Abstract
Human tuberculosis disease (TB), caused by Mycobacterium tuberculosis (Mtb), is a complex disease, with a spectrum of outcomes. Genomic, transcriptomic and methylation studies have revealed differences between Mtb lineages, likely to impact on transmission, virulence and drug resistance. However, so far no studies have integrated sequence-based genomic, transcriptomic and methylation characterisation across a common set of samples, which is critical to understand how DNA sequence and methylation affect RNA expression and, ultimately, Mtb pathogenesis. Here we perform such an integrated analysis across 22 M. tuberculosis clinical isolates, representing ancient (lineage 1) and modern (lineages 2 and 4) strains. The results confirm the presence of lineage-specific differential gene expression, linked to specific SNP-based expression quantitative trait loci: with 10 eQTLs involving SNPs in promoter regions or transcriptional start sites; and 12 involving potential functional impairment of transcriptional regulators. Methylation status was also found to have a role in transcription, with evidence of differential expression in 50 genes across lineage 4 samples. Lack of methylation was associated with three novel variants in mamA, likely to cause loss of function of this enzyme. Overall, our work shows the relationship of DNA sequence and methylation to RNA expression, and differences between ancient and modern lineages. Further studies are needed to verify the functional consequences of the identified mechanisms of gene expression regulation.
Introduction
Human tuberculosis disease (TB), caused by Mycobacterium tuberculosis (Mtb), is a major global public health issue1. A deeper understanding of the biology of Mtb should reveal new insights that may help to improve diagnostics, treatments, vaccines and other much needed control measures. Mtb belongs to the M. tuberculosis complex (MTC), which consists of seven main lineages classified into modern (lineages 2–4), ancient (lineages 1, 5 and 6), and intermediate (lineage 7) strains2. The lineages vary in their geographic distribution and spread, with lineage 2 being particularly mobile with evidence of recent spread from Asia to Europe and Africa. Lineage 4 is common in Europe and southern Africa, coinciding with regions of high TB incidence and high levels of HIV co-infection. The lineages may vary in their propensity to transmit and to cause disease, and in the site and severity of disease3–5. A set of SNPs in the Mtb genome (size 4.4 Mb) has been identified that can be used to barcode sub-lineages6, leading to informatic tools that position sequenced samples within a global phylogeny7.
Genetic diversity, accessible through whole genome sequencing, plays an important role also in transcription. Gene expression differences have been observed, with 15% of the genes found to be differentially expressed among different Mtb clinical isolates8, and lineage-specific transcriptome differences have been observed in vitro and during survival in macrophages9,10. The mechanisms controlling expression of candidate genes, such as the upregulation of the dosR operon specific to Beijing strains, have been broadly investigated11–13. However, little is known about the effect of genomic variation on transcription at a whole genome scale. These effects can be explored through an association analysis of polymorphisms, such as single nucleotide polymorphisms (SNPs), and gene expression levels to determine expression quantitative trait loci (eQTL). eQTLs are genetic variants that explain variation in gene expression levels, and can be classified as cis or trans depending on the physical distance from the gene they regulate14. In Mtb, one previous study focusing on lineage 1 and 2 strains, highlighted two types of mechanisms where polymorphisms may change gene expression: through impairment of transcriptional regulators or by affecting the promoter regions10.
In addition to genomic variants, epigenetic mechanisms such as DNA methylation have an effect on gene expression. Several lines of evidence have revealed N6-methyladenine (m6A) and 5-methylcytosine (m5C) methylation mechanisms within Mtb genomes, and these can be characterised using single-molecule real time (SMRT) sequencing from Pacific Biosciences technology15,16. Motifs within three DNA methyltransferases (MTases), mamA, mamB, and hsdM are responsible for m6A modification15–17. In Mtb it has been shown that the loss of mamA MTase can decrease gene expression and affect survival during hypoxia17. Methylation sites have been found to overlap with sigma factor binding sites, suggesting that if methylation affects sigma factor binding, methylation status may play a role in transcription17. Lineage-specific methylation patterns have been reported for Mtb strains16, which indicates the potential for novel functional differences between them. In eukaryotic cells, DNA methylation is often associated with repression of gene expression; however, in prokaryotes, methylation has been associated with both induction and repression of gene expression17,18.
To date, no studies have integrated sequence-based genomic, transcriptomic and methylation characterisation across a common set of samples. This integration is critical to understand how DNA sequence and methylation affect RNA expression and, ultimately, Mtb pathogenesis. Here we seek to investigate the relationship between the genome, transcriptome and methylome in a panel of 22 Mtb isolates, belonging to the Karonga Prevention Study, a longitudinal epidemiological project focused on mycobacterial disease19. We present a differential gene expression study correlated with lineage, as well as an eQTL study linked with SNPs and methylated bases at a whole genome scale. Differential transcription between lineages was found, and genetic variants revealed as potential candidate eQTLs. Methylation status was also found to have a potential role in transcription, with evidence of differential gene expression between samples with non-methylated and methylated genes.
Results
Genomic analysis
Mtb was isolated from 22 sputum samples from 22 different TB patients collected between 2003 and 2009 in Karonga, a northern district of Malawi. The majority of individuals were HIV positive (16/22). Genomic DNA was extracted and sequenced using PacBio single-molecule real time (SMRT) and Illumina sequencing technologies. One ancient (L1, n = 8) and two modern lineages (L2 and L4, n = 14) were represented (Supplementary Table S1). For each isolate, the raw sequence data was aligned to the H37Rv reference genome, leading to >100-fold average coverage. Across all samples 9,384 unique SNPs were characterised, with ~40% of them identified in single isolates. Only 1,446 of the 9,384 SNPs were located in intergenic regions. The average number of SNPs per isolate varied by lineage (L1: 2,613; L2: 1,675; L4: 1,101); the sub-lineage 4.9 (H37Rv-like) was the least polymorphic (~600 variants). Using the 9,384 SNPs, a maximum-likelihood phylogenetic tree was constructed (Fig. 1) and the isolates clustered by lineage as expected.
Figure 1.
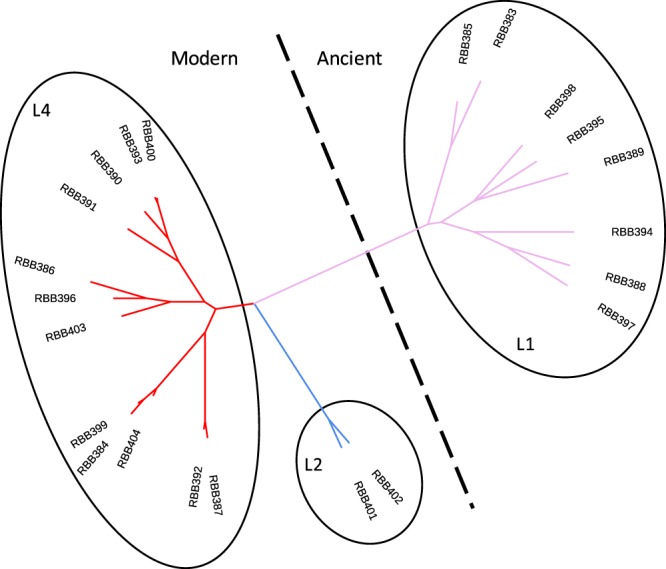
Phylogenetic tree of the 22 Karonga strains. Maximum-likelihood phylogenetic tree of the 22 isolates analysed, covering lineages 1 (L1), 2 (L2) and 4 (L4).
Transcriptomic analysis and lineage-specific expression
Mtb RNA was extracted from the 22 clinical isolates following liquid culture at mid-log phase growth and sequenced using Illumina HiSeq technology. Short reads were aligned to the H37Rv reference genome and counts per gene were obtained. A total of 3,987 genes were transcribed in at least two clinical isolates with a minimum of 10 counts. The average number of transcripts in the sample set is 3,864. A differential expression test was performed by clade, between the ancient (L1; n = 8) and the modern (L2 and L4; n = 14) strains in our sample set (Supplementary Fig. S1A). At a significance level of p < 1.24 × 10−5 (corresponding to a Bonferroni adjusted p < 0.05), 105 genes were revealed as differentially expressed (Fig. 2, Supplementary Table S2). Five of them (Rv1524-wbbL2, Rv2652c-Rv2653c-Rv2658c) correspond to known deletions in ancient isolates. PE_PGRS57 was also absent in ancient genomes of our samples, which has also been observed to be deleted in other ancient (L5; M. Africanum) strains in other studies20,21. As expected, Rv1524-wbbL2, Rv2652c-Rv2653c-Rv2658c and PE_PGRS57 transcripts were down-regulated in ancient strains. Forty-eight of the 105 (45.7%) genes found to be differentially expressed by clade have been reported in previous transcriptomic analyses performed between ancient and modern strains or L1 and L29,10, leading to 57 newly described genes here. The main functional ontological categories for the 105 identified genes were conserved hypotheticals and intermediary metabolism and respiration. Enrichment in nitrogen metabolism (p = 2.75 × 10−5) and PE-PGRS (p = 7.2 × 10−3) associated genes was found. Within clade-specific patterns, genes associated with transcriptional regulation were also identified. For ancient strains, Rv0273c, Rv0275c, and Rv2160A were the most under-expressed, whilst pknH, Rv2282c, virS, and Rv3167c, were over-expressed. In addition, several of the 105 differentially expressed genes were associated with virulence. Three of them belonged to the vapBC toxin-antitoxin system (vapB10, vapC10, vapB22), which were up- or down-regulated in ancient strains. Also, the mce4A gene, involved in cholesterol uptake during macrophage survival and associated with long term persistence22, and yrbE4B, forming part of the mce4 operon, were found over-expressed in ancient isolates. Finally, genes associated with drug resistance, such as the efflux pump Rv2994 and the isoniazid related iniA and iniB genes, were revealed as differentially expressed between the ancient and modern lineages studied. Rv2994 has found to be over-expressed in multi-drug resistant isolates23, and the iniA and iniB genes are related with higher persistence under isoniazid conditions24,25.
Figure 2.
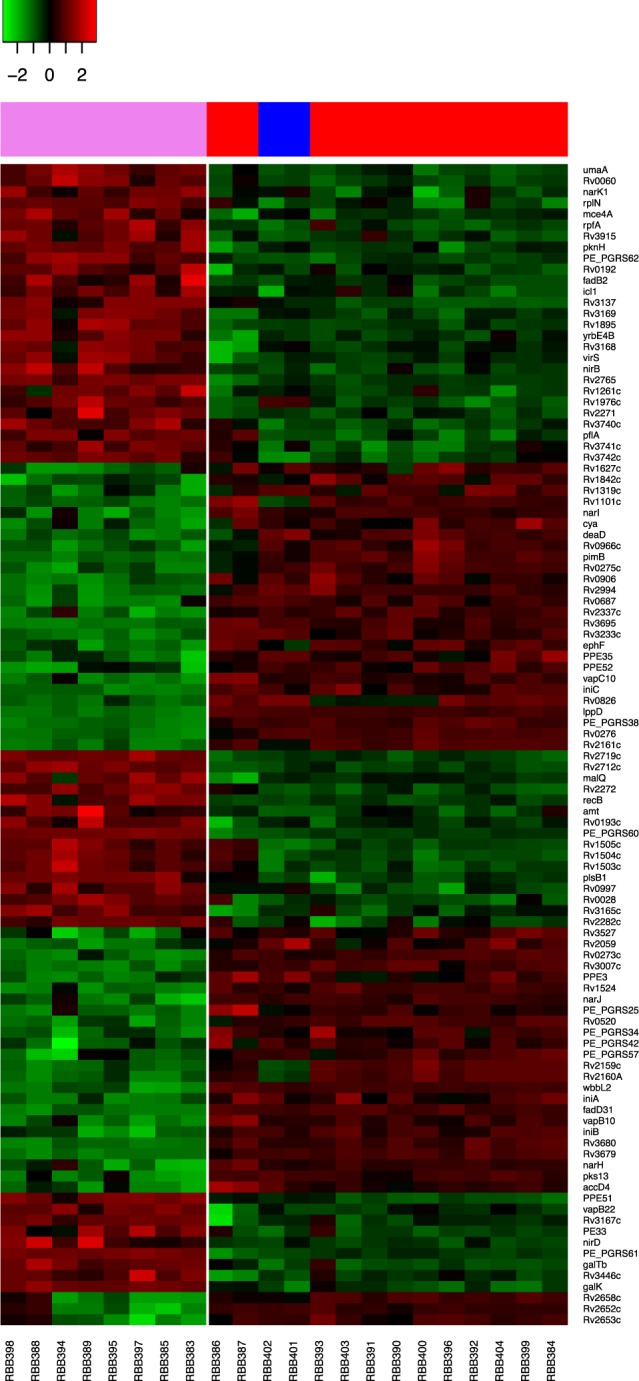
Gene expression differences between modern (lineage 2 and 4) and ancient (lineage 1) strains. A heatmap showing the 105 genes differentially expressed between ancient and modern strains, constructed with the gene expression distances between rows. Rows and columns are ordered based on row or column means. Over-expressed genes are coloured in red whilst under-expressed ones in green. Ancient strains (n = 8) represented on the left of the white vertical line and modern strains (n = 14) on the right. Lineage 1 represented in violet, Lineage 2 in blue and Lineage 4 in red.
Identification of Expression Quantitative Trait Loci (eQTL)
An eQTL analysis was performed at a whole genome scale across the 22 isolates, and we attempted to associate SNP alleles with differential transcription signal. Association testing was performed between 9,384 SNPs and 3,987 transcripts using a linear regression modelling approach (Supplementary Fig. S1B). We identified potential eQTLs from the 38,949 significant associations between 5,608 SNP positions and 118 differential transcribed genes (p < 1.32 × 10−9; adjusted p < 0.05). The 5,608 SNPs considered as eQTLs were located in 2,279 genes and intergenic regions. Forty-two of the 118 (35.6%) genes were differentially expressed due to large deletions and were subsequently excluded from further analysis (Supplementary Table S3), leaving 76 genes as potentially affected by SNP eQTLs (Supplementary Table S4). More than half of these 76 genes had a lineage or sub-lineage-specific expression profile. Moreover, a large number of the eQTLs associations were due to both lineage-specific SNPs and expressed genes. Thereby, a group of 790 common SNPs across all ancient isolates was associated with the expression of 24 genes; a group of 169 SNPs present in all L1 and L2 isolates was associated with the expression of 9 genes, and 584 SNPs present in Beijing (L2) isolates were associated with the expression of 3 genes (Supplementary Table S4). To assign the most likely causative genetic variation of the eQTLs, we investigated SNPs with a potential cis regulatory function and those within transcriptional regulatory proteins.
Cis-regulatory eQTLs
A cis-eQTL analysis was performed at SNPs, within each gene or < 200 bp upstream from their start codon, tested for differential expression (Supplementary Fig. S1C). This analysis identified 99 potential cis-eQTLs associated with the differential expression of 83 genes (p < 4.04 × 10−6, adjusted p < 0.05), involving 92 SNPs (Supplementary Table S5). The majority (65/92) of these candidate cis-eQTL SNPs were located within the gene, 15 were located in the upstream intergenic region and 8 within the upstream gene. Among those in the upstream intergenic region, 8 were in predicted promoter regions. Eleven upstream SNPs (11/15) were common (allele frequency >5%) in a global set of strains (n = 6,218)26. Also, 6 SNPs within the upstream gene (6/8) were common (Table 1). Among them, the antitoxin vapB22, is known to be over-expressed in ancient isolates when compared to modern strains, and was found to harbour a SNP in its promoter (T3137237C) in all ancient isolates, thereby providing a possible explanation for the change in expression. Further, all the SNPs identified as potential cis-eQTLs were aligned to a map of transcriptional start sites (TSS)27. We found that three were located within the TSS of three genes shown to be differentially expressed in L1 compared to modern strains, with PE_PGRS38 (A2424864G) and fadD31 (T2177073C) under-expressed, and virS (A3447480C) over-expressed in ancient isolates. Overall, five SNPs present in ancient strains identified in this study as potential cis-eQTLs have already been reported as potentially associated with variation in gene transcription10, giving us confidence in our approach.
Table 1.
Putative functional SNPs associated with expression (cis-eQTLs with allele frequencies >5%; adjusted p < 0.05).
| Transcript differentially expressed | Annotation | SNP | Position SNP | Regulation | Strain Lineage |
Allele frequency** | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Distance (bp) from start codon | Promoter (P)/TSS | Ancient | Modern | ||||||
| SNPs in upstream region | Rv0193c | 1 | G226676A | IGR | −105 | — | Up | 1 | 0.973 | 0 |
| Rv0326 | — | T392261C | Rv0325 | −12 | — | Up | 1,2 | 0.978 | 0.324 | |
| Rv0377 | 6 | T454295C | Rv0376c | −126 | — | Up | 1,2,4.1,4.3.4, 4.8,4.9 | 1 | 0.994 | |
| gpdA1 | 4 | T655986G | IGR | −37 | P | Up | 1,2 | 0.976 | 0.324 | |
| mce2D | 6 | A690450C | mce2C | −51 | — | Up | 1,2 | 0.976 | 0.324 | |
| Rv0669c | 3 | T769663G | IGR | −66 | P | Down | 4.3.3 | 0 | 0.050 | |
| Rv0958 | 3 | C1069871T | IGR | −12 | P | Up | 1.1.3 | 0.220 | 0 | |
| Rv1096 | 3 | T1224367C | IGR | −18 | P | Down | 1,2,4.1,4.3,4.8 | 1 | 0.976 | |
| Rv1503c | 1 | A1694547C | IGR | −3 | — | Up | 1 | 0.973 | 0 | |
| fadD31 | 4 | T2177073C | IGR | −14 | TSS/P | Down | 1 | 0.973 | 0 | |
| Rv2036 | 3 | C2282058T | Rv2035 | −41 | — | Up | 1.2.2* | 0.157 | 0 | |
| Rv2159c | 1 | A2421816G | Rv2160A | −151 | — | Down | 1,2 | 0.977 | 0.323 | |
| PE_PGRS38 | 7 | A2424864G | IGR | −18 | TSS | Down | 1 | 0.973 | 0 | |
| Rv2712c | 1 | C3025431T | IGR | −103 | P | Up | 1 | 0.971 | 0 | |
| vapB22 | 5 | T3137237C | IGR | −13 | P | Up | 1 | 0.973 | 0 | |
| yrbE4B | 5 | G3920109T | yrbE4A | −47 | — | Up | 1 | 0.971 | 0 | |
| Rv3695 | 2 | T4137190C | IGR | −16 | — | Down | 1 | 0.973 | 0 | |
Table showing the candidate transcripts differentially expressed due to SNPs in upstream intergenic regions (IGRs) or within the upstream gene. Annotation of the transcript differentially expressed: 1 – Conserved hypotheticals, 2 – Cell wall and cell processes, 3 – Intermediary metabolism and respiration, 4 – Lipid metabolism, 5 – Virulence, detoxification, adaptation, 6 – Regulatory proteins, 7 – PE/PPE, 8 – information pathways. Distance of the SNP location from the start codon of the transcript is showed as negative when it is upstream and positive when it is located within the gene. TSS = Transcriptional Start Site. *Only one or two samples from the lineage out of the 3 analysed. **Allele frequency refers to the fraction of strains harbouring the SNP in a larger data set (n = 6,218)50; “—“ when not available.
Transcriptional regulatory proteins
We next considered candidate SNP eQTLs with non-synonymous mutations in transcriptional regulatory proteins (Supplementary Fig. S1D). These mutations could affect the DNA binding function of the protein. In total, 46 SNPs in 38 different transcriptional regulatory proteins (Table 2) were associated in the eQTL analysis with the differential transcription of 56 genes, accounting for a total of 376 potential eQTL associations. Ten of these 46 SNPs have been previously reported as having a potential effect in transcriptional regulation10. Functional effects were investigated through the SIFT algorithm, and 16 of the 38 (42.1%) transcriptional regulators were predicted to have SNP mutations affecting functional impairment. For the majority of the regulatory genes (20/38; 52.6%), the SIFT software did not predict a functional consequence of the mutations, due to the lack of homology with sequences in its database.
Table 2.
Non-synonymous variants in transcriptional regulatory genes with eQTL associations, with potential functional impairment.
| Gene | Mutation | Family | Lineage of strains carrying mutation | Allele frequency | |
|---|---|---|---|---|---|
| Ancient | Modern | ||||
| whiB5 | S21G | whiB | 1.2.2** | 0.021 | 0 |
| Rv0023 | G217D | 4.9** | 0 | 0.001 | |
| Rv0042c | L186R* | MarR | 4.9** | 0 | 0 |
| Rv0144 | P36L* | tetR | 4.9** | 0 | 0 |
| Rv0195 | C41STOP | LuxR | 1.2.2** | 0.021 | 0 |
| Rv0275c | S24L | tetR | 1 | 0.973 | 0 |
| iniR | E23K | 1.2.2** | 0.019 | 0 | |
| Rv0377 | P302R* | LysR | 1 | 0.973 | 0 |
| Rv0386 | L475R* | LuxR/UhpA | 4.1.1.3 | 0 | 0.003 |
| ramB | P91Q | 1 | 0.973 | 0 | |
| T118A | 4.9** | 0 | 0.001 | ||
| Q121R | 1 | 0.973 | 0 | ||
| Rv0576 | R233H* | ArsR | 1,2 | 0.978 | 0.334 |
| Rv0691c | A140T | 2 | 0.003 | 0.114 | |
| Rv0818 | P227L* | 4.1.1.3 | 0 | 0.003 | |
| E246K* | 4.1.2 | 0 | 0.009 | ||
| narL | G169R* | 2 | 0.003 | 0.147 | |
| Rv0890c | E234G* | LuxR | 2 | 0.003 | 0.111 |
| E303K* | 4.1.2 | 0 | 0.009 | ||
| Rv0891c | V37G* | 1,2,4.1,4.3,4.8 | 1 | 0.974 | |
| kdpE | G60S* | KDPD/KDPE | 2 | 0.003 | 0.111 |
| Rv1219c | R11T | 1.2.2** | 0.148 | 0 | |
| embR | A70S | 4.1.2 | 0 | 0.009 | |
| C110Y | 1 | 0.973 | 0 | ||
| Rv1453 | D208N | 1.1.3 | 0.230 | 0 | |
| D218N | 1.2.2** | 0.021 | 0 | ||
| P405Q | 1,2,4.1,4.3,4.8 | 1 | 0.974 | ||
| Rv1674c | E189G* | 4.3 | 0.014 | 0.281 | |
| cmr | V59A | CRP/FNR | 1 | 0.974 | 0 |
| A125S | 1.1.3* | 0.072 | 0 | ||
| Rv1776c | R154S | 1.2.2** | 0.019 | 0 | |
| blaI | L57R | 1 | 0.970 | 0 | |
| mce3R | D148Y* | tetR | 1.1.3** | — | — |
| Rv2017 | A262E | 1,2,4.1,4.3,4.8 | 0.998 | 0.973 | |
| Rv2160A | C155R | tetR | 1,2 | 0.977 | 0.323 |
| zur | H64R* | 1 | 0.973 | 0 | |
| Rv2488c | D184Y* | LuxR | 1.2.2** | 0.018 | 0 |
| Rv2621c | A110V | 2 | 0.003 | 0.148 | |
| sirR | Q131STOP | 1 | 0.973 | 0 | |
| Rv3060c | G420D | GntR | 4.1.2 | 0 | 0.009 |
| virS | L316R* | AraC/XylS | 1 | 0.973 | 0 |
| Rv3167c | P17Q | tetR | 1 | 0.973 | 0 |
| Rv3249c | T154A | tetR | 4.1.1.3 | 0.003 | 0.049 |
| whiB4 | S2L | whiB | 1.1.3 | 0.223 | 0 |
| Rv3736 | G144R* | AraC/XylS | 1 | 0.971 | 0 |
| whiB6 | G71D | whiB | 1.2.2** | 0.014 | 0 |
Table showing non-synonymous mutations in transcriptional regulatory genes found as potential eQTLs. *Sorting Intolerant from tolerant (SIFT) predicted scores (p value) < 0.05 and considered to have functional impact; whilst for the others the SIFT software was unable to predict functional effects of mutations; **Only one or two samples available from the lineage. Allele frequency refers to the fraction of strains harbouring the SNP in a larger data set (n = 6,218)26.
Mutations in the sirR and Rv0195 genes resulted in stop codons and led to truncated proteins. The stop codon in sirR, a manganese-dependent transcriptional repressor28, was observed in all L1 samples. While, mutations in Rv0195, a LuxR family regulatory gene, were observed in one L1 sample. Some of the 38 transcriptional regulators belonged to other known regulatory families such as TetR. The TetR family of transcriptional regulators (TFTRs) are one-component prokaryotic signal transduction systems controlling different biochemical functions. Although they were thought to be expression repressors, work in other bacteria has shown that they can act also as activators29. The TFTR Rv2160A carried a SNP (C155R) and an insertion (304insGGAA) causing a change in the reading frame in isolates from L1 and L2. Rv2160A is likely to form part of the operon Rv2159c/Rv2160A/Rv2161c. In our analysis, Rv2159c and Rv2161c were revealed as highly down-regulated in ancient strains compared to modern ones, and marginally down-regulated in L2 compared to L4 isolates. These observations suggest the operon may act as an activator, and that the mutations may lead to a loss of its function.
In Streptomyces it has been shown that TFTRs can regulate divergently oriented neighbouring genes30, and previous studies in Mtb10,31 have found differential expression of genes adjacent to TFTRs. We looked for similar effects in Mtb TFTRs carrying potential eQTLs. Rv0275c is a potential regulator of its divergent oriented neighbouring gene Rv0276. The ancient strains carried a mutation (S24L) in Rv0275c, which was associated with the under-expression of Rv0276. Similarly, Rv3167c is a potential regulator of its divergent oriented neighbour gene Rv3168. Although, the ancient strains carried a mutation (P17Q) in Rv3167c, and Rv3168 appeared slightly over-expressed, this effect did not reach the stringent significance cut off imposed in the eQTL analysis.
In order to study the consequential effects of mutations in the transcriptional regulators of the genes found as being differentially expressed, network gene regulation was analysed through the Environment and Gene Regulatory Influence Network (EGRIN) model from the MTB Network Portal32 and the regulatory network map from the TB database33. We compared the predicted induced and repressed genes by the transcriptional regulators harbouring non-synonymous SNPs with the differentially expressed genes in our samples. This analysis revealed the association of genes differentially expressed with five of our candidate transcriptional regulators (Supplementary Table S6). Rv0275c, which is predicted to auto-induce its expression, was found to be down-regulated in ancient strains (with S24L mutation), although this effect did not reach the statistical significance cut-off. In addition to the under-expression of Rv0276, discussed above, three other genes (Rv0520, Rv2162c and Rv0826) were found to be under-expressed in ancient strains and are predicted to be regulated by Rv0275c. Genes regulated by ramB, were up- or down-regulated in ancient strains carrying ramB P91Q and Q121R mutations. Other genes were regulated by the transcriptional regulators Rv1776c, Rv3167c and Rv3249c, which harboured potential impairment mutations, leading to under- or over-expression in those isolates carrying the mutations. For the remaining regulators within known control networks, no statistically significant associations of variable gene expression with mutations were found.
Sigma and anti-sigma factors are critical to the gene expression regulatory network34, and here we hypothesised that polymorphisms in these factors might affect the transcription of those genes regulated by them. We found three anti-sigma factors (rseA, rskA and rsfA) harbouring non-synonymous SNPs that were considered as potential eQTLs (adjusted p < 0.05) associated with six genes differentially expressed between the isolates carrying and not carrying the mutations (Supplementary Table S7).
Methylation analysis
Motif and methylation finding was performed through the Modification and Motif Analysis pipeline provided by the SMRT portal (https://github.com/PacificBiosciences/SMRT-Analysis). By analysing the kinetic variation through the inter-pulse duration ratio (IPD) at each nucleotide in the genome, a large number of modifications were identified. Only high quality 6-methyl-adenine (m6A) levels were found within motifs, where m6A is a well characterised epigenetic regulator in other prokaryotes35,36. The three motifs previously reported in Mtb15–17 were identified: CTCCAG and GATN4RTAC and their partner motifs (CTGGAG and GTAYN4ATC, respectively), and the hemi-methylated CACGCAG. The distribution and numbers of the different motifs were similar across the samples regardless of lineage and sub-lineage, with an average number of 1,934 for CTCCAG, 357 for GATN4RTAC and 813 for CACGCAG. However, the fraction of methylated motifs varied across isolates and (sub-)lineage patterns (Supplementary Table S8), consistent with a previous report16. In particular, within L4, two sub-lineages patterns were found with methylation in GATN4RTAC and CACGCAG motifs. Moreover, the CTCCAG motif was not methylated in either of the two L2 isolates. Among L1, methylation in CTCCAG and CACGCAG motifs was absent in some samples. When methylated, the percentage of motifs modified across all the samples varied from 50% to ~100%.
To explain the lack of methylation observed in some isolates, the presence of SNPs in the MTases genes was investigated. Three SNP mutations were identified: (i) E270A in mamA in L2, (ii) P306L in hsdM in sub-lineages 4.3, 4.8 and 4.9, and (iii) S253L in mamB in sub-lineage 1.1.3; which have been reported previously to be associated with the loss of function of the enzymes15,16 (Supplementary Table S9). Two novel mutations (Q340K and G152S) and a deletion (1232delG) were also identified in mamA, potentially associated with the lack of methylation of CTCCAG in two isolates belonging to L1 and L4. For the remaining samples with an absence of methylation in any of the three motifs, there were no SNPs uniquely found in these samples that could be correlated with the loss of function of the enzyme.
Differential gene expression linked with methylation
In order to understand how the methylation status of the genes affects their expression, a differential transcription analysis was performed on the L1 and L4 strains (n = 20) (Supplementary Fig. S1E). The analysis involved stratifying by lineage to overcome the lineage-specific transcriptional profiles seen above. L2 was discarded due to the low number of clinical isolates represented. Firstly, 5,326 different intragenic methylation sites were used. A linear regression analysis was applied to obtain the correlation between methylation status and gene expression level at a whole-genome scale. Across L4, 44 genes were found to be differentially expressed (Benjamini-Hochberg (BH) adjusted p < 0.05), whose over- or under-expression was potentially associated with their methylation status. Twenty-eight (of the 44; 63.6%) genes, mostly down-regulated, were deficient in methylation only in the CTCCAG motif in one sample, which was associated with the presence of the mutation G152S in mamA (Supplementary Fig. S2). These genes were enriched for metabolic pathways (p < 0.05). The remaining 16 genes differentially expressed in L4 were non-methylated in >1 isolate and mostly in the CTCCAG motif (Supplementary Fig. S3). For L1, none of the genes that were found to be differentially expressed were significantly associated with methylation status. Methylation of the upstream intergenic regions may have a role in gene expression, and we performed a lineage-stratified cis-eQTL analysis with the 393 unique methylation sites located within 200 bp upstream from the start codons of the genes. In L4, seven eQTLs (BH adjusted p < 0.05) for 8 genes differentially expressed were revealed (Table 3, Supplementary Fig. S4), including one located in the predicted promoter region and overlapping with the TSS. Among ancient strains, none of the genes that were found to be differentially expressed were significantly associated with methylation of upstream regions.
Table 3.
cis-eQTLs located in upstream intergenic regions linked with methylation in Lineage 4 strains.
| Gene | Position | strand | Motif | Distance from start codon (bp) | Promoter/TSS | Regulation in non-methylated samples |
|---|---|---|---|---|---|---|
| Rv0565c | 657533 | − | CTGGAG | −63 | − | Down |
| ompA | 1002711 | + | CTCCAG | −101 | − | Down |
| Rv1371 | 1543277 | + | CTCCAG | −82 | − | Up |
| scpB | 1938088 | + | CTCCAG | −58 | P, TSS | Up |
| moaC3 | 3710411 | − | CTCCAG | −163 | − | Up |
| Rv3324A | 3710411 | − | CTCCAG | −32 | − | Up |
| Rv3325 | 3710408 | + | CTGGAG | −25 | − | Down |
| PE_PGRS60 | 4093563 | + | CTGGAG | −69 | − | Down |
Table showing genes differentially expressed potentially due to the lack of methylation in the upstream region. The name of the gene, the position of the eQTL (methylation site), strand, motif, distance of the methylated base from start codon of the transcript (negative shown as upstream), prediction of promoter or TSS (P = promoter region, TSS = Transcriptional Start Site), and type of regulation of the gene in non-methylated samples is shown.
Overlap between eQTLs linked with SNPs and methylation
Finally, we assessed whether there is a link between the SNPs and methylated motifs associated with the differentially expressed genes identified. To this end, we evaluated the degree of overlap between the different associations (Fig. 3). We considered three types of association: (i) genes differentially expressed due to SNPs in promoter regions, TSS or within the gene, denoted as cis-eQTLs; (ii) genes differentially expressed due to potential impairing mutations in transcriptional regulators that are predicted to control their expression, denoted as tr-eQTLs; and (iii) genes differentially expressed as a consequence of methylation of either the promoter, TSS, upstream region or the gene, denoted as mod-eQTLs. We found that 5 genes with variable transcription were associated with both, mod-eQTLs and cis-eQTLs, and another 9 were associated with cis-eQTLs and tr-eQTLs. There was no overlap between genes differentially expressed due to tr-eQTLs and mod-eQTLs, and the majority of the genes were uniquely assigned to one of the mechanisms responsible for their differential expression.
Figure 3.
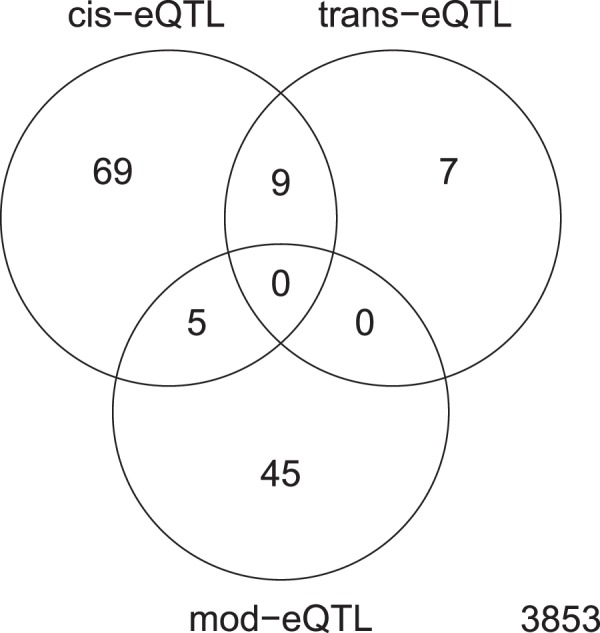
Venn diagram showing the overlap of genes differentially expressed (from the 3,987 investigated) associated with the different eQTL types (cis, trans and modified). The numbers represent the number of genes differentially expressed associated with the different types of eQTLs: cis-eQTLs, SNPs in promoter regions, transcriptional start sites (TSS), upstream (up to −200 bp) or within the gene; tr-eQTLs, potentially impairing non-synonymous SNPs located in transcriptional regulators; and mod-eQTLs, methylated bases located either within the gene or upstream including promoter regions and TSS.
Discussion
Genetic mutations and variations in gene expression have an important impact on MTC virulence and pathogenicity4,5. Previous studies have shown how genomic variants or methylation can affect the level of gene expression9,10,17, but have not shown how one analysis may influence another. In this study, for the first time, we performed an integrated analysis of the genome, methylome and transcriptome, across 3 major Mtb lineages. We have revealed clade-specific differences in the core transcriptomes between ancient and modern strains, as previously observed9, but in addition our analysis has revealed genes linked to virulence and pathogenicity (e.g. vapBC family), drug resistance and efflux pumps (e.g. Rv299423 or iniA and iniB24,25). An eQTL analytical approach revealed 5,608 SNPs associated with differential gene expression (a total of 38,949 candidate eQTLs) and reinforced the lineage-specific genetic diversity and its effects on transcriptomes. To achieve improved resolution, cis-eQTLs based on regions upstream or within the genes differentially expressed were considered. This approach revealed ten SNPs within the promoter regions or TSS of genes differentially expressed, as well as others within coding regions of the genes, doubling the number of previously reported associations10. Among these variants, lineage-specific SNPs were associated with the genes differentially expressed, thereby revealing a potential explanation for the differential core transcription.
The high proportion of non-synonymous mutations present in coding regions in Mtb has been suggested to have a functional impact4, with consequences for transcription when found within transcriptional regulators10. In our study, functional impairment was predicted for sixteen of the transcriptional regulators found among the 38,949 potential eQTLs, including in sirR and Rv0195 that contained premature stop codons. The number of regulators found is likely to be an under-estimate, as databases accessible to SIFT are incomplete, leading to no prediction for the vast majority of loci. Most of the potential impairing mutations were found to be lineage-specific. In particular, we identified a mutation and an insertion in L1 and L2 strains in Rv2160A, which act as a transcriptional activator of the adjacent genes Rv2159c and Rv2161c, with which it likely forms an operon29. Similarly, the protein encoded by Rv3167c was predicted to function as a repressor of its contiguous gene Rv3168, over-expressed in ancient samples with the P17Q mutation. Whilst Rv0275c was shown as a candidate activator of the adjacent gene Rv0276, and under-expressed in the L1 strains with the S24L mutation, consistent with previously reported associations10,31. The analysis of the regulatory networks of the transcriptional regulators was performed in order to look for trans-eQTLs, and found 11 of the genes differentially expressed from the primary eQTL analysis were regulated by one of the transcriptional regulators harbouring potential impairing mutations. Three mutations affecting the function of three anti-sigma factors (rseA, rskA and rsfA) were associated with the up-regulation of 6 genes. This result suggests that the functional impairment of sigma and anti-sigma factors can be the cause of variable gene expression.
Our study confirmed the same motifs and patterns of methylation as previously reported15,16 but in addition identified three novel variants (Q340K, 121delG and G152S) in mamA, which could explain the lack of methylation in the CTCCAG motif in the samples harbouring them. DNA methylation has been hypothesised to affect gene expression in bacteria35, and the disruption of mamA in Mtb has been shown to result in altered gene expression17. In E. coli it has been suggested that an overrepresented motif in the genome is more likely to be involved in gene expression regulation mediated by methylation37. Different hypotheses concerning the control of gene expression by dam MTase have been proposed, including regulation by motifs found in promoter38 and coding regions39. Further, it has been suggested that DNA methylation is a mechanism of switching regulatory states in phase variation systems37. Across the three lineages studied here, CTCCAG was the most abundant motif and was predominantly found in coding regions. An investigation of the relationship between the methylation status and gene expression levels revealed that the CTCCAG motif has the highest impact. In L4, the differential expression of 38 genes was potentially associated with CTCCAG methylation status, compared to 4 and 2 genes associated with CACGCAG and GATN4RTAC methylation, respectively. A subset of these genes (28/44), mostly down-regulated, were found to be uniquely non-methylated in the sample with the mamA G152S mutation. These included genes associated with metabolic pathways or regulatory proteins (e.g. Rv0348, virS or Rv1359), and from the pe/ppe families (e.g. PE17, PPE17 or PE_PGRS2). We also found that non-methylated CTCCAG motifs in upstream regions and TSS have an effect on gene expression, which is consistent with previous work17. In L1 no genes significantly associated with methylation were found. Overall our results show that methylation in the promoter regions and coding regions is likely to be involved in gene expression, with the CTCCAG motif as the main candidate with a role in regulation.
The functional impairment of MTases may have implications in biological processes of the Mtb controlled by genes whose expression is affected by the methylation status. This could eventually influence the Mtb’s virulence, pathogenicity or drug resistance. For instance, variable methylation status was found to be related to the differential transcription of genes associated with metabolic pathways, among others, which suggests the potential role of methylation on regulation of biological processes related with growth or persistence. However, further work is needed to understand how methylation regulates gene expression under different environmental cues including those encountered by Mtb inside the host.
In Mtb, virulence and the ability to become drug resistant vary across lineages40,41. Hence, the study of lineage-specific transcriptomic profiles and the mechanisms that regulate gene expression can give insights into mechanisms underlying these biological differences. Such insights will be useful to identify potential targets for the development of new anti-tuberculosis drugs or vaccines. The small sample size is a potential limitation of the study, but our integrated analysis has detected known variants and methylated motifs, and putative candidate eQTLs for follow-up experiments. Future studies should consider larger sample sizes, including more lineages (e.g. other ancient lineages, such as L5 and L6), in order to confirm the candidate associations found in this analysis. In addition, there is a need for complementary proteomic analyses, to perform a comprehensive integrated study of Mtb genetic and epigenetic mechanisms of gene expression control. Overall, our data has identified common functional variants that affect transcriptional control, which gives further support to differential pathophysiology in ancient and modern Mtb lineages.
Materials and Methods
Bacterial strains, DNA and RNA sequencing
All 22 Mtb isolates listed in Supplementary Table S1 were sourced from 22 TB patients from Karonga (Malawi) between 2003 and 2009, and cultured in the LSHTM. Mtb isolates were grown by liquid culture (in the absence of antimicrobial drugs) from frozen stocks of Lowenstein-Jensen or liquid cultures derived from patient’s sputum specimens already isolated. Mtb strains were grown to mid-log phase (OD = 0.6–0.8) in Middlebrook 7H9 supplemented with 0.05% Tween 80 and 10% albumin-dextrose-catalase (ADC) at 37 °C in standing 25 cm2 vented tissue culture flasks and subcultured in 75 cm2 vented tissue culture flasks. DNA and RNA were extracted from the same cultures (passage 3–4 from original sputum sample) using the phenol-chloroform-isoamyl alcohol method and the trizol method with bead-beating as previously described42,43. The samples were sequenced at the Genome Institute of Singapore. Single-molecule real time (SMRT) sequencing from Pacific Biosciences (PacBio) RSII long read technology was used with the parameter of 6 hours per SMRTcell (PacBio RS II SMRT Cells 8Pac). The library preparation involved the use of the template prep kit 1.0, and the binding chemistry involved the use of DNA/Polymerase binding kit P6. The sequencing kit used was the DNA Sequencing Reagent Kit 4.0.
For RNA sequencing, total RNA extracts were run on the Agilent 4200 Tapestation System (Agilent Technologies, Santa Clara, CA, USA) using the RNA Tapestation Assay to determine the RNA integrity values. TruSeq Stranded mRNA sample preparation was used according to the manufacturer’s instructions for next generation library preparation. Briefly, library preparation started with purification of mRNA using poly-T oligo attached magnetic beads, fragmentation of mRNA, 1st and 2nd strand cDNA synthesis, A-tailing and ligation of adapters with multiplex indexes. Samples were enriched with 15 PCR cycles followed by Agencourt AMPure XP magnetic bead (Beckman Coulter, Brea, CA, USA) clean up as per the manufacturer’s instructions. Quality of cDNA libraries was checked with Agilent D1000 Tapestation Assay (Agilent 4200 Tapestation System, Agilent Technologies, Santa Clara, CA, USA). Next generation sequencing was performed using Illumina Hiseq4000 flow cell, with 2 × 151 base pair-end runs. PhiX was used as a control.
Bioinformatic and association analysis
PacBio long reads were analysed using the pipelines provided by the SMRT Portal software. Briefly, raw sequence data were aligned to the H37Rv (GCA_000195955.2) reference genome and small variants (SNPs and indels) were called over the consensus sequences. Single nucleotide polymorphisms (SNPs) were used to build the maximum-likelihood phylogenetic tree using RAxML software44. The Modification and Motif Analysis pipeline was used then for the methylation study and motif finding. Detection of base modification was performed with a minimum QV score of 30 and coverage of 20-fold. Six-methyl-adenine (m6A) was determined within motifs with an inter-pulse duration ratio (IPD ratio) between 3 and 10. Statistical enrichment analysis was performed using DAVID software45. Functional impairment prediction for proteins harbouring non-synonymous mutations was performed using the Sorting Intolerant from tolerant (SIFT) algorithm46.
Pair-end short reads generated by Illumina HiSeq technology for RNA sequencing were assessed for quality and trimmed using Trimmomatic v0.3647. High quality reads were mapped to the H37Rv reference genome (GCA_000195955.2) using the Burrows-Wheeler Alignment (BWA-mem) v0.7.15 tool48. HTSeq. 0.9.149 was used to quantify the number of reads per transcript. Lowly expressed genes were filtered out by a minimum count per million (CPM) value of 0.6, equivalent to 10 counts. For differential transcription analysis, counts were then normalised using the trimmed mean of M-values normalization (TMM) method50. To compare expression levels between ancient and modern strains as well as for the eQTL studies linked with SNPs and methylation, significant differences were obtained through linear regression tests. Adjusted p values for multiple testing were calculated through the Bonferroni and Benjamini-Hochberg corrections for statistical significance. The prediction of promoter regions was performed using Neural Network Promoter Prediction (http://www.fruitfly.org/seq_tools/promoter.html). The EGRIN model from the MTB Network Portal32 and the regulatory network map from the TB Database33 were used for the study of the association between transcriptional regulators and genes differentially expressed. The allele frequencies of variants identified in the eQTL analysis were calculated in an independent set of ancient and modern strains using a large published dataset (n = 6,218), described previously26.
Supplementary information
Acknowledgements
We thank Teresa Cortes for useful comments. P.J.G.-G. is funded by an MRC-LID PhD studentship. J.P. is funded by a Newton Institutional Links Grant (British Council. 261868591). T.G.C. is funded by the Medical Research Council UK (Grant Nos MR/M01360X/1, MR/N010469/1, MR/R025576/1, and MR/R020973/1) and BBSRC (Grant No. BB/R013063/1). S.C. is funded by Medical Research Council UK grants (MR/M01360X/1, MR/R025576/1, and MR/R020973/1). We gratefully acknowledge the Scientific Computing Group for data management and compute infrastructure at Genome Institute of Singapore for their help. The MRC eMedLab computing resource was used for bioinformatics and statistical analysis. The authors declare no conflicts of interest.
Author Contributions
M.L.H. and T.G.C. conceived and directed the project. A.C.C. and J.R.G. coordinated sample collection. N.A. undertook sample processing and DNA/RNA extraction. N.A., P.F.d.S. and M.L.H. coordinated sequencing. P.J.G.-G. performed bioinformatic and statistical analyses under the supervision of M.L.H. and T.G.C. P.J.G.-G., J.E.P., S.C., P.D.B., M.L.H. and T.G.C. interpreted results. P.J.G.-G. wrote the first draft of the manuscript with inputs from T.G.C. and M.L.H. All authors commented and edited on various versions of the draft manuscript and approved the final manuscript. P.J.G.-G., M.L.H. and T.G.C. compiled the final manuscript.
Data Availability
All pathogen raw sequencing data is available from the ENA short read archive (accession number PRJEB29197).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Martin L. Hibberd and Taane G. Clark jointly supervised this work.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41692-2.
References
- 1.WHO. Global Tuberculosis Report 2017. WHO, WHO/HTM/TB/2017.23 (2017).
- 2.Brosch R, et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. 2002;99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koser CU, Feuerriegel S, Summers DK, Archer JAC, Niemann S. Importance of the Genetic Diversity within the Mycobacterium tuberculosis Complex for the Development of Novel Antibiotics and Diagnostic Tests of Drug Resistance. Antimicrob. Agents Chemother. 2012;56:6080–6087. doi: 10.1128/AAC.01641-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershberg R, et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 2008;6:2658–2671. doi: 10.1371/journal.pbio.0060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coscolla M, Gagneux S. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin Immunol. 2014;26:431–444. doi: 10.1016/j.smim.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coll F, et al. PolyTB: A genomic variation map for Mycobacterium tuberculosis. Tuberc. 2014;94:346–354. doi: 10.1016/j.tube.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benavente ED, et al. PhyTB: Phylogenetic tree visualisation and sample positioning for M. tuberculosis. BMC Bioinformatics. 2015;16:155. doi: 10.1186/s12859-015-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Q, et al. Gene expression diversity among Mycobacterium tuberculosis clinical isolates. Microbiology. 2005;151:5–14. doi: 10.1099/mic.0.27539-0. [DOI] [PubMed] [Google Scholar]
- 9.Homolka S, Niemann S, Russell DG, Rohde KH. Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: Delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog. 2010;6:1–17. doi: 10.1371/journal.ppat.1000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose G, et al. Mapping of genotype-phenotype diversity among clinical isolates of Mycobacterium tuberculosis by sequence-based transcriptional profiling. Genome Biol. Evol. 2013;5:1849–1862. doi: 10.1093/gbe/evt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed MB, Gagneux S, DeRiemer K, Small PM, Barry CE. The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J. Bacteriol. 2007;189:2583–2589. doi: 10.1128/JB.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallow A, Domenech P, Reed MB. Strains of the East Asian (W/Beijing) lineage of Mycobacterium tuberculosis are DosS/DosT-DosR two-component regulatory system natural mutants. J. Bacteriol. 2010;192:2228–2238. doi: 10.1128/JB.01597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domenech P, et al. Unique regulation of the DosR regulon in the Beijing lineage of Mycobacterium tuberculosis. J. Bacteriol. 2017;199:1–19. doi: 10.1128/JB.00696-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nica AC, Dermitzakis ET. Expression quantitative trait loci: present and future. Philos. Trans. R. Soc. B Biol. Sci. 2013;368:20120362. doi: 10.1098/rstb.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L, et al. Precision methylome characterization of Mycobacterium tuberculosis complex (MTBC) using PacBio single-molecule real-time (SMRT) technology. Nucleic Acids Res. 2016;44:730–743. doi: 10.1093/nar/gkv1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelan J, et al. Methylation in Mycobacterium tuberculosis is lineage specific with associated mutations present globally. Sci. Rep. 2018;8:1–7. doi: 10.1038/s41598-017-18188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shell SS, et al. DNA Methylation Impacts Gene Expression and Ensures Hypoxic Survival of Mycobacterium tuberculosis. PLoS Pathog. 2013;9:24–28. doi: 10.1371/journal.ppat.1003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balbontín R, et al. DNA adenine methylation regulates virulence gene expression in Salmonella enterica serovar typhimurium. J. Bacteriol. 2006;188:8160–8168. doi: 10.1128/JB.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crampin AC, Glynn JR, Fine PEM. What has Karonga taught us? Tuberculosis studied over three decades. Int. J. Tuberc. Lung Dis. 2009;13:153–164. [PMC free article] [PubMed] [Google Scholar]
- 20.Roetzer A, et al. Whole Genome Sequencing versus Traditional Genotyping for Investigation of a Mycobacterium tuberculosis Outbreak: A Longitudinal Molecular Epidemiological Study. PLoS Med. 2013;10:e1001387. doi: 10.1371/journal.pmed.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winglee K, et al. Whole Genome Sequencing of Mycobacterium africanum Strains from Mali Provides Insights into the Mechanisms of Geographic Restriction. PLoS Negl. Trop. Dis. 2016;10:1–28. doi: 10.1371/journal.pntd.0004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha R, et al. Methyl-accepting chemotaxis like Rv3499c (Mce4A) protein in Mycobacterium tuberculosis H37Rv mediates cholesterol-dependent survival. Tuberculosis. 2018;109:52–60. doi: 10.1016/j.tube.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Li G, et al. Efflux pump gene expression in multidrug-resistant Mycobacterium tuberculosis clinical isolates. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colangeli R, et al. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol. Microbiol. 2005;55:1829–1840. doi: 10.1111/j.1365-2958.2005.04510.x. [DOI] [PubMed] [Google Scholar]
- 25.Li, Y., Zeng, J., Zhang, H. & He, Z. G. The characterization of conserved binding motifs and potential target genes for M. tuberculosis MtrAB reveals a link between the two-component system and the drug resistance of M. smegmatis. BMC Microbiol. 10 (2010). [DOI] [PMC free article] [PubMed]
- 26.Coll, F. et al. Genome-wide analysis of multi- and extensively drug-resistant Mycobacterium tuberculosis. Nat. Genet. 50 (2018). [DOI] [PubMed]
- 27.Cortes T, et al. Genome-wide Mapping of Transcriptional Start Sites Defines an Extensive Leaderless Transcriptome in Mycobacterium tuberculosis. Cell Rep. 2013;5:1121–1131. doi: 10.1016/j.celrep.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey R, et al. MntR(Rv2788) a transcriptional regulator that controls manganese homeostasis in Mycobacterium tuberculosis. Mol. Microbiol. 2015;98:1168–1183. doi: 10.1111/mmi.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balhana RJC, Singla A, Sikder MH, Withers M, Kendall SL. Global analyses of TetR family transcriptional regulators in mycobacteria indicates conservation across species and diversity in regulated functions. BMC Genomics. 2015;16:1–12. doi: 10.1186/s12864-015-1696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn SK, Cuthbertson L, Nodwell JR. Genome Context as a Predictive Tool for Identifying Regulatory Targets of the TetR Family Transcriptional Regulators. PLoS One. 2012;7:e50562. doi: 10.1371/journal.pone.0050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley J, et al. The cell wall lipid PDIM contributes to phagosomal escape and host cell exit of Mycobacterium tuberculosis. MBio. 2017;8:1–12. doi: 10.1128/mBio.00148-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turkarslan S, et al. A comprehensive map of genome-wide gene regulation in Mycobacterium tuberculosis. Sci. Data. 2015;2:1–10. doi: 10.1038/sdata.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galagan JE, et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature. 2013;499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chauhan, R. et al. Reconstruction and topological characterization of the sigma factor regulatory network of Mycobacterium tuberculosis. Nat. Commun. 7 (2016). [DOI] [PMC free article] [PubMed]
- 35.Casadesús J, Low D. Epigenetic gene regulation in the bacterial world. Microbiol. Mol. Biol. Rev. 2006;70:830–56. doi: 10.1128/MMBR.00016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki MM, Bird A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 37.Adhikari S, Curtis PD. DNA methyltransferases and epigenetic regulation in bacteria. FEMS Microbiol. Rev. 2016;40:575–591. doi: 10.1093/femsre/fuw023. [DOI] [PubMed] [Google Scholar]
- 38.Oshima T, et al. Genome-wide analysis of deoxyadenosine methyltransferase-mediated control of gene expression in Escherichia coli. Mol. Microbiol. 2002;45:673–695. doi: 10.1046/j.1365-2958.2002.03037.x. [DOI] [PubMed] [Google Scholar]
- 39.Hénaut A, Rouxel T, Gleizes A, Moszer I, Danchin A. Uneven distribution of GATC motifs in the Escherichia coli chromosome, its plasmids and its phages. J. Mol. Biol. 1996;257:574–585. doi: 10.1006/jmbi.1996.0186. [DOI] [PubMed] [Google Scholar]
- 40.Merker M, et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat. Genet. 2015;47:242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parwati I, van Crevel R, van Soolingen D. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect. Dis. 2010;10:103–111. doi: 10.1016/S1473-3099(09)70330-5. [DOI] [PubMed] [Google Scholar]
- 42.Benjak, A., Sala, C. & Hartkoorn, R. C. Whole-Genome Sequencing for Comparative Genomics and De Novo Genome Assembly. In 1–16, 10.1007/978-1-4939-2450-9_1 (2015). [DOI] [PubMed]
- 43.Tischler AD, Leistikow RL, Kirksey MA, Voskuil MI, McKinney JD. Mycobacterium tuberculosis requires phosphate-responsive gene regulation to resist host immunity. Infect. Immun. 2013;81:317–328. doi: 10.1128/IAI.01136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 46.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1082. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 47.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anders S, Pyl PT, Huber W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All pathogen raw sequencing data is available from the ENA short read archive (accession number PRJEB29197).


