonization of the animal host. Mutation may thus permit short-term adaptation to the gall bladder at the expense of losing fitness for transmission to new hosts. In contrast, non mutational adaptation may have evolved as a fitness-preserving strategy. Failure of RpoS− mutants to colonize the gall bladder supports the involvement of the general stress response in non mutational adaptation.
Introduction
Variation of mutation rates in response to environmental factors is an old prediction of population genetics1, and has been confirmed by studies in bacterial populations2–6. Different kinds of stress induce different types of mutations, thus supporting the view that increase of the mutation rate under stress is an adaptive response indeed7.
A drawback of mutational adaptation is that mutations are often deleterious, and their accumulation in a population can decrease fitness8,9. In fact, bacterial populations with high mutation rates adapt quickly to a given environment but are outcompeted by non mutators in the long run10. This loss of long term fitness may contribute to explain the evolutionary emergence of non mutational strategies that adapt bacterial populations to cope with environmental challenges11–13.
Here, we have investigated the contribution of mutational and non mutational adaptation to bacterial survival in a natural environment, the gall bladder of the house mouse, Mus musculus L. The gall bladder is an infamous environment for bacteria due to the presence of high concentrations of bile salts that cause membrane disruption, protein denaturation, and DNA damage14,15. However, certain bacterial pathogens are able to colonize the gall bladder, and a relevant example is Salmonella enterica14. Invasion of the gall bladder epithelium and formation of biofilms can protect Salmonella from bile; however, planktonic Salmonella cells are also found in the gall bladder lumen15–17. Studies in vitro have shown that S. enterica can survive in the presence of a lethal concentration of bile salts by both mutational and non mutational mechanisms18. Among the mutations found to confer bile resistance, one class affects lipopolysaccharide transport genes18, an observation consistent with the role played by the LPS as an envelope barrier to bile salt uptake19. In turn, non mutational adaptation in vitro has been shown to involve upregulation of the RpoS-dependent general stress response18. Additional stress responses may be also involved, including activation of efflux systems and remodeling of the cell envelope18,20,21.
Our investigation of adaptation of S. enterica to the gall bladder offers the obvious advantage that the setting is a natural environment. A disadvantage, however, is that statistical analysis is hampered by the ignorance of bacterial population sizes inside the gall bladder, of bacterial cell death rates, and of other parameters. Despite this limitation, we present bona fide evidence that high mutation rates may occur in the gall bladder, thus suggesting the occurrence of stress-induced mutation. However, a significant fraction of mutations that permit survival in the gall bladder appear to impair other virulence-related traits. We also show that non mutational adaptation to bile is a common phenomenon, as previously observed in laboratory trials18. Furthermore, we tentatively propose that the RpoS-dependent general stress response may play a crucial role in gall bladder colonization.
Results
Recovery of Salmonella enterica populations from murine gall bladders
Salmonella infection was performed on two strains of mice: BALB/c, an immunodeficient breed suitable to monitor systemic, acute infection22–24 and 129S2/SvPasCrl, an immunoproficient breed that permits monitoring of long lasting, persistent infection25. Both types of mice were infected by the oral route.
Two days after oral infection, feces were homogenized in LB to obtain a final concentration of 1 g/l, and aliquots were spread on LB with ox bile. Bile-resistant colonies were not detected, indicating that bile-resistant mutants were rare or absent in the intestine of infected animals.
The nineteen BALB/c mice showed customary disease symptoms after five days, and were euthanized. Gall bladders were recovered, homogenized, and serial dilutions (10−1, 10−2, 10−3, and 10−4) were prepared in 0.9% NaCl. To select Salmonella isolates avoiding growth of other intestinal bacteria, the dilutions were plated on LB agar with streptomycin and incubated overnight at 37 °C (strain SL1344 is intrinsically resistant to streptomycin). The following day, the colonies were counted. Table 1 shows the total number of colony-forming-units (CFU) recovered from the gall bladder of each BALB/c mouse (applying dilution factors to the calculations when necessary). The same table shows the number of CFU detected upon replica-printing onto plates containing increasing concentrations of ox bile (12–20%). Because the MIC of ox bile for the wild type is 12%, only isolates with stable bile resistance (in other words, mutants) can grow on higher concentrations of bile. Substraction of the number of mutants from the total number of CFU provides the number of isolates that had undergone non mutational adaptation.
Table 1.
Number of Salmonella CFU recovered from the gall bladder of BALB/c mice.
| Mouse | Total number of CFU recovered from the gall bladder | Number of bile-resistant CFU after non selective growth |
|---|---|---|
| 1 | 63 | 2 |
| 2 | 548 | 8 |
| 3 | 105 | 1 |
| 4 | 475 | 0 |
| 5 | 6,694 | 1 |
| 6 | 51 | 0 |
| 7 | 31,204* | 1 |
| 8 | 37 | 0 |
| 9 | 1,042 | 1 |
| 10 | 222 | 40 |
| 11 | 2,858 | 50 |
| 12 | 203,400* | 32 |
| 13 | 182 | 11 |
| 14 | 422 | 16 |
| 15 | 202,500* | 49 |
| 16 | 1,599,000* | 122 |
| 17 | 1,018 | 20 |
| 18 | 2,783 | 17 |
| 19 | 11,203* | 29 |
*Inferred from dilution and plate counts.
To analyze the S. enterica populations that colonized the gall bladder upon long lasting infection, twenty-five 129S2/SvPasCrl mice were infected. Because the mice did not show clinical disease symptoms after infection, mouse feces were periodically collected to confirm ongoing infection26. These surveys were performed weekly during the first month, and two-weekly afterwards. The presence of the inoculated Salmonella strain was confirmed by observing colony formation on LB agar containing streptomycin. Plating of culture aliquots to LB agar containing 13% ox bile failed to detect bile-resistant mutants in feces. Seven 129S2/SvPasCrl mice cleared the Salmonella infection during the first four weeks, and four additional mice during the second month after infection. The remaining fourteen 129S2/SvPasCrl mice were euthanized after 10 weeks of infection. Gall bladders were recovered and homogenized, and Salmonella CFU were recovered. Discrimination between bile-resistant mutants and unstable bile-resistant isolates was performed as above. Table 2 shows the total number of CFU and the number of stable bile-resistant CFU present in the gall bladder of each 129S2/SvPasCrl mouse.
Table 2.
Numbers of Salmonella CFU recovered from the gall bladder of 129S2/SvPasCrl mice.
| Mouse | Total number of CFU recovered from the gall bladder | Number of bile-resistant CFU after non selective growth |
|---|---|---|
| 1 | 556 | 8 |
| 2 | 14 | 0 |
| 3 | 5 | 0 |
| 4 | 78 | 3 |
| 5 | 23 | 0 |
| 6 | 8 | 1 |
| 7 | 27 | 0 |
| 8 | 720 | 4 |
| 9 | 45 | 0 |
| 10 | 236 | 10 |
| 11 | 231 | 1 |
| 12 | 49 | 0 |
| 13 | 89 | 4 |
| 14 | 16 | 0 |
Relevant observations from these trials were as follows:
-
(i)
The numbers of CFU found in the gall bladder showed high variability from one mouse to another (Tables 1 and 2). Note that all mice from each strain (BALB/c or 129S2/SvPasCrl) were genetically identical and that inoculation was performed with identical numbers of cells. High variability suggests that gall bladder colonization may be under the influence of multiple factors, probably with stochastic components including clonal expansion.
-
(ii)
The numbers of Salmonella colony-forming-units found in the gall bladders of 129S2/SvPasCrl mice were lower than in BALB/c mice. Furthermore, gall bladders that did not appear to contain Salmonella cells were detected in 129S2/SvPasCrl mice but not in BALB/c mice (Tables 1 and 2). Clearance of infection was therefore significant in immunocompetent mice, and absent in immunodeficient mice.
A relevant conclusion from the data shown in Tables 1 and 2 is that the majority of isolates recovered from mice gall bladders appear to derive from Salmonella cells that had survived in the gall bladder without mutation. Bile-resistant mutants were not even obtained in certain cases (BALB/c mice #4, #6 and #8, and 129S2/SvPasCrl mice #2, #3, #5, #7, #9, #12, and #14). These observations are in agreement with a previous study of bile resistance in vitro describing that non-mutational adaptation to bile was more frequent than mutation18. However, the number of bile-resistant mutants was remarkably high. An extreme case was BALB/c mouse #10, which yielded 40 bile-resistant mutants vs 182 isolates with unstable bile resistance. Mutant frequencies cannot be calculated due to our ignorance of critical numbers: the bacterial population size, the number of cell divisions within the gall bladder, the bacterial death rate, etc. Furthermore, bile-resistant isolates from the same gall bladder may be siblings. However, the relative abundance of bile-resistant mutants may suggest the occurrence of adaptive mutations in response to the antimicrobial and mutagenic effects of bile27,28.
Whole-genome sequencing in bile-resistant mutants isolated from gall bladders
Ten bile-resistant mutants of independent origin (each isolated from a different BALB/c mouse gall bladder) where chosen for further study. The bile resistant phenotype of the mutants was confirmed by determination of the minimal inhibitory concentration of sodium deoxycholate (DOC) (Table 3). Full genome sequencing was then performed, including the genome of the laboratory stock of S. enterica SL1344 as a control. DNA sequencing data were validated by sequencing PCR products generated with high fidelity DNA polymerase. The oligonucleotides used to amplify appropriate DNA regions from each mutant are listed in Table S1. Alignment with the S. enterica SL1344 genome sequence (ASM21085v2) was used to identify DNA sequence differences. The alignment tool used was the BWA-MEM algorithm29,30. Relevant observations were as follows (see also Table 3):
-
(i)
Mutant #1 harbored a transversion in rlpB, a gene that encodes a lipoprotein B precursor in E. coli and is involved in LPS transport31. A point mutation in rlpB had been previously described in a S. enterica bile-resistant mutant isolated in vitro18. Interestingly, rlpB has been reported to be upregulated by bile in S. enterica32.
-
(ii)
Mutant #2 harbored a transition in pbgA, which encodes a transmembrane protein involved in transport of acidic diphosphatidylglycerols (also known as cardiolipins) to the outer membrane33. Transcription of pbgA is upregulated by bile32.
-
(iii)
Mutant #3 harbored a transversion in the dipZ gene. In E. coli, this gene encodes a protein involved in the formation of disulfide bonds in proteins that are translocated across the cytoplasmic membrane34,35. Evidence exists that DipZ may play a role in copper tolerance36.
-
(iv)
Mutants #4 and #7 harbor mutations in genes that encode cell division factors (ftsQ and ftsK) and show increased expression in the presence of bile32. In mutant #4, a transversion changes the ftsQ gene start codon from ATG to TTG, which is the second most used start codon in E. coli37. Mutant #7 presents an in-frame deletion of 36 nt that results in loss of 12 amino acids. Studies in E. coli have shown that both FtsQ and FtsK are essential membrane proteins that are recruited to the Z ring and participate in septum formation38,39. Interestingly, E. coli FtsQ has been shown to interact with DamX, a septal protein involved in bile resistance in both Salmonella and E. coli40,41.
-
(v)
Four independent mutants (#5, #7, #9 and #10) harbored mutations of various types in intergenic regions. Interestingly, in mutants #5 and #10 the mutations are located in genome regions that are highly induced by bile and include the small RNAs STnc400 and GlmZ32.
-
(vi)
Mutant #6 shows a nucleotide substitution in the DNA adenine methylase gene, dam. This result was unexpected and difficult to interpret because dam mutations usually cause bile sensitivity27,42–44.
-
(vii)
Mutant #7 presents mutations in two unknown loci, STM1268 and ygcF. A homolog of ygcF has been shown to be activated by copper shock in the plant pathogen Erwinia amylovora, and its deletion renders a copper-sensitive phenotype45.
-
(viii)
Mutant #8 harbors a transition in the yhbG gene (also known as lptB, lipopolysaccharide transport protein). In E. coli, this gene encodes an ABC transporter that may play a role in envelope integrity46–49. The S. enterica yhbG gene was found to be upregulated in a pig model of S. enterica persistence50. Interestingly, alteration of LPS transport in S. enterica has been previously shown to play a role in bile resistance18.
Table 3.
Mutations present in the genome of bile resistant derivatives of S. enterica SL1344 isolated from gall bladders.
| Mutant # | MIC of DOC (%)a | Genome position | Locus affected | Mutation | Predicted change | Cellular function affected |
|---|---|---|---|---|---|---|
| 1 | 14 | 708234 | rlpB | G → T | Ala → Glu | Lipopolysaccharide transport |
| 2 | 13 | 2326694 | pbgA | A → T | His → Leu | Outer membrane maintenance |
| 3 | 13 | 4589340 | dipZ | C → A | Ala → Ser | Copper tolerance and cytochrome c biogenesis |
| 4 | 13 | 153536 | ftsQ | A → T | Met → Leu (start codon change) | Cell division |
| 5 | 14 | 1072585 | Intergenic region STnc400 (sRNA) and STM3845 (hypothetical protein) | Deletion of 1 nt (A) | Unknown | Unknown |
| 6 | 14 | 3660275 | dam | T → A | Synonym amino acid change | DNA methylation |
| 7 | 14 | 994451 | ftsK | Deletion of 36 nt | Loss of 12 amino acids | Cell division |
| 1306516 | STM1268 | C → T | Ala → Val | Unknown | ||
| 3118294 | ygcF | A → C | Val → Gly | Unknown | ||
| 3216955 | Intergenic region STM3034 (hypothetical virulence protein) and STM3036 (hypothetical protein) | G → A | Unknown | Unknown | ||
| 8 | 14 | 3505277 | yhbG (lptB) | G → A | Ala → Thr | Lipopolysaccharide transport |
| 9 | 13 | 2728613 | Intergenic region gogB (effector protein) and STM2585 (hypothetical transposase) | Insertion of 6 nt | Unknown | Unknown |
| 10 | 14 | 4162532 | Intergenic region yifK (probable amino acid permease) and GlmZ (sRNA) | Deletion of 15 nt | Unknown | Unknown |
aThe MIC of DOC for the wild type is 7%.
Growth of bile-resistant mutants
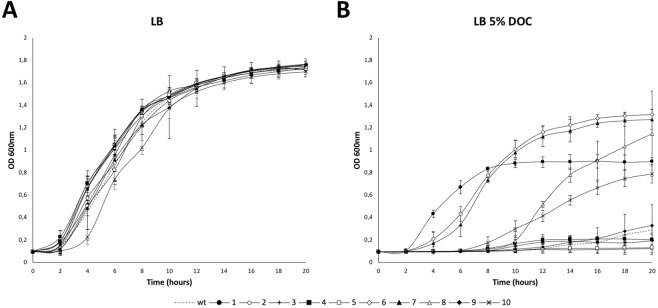
Because slow growth of certain mutants was observed during MIC determinations, we monitored growth of the 10 bile-resistant mutants under study in LB broth and LB broth supplemented with a sublethal concentration of DOC, including the wild type as control. While the 10 mutants under study grew normally in LB, half of the mutants (#1, #6, # 7, #8 and #10) showed faster growth than the wild type in the presence of DOC, four of the mutants showed slower growth (#2, #3, #4 and #5), and only one mutant (#9) showed a growth rate similar to the wild type (Fig. 1). Growth restraint in specific host niches has been shown to be an adaptive response of bacterial pathogens including Salmonella51–54. Hence, the possibility that slow growth may contribute to bile resistance may be considered. Albeit speculative, this hypothesis is supported by the observation that non-dividing cultures of wild type S. enterica show increased resistance to bile salts55.
Figure 1.
Growth of bile-resistant mutants. Growth curves of S. enterica bile-resistant mutants isolated from murine gall bladders in LB (A) and LB with 5% sodium deoxycholate (B). The wild type is included as a control in both graphs. Error bars represent the standard error of the mean from 4 independent replicates.
Analysis of virulence-related traits in bile-resistant mutants
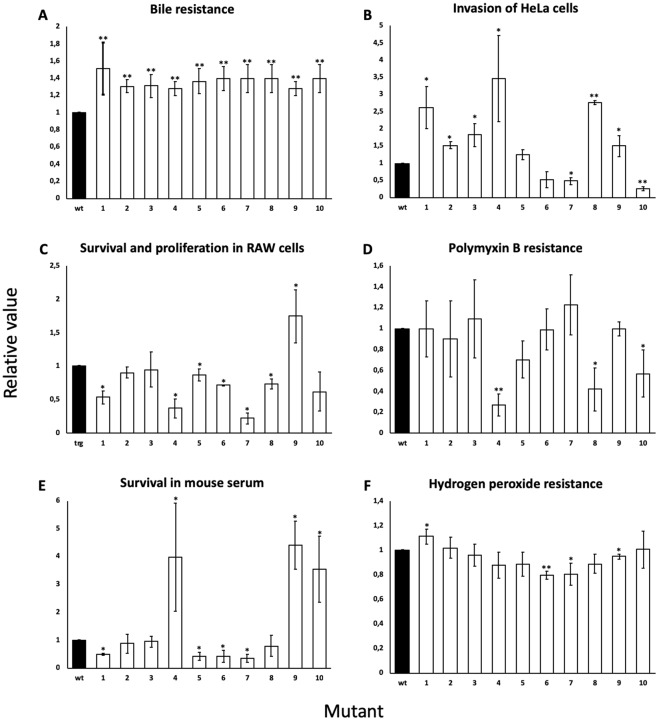
A potential drawback of mutation as an adaptive strategy is impairment of fitness upon environmental change56. Because the bile-resistance mutations identified in this study mapped in loci potentially involved in multiple physiological activities, we examined whether they affected virulence-related traits other than bile resistance. For this purpose, we examined the ability of the 10 mutants under study to invade HeLa epithelial cells, to survive and proliferate inside macrophages in vitro, to resist polymyxin B (a cationic antimicrobial peptide that interacts with the cellular envelope and is similar to those found in the intestine epithelium), to survive exposure to mouse serum (a test that provides information about the ability of the pathogen to survive the bactericidal activity of the complement), and to resist hydrogen peroxide, an antibacterial molecule produced inside phagocytes. As a control, resistance to ox bile was also tested (Fig. 2A). Relevant observations can be summarized as follows:
-
(i)
Six out of 10 mutants were able to invade epithelial cells more efficiently than the wild type (#1, #2, #3, #4, #8 and #9). Two mutants invaded less efficiently (#7 and #10), and another two mutants (#5 and #6) invaded like the wild type (Fig. 2B).
-
(ii)
Survival and/or proliferation inside macrophages were found to be impaired in 5/10 mutants (#1, #4, #6, #7 and #8). Four mutants (#2, #3, #5 and #10) behaved similar to the wild type, and mutant #9 showed increased survival (Fig. 2C).
-
(iii)
Seven mutants (#1, #2, #3, #6, #7 and #9) showed levels of polymyxin resistance similar to that of the wild type; the remaining mutants (#4, #5, #8, and #10) showed lower levels of resistance (Fig. 2D)
-
(iv)
Survival to mouse serum was found to be lower in 4 mutants (#1, #5, #6, and #7). Three mutants (#4, #9, and #10) showed higher survival and another three (#2, #3, and #8) behaved like the wild type (Fig. 2E).
-
(v)
Wild type (or near-wild type) levels of peroxide resistance were found in most mutants. Exceptions were #1 and #6 (Fig. 2F).
Figure 2.
Assessment of virulence-related fitness. Resistance to bile (A), invasion of Hela epithelial cells (B), survival and proliferation inside macrophages (C), resistance to polymyxin B (D), survival in mouse serum (E) and resistance to hydrogen peroxide (E) of the bile-resistant mutants under study. The values obtained for each mutant and condition were normalized to those of the wild type (which was set as 1 for each condition). Absolute chemical concentrations were: DOC, 7%; polymixin B, 0.4 μg/ml; hydrogen peroxide, 0.002%. Standard deviations of 3 independent experiments are shown. Bars with asterisks are significantly different according to the two-tailed t test (*P < 0.05; **P < 0.01).
A noteworthy observation is that 7/10 bile-resistant mutants (#1, #4, #5, #6, #7, #8, and #10) showed one or more virulence-related defects. In such cases, one may tentatively conclude that resistance to bile is achieved at the expense of virulence impairment. On the other hand, it is conceivable that increased invasiveness of epithelial cells, especially in mutants #1, #4, and #8 might contribute to gall bladder colonization16,17. However, these mutants might be anyway avirulent as they are deficient in one or more virulence traits (Fig. 2). Altogether, the above observations suggest that mutational adaptation to the gall bladder may involve fitness tradeoffs in certain cases. Albeit speculative, this view is in agreement with the evidence that within-host evolution of pathogens can result in loss of fitness for transmission9.
Role of the RpoS general stress response in non mutational adaptation to bile
A previous study in vivo provided evidence that activation of the RpoS-dependent general stress response is a major mechanism for non mutational adaptation to bile18. Based on this antecedent, we tested the ability of an RpoS− strain to colonize the gall bladder. For this purpose, we co-infected five BALB/c mice with two strains, SV5561 (RpoS−) and SV4898 (wild type). Infection was performed by the oral route, and mice were euthanized after 7–10 days. Gall bladders were then recovered, and bacterial cells were plated onto LB agar + kanamycin (to distinguish the wild type) and LB agar + ampicillin (to distinguish the RpoS− strain). Kmr and Apr colonies were then replica-printed to LB + ox bile to determine whether bile-resistant mutants were present. The RpoS− strain largely failed to colonize the gall bladder (Table 4), and bile-resistant mutants were a significant fraction of the rare RpoS− isolates recovered. Failure of the RpoS− mutant to colonize the gall bladder is in agreement with the inefficient colonization of other organs by RpoS− strains57,58 and with the sensitivity of RpoS− mutants to bile18,21.
Table 4.
Numbers of CFU recovered from gall bladders of BALB/c mice co-infected with RpoS+ and RpoS− strains.
| Mouse | Number of RpoS+ (Kmr) CFU recovered from the gall bladder | Number of RpoS− (Apr) CFU recovered from the gall bladder | ||
|---|---|---|---|---|
| Bile-sensitive | Bile-resistant | Bile-sensitive | Bile-resistant | |
| 1 | 203 | 7 | 0 | 1 |
| 2 | 1,788 | 141 | 5 | 6 |
| 3 | 4,661 | 194 | 14 | 21 |
| 4 | 1,507 | 11 | 7 | 12 |
| 5 | 55,000* | 1,106 | 9 | 13 |
*Inferred from dilution and plate counts.
Discussion
Recovery of Salmonella isolates from murine gall bladders yields bile-resistant mutants and isolates that lose bile resistance after non selective growth. Their absolute and relative numbers fluctuate widely, suggesting that colonization of the gall bladder may involve multiple factors, perhaps including stochastic components. This view is supported by a previous study in vitro indicating that S. enterica cultures contain cells that are preadapted to survive bile by two types of stochastic mechanisms: mutation and “noisy” activation of stress responses18.
Bile-resistant mutants were recovered at high frequency from gall bladders (Tables 1 and 2). Unfortunately, several hurdles impede the estimation of mutation rates: ignorance of the size of the Salmonella populations that had colonized the gall bladder, of the number of cell divisions undergone within the gall bladder, and of the rate of cell death. However, if the populations that colonize the gall bladders of mice are made of less than 50 bacterial cells as shown in other organs59, the frequency of mutants may be considered extremely high. The possibility that the bile-resistant isolates recovered from the gall bladder derived from spontaneous mutants present in the inoculum seemed unlikely as bile-resistant mutants were not detected in fecal samples. Occurrence of stress-induced mutation in the gall bladder may thus be tentatively proposed. This tentative conclusion is supported by a previous study showing the occurrence of mutations that improved Salmonella colonization of the intestine, and potentially of the liver and the spleen60.
The nature of the bile resistance mutations found (Table 3) permits tentative interpretation of their consequences. Most mutations (7 out of 13) map in loci that encode envelope-related structures (rlpB, pbgA, dipZ, yhbG [lptB], ftsQ, ftsK, and the intergenic region between yifK and glmZ), in agreement with previous studies that underline the relevance of the bacterial envelope as a barrier to bile28,61–65. Two such mutations (rlpB and yhbG) map in genes involved in LPS transport, previously shown to play a role in bile resistance18. Curiously enough, a mutation in rlpB was also found to confer bile resistance in a study that selected bile resistant-mutants in vitro18. In turn, the discovery of mutations in cell division factors is in agreement with the upregulation of the cell division factor gene zapB by bile18 and with the occurrence of peptidoglycan remodeling in the presence of bile66. A side but interesting observation is that a significant fraction of the loci affected (yhbG, pbgA, ftsQ, ftsK, STnc400, rlpB, and glmZ) are known to be upregulated by bile32.
Because a fraction of bile resistant mutants under study grew slowly during MIC tests, their growth patterns were monitored. These surveys were prompted by the well known fact that certain bacterial pathogens reduce or arrest growth in host environments, a strategy often known as “dormancy”67. In Salmonella, dormant-like states have been shown to occur inside phagocytes51 and fibroblasts52,53. While all the mutants under study showed similar or identical growth rates in LB (Fig. 1), 4 out of 10 mutants showed growth rate reduction in the presence of a sublethal concentration of sodium deoxycholate. Slow growth rate is in sharp contrast with their ability to survive in the presence of a lethal concentration of DOC in MIC assays (>12% in all cases), and may support the speculation that a dormant-like state contributes to bile resistance in certain cases.
A notion well established in the literature is that mutations that confer rapid adaptation in a given environment may not necessarily be beneficial in the long term9. In the case of Salmonella, which thrives in multiple environments inside and outside hosts, shedding of a virulent population to the environment is crucial to warrant transmission to new hosts68. On these grounds, we tested virulence-relevant traits of the bile-resistant mutants under study by performing reductionist assays in vitro (Fig. 2). To our surprise, most mutants characterized in this study (7 out of 10) turned out to be affected in one or more virulence-related traits. The ability of such mutants to thrive inside the gall bladder may thus be accompanied by deleterious consequences in other environments (and the list may fall short to describe other potential long term defects as out-of-host challenges were not tested). Stress-induced mutation in the gall bladder may thus provide genotypic diversity to survive in a harsh niche. However, increased mutation rates may also lead the pathogen towards a dead end in the pursuit of short-term adaptation9.
Inefficient colonization of the gall bladder by RpoS− mutants supports the view that non mutational adaptation involves upregulation of the general stress response, a phenomenon previously documented in vitro18. Additional and/or alternative physiological adjustments may further contribute to survival of planktonic cells in the bile-laden gall bladder lumen15. Whatever the molecular mechanisms involved, the frequent occurrence of non mutational adaptation suggests that it may have selective value, and one potential advantage may be avoidance of payoffs associated to mutation.
Methods
Bacterial strains
Strains of Salmonella enterica serovar Typhimurium (often abbreviated as S. enterica) derive from the mouse-virulent, streptomycin resistant strain SL134469. Strain SV4898 (trg::MudJ KmR) was used as wild type during assays of virulence in mice and of proliferation and survival in macrophages. The mutation trg::MudJ KmR is neutral for virulence70. SV5561 is a RpoS− mutant, and the mutation is null18.
Culture media and growth conditions
Lysogeny broth (LB) was used as standard liquid medium71. LB plates contained agar at 15 g/l and streptomycin at 0.2 g/l as final concentrations. To assay S. enterica bile resistance levels, sodium choleate (ox bile extract, Sigma Aldrich) plates were prepared with stocks of LB agar containing 12, 14, 16, 18 and 20% ox bile extract. In most experiments, cultures were grown at 37 °C with shaking at 250 rpm in a New Brunswick Innova 3100 waterbath. For oral infection of mice, bacterial cultures were grown overnight in LB at 37 °C without shaking. For invasion assays, bacteria were grown overnight in LB broth with 0.3 M NaCl at 37 °C without shaking. For survival/proliferation assays, bacteria were grown 20–24 h in LB broth at 37 °C with shaking as described in Segura et al.70. Recovery of Salmonella from feces was performed as described elsewhere25.
Growth curves
Overnight cultures of the wild type strain and of bile-resistant mutants were diluted 1:25 in LB and incubated at 37 °C with shaking at 250 rpm for 1 h. Aliquots containing around 3 × 102 colony-forming-units (CFU) were transferred to U-bottom 96-well polypropylene microtiter plates (Greiner Bio One) containing either LB or LB with 5% sodium deoxycholate in a final volume of 0.2 ml. The plates were incubated at 37 °C with shaking on an automated microplate reader (Synergy HTX Multi-Mode Reader, Biotek) and the absorbance at 600 nm for each well was measured every 30 min. The duration of each assay was 20 h. The assays were performed in triplicate.
Isolation and characterization of isolates from mice gall bladders
Eight-week-old female mice belonging to strains BALB/c and 129S2/SvPasCrl, (Charles River Laboratories, Santa Perpetua de Mogoda, Spain), were inoculated with appropriate S. enterica strains. Information on BALB/c and 129S2/SvPasCrl mice strains can be found at the Mouse Genome Informatics page, http://www.informatics.jax.org/. Bacterial cultures were previously grown overnight at 37 °C in LB without shaking. Oral inoculation was performed by feeding the mice with 25 μl of NaCl 0.9% containing 0.1% lactose and 108 bacterial colony-forming units (CFU). Bacterial cells were recovered from the gall bladder of BALB/c mice 5–10 days after oral infection. Recovery from the gall bladder of 129S2/SvPasCrl mice was performed 70–80 days after the infection. Gall bladder extracts were plated on LB + streptomycin and grown overnight at 37 °C. The following day, the plates were replicated onto LB agar that contained increasing concentrations of ox bile (12, 14, 16, 18, and 20%), and incubated overnight at 37 °C. Bile-resistant mutants were isolated and purified, and the stability of their bile-resistant phenotype was confirmed by determining the MIC of DOC in 96-well plates. Isolates from the gall bladders of mice co-infected with RpoS+ and RpoS− strains were recovered by plating identical amounts of gall bladder homogenate on LB kanamycin and LB ampicillin. After 24 incubation, colonies were replica-plated on LB agar containing 12–16% ox bile.
Determination of minimal inhibitory concentrations (MICs)
Exponential cultures in LB broth were prepared, and samples containing around 3 × 10² colony-forming-units (CFU) were transferred to polypropylene microtiter plates (Soria Genlab) containing known amounts of sodium deoxycholate (DOC) (Sigma Aldrich), the archetypal and most abundant bile salt72. Growth was visually monitored after 12–36 h. Similar protocols were used for determination of MICs of polymyxin B sulfate salt (Sigma Aldrich) and hydrogen peroxide. The Student’s t test was used to determine whether the differences in MIC values were significant.
Extraction of genomic DNA
For the extraction of genomic DNA, 5 ml of cells grown to late exponential phase were collected and re-suspended in 0.4 ml of lysis buffer (Tris-HCl 50 mM pH 8, EDTA 10 mM, NaCl 100 mM, SDS 0.2%). Four μl of RNAse (10 mg/ml) were added and the mixture was incubated at 37 °C for 30 min. Twenty μl of a preparation of proteinase K (20 mg/ml) was added and the sample was incubated for 2 h at 65 °C. Finally, 3 or 4 extractions were performed with phenol:chloroform-isoamyl alcohol in a 2:1 proportion. One last extraction was performed with chloroform:isoamyl alcohol (24:1). DNA was precipitated at −20 °C by adding 1/10 volume of sodium acetate 3 M and 2.5 volumes of ethanol. After precipitation, genomic DNA was washed with 70% ethanol and re-suspended in 20 μl of TER buffer (Tris-HCl 100 mM pH 7.5, EDTA 1 mM pH 8, RNAse 20 μg/ml).
Whole genome DNA sequencing and analysis
Bacterial genome sequencing was performed at the DNA Sequencing Service of the University of Seville (Servicio General de Biología, CITIUS, Universidad de Sevilla, Spain). Sample preparation included gDNA isolation by phenolic extraction and ethanol precipitation. Genome sequencing employed Roche 454 FLX + technology on a GS FLX titanium system. Emulsion PCR and 454 pyrosequencing were performed and 1,031,375 reads with an average read length of 810 base pairs, totaling 1000 Mb. Whole genome sequences were analyzed using the Burrow-Wheeler Alignment tool (BWA), specifically the BWA-MEM algorithm29,30. As a reference, the genome sequence of the laboratory stock of S. enterica SL1344 was also analyzed. Genomes were submitted to NCBI GenBank and are available as SUB3834111 “Salmonella typhimurium isolates from Balb/c mice gall bladders (Urdaneta & Casadesus)”. PCR amplification of regions harboring mutations was performed using the primers listed in Table S1. Chromosomal DNA samples obtained by PCR were sequenced by Stab Vida (Caparica, Portugal).
Eukaryotic cell lines
HeLa cells (ATCC CCL2) and macrophages (RAW264.7) were used as models of nonphagocytic and phagocytic cells, respectively. Cells were routinely cultured at 37 °C with 5% CO2 in DMEM medium (Biowest) containing 10% fetal calf serum (Biowest), 4 mM L-glutamine (Biowest) and 1X penicillin-streptomycin solution (Biowest).
Invasion assays
Invasion assays followed a standard protocol70 with slight modifications. HeLa cells resuspended in DMEM were seeded and incubated 24 h before infection in 24 well plates (Thermo Scientific, Denmark) at a concentration of 1 × 105 cells/well. Bacteria were grown overnight (14–16 h) under invasive conditions: LB broth with 0.3 M NaCl at 37 °C without shaking. Bacteria diluted in DMEM were added to reach a MOI of 50:1 bacteria/HeLa cell. Thirty minutes after infection, the cells were washed twice with PBS and incubated in fresh DMEM containing 100 µg/ml gentamicin. Ninety minutes later cells were washed twice with PBS. Numbers of viable intracellular bacteria were obtained after lysis of infected cells with 1% Triton 100-X (prepared in PBS) and plating on LB agar plates. Infections were carried out in triplicate. Invasion rates were determined as the ratio between viable intracellular bacteria and viable bacteria added to infect the HeLa cells. The Student’s t test was used to determine whether the differences in invasion rates were significant.
Survival within macrophages
Survival/proliferation assessment, competitive index (CI) assays with S. enterica strains were performed as described elsewhere70. RAW264.7 macrophages were incubated on 24-well plates (Thermo Scientific, Denmark) 24 h before infection using a concentration of 1 × 105 cells/well. Bacteria were grown for 20–24 h in LB broth at 37 °C with shaking. A 1:1 mixture of the bacterial strains was prepared in DMEM without antibiotics, and added to cultured macrophages to reach a multiplicity of infection (MOI) of 50:1 bacteria/macrophage. Fifteen minutes after infection, cells were washed twice with PBS and incubated in fresh DMEM containing 100 µg/ml gentamicin. Sixty minutes later the concentration of gentamicin was lowered to 16 µg/ml. Numbers of viable intracellular bacteria were calculated by plating on LB X-Gal agar plates after cell lysis with 1% Triton X-100 at two time points: 1 h 15 min after infection and 24 h after infection. Infections were carried out in triplicate. The competitive index in proliferation (CIP) was calculated as described elsewhere70. The Student’s t test was used to determine whether the differences in competitive indexes were significant.
Survival in mouse serum
Ten μL of bacterial suspensions containing 1 × 104 bacteria/ml were incubated at 37 °C in 150 μL of either mouse serum (Sigma) with MgCl2 1.3 mM or PBS with MgCl2 1.3 mM. After 2 h incubation, 100 μL aliquots were plated on LB agar. Survival rates were determined as the ratio between viable bacteria treated with mouse serum and viable bacteria incubated in PBS. The Student’s t test was used to determine whether the differences in survival rates were significant.
Virulence assays in mice
BALB/c mice (Charles River Laboratories, Santa Perpetua de Mogoda, Spain) were inoculated with a 1:1 mixture of strains SV5561 and SV4898. Bacterial cultures were previously grown overnight at 37 °C in LB without shaking. Oral inoculation was performed by feeding the mice with 25 µl of PBS containing 0.1% lactose and 108 bacterial CFU. Bacteria were recovered from the gall bladder 7 days post-infection. A competitive index (CI) was calculated as described by Beuzón and Holden73.
Ethics statement
Animal research adhered to the principles mandatory in the European Union, as established in the Legislative Act 86/609 CEE (November 24, 1986) and followed the specific protocols established by the Royal Decree 1201/2005 of the Government of Spain (October 10, 2005). The protocols employed in the study were reviewed by the Comité Ético de Experimentación de la Universidad de Sevilla, and were approved on January 16, 2010 (permit number 59-A-2010).
Supplementary information
Acknowledgements
This study was supported by grants BIO2013-44220-R, PCIN-2015-131 and BIO2016-75235-P from the Ministerio de Economía y Competitividad (MINECO) of Spain and the European Regional Fund. V.U. was supported by a postdoctoral contract associated to grant CVI-5879 from the Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía, Spain. We are grateful to Jay Hinton and Al Darby, University of Liverpool, for help with the analysis of whole genome sequences, and to Ana I. Prieto, Francisco Ramos-Morales, Graciela Pucciarelli, and Francisco García-del Portillo for advice in mouse infections and Salmonella recovery from gall bladders. We appreciate the assistance provided by Modesto Carballo, Laura Navarro, and Cristina Reyes of the Servicio de Biología of the Centro de Investigación, Tecnología e Innovación de la Universidad de Sevilla (CITIUS).
Author Contributions
V.U. and S.B.H. carried out the experiments; V.U., S.B.H. and J.C. designed the experiments, interpreted results, and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41600-8.
References
- 1.Fitch WM. The challenges to darwinism since the last centennial and the impact of molecular studies. Evolution. 1982;36:1133–1143. doi: 10.1111/j.1558-5646.1982.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 2.Denamur E, Matic I. Evolution of mutation rates in bacteria. Mol. Microbiol. 2006;60:820–827. doi: 10.1111/j.1365-2958.2006.05150.x. [DOI] [PubMed] [Google Scholar]
- 3.Galhardo RS, Hastings PJ, Rosenberg SM. Mutation as a stress response and the regulation of evolvability. Crit. Rev. Biochem. Mol. Biol. 2007;42:399–435. doi: 10.1080/10409230701648502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean MM, Chang Y, Dhar G, Heiss JK, Johnson RC. Multiple interfaces between a serine recombinase and an enhancer control site specific DNA inversion. eLife. 2013;2013:1–21. doi: 10.7554/eLife.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald DM, Hastings PJ, Rosenberg SM. Stress-induced mutagenesis: Implications in cancer and drug resistance. Annu. Rev. Cancer Biol. 2017;1:119–140. doi: 10.1146/annurev-cancerbio-050216-121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster PL. Stress-induced mutagenesis in bacteria. Crit. Rev. Biochem. Mol. Biol. 2007;42:373–397. doi: 10.1080/10409230701648494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maharjan RP, Ferenci T. A shifting mutational landscape in 6 nutritional states: Stress-induced mutagenesis as a series of distinct stress input–mutation output relationships. PLoS Biol. 2017;15:1–22. doi: 10.1371/journal.pbio.2001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth JR, Kugelberg E, Reams AB, Kofoid E, Andersson DI. Origin of mutations under selection: The adaptive mutation controversy. Annu. Rev. Microbiol. 2006;60:477–501. doi: 10.1146/annurev.micro.60.080805.142045. [DOI] [PubMed] [Google Scholar]
- 9.Tanner JR, Kingsley RA. Evolution of Salmonella within hosts. Trends Microbiol. 2018;26:1–13. doi: 10.1016/j.tim.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turrientes MC, et al. Normal mutation rate variants arise in a mutator (MutS) Escherichia coli population. PLoS One. 2013;8:e72963. doi: 10.1371/journal.pone.0072963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez-Romero MA, Casadesús J. Contribution of phenotypic heterogeneity to adaptive antibiotic resistance. Proc. Natl. Acad. Sci. 2014;111:355–360. doi: 10.1073/pnas.1316084111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnoldini M, et al. Bistable expression of virulence genes in Salmonella leads to the formation of an antibiotic-tolerant subpopulation. PLoS Biol. 2014;12:e1001928. doi: 10.1371/journal.pbio.1001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cota I, et al. Epigenetic control of Salmonella enterica O-antigen chain length: A tradeoff between virulence and bacteriophage resistance. PLoS Genet. 2015;11:e1005667. doi: 10.1371/journal.pgen.1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bäumler AJ, Winter SE, Thiennimitr P, Casadesús J. Intestinal and chronic infections: Salmonella lifestyles in hostile environments. Environ. Microbiol. Rep. 2011;3:508–517. doi: 10.1111/j.1758-2229.2011.00242.x. [DOI] [PubMed] [Google Scholar]
- 15.Urdaneta V, Casadesús J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front. Med. 2017;4:1–13. doi: 10.3389/fmed.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menendez A, et al. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J. Infect. Dis. 2009;200:1703–1713. doi: 10.1086/646608. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Escobedo G, Gunn JS. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect. Immun. 2013;81:2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández SB, Cota I, Ducret A, Aussel L, Casadesús J. Adaptation and preadaptation of Salmonella enterica to bile. PLoS Genet. 2012;8:e1002459. doi: 10.1371/journal.pgen.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picken R, Beacham I. Bacteriophage-resistant mutants of Escherichia coli K12. Location of receptors within the lipopolysaccharide. J. Gen. Microbiol. 1977;102:305–318. doi: 10.1099/00221287-102-2-305. [DOI] [PubMed] [Google Scholar]
- 20.Prouty AM, Brodsky IE, Falkow S, Gunn JS. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology. 2004;150:775–783. doi: 10.1099/mic.0.26769-0. [DOI] [PubMed] [Google Scholar]
- 21.Urdaneta V, Casadesús J. Adaptation of Salmonella enterica to bile: Essential role of AcrAB-mediated efflux. Environ. Microbiol. 2018;20:1405–1418. doi: 10.1111/1462-2920.14047. [DOI] [PubMed] [Google Scholar]
- 22.Canonne-Hergaux F, Gruenheid S, Govoni G, Gros P. The Nramp1 protein and its role in resistance to infection and macrophage function. Proc. Assoc. Am. Physicians. 1999;111:283–289. doi: 10.1046/j.1525-1381.1999.99236.x. [DOI] [PubMed] [Google Scholar]
- 23.Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsolis RM, et al. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 1999;473:261–274. doi: 10.1007/978-1-4615-4143-1_28. [DOI] [PubMed] [Google Scholar]
- 25.Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J. Exp. Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Quintanilla M, Ramos-Morales F, Casadesús J. Conjugal transfer of the Salmonella enterica virulence plasmid in the mouse intestine. J. Bacteriol. 2008;190:1922–1927. doi: 10.1128/JB.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prieto AI, Ramos-Morales F, Casadesús J. Bile-induced DNA damage in Salmonella enterica. Genetics. 2004;168:1787–1794. doi: 10.1534/genetics.104.031062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. eprint arXiv:1303.3997 (2013).
- 31.Wu T, et al. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA. 2006;103:11754–11759. doi: 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kröger C, et al. An infection-relevant transcriptomic compendium for Salmonella enterica serovar Typhimurium. Cell Host Microbe. 2013;14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Dalebroux ZD, et al. Delivery of cardiolipins to the Salmonella outer membrane is necessary for survival within host tissues and virulence. Cell Host Microbe. 2015;17:441–451. doi: 10.1016/j.chom.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart EJ, Katzen F, Beckwith J. Six conserved cysteines of the membrane protein DsbD are required for the transfer of electrons from the cytoplasm to the periplasm of Escherichia coli. EMBO J. 1999;18:5963–5971. doi: 10.1093/emboj/18.21.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon EH, Page MD, Willis AC, Ferguson SJ. Escherichia coli DipZ: anatomy of a transmembrane protein disulphide reductase in which three pairs of cysteine residues, one in each of three domains, contribute differentially to function. Mol. Microbiol. 2000;35:1360–1374. doi: 10.1046/j.1365-2958.2000.01796.x. [DOI] [PubMed] [Google Scholar]
- 36.Gupta SD, Wu HC, Rick PD. A Salmonella typhimurium genetic locus which confers copper tolerance on copper-sensitive mutants of Escherichia coli. J. Bacteriol. 1997;179:4977–4984. doi: 10.1128/jb.179.16.4977-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blattner FR, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 38.Chen JC, Beckwith J. FtsQ, FtsL and Ftsl require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 2001;42:395–413. doi: 10.1046/j.1365-2958.2001.02640.x. [DOI] [PubMed] [Google Scholar]
- 39.Buddelmeijer N, Beckwith J. A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol. Microbiol. 2004;52:1315–1327. doi: 10.1111/j.1365-2958.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 40.López-Garrido J, Cheng N, García-Quintanilla M, García-del Portillo F, Casadesús J. Identification of the Salmonella enterica damX gene product, an inner membrane protein involved in bile resistance. J. Bacteriol. 2010;192:893–895. doi: 10.1128/JB.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Garrido, J. & Casadesús, J. The DamX protein of Escherichia coli and Salmonella enterica. 1, 285–288 (2010). [DOI] [PMC free article] [PubMed]
- 42.Heithoff DM, et al. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 2001;69:6725–6730. doi: 10.1128/IAI.69.11.6725-6730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pucciarelli MG, Prieto AI, Casadesus J, Garcia-del Portillo F. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology. 2002;148:1171–1182. doi: 10.1099/00221287-148-4-1171. [DOI] [PubMed] [Google Scholar]
- 44.Giacomodonato MN, Sarnacki SH, Caccuri RL, Sordelli DO, Cerquetti MC. Host response to a dam mutant of Salmonella enterica serovar Enteritidis with a temperature-sensitive phenotype. Infect. Immun. 2004;72:5498–5501. doi: 10.1128/IAI.72.9.5498-5501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Águila-Clares B, et al. Transcriptional response of Erwinia amylovora to copper shock: in vivo role of the copA gene. Mol. Plant Pathol. 2018;19:169–179. doi: 10.1111/mpp.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperandeo P, Pozzi C, Dehò G, Polissi A. Non-essential KDO biosynthesis and new essential cell envelope biogenesis genes in the Escherichia coli yrbG – yhbG locus. Res. Microbiol. 2006;157:547–558. doi: 10.1016/j.resmic.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Saurin W, Hofnung M, Dassa E. Getting in or out: early segregation between importers and exporters in the evolution of ATP-binding cassette (ABC) transporters. J. Mol. Evol. 1999;48:22–41. doi: 10.1007/PL00006442. [DOI] [PubMed] [Google Scholar]
- 48.Dassa E, Bouige P. The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001;152:211–229. doi: 10.1016/S0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- 49.Sperandeo P, Dehò G, Polissi A. The lipopolysaccharide transport system of Gram-negative bacteria. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2009;1791:594–602. doi: 10.1016/j.bbalip.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 50.van Parys A, et al. Tissue-specific Salmonella typhimurium gene expression during persistence in pigs. PLoS One. 2011;6:e24120. doi: 10.1371/journal.pone.0024120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helaine S, et al. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science. 2014;343:204–208. doi: 10.1126/science.1244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cano DA, et al. Salmonella enterica serovar Typhimurium response involved in attenuation of pathogen intracellular proliferation. Infect. Immun. 2001;69:6463–6474. doi: 10.1128/IAI.69.10.6463-6474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cano DA, Pucciarelli MG, Martínez-Moya M, Casadesús J, García-Del Portillo F. Selection of small-colony variants of Salmonella enterica serovar Typhimurium in nonphagocytic eucaryotic cells. Infect. Immun. 2003;71:3690–3698. doi: 10.1128/IAI.71.7.3690-3698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 55.Pucciarelli MG, Prieto AI, Casadesús J. & García-del Portillo, F. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology. 2002;148:1171–1182. doi: 10.1099/00221287-148-4-1171. [DOI] [PubMed] [Google Scholar]
- 56.Bataillon T, Bailey SF. Effects of new mutations on fitness: Insights from models and data. Ann. N. Y. Acad. Sci. 2014;1320:76–92. doi: 10.1111/nyas.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhlmurium mutants deficient in the expression of the RpoS (σs) regulon. Mol. Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 58.Wilmes-Riesenberg MR, Foster JW, Curtiss R. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect. Immun. 1997;65:203–210. doi: 10.1128/iai.65.1.203-210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim CH, et al. Independent bottlenecks characterize colonization of systemic compartments and gut lymphoid tissue by. Salmonella. PLoS Pathog. 2014;10:e1004270. doi: 10.1371/journal.ppat.1004270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Søndberg E, Jelsbak L. Salmonella typhimurium undergoes distinct genetic adaption during chronic infections of mice. BMC Microbiol. 2016;16:1–11. doi: 10.1186/s12866-016-0646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prieto AI, et al. Roles of the outer membrane protein AsmA of Salmonella enterica in the control of marRAB expression and invasion of epithelial cells. J. Bacteriol. 2009;191:3615–3622. doi: 10.1128/JB.01592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merritt ME, Donaldson JR. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J. Med. Microbiol. 2009;58:1533–1541. doi: 10.1099/jmm.0.014092-0. [DOI] [PubMed] [Google Scholar]
- 63.Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2:907–913. doi: 10.1016/S1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 64.Raivio TL. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 2005;56:1119–1128. doi: 10.1111/j.1365-2958.2005.04625.x. [DOI] [PubMed] [Google Scholar]
- 65.Álvarez-Ordóñez A, et al. Salmonella spp. survival strategies within the host gastrointestinal tract. Microbiology. 2011;157:3268–3281. doi: 10.1099/mic.0.050351-0. [DOI] [PubMed] [Google Scholar]
- 66.Hernández SB, Ayala JA, Rico-Pérez G. García-del Portillo, F. & Casadesús, J. Increased bile resistance in Salmonella enterica mutants lacking Prc periplasmic protease. Int. Microbiol. 2013;16:87–92. doi: 10.2436/20.1501.01.183. [DOI] [PubMed] [Google Scholar]
- 67.Balaban NQ, Gerdes K, Lewis K, McKinney JD. A problem of persistence: still more questions than answers? Nature Rev. Microbiol. 2013;11:587–591. doi: 10.1038/nrmicro3076. [DOI] [PubMed] [Google Scholar]
- 68.Monack DM. Salmonella persistence and transmission strategies. Curr. Opin. Microbiol. 2012;15:100–107. doi: 10.1016/j.mib.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 70.Segura I, Casadesús J, Ramos-Morales F. Use of mixed infections to study cell invasion and intracellular proliferation of Salmonella enterica in eukaryotic cell cultures. J. Microbiol. Methods. 2004;56:83–91. doi: 10.1016/j.mimet.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic. Escherichia coli. J. Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofmann AF, Hagey LR. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beuzón CR, Holden DW. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 2001;3:1345–1352. doi: 10.1016/S1286-4579(01)01496-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.