Abstract
Evidence is accumulating of the clinical utility of single nucleotide polymorphisms to effectively stratify risk of breast cancer. Yet for this personalized polygenic information to be translated to clinical practice, consideration is needed about how this personalized risk information should be communicated and the impact on risk perception. This study examined the psychosocial implications and the impact on risk perception of communicating personalized polygenic breast cancer risk to high-risk women. High-risk women with a personal history of breast cancer and an uninformative BRCA1/2 result were genotyped in the Variants in Practice study for 22 breast cancer single nucleotide polymorphisms. Participants in the highest quartile of polygenic breast cancer risk were invited to receive their individual research results. Two personalized visual risk communication tools were used to facilitate communication of the polygenic information. Participants subsequently undertook a semi-structured interview examining their experience of receiving their polygenic breast cancer risk and their breast cancer risk perception. Thirty-nine women opted to receive their results and were interviewed. The women described the risk communication tools as helpful as the tool enabled comparison of their personalized breast cancer risk to the general population. Participants incorporated the polygenic risk information into their breast cancer risk perception, which for some reawakened feelings of being at risk years after an uninformative BRCA1/2 result. However, few reported any detrimental emotional impact. The delivery of personalized polygenic breast cancer risk to high-risk women informed and modified their breast cancer risk perception with little emotional impact.
Keywords: Breast cancer risk, Polygenic risk, Single nucleotide polymorphism, Risk perception, Psychosocial
Introduction
Research efforts to identify significant heritable factors to further explain the etiology of breast cancer have more recently focused on single nucleotide polymorphisms (SNPs) (Li et al. 2016; Mavaddat et al. 2015). Genome-wide association studies have identified over 90 SNPs that collectively explain ~ 16% of familial breast cancer risk (Michailidou et al. 2015; Michailidou et al. 2013). Studies combining polygenic risk with existing risk prediction models demonstrate improvements in breast cancer risk prediction tools that could be effective in the stratification of breast cancer risk for women with and without family history (Li et al. 2016; Mavaddat et al. 2015; Maxwell and Nathanson 2013; Sawyer et al. 2012; Vachon et al. 2015). Despite this evidence, translation of combined SNP testing to clinical genetics practice has been slow, possibly due to a continued focus on single gene disorders and only recent evidence of SNP clinical utility (Shieh et al. 2016).
Research examining hypothetical interest towards SNP testing consistently indicates that participants with and without personal and family histories of cancer possess positive attitudes towards being offered genomic testing for cancer risk (Graves et al. 2011; Hall et al. 2015; Henneman et al. 2011; Leventhal et al. 2013). More recently, cancer SNP testing has been offered to research participants to examine the psychosocial implications of genomic risk profiling and provide evidence for future translation of this testing from research to clinical settings (Bancroft et al. 2015; Nusbaum et al. 2013; Yanes et al. 2017; Young et al. 2017). Participants in a prostate cancer screening study who all had a family history of prostate cancer could accurately recall their personalized risk result, did not experience distress, and were reassured by their risk profile information (Bancroft et al. 2014; Bancroft et al. 2015). Similarly, all participants recruited to a pilot study in a primary care setting accepted SNP testing for colon cancer due to their curiosity, to improve the accuracy of their personal medical information, and because of the perceived implications of SNP results for future generations (Nusbaum et al. 2013). The participants from both these studies reported their personalized result did not influence their screening intentions, which is reassuring given the SNP testing offered by both these studies did not have any proof of clinical utility (Bancroft et al. 2014; Bancroft et al. 2015; Nusbaum et al. 2013).
The translation of SNP testing from research to clinical settings requires consideration about how personalized risk should be communicated and how this information may impact patients’ risk perception (Chowdhury et al. 2013; Sawyer et al. 2012). Austin and Honer (2008) describe a psychiatric genetic counseling model that incorporates a visual aid to facilitate understanding about the genetic contribution towards the development of psychiatric conditions. While participants were not specifically asked to give feedback about the visual aid, they all reported their understanding of the genetic and environmental causes of psychiatric illness had increased when followed up 1 month after the genetic counseling session (Austin and Honer 2008). Furthermore, using personalized visual risk communication tools is preferred by the general community when considering the communication of polygenic cancer risk (Smit et al. 2016), and when used are demonstrated to improve understanding of risk (Gigerenzer and Edwards 2003). The use of visual aids or tools to assist with understanding and perception of risk is one of many approaches established in genetic counseling practice for cancer genetics, although risk tends to be verbally communicated (Julian-Reynier et al. 2003; Lobb et al. 2003).
As evidence is accumulating of the clinical utility of breast cancer SNPs, translation of this information from research to the clinic is becoming more realistic (Li et al. 2016). Nevertheless, there is a lack of critical examination of the psychosocial implications of communicating polygenic breast cancer risk information or empirical evidence of the use of personalized risk communication tools to facilitate this disclosure. This study invited research participants with a personal history of breast cancer and an uninformative BRCA1/2 result, to receive their polygenic breast cancer risk information. Disclosure of this personalized breast cancer risk information was facilitated using two visual risk communication tools. This study aims to examine women’s experiences of receiving polygenic breast cancer risk information and explores how this information impacted their breast cancer risk perception.
Methods
Study population
This study was approved by the Human Research Ethics Committee at the Peter MacCallum Cancer Centre, Victoria, Australia (ID 11.43, 11.08). Participants were women enrolled in the Victorian Familial Breast and Ovarian Cancer Cohort (VFBOCC). This cohort comprises women at high risk of breast and ovarian cancer based on a personal and family history of cancer who did not have a BRCA1/2 mutation detected during clinical genetic testing and controls who were women selected from the Australian voting register (Sawyer et al. 2012). The VFBOCC participants were genotyped for 22 breast cancer SNPs, from which a PRS was calculated with corresponding relative and absolute risks for breast cancer (Sawyer et al. 2012). At the time of genotyping, moderate-risk genes (e.g., CHEK2, PALB2, ATM, and BRIP) were not included in the research protocol. The high-risk women were stratified into quartiles according to PRS, with significant clinical differences observed between the lowest and highest quartiles (Sawyer et al. 2012). These differences included earlier age of breast cancer diagnoses and an increase in the number of second primary breast cancers in women in the highest quartile (Sawyer et al. 2012).
Sixty-eight participants with a PRS in the highest breast cancer risk quartile were identified as potential participants for this psychosocial study. Of these, three were excluded because they were not English speakers or had a psychiatric diagnosis. Of the 65 women who were sent invitation letters, 19 declined to participate, one was deceased, and six accepted then dropped out of the study. After consenting, participants were invited to receive their research results, i.e., SNP profile, in-person from the coauthors PJ or MAY, both of whom also conduct clinical genetics sessions, at the Familial Cancer Centre at the Peter MacCallum Cancer Centre, Australia. For the purposes of returning the results, there was no further stratification of participants, beyond having a PRS in the highest quartile. During the feedback session, participants’ personalized breast cancer risk was disclosed in terms of relative and absolute risk, and contextualized in comparison to the general population to demonstrate their increased risk status. In addition to disclosing participants’ polygenic risk, the inheritance of SNPs and risk to family members were also discussed during the feedback session. Participants’ understanding of the inheritance of SNPs and risk to family members has been described in a companion paper (Young et al. 2017).
Returning breast cancer risk results using visual risk communication tools
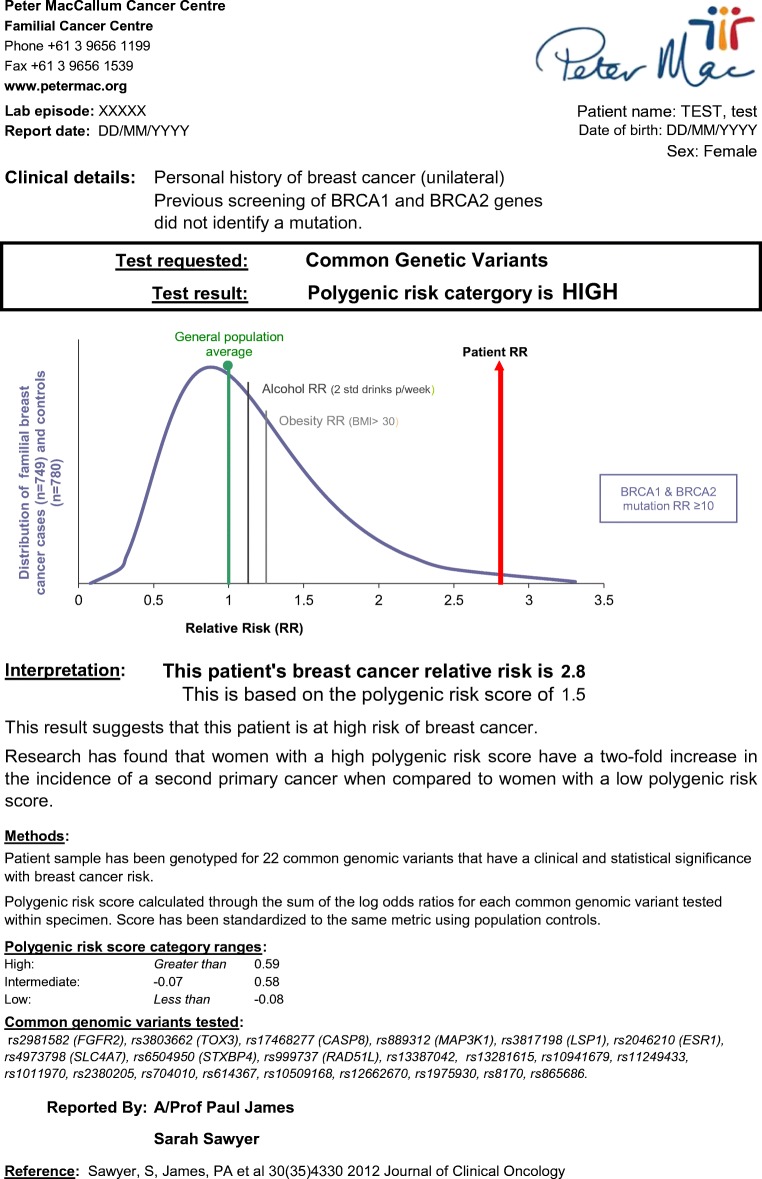
Two visual risk communication tools were annotated for each participant with their risk figures and used with every participant to assist the delivery of the breast cancer risk information (Sawyer et al. 2012). The first tool consisted of a graph that presented the skewed normal distribution curve of breast cancer relative risks for familial breast cancer cases and controls (Fig. 1). The graph included an indication of the average breast cancer risk for the Australian general population (RR = 1.0), the individual participants’ personalized relative risk, and lifestyle risk factors: alcohol consumption (RR = 1.13) and body mass index above 30 (RR = 1.25) (Hamajima et al. 2002; Parkin and Boyd 2011). The relative risk of breast cancer for BRCA1/2 mutation carriers (RR > 10) was greater than the range derived from SNP data (highest relative risk was 4.2) and was indicated separately on the bottom right side of the graph (Antoniou et al. 2003). This risk communication tool was developed specifically by the coauthors SS and PJ to deliver the personalized risk information to the participants.
Fig. 1.
Example of clinical report presenting relative risk of breast cancer based on common genomic variants
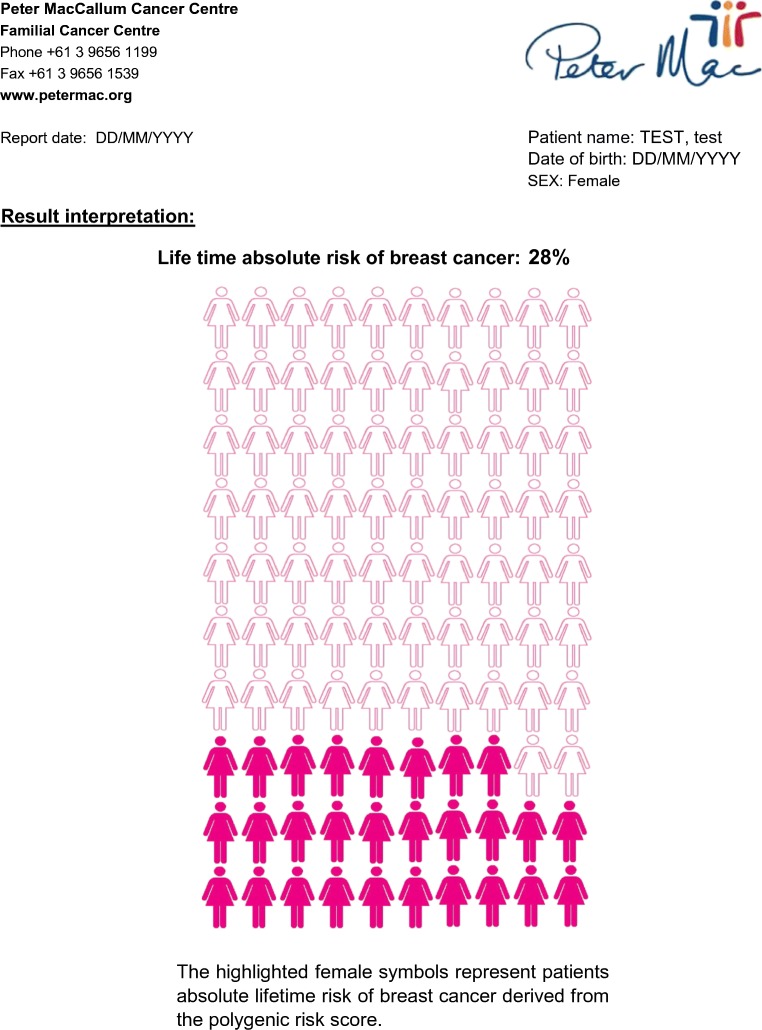
The second risk communication tool consisted of a pictograph, or icon array, with 100 women and was used to present absolute risk of breast cancer (Galesic et al. 2009). Participants’ breast cancer risks were indicated by the number of women colored pink (Fig. 2).
Fig. 2.
Example of clinical report presenting absolute risk of breast cancer based on common genomic variants
Data collection
Participants undertook a qualitative semi-structured interview examining their experience of receiving their personalized research-based polygenic breast cancer risk result in a clinical setting, approximately 1 week after receiving their PRS. The semi-structured interviews were conducted by investigators SS and LF either in person or via telephone and were audio-recorded and then transcribed verbatim. Transcripts were de-identified, participants assigned pseudonyms, and the data were thematically analyzed. The analysis was informed by grounded theory; transcripts were analyzed iteratively taking an inductive approach using constant comparison to stimulate the emergence of ideas, categories, and concepts, which were then organized into themes (Glaser and Strauss 1967; Strauss and Corbin 1994).
Results
Thirty-nine women received their PRS and participated in a follow-up interview. Fourteen were interviewed in-person, with the remainder conducted via telephone due to participants’ geographic location. Interviews ranged from 19 to 88 min in length. Overarching key themes were identified that illustrate participants’ understanding of SNPs and perception of their polygenic breast cancer risk. Findings describing participants’ understanding of the inheritance of polygenic information and perceived utility of personalized genetic profiling have been published in a companion paper (Young et al. 2017). This paper presents findings from the interviews describing how the visual risk communication tools and receipt of the polygenic results influenced participants’ risk perception. Specifically, how the use of two visual risk communication tools in the feedback session facilitated objective aspects of risk perception, and how receiving polygenic breast cancer risk information impacted participants’ subjective risk perception. This paper presents two key themes: (1) the influence of visual risk communication tools on breast cancer risk perception and (2) forewarning or reawakening subjective risk perception.
Participant description
Participants were aged 38–83 years and all had a previous diagnosis of breast cancer with an average age of onset of 46.7 years (range 28–66 years) (Table 1). Participants were receiving their SNP results an average of 7.3 years after they attended the clinic to receive their BRCA1/2 genetic test results. Participants’ PRS ranged from 0.62 to 2.10, and the range of corresponding breast cancer RR was 1.4–4.2. Six (15%) participants had no close family members with a breast cancer diagnosis and two participants each had one male family member with a breast cancer diagnosis (Table 2). As a result of their previous diagnosis, all participants had undergone breast cancer surgery but eight participants could not recall their breast cancer surgery type (Table 3). Twelve participants had also undergone a bilateral salpingo-oophorectomy (BSO). Participants of this study had an average breast cancer relative risk of 1.96 (range 1.4–4.2).
Table 1.
Participant socio-demographics and cancer history
| Participant description | n |
|---|---|
| Total number of participants | 39 |
| Age at interview (years) | |
| Mean (range) | 59.8 (38–83) |
| Relationship status | |
| Partnered | 26 |
| Not partnered at time of interview | 11 |
| Unknown | 2 |
| Children | |
| No. participants who have children | 34 |
| Mean no. of children | 2.2 |
| No. participants who have daughters | 29 |
| Mean no. of daughters | 1.1 |
| Ethnicity | |
| Caucasian | 37 |
| Other | 2 |
| Residence | |
| Metropolitan | 25 |
| Non-metropolitan | 14 |
| Employment | |
| Employed | 28 |
| Retired/unable to work | 11 |
| Previous breast cancer diagnosis | 39 |
| Age at diagnosis (years) | |
| Mean (range) | 46.7 (28–66) |
| Time since breast cancer diagnosis (years) | |
| Mean (range) | 13.2 (4–36) |
| Time since receiving BRCA1/2 results (years) | |
| Mean (range) | 7.3 (4–13) |
Table 2.
Participants’ family history
| Family history | |
|---|---|
| No. 1st–3rd degree relatives | |
| Total | 1176 |
| Range (min–max) | 9–66 |
| Mean | 30.2 |
| Female 1st–3rd degree relatives with unilateral breast cancer | |
| Total | 73 |
| Range (min–max) | 0–6 |
| Mean | 1.9 |
| No. participants (%) ≥ 2 | 22 (56.4) |
| Female 1st–3rd degree relatives with bilateral breast cancer | |
| Total | 3 |
| Range (min–max) | 0–1 |
| Mean | 0.1 |
| No. participants (%) = 1 | 3 (8.0) |
| Female 1st–3rd degree relatives with ovarian cancer | |
| Total | 13 |
| Range (min–max) | 0–2 |
| Mean | 0.3 |
| No. participants (%) ≥ 1 | 12 (30.7) |
| Male 1st–3rd degree relatives with breast cancer | |
| Total | 2 |
| Range (min–max) | 0–1 |
| Mean | 0.05 |
| No. participants (%) = 1 | 2 |
Table 3.
Participant-reported breast and ovarian cancer management
| n (%) | |
|---|---|
| Breast cancer risk management | |
| Surgery after breast cancer diagnosis | 39 (100) |
| Type of breast surgery not specified by participant | 6 (15) |
| Lumpectomy | 2 (5) |
| Mastectomy | |
| Affected breast only (unilateral) | 16 (41) |
| Bilateral | 15 (38) |
| Ovarian cancer management | |
| Bilateral salpingo-oophorectomy | 12 (31) |
Influence of visual risk communication tools on breast cancer risk perception
The two visual risk communication tools facilitated participants’ perception of their risks by supporting them to translate their visually represented risk to a personalized numerical risk, compare themselves to the breast cancer risk of the general population, and contextualize their genetic risk with lifestyle factors that infer increased breast cancer risk.
Visualizing and comparing polygenic breast cancer risk
Participants said they found the two risk communication tools “helpful” when they received their polygenic breast cancer risk results. The tools were described as enabling them to “see” where their risk was on the graph, and the number of women “highlighted” indicating their personal risk was described by participants as “clear as anything” (Saffron, RR 2.3). However, it was the inclusion of two lifestyle breast cancer risk factors that was seen as providing participants with context and a point of comparison to their genetic risk.
It [graph] has shown me the average population, it has shown me what the risk is for the people who are overweight, also the risk for the people who drink too much, and what is the risk to me. So there was a comparison on the graph. (Andrea, RR 1.4)
…the curve goes like this and there’s the norm and there’s the two lifestyle factors and then there was my line. So clearly genetics was playing a major, more of a role. (Victoria, RR 1.5)
Nevertheless, some participants found the depiction of their relative risk on the graph “confusing”. Whereas the “second page where [genetic health professional] had the pictures of the hundred women and it had the 14 women colored in” (Diane, RR 1.4) was easier to understand.
Translating visual representation to numerical breast cancer risk
Participants were able to translate the visual representation of their breast cancer risk, referencing the 100 women figure, to an objective, numerical description of their personal risk and describe how this compares to the general population. As Sally (RR 1.8) explained, risk of breast cancer for the general population is “usually about ten in one hundred but I’m eighteen in one hundred.” In contrast, fewer participants were also able to compare and contrast their relative risk with the general population risk of breast cancer. A participant who could do this said:
Well, [genetic health professional] numerically described it was 1.9 risk factor something… If it’s one in ten is the average and I’m 1.9 more which means I’m about double the… I have [a]bout twice the risk of your average. (Daisy, RR 1.9)
However, for some others, the numerical description of breast cancer risk was confusing or incomprehensible.
there’s a little bit of confusion about the difference between me having … double the risk of the norm and that risk being 20%. …so those figures and I’m not great with figures … feel like they’re contradictory. You know, 20%, how does 20% add up to double and even though I know, well if 10% is the normal risk then 20% is double. But double feels bigger than 20% somehow, if that makes sense? (Alexandra, RR 1.8)
Rose could describe her absolute risk referring to the figure: “that’s my absolute risk, and so that’s obviously a hundred women, there’s 24 pink ones” but struggled to marry this risk with her PRS of 1.27 described below the graph:
But the risk of 1.27, I’m not sure what that means now. So, or maybe that’s where most of the people of polygenic risk are: 1.27 of the average, above the average – 0.27 above 1 – I don’t know. (Rose, RR 2.4)
Forewarning or reawakening: modifying subjective perception of breast cancer risk
Receiving their polygenic result stimulated participants’ recall of their uninformative BRCA1/2 result in two contrasting ways. For some, the receipt of their polygenic result was regarded as unsurprising because they recalled being forewarned when they received their uninformative BRCA1/2 result about the possibility of “medical science” identifying another genetic cause of their breast cancer in the future. Other participants experienced a reawakening of feelings of being at-risk again, feelings they had earlier dismissed after receiving an uninformative BRCA1/2 test result. Regardless of whether participants felt forewarned about their risk or experienced a reawakening of their risk perception, few reported experiencing any detrimental emotional impact by receiving their polygenic risk result.
Awareness of “other” genetic factors causing breast cancer risk
Despite receiving an uninformative BRCA1/2 result in the past, many participants said they were aware that there may have been “other” genetic factors causing their personal and family history of breast cancer.
At the time, I had my initial counseling, the doctor who spoke to me … said … that with the family history even though we didn’t have BRCA1 and 2, she believed looking at my family history that there was probably a strong likelihood that sometime in the future they may find other genes or genetic mutations or mutations that acted together to increase my risk of breast cancer. (Pia, RR 1.5)
Many participants recalled how “pleased” they were when they learnt that they did not have a BRCA1/2 mutation, but remembered being informed “that there could be other reasons which the medical science is unable to find at that moment” (Andrea, RR 1.4). This caveat from genetic health professionals at the time of BRCA1/2 testing forewarned participants that progressive development in genetic technologies might find a reason for their personal and family history of breast cancer in the future.
When we’d finished and they’d said that we didn’t have the BRCA gene, they did say and … it’s something I’ve kept in my mind … that it’s most probably something that they haven’t found yet. If there was a group of people in a family that had things that seemed to be related, it was most probably something that hadn’t been found yet. (Clementine, RR 1.9)
Reawakening of breast cancer risk perception
Other participants experienced a reawakening of the feeling of being at-risk after they received their polygenic result. These women described a period of time between receiving their BRCA1/2 result and their polygenic result, where they perceived their risk of breast cancer to have been reduced and often assumed they were “no more likely than anyone else … to get another cancer” (Patricia, RR 1.4) or were “just like” other women in the general population (Julie, RR 1.5).
…once my sister got sick [breast cancer] … I knew something was wrong with the BRCA1 and 2, … in ‘97 it wasn’t that I was lead to believe, but it seemed very clear cut: you either carry the gene or not. So my assumption was I was just like anyone else in the general population for that small period (Julie, RR 1.5)
For these women, receiving their polygenic result brought the realization that their breast cancer risk still existed. Mary described her changing perception by likening her breast cancer risk to school grades and changing from being an “A” grade student to a “C” grade student:
Before I was getting an A [after receiving her BRCA1/2 result] and now I’m getting a C [after receiving polygenic risk result] (Mary, RR 2.9)
Personal impact of polygenic breast cancer risk
For many participants, regardless of whether their risk perception was reawakened or not, the presentation of breast cancer risks based on common variant information did not appear to profoundly impact their emotional state. Some demonstrated acceptance saying “you’ve just got to live with it” (Mia, RR 1.6) or “it’s just something you accept really” (Clementine, RR 1.9). Alexandra (RR 1.8) described herself as “naively optimistic” because her “risk doesn’t feel risky,” but acknowledged that she may not fully appreciate the possibility that she could develop a second primary breast cancer, hence her positive outlook and risk perception. Other participants compared their personalized risk to the general population and still concluded that it was not something to worry about.
I still think 14 [%] is pretty low on the scale of things. When you’re talking about the general population, everybody walking around is 10% [risk of breast cancer] and I have another 5% because of, okay, some gene, a different gene. I’m not sure that’s enough to put you in a state of panic or worry or change or anything like that. (Margaret, RR 1.5)
Emily, who was in her eighties, was similarly unaffected by her polygenic breast cancer risk and described herself as “sanguine.” She acknowledged that she had lived through much of her lifetime risk without a second primary breast cancer diagnosis, and noted that:
… at this stage of my life I could die of any number of things other than cancer, so why would I worry too much about that? … I think I was just fairly sanguine about it [breast cancer risk] because as I said before, at 83 … I’ve been free of cancer for all those years … it really didn’t affect me. (Emily, RR 1.4)
Another participant, Victoria, was similarly unperturbed by her polygenic risk and stated that she would not change her breast screening practices or base any decisions about risk reduction strategies, such as mastectomy, on this genetic information.
I don’t think it’s going to keep me awake at night, I don’t think there’s anything much I can do beyond what I’m currently doing – I’m certainly not going to have prophylactic surgery on the basis of that sort of risk (Victoria, RR 1.5)
Discussion
This study presents the first opportunity to describe how the delivery and receipt of polygenic risk scores for breast cancer influenced women’s risk perception. Each participant’s polygenic breast cancer risk information was delivered using two personalized visual risk communication tools: one displaying relative risk derived from the participant’s PRS and marked on a graph of the population distribution of risk (Fig. 1) and the other employing a natural frequency format of a grid comprised of 100 women displaying absolute lifetime risk (Fig. 2) (Gigerenzer and Hoffrage 1995). Some participants found the numerical risk information represented by one tool more accessible compared to the other, while there were only a few participants who appeared to integrate both forms of information into their risk perception. Nevertheless, the frequency grid format appeared to be more easily understood, a commonly demonstrated phenomena (Kurzenhauser and Dieckmann 2009), because unlike conditional probabilities or relative frequencies, natural frequency refers to the same class of observations, enabling participants to compare their personal breast cancer risk to the general population’s risk of breast cancer (e.g., 18% compared to 9%) (Gigerenzer and Edwards 2003). In contrast, the PRS presented on the graph as a ranking of relative risks (e.g., 1.27) was more challenging for some to comprehend, where they were particularly unable to marry the numerical risks presented in the two tools.
Objective and subjective risk perception
Risk perception and the meanings individuals ascribe to risk information are a marriage between subjective experience and objective numerical probabilities (d’Agincourt-Canning 2005). It is possible that the inclusion of the lifestyle risk factors, body mass index over 30 and alcohol consumption, drew on participants’ subjective experiences providing a counterpoint to the more abstract idea of SNPs to inform their breast cancer risk perception. Indeed, other studies have demonstrated that perceived risk of cancer can be more influenced by subjective experiences such as family history of cancer than numerical risk factors (Kenen et al. 2003; McAllister 2003).
There are many factors that influence and contribute towards the development of risk perception, including personal and familial experiences, contextual aspects, and delivery of new information (d’Agincourt-Canning 2005). As experiences are accumulated throughout life and context changes, so does risk perception (McAllister 2003). In this way, risk perception can be modified (Austin 2010). All the participants in this study previously had breast cancer and had undergone genetic counseling and testing for a BRCA1/2 mutation. These experiences may have contributed to a period of time where they considered the possibility that they might have a BRCA1/2 mutation and considered the significant, implied cancer risk for themselves and their family members. While all participants’ genetic test results were uninformative, “embodied” lived experiences such as these have been demonstrated to inform risk perception (Abel and Browner 1998). For example, some participants juxtaposed their experience of receiving an uninformative BRCA1/2 test result with their personal and family cancer history to explain how they felt forewarned because “other genes or genetic mutations” may be identified in the future and were, therefore, generally unsurprised by the existence of their polygenic breast cancer risk. Whereas, others described a reawakening of their breast cancer risk after receiving their polygenic result, demonstrating how their risk perception was modified from being similar to women in the general population to an increased level.
Implications for genetic counseling practice
Determining women’s polygenic risk, particularly those who are clinically high-risk yet have received an uninformative BRCA1/2 test result, offers more detailed and personalized breast cancer risk stratification than is currently available. Delivering information about the inheritance of SNPs has been proven to be acceptable to women, who understood the personal rather than familial nature of the information, the mode of inheritance, and the inferred risk of breast cancer but not ovarian cancer (Young et al. 2017). However, in order for this SNP information to be useful, the polygenic risk must be communicated to women in a manner that facilitates understanding and informs risk perception. Indeed, participants reported that the use of the two visual risk communication tools in this study informed their risk perception by providing objective and personalized numerical information in context with more subjective lifestyle risk information. The findings suggest that if and when SNP testing is integrated into clinical genetics practice, visual communication tools may be useful aids for genetic health professionals to incorporate into their practice when delivering polygenic results. Further research is required to formally evaluate and compare visual communication tools.
Limitations
This study is limited by the sample comprised predominantly of Caucasian, metropolitan women who had previously received genetic counseling and testing at the Familial Cancer Centre at the Peter MacCallum Cancer Centre. The women who responded to recruitment may have been women who were more receptive to learning new genetic information and were therefore less likely to negatively experience the receipt of this information. There are likely to be women who actively or passively declined to participate for whom the polygenic information would have had a more detrimental impact on their emotional and psychological well-being. If polygenic testing is to be successfully integrated into clinical practice, then we need to ensure these women are appropriately supported to assimilate the polygenic results into their risk perception. Furthermore, this study only included women with a personal history of breast cancer. It is unknown how women from high-risk families who do not have a personal history of breast cancer will perceive their polygenic risk if it falls in the highest group. For these reasons, a mixed methods psychosocial study is now being conducted to compare acceptability of the delivery and receipt of polygenic information to women.
Conclusion
In this study, the use of two personalized visual risk communication tools to aid the delivery of polygenic breast cancer risk information informed and modified women’s breast cancer risk perception. Women were able to incorporate this information into their perceived risk of breast cancer with little emotional impact. This study demonstrates the successful delivery of polygenic information to women with a personal and familial history of breast cancer, aided by two personalized visual risk communication tools. Further research is required to examine whether women who are not at high risk of breast cancer are receptive to receiving polygenic breast cancer risk information and examine the associated psychosocial repercussions.
Acknowledgements
The authors would like to acknowledge all the women who took part in this study and gave their time so generously to participate in an interview. We would also like to acknowledge and thank the team in the Parkville Familial Cancer Centre at Peter MacCallum Cancer Centre for accommodating the processes involved in this study. Many thanks to Rowan Forbes Shepherd, Maatje Scheepers-Joynt, and Victoria Rasmussen for their feedback on the manuscript.
Funding
Dr. Laura Forrest is funded by a postdoctoral fellowship from the National Breast Cancer Foundation, Australia.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Abel EK, Browner CH. Selective compliance with biomedical authority and the uses of experiential knowledge. In: Lock M, Kaufert PA, editors. Pragmatic women and body politics. Cambridge: Cambridge Studies in Medical Anthropology. Cambridge University Press; 1998. pp. 310–326. [Google Scholar]
- Antoniou A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JC. Re-conceptualizing risk in genetic counseling: implications for clinical practice. J Genet Couns. 2010;19:228–234. doi: 10.1007/s10897-010-9279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JC, Honer WG. Psychiatric genetic counselling for parents of individuals affected with psychotic disorders: a pilot study. Early Interv Psychiatry. 2008;2:80–89. doi: 10.1111/j.1751-7893.2008.00062.x. [DOI] [PubMed] [Google Scholar]
- Bancroft EK, et al. “It’s all very well reading the letters in the genome, but it’s a long way to being able to write”: men’s interpretations of undergoing genetic profiling to determine future risk of prostate cancer. Fam Cancer. 2014;13:625–635. doi: 10.1007/s10689-014-9734-3. [DOI] [PubMed] [Google Scholar]
- Bancroft EK, et al. The psychological impact of undergoing genetic-risk profiling in men with a family history of prostate cancer. Psychooncology. 2015;24:1492–1499. doi: 10.1002/pon.3814. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, et al. Incorporating genomics into breast and prostate cancer screening: assessing the implications. Genet Med. 2013;15:423–432. doi: 10.1038/gim.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Agincourt-Canning L. The effect of experiential knowledge on construction of risk perception in hereditary breast/ovarian cancer. J Genet Couns. 2005;14:55–69. doi: 10.1007/s10897-005-1500-0. [DOI] [PubMed] [Google Scholar]
- Galesic M, Garcia-Retamero R, Gigerenzer G. Using icon arrays to communicate medical risks: overcoming low numeracy. Health Psychol. 2009;28:210–216. doi: 10.1037/a0014474. [DOI] [PubMed] [Google Scholar]
- Gigerenzer G, Edwards A. Simple tools for understanding risks: from innumeracy to insight. Br Med J. 2003;327:741–744. doi: 10.1136/bmj.327.7417.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigerenzer G, Hoffrage U. How to improve bayesian reasoning without instruction: frequency formats. Psychol Rev. 1995;102:684–704. doi: 10.1037/0033-295X.102.4.684. [DOI] [Google Scholar]
- Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. New Brunswick: Aldine Transaction; 1967. [Google Scholar]
- Graves KD, Peshkin BN, Luta G, Tuong W, Schwartz MD. Interest in genetic testing for modest changes in breast cancer risk: implications for SNP testing. Public Health Genomics. 2011;14:178–189. doi: 10.1159/000324703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MJ, Ruth KJ, Chen DY, Gross LM, Giri VN. Interest in genomic SNP testing for prostate cancer risk: a pilot survey. Hered Cancer Clin Pract. 2015;13:11. doi: 10.1186/s13053-015-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima N, et al. Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman L, Timmermans DR, Bouwman CM, Cornel MC, Meijers-Heijboer H. ‘A low risk is still a risk’: exploring women’s attitudes towards genetic testing for breast cancer susceptibility in order to target disease prevention. Public Health Genomics. 2011;14:238–247. doi: 10.1159/000276543. [DOI] [PubMed] [Google Scholar]
- Julian-Reynier C, Welkenhuysen M, Hagoel L, Decruyenaere M, Hopwood P. Risk communication strategies: state of the art and effectiveness in the context of cancer genetic services. Eur J Hum Genet. 2003;11:725–736. doi: 10.1038/sj.ejhg.5201037. [DOI] [PubMed] [Google Scholar]
- Kenen R, Arden-Jones A, Eeles R. Family stories and the use of heuristics: women from suspected hereditary breast and ovarian (HBOC) families. Sociol Health Illn. 2003;25:838–865. doi: 10.1046/j.1467-9566.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- Kurzenhauser S, Dieckmann A (2009) Risk communication: simple tools to foster understanding. In: Smoller JW, Sheidley BR, Tsuang MT (eds) Psychiatric genetics: applications in clinical practice. American Psychiatric Publishing, pp 47–65
- Leventhal KG, et al. “Is it really worth it to get tested?”: primary care patients’ impressions of predictive SNP testing for colon cancer. J Genet Couns. 2013;22:138–151. doi: 10.1007/s10897-012-9530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. Breast cancer risk prediction using a polygenic risk score in the familial setting: a prospective study from the Breast Cancer Family Registry and kConFab. Genet Med. 2016;19:30. doi: 10.1038/gim.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb EA, et al. Women’s preferences and consultants’ communication of risk in consultations about familial breast cancer: impact on patient outcomes. J Med Genet. 2003;40:e56. doi: 10.1136/jmg.40.5.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavaddat N et al (2015) Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst 107. 10.1093/jnci/djv036 [DOI] [PMC free article] [PubMed]
- Maxwell KN, Nathanson KL. Common breast cancer risk variants in the post-COGS era: a comprehensive review. Breast Cancer Res. 2013;15:212. doi: 10.1186/bcr3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister M. Personal theories of inheritance, coping strategies, risk perception and engagement in hereditary non-polyposis colon cancer families offered genetic testing. Clin Genet. 2003;64:179–189. doi: 10.1034/j.1399-0004.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- Michailidou K, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–361. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidou K, et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47:373–380. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum R, et al. Translational genomic research: protocol development and initial outcomes following SNP testing for colon cancer risk. Transl Behav Med. 2013;3:17–29. doi: 10.1007/s13142-012-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Boyd L. 8. Cancers attributable to overweight and obesity in the UK in 2010. Br J Cancer. 2011;105:S34–S37. doi: 10.1038/bjc.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S, Mitchell G, McKinley J, Chenevix-Trench G, Beesley J, Chen XQ, Bowtell D, Trainer AH, Harris M, Lindeman GJ, James PA. A role for common genomic variants in the assessment of familial breast cancer. J Clin Oncol. 2012;30:4330–4336. doi: 10.1200/JCO.2012.41.7469. [DOI] [PubMed] [Google Scholar]
- Shieh Y, et al. Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat. 2016;159:513–525. doi: 10.1007/s10549-016-3953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AK, Keogh LA, Hersch J, Newson AJ, Butow P, Williams G, Cust AE. Public preferences for communicating personal genomic risk information: a focus group study. Health Expect. 2016;19:1203–1214. doi: 10.1111/hex.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A, Corbin J. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. California: Thousand Oaks Sage Publications; 1994. pp. 273–285. [Google Scholar]
- Vachon CM, Pankratz VS, Scott CG, Haeberle L, Ziv E, Jensen MR, Brandt KR, Whaley DH, Olson JE, Heusinger K, Hack CC, Jud SM, Beckmann MW, Schulz-Wendtland R, Tice JA, Norman AD, Cunningham JM, Purrington KS, Easton DF, Sellers TA, Kerlikowske K, Fasching PA, Couch FJ. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015;107:107. doi: 10.1093/jnci/dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes T, Meiser B, Young MA, Kaur R, Mitchell G, Barlow-Stewart K, Roscioli T, Halliday J, James P. Psychosocial and behavioral impact of breast cancer risk assessed by testing for common risk variants: protocol of a prospective study. BMC Cancer. 2017;17:491. doi: 10.1186/s12885-017-3485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MA et al (2017) Making sense of SNPs: women’s understanding and experiences of receiving a personalized profile of their breast cancer risks. J Genet Couns. 10.1007/s10897-017-0162-z [DOI] [PubMed]