Abstract
Background
The vasoactive-inotropic score (VIS) predicts mortality and morbidity after paediatric cardiac surgery. Here we examined whether VIS also predicted outcome in adults after cardiac surgery, and compared predictive capability between VIS and three widely used scoring systems.
Methods
This single-centre retrospective cohort study included 3213 cardiac surgery patients. Maximal VIS (VISmax) was calculated using the highest doses of vasoactive and inotropic medications administered during the first 24 h post-surgery. We established five VISmax categories: 0–5, >5–15, >15–30, >30–45, and >45 points. The predictive accuracy of VISmax was evaluated for a composite outcome, which included 30-day mortality, mediastinitis, stroke, acute kidney injury, and myocardial infarction.
Results
VISmax showed good prediction accuracy for the composite outcome [area under the curve (AUC), 0.72; 95% confidence interval (CI), 0.69–0.75]. The incidence of the composite outcome was 9.6% overall and 43% in the highest VISmax group (>45). VISmax predicted 30-day mortality (AUC, 0.76; 95% CI, 0.69–0.83) and 1-yr mortality (AUC, 0.70; 95% CI, 0.65–0.74). Prediction accuracy for unfavourable outcome was significantly better with VISmax than with Acute Physiology and Chronic Health Evaluation II (P=0.01) and Simplified Acute Physiological Score II (P=0.048), but not with the Sequential Organ Failure Assessment score (P=0.32).
Conclusions
In adults after cardiac surgery, VISmax predicted a composite of unfavourable outcomes and predicted mortality up to 1 yr after surgery.
Keywords: acute kidney injury, cardiac surgery, cardiovascular system, mortality, myocardial infarction, stroke, risk assessment scoring system, postoperative outcome
Editor's key points.
-
•
Traditional ICU scoring systems have not been developed for patients after cardiac surgery, and typically do not consider or emphasise dose rates of inotropes and vasopressors.
-
•
The vasoactive-inotropic score is derived from summing the maximum dose rates—using correction factors to account for differential units of measurement—of inotropes and vasopressor medications administered in the first 24 h after cardiac surgery.
-
•
In this study, the maximum vasoactive-inotropic score in the first 24 h after cardiac surgery was a good independent predictor for adults of morbidity and mortality up to 1 yr after their surgery.
-
•
It might be useful to include the maximum vasoactive-inotropic score as well as traditional ICU risk metrics when assessing prognosis after heart surgery.
ICU scoring systems are useful for estimating the severity of critical illness and predicting outcomes. However, they were not developed for postoperative cardiac surgery patients, and thus do not consider surgical success or patients' responses to surgery and cardiopulmonary bypass.1 In the ICU, mortality and morbidity risk are often estimated using scoring systems such as the Simplified Acute Physiological Score (SAPS II/III),2, 3 Sequential Organ Failure Assessment (SOFA), and Acute Physiology and Chronic Health Evaluation II (APACHE II).1, 4, 5 Although cardiac surgery patients were excluded during their development, these instruments have been studied for risk assessment after cardiac surgery.1, 2, 3, 6 Specific heart surgery risk scores—such as the EuroScore II, Society of Thoracic Surgeons (STS) risk score, and Parsonnet score —were developed to estimate the risk of cardiac surgery, mainly using preoperative health data.7, 8, 9, 10, 11, 12
The vasoactive-inotropic score (VIS) is calculated as a weighted sum of all administered inotropes and vasoconstrictors, reflecting pharmacological support of the cardiovascular system.13 Studies in paediatric populations have shown that higher VIS predicts unfavourable outcomes, including morbidity and mortality, after cardiac surgery.13, 14, 15, 16, 17 The maximum VIS value within 24 h after ICU admission (VISmax) has been assessed as a good predictor of unfavourable outcomes after paediatric cardiac surgery.13, 15 Higher VIS values have also been associated with worse outcomes in septic paediatric patients.18 The predictive performance of the VIS in adult populations has not been assessed.
In the present retrospective cohort study, we investigated whether VISmax predicted unfavourable outcomes after cardiac surgery in an adult population. We hypothesised that VISmax would predict short-and intermediate-term morbidity and mortality. We assessed the predictive ability of VISmax compared with that of the SOFA, APACHE II, and SAPS II scores. We also determined whether patients with the highest maximal cardiovascular failure score (value 4) according to SOFA were also in the highest risk group for unfavourable outcome according to VISmax.
Methods
Study population
We performed a single-centre retrospective cohort study including all cardiac surgery patients admitted to the ICU (n=26 beds) at Kuopio University Hospital, Kuopio, Finland, between January 2010 and July 2014. The included patients underwent coronary artery bypass grafting (CABG) surgery, valve replacement or repair, atrial septal defect or ventricular septal defect repair, surgery for intracardiac tumours, or combined CABG and valvular or ascending aortic procedures. Patients who underwent catheter-based valvular (transapical or transfemoral aortic), aortic arch, or descending aortic procedures were excluded.
This clinical study was approved by the Research Ethic Committee of the Northern Savo Hospital District (no: 131/2015). It was conducted in full compliance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice Guidelines, and the applicable local regulatory requirements. The requirement to obtain patient consent was waived by the ethics committee because the data analysis was retrospective.
Treatment protocol
After cardiac surgery, the patients were treated in the ICU. Patients received postoperative red blood cell transfusion if haemoglobin was <80 g L−1, fresh frozen plasma if the international normalised ratio was >1.5, and platelets if platelet count was <50×109 L−1, or if bleeding was because of platelet dysfunction and the patient had received preoperative acetylsalicylic acid or clopidogrel.
Because of the retrospective nature of the study, there was no standardised haemodynamic treatment protocol. According to our institutional protocol, inotropic medication was initiated if the cardiac index was <1.8 L m−2 min−1 or <2.0 L m−2 min−1 with simultaneous hypotension, with MAP<65 mm Hg. Dobutamine was the first-line inotropic medication, and the second-line option was to add milrinone to dobutamine. However, if the combination of dobutamine and milrinone was insufficient to increase the cardiac output and arterial pressure, then dobutamine was replaced by norepinephrine. Dobutamine was started at 2 μg kg−1 min−1 and was titrated up to 10 μg kg−1 min−1. In the case of clinically significant tachycardia (HR>110 min−1 or >15% increase over predosing values), dobutamine was decreased. Milrinone was administered after dobutamine, if cardiac output remained low, pulmonary pressures were high, or both. Milrinone was started with or without a bolus administration (50 μg kg−1 over 10 min), and was continued with dose 0.375–0.5 μg kg−1 min−1. If dobutamine and milrinone failed to achieve the target cardiac output, epinephrine was started at 0.04 μg kg−1 min−1. The epinephrine dose rate was titrated up to 0.1 μg kg−1 min−1.
MAP was maintained at >65 mm Hg with norepinephrine infusion if needed. Norepinephrine was started at 0.04 μg kg−1 min−1 and was titrated up to 0.5 μg kg−1 min−1. Use of vasopressin was at the clinicians' discretion, if hypotension was not responsive to norepinephrine. Vasopressin was combined with norepinephrine, with a starting dose rate of 1 IU h−1. The dose rate was titrated up to 4 IU h−1.
Pulmonary capillary wedge pressure (PCWP) was maintained between 8 and 12 mm Hg, or between 10 and 15 mm Hg in cases of low output syndrome. Low PCWP was treated with additional fluids after a physician's evaluation.
Responses to vasoactive and inotropic medications were tracked, and infusions of these medications were titrated to the lowest required dose rates to maintain adequate cardiac output and MAP.
If weaning from cardiopulmonary bypass failed with inotropes and vasopressors 15–30 min after aortic occlusion clamp removal, an intra-aortic balloon pump was placed, extracorporeal membrane oxygenator therapy was initiated, or both. Sedation was continued until the patient exhibited a normal temperature, chest tube drainage of <100 ml h−1, SpO2>90%, and haemodynamic stability. Sedation was then discontinued, and the patient was weaned from mechanical ventilation. ICU discharge was at the physician's discretion.
Data collection methods
From the ICU critical care information system (Centricity™ Critical Care Clinisoft version 8.1, GE Healthcare, Barrington, IL, USA), we extracted patient characteristic and clinical data, and SOFA, SAPS II, and APACHE II values during the first 24 h after ICU arrival. SAPS II and APACHE II evaluate cardiovascular status using HR and BP. SOFA assesses cardiovascular failure based on inotrope and vasoactive medication dosing rates, defined as 0.1 μg min−1 kg−1 norepinephrine or epinephrine (equal to VISmax of 10).
VISmax was calculated (VIS=dopamine dose [μg kg−1 min−1]+dobutamine [μg kg−1 min−1]+100×epinephrine dose [μg kg−1 min−1]+50×levosimendan dose [μg kg−1 min−1]+10×milrinone dose [μg kg−1 min−1]+10 000×vasopressin [units kg−1 min−1]+100×norepinephrine dose [μg kg−1 min−1]) using the maximum dosing rates of vasoactive and inotropic medications (μg kg−1 min−1 or IU kg−1 min−1) during the first 24 h after postoperative ICU admission, which were retrieved from the ICU critical care information system with separate database queries.13, 19 We selected VISmax cut-off values to define five groups for an adult population instead of using values of a paediatric population.13
Radiological imaging data were collected from our hospital radiological image archiving and communication system (Sectra, PACS, Linköping, Sweden). CT images were interpreted by a consultant radiologist to evaluate cerebral haemorrhage or infarction, or mediastinitis.
All data were reviewed and validated by ICU doctors and research nurses, and then stored by the Finnish Intensive Care Quality Consortium. Dates of patients' deaths were acquired from the national Causes of Death Register of Statistics Finland (Official Statistics of Finland, Helsinki, Finland).
Outcome definitions
The primary outcome was a composite of unfavourable outcomes, including 30-day mortality, mediastinitis, cerebral infarction, cerebral haemorrhage, kidney injury, and myocardial infarction. Earlier studies of VISmax have similarly defined outcome.13, 15 Secondary outcomes were the in-ICU, 30-, 90-, 180-day, and 1-yr death rates, and prolonged ICU stay.
Mediastinitis was defined as a post-surgical mediastinum infection confirmed by CT imaging up to 1 yr after surgery, consistent with the European Association of Cardio-Thoracic Surgery definition.20 Cerebral infarction and cerebral haemorrhage were defined as new neurological deficits with new radiological evidence within 24 h after cardiac surgery.21 Postoperative acute kidney injury was defined as newly initiated renal replacement therapy on the ICU.22 Myocardial infarction was defined as creatine kinase MB isoenzyme mass of >70 μg ml−1 on the operation day, or >100 μg ml−1 on the 1st postoperative morning.23, 24 Prolonged ICU stay was defined as more than 2.1 days (90th percentile), similar to in earlier studies.25
Statistical analysis
Descriptive statistics are presented as median [inter-quartile range (IQR)] or mean (standard deviation) for continuous variables, and as n (%) for categorical variables. Patient characteristics and clinical characteristics of outcome groups were compared by the t-test or Mann–Whitney U-test for continuous variables, and by the Z-test or χ2 test for categorical variables. Multiple categories comparison was executed separately for each pair of groups. Bonferroni adjustments were used to adjust the P values. VISmax classifications were determined using a χ2 automatic interaction detection decision tree.
The Hosmer-Lemeshow goodness-of-fit test was used to confirm no significant discrepancy between predicted and observed unfavourable outcomes. Good calibration was indicated by a low χ2 and a high P value >0.05. The discriminative powers of VISmax, SOFA, APACHE II, and SAPS II regarding unfavourable outcome and mortalities were assessed by the area under the curve (AUC) of receiver operating characteristics (ROC) curves, which were compared using the non-parametric DeLong method.26
Logistic regression modelling was used to assess associations between predictive factors and the primary outcome. Multivariable Cox regression analysis was used to assess the relationship between VISmax and other candidate predictive factors and the length of ICU stay. Data are presented as odds ratios (ORs), hazard ratios, and 95% confidence intervals (CIs). Cumulative survival curves, as a function of time, were generated using the Kaplan-Meier approach, and compared by log-rank test. The χ2 test was performed to assess whether VISmax predicted unfavourable outcome among patients with the most severe cardiovascular dysfunction/failure according to the SOFA score.
Statistical analyses were performed using IBM SPSS 24.0 (SPSS Inc., Chicago, IL, USA); the DeLong method was executed in R 3.2.1 (The R Foundation for Statistical Computing, Vienna, Austria) using the pROC 1.10.0 library. A P value <0.05 was considered to indicate statistical significance.
Results
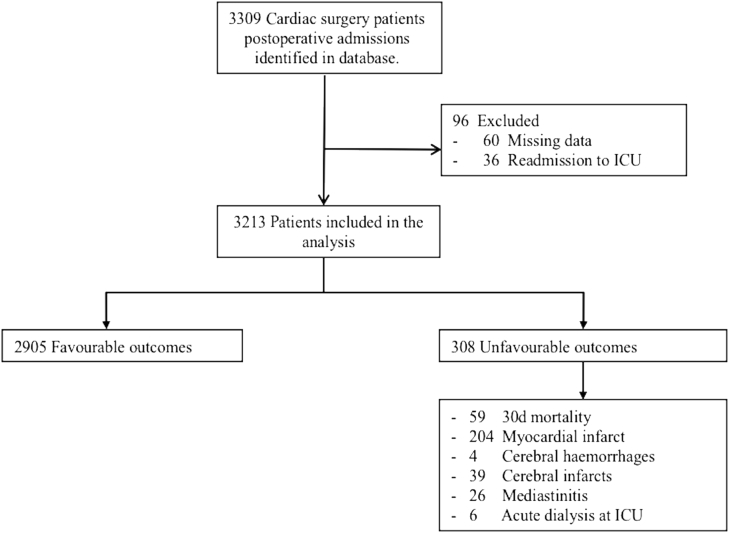
During the observation period, 3309 consecutive cardiac surgery patients were evaluated, of whom 96 were excluded. Thus, 3213 patients were included in the analyses (Fig. 1), among whom 1930 (60%) received vasoactive or inotrope support within 24 h after ICU admission. The median VISmax was 4.0 (IQR, 0.0–14.6) overall, and 12.0 (IQR, 6.0–21.8) among patients who received vasoactive or inotropic medication. Inotrope or vasopressor medication was administered to 985 (31%) patients at arrival to the ICU, and 130 (13%) of the patients receiving vasoactive or inotropic medication met criteria for unfavourable outcome. After 24 h, 157 (16%) of the patients receiving vasoactive or inotropic medication when arriving in the ICU were still receiving these drugs, and 56 (36%) of the patients receiving vasoactive or inotropic medication after 24 h at the ICU had unfavourable outcome.
Fig 1.
Study flowchart. Each patient was included only once, even when they had multiple re-admissions. Incidence of each adverse event is presented separately. ICU, Intensive care unit.
Overall, 308 patients (9.6%) had an unfavourable outcome—most commonly a myocardial infarction (Fig. 1). When compared with patients with favourable outcomes, patients with unfavourable outcomes were older, more often female, had more commonly undergone an emergency procedure, had higher EuroScore II, SOFA, SAPS II, and APACHE II scores, and had lower preoperative ejection fractions and longer cardiopulmonary bypass times (Table 1). The majority of operations were isolated CABG. A higher proportion of valvular, combined, and other procedures were associated with unfavourable outcomes compared to isolated CABG (Table 1).
Table 1.
Patient and clinical characteristics according to unfavourable outcome. Data are presented as median (inter-quartile range) or median (range), mean (standard deviation), or n (%). SOFA, SAPS II, and APACHE II scores were calculated during the first 24 h after arrival at the ICU. Combined procedures include concomitant coronary artery bypass graft and valve procedures, multivalve surgery, or both. Other cardiac surgery includes atrial septal defect or ventricular septal defect repair, or for intracardiac tumours. APACHE II, Acute Physiology and Chronic Health Evaluation Score II; AO, aortic occlusion; CABG, coronary artery bypass grafting; CBP, cardiopulmonary bypass; EF, ejection fraction; EuroScore II, European System for Cardiac Operative Risk Evaluation II; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure; TIA, transient ischemic attack; VISmax, maximum vasoactive inotropic score in the first postoperative 24 h. * t-test for normally distributed variables, Mann–Whitney independent samples test for non-normally distributed variables. †χ2 test for operation characteristics between outcome and operation groups. ‡Preoperative renal insufficiency defined as glomerular filtration rate <60 ml kg−1 1.73 m−2. ¶Chronic obstructive pulmonary disease or asthma. §Preoperative anaemia was defined as <130 g L−1 for male and <120 g L−1 for female. ||Preoperative treatment with warfarin, low molecular weight heparin, or clopidogrel etc. #Earlier transient ischaemic attack. **Earlier cerebral infarct or haemorrhage
| All patients (n=3213) | Unfavourable outcome |
|||
|---|---|---|---|---|
| No (n=2905) | Yes (n=308) | P-value* | ||
| Age (yr) | 68 (19–90) | 67 (19–90) | 67 (19–87) | 0.522 |
| Male sex | 2345 (73) | 2130 (73) | 216 (70) | 0.230 |
| Emergency | 203 (6) | 146 (5) | 57 (19) | 0.000 |
| Renal insufficiency‡ | 565 (18) | 520 (18) | 64 (21) | 0.213 |
| Lung disease¶ | 421 (13) | 375 (13) | 46 (15) | 0.316 |
| Preoperative anaemia§ | 699 (21) | 605 (21) | 94 (31) | <0.001 |
| Anticoagulation|| | 946 (29) | 830 (29) | 116 (38) | 0.001 |
| TIA# | 134 (4) | 114 (4) | 20 (6) | 0.032 |
| Stroke** | 126 (4) | 110 (3) | 16 (5) | 0.226 |
| ICU stay (days) | 1.0 (0.8–1.0) | 0.9 (0.9–1.0) | 1.8 (0.9–4.0) | <0.001 |
| SOFA | 5.0 (4–6) | 5.0 (4.0–6.0) | 7.0 (5.0–9.0) | <0.001 |
| SAPS II | 31 (25–35) | 29 (25–34) | 35 (29–43) | <0.001 |
| APACHE II | 16 (12–18) | 15 (12–18) | 18 (14–22) | <0.001 |
| EuroScore II | 2.1 (1.2–4.0) | 2.0 (1.1–3.7) | 3.2 (1.7–6.4) | <0.001 |
| Preoperative EF (%) | 56 (12) | 56 (12) | 55 (12) | 0.111 |
| CBP time (min) | 110 (59) | 104 (53) | 149 (82) | <0.001 |
| AO time (min) | 91 (45) | 84 (41) | 127 (61) | <0.001 |
| VISmax | 4 (0–15) | 3 (0–13) | 15 (5–35) | <0.001 |
| CABG | 1810 (56) | 1694 (58) | 116 (38) | <0.001† |
| Valve surgery | 869 (27) | 761 (26) | 108 (35) | <0.001† |
| Combined procedures | 446 (14) | 369 (13) | 77 (25) | <0.001† |
| Other cardiac surgery | 88 (3) | 81 (3) | 7 (2) | 0.604† |
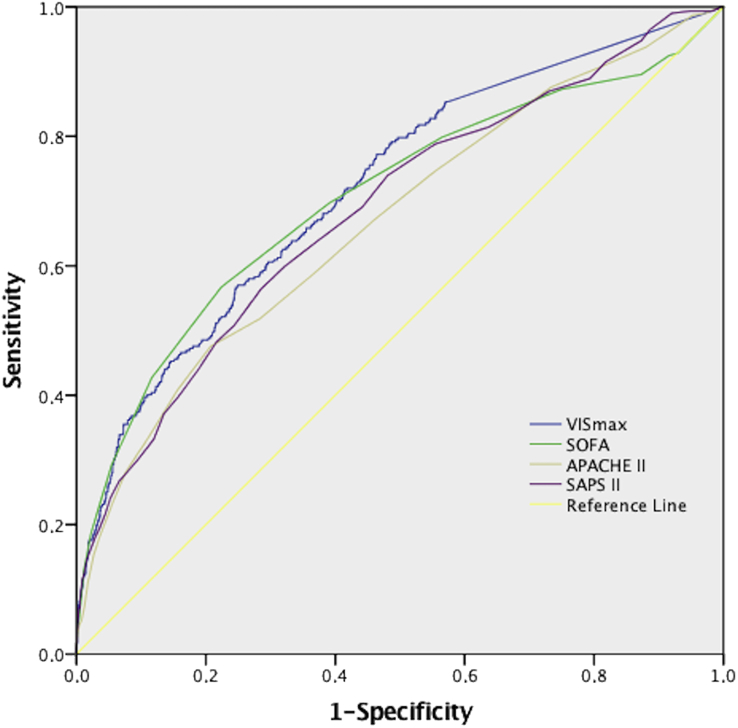
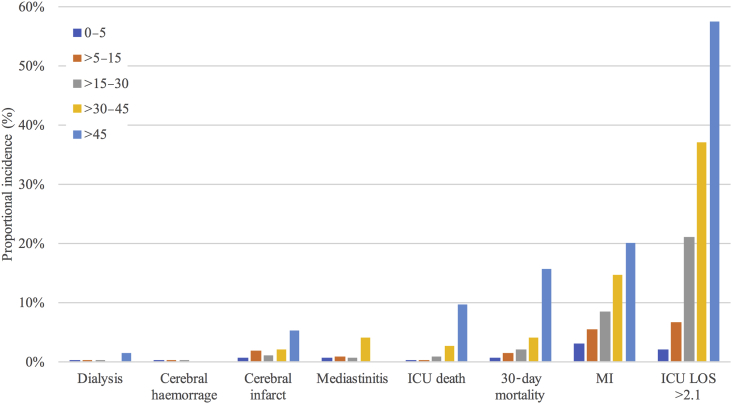
VISmax predicted unfavourable outcome (AUC, 0.72; 95% CI, 0.69–0.75; P<0.001; Fig. 2). The VISmax groups were as follows: 0–5, >5–15, >15–30, >30–45, and >45 points (Supplementary Table S1 presents the sensitivity and specificity of the VISmax at each group). VISmax had significant predictive ability for 30-day mortality, prolonged ICU stay, and myocardial infarction (Fig. 3). It was impossible to test whether VISmax independently predicted kidney insufficiency, cerebral haemorrhage, cerebral infarct, or mediastinitis because of the low incidences (Fig. 1).
Fig 2.
Receiver operating curves (ROC) of unfavourable outcome on VISmax, SOFA, SAPS II, and APACHE II. VISmax showed better discrimination capability than APACHE II, and SAPS II (Delong-method with P<0.05) and similar to SOFA (P=0.31). APACHE II, Acute Physiology and Chronic Health Evaluation II score; SAPS II, Simple Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment score; VISmax, maximum vasoactive-inotropic score.
Fig 3.
Proportional incidence of outcomes in each VISmax group. The 0–5 and >45 groups significantly differed from the other groups with regards to 30-day mortality (χ2 test, P<0.05). The groups 0–5, 5–30, and 30–45 significantly differed from one another in ICU length of stay (LOS) and myocardial infarct (MI) (χ2 test, P<0.05). VISmax maximum vasoactive-inotropic score.
In binary logistic regression analysis, VISmax independently predicted unfavourable outcome when the model included age, sex, emergency procedure, cardiopulmonary bypass time, and preoperative ejection fraction (Table 2). Calibration with the Hosmer-Lemeshow Test showed a good fit (χ2=7.0, P=0.53). Overall Correct Classification (Supplementary Table S1 presents the sensitivity and specificity of the VISmax at each group) was 91.0%, with a cut-off value of 0.5. The ORs for unfavourable outcome increased linearly with increasing VISmax groups (Table 2).
Table 2.
Logistic regression analysis of postoperative factors for unfavourable outcome. All were predictors of increased risk, except age, sex, and preoperative ejection fraction. *The odds ratios are shown as a 1-min change in cardiopulmonary bypass and aortic occlusion time. AO, aortic occlusion; CBP, cardiopulmonary bypass; VISmax, maximum vasoactive-inotropic score
| Variable | Beta coefficient | Standard error | Odds ratio (95% confidence interval) | P |
|---|---|---|---|---|
| Age | ||||
| 1-yr increment | −0.01 | 0.01 | 0.99 (0.98–1.00) | 0.184 |
| Sex (female) | ||||
| Male | 1 (reference group) | – | ||
| Female | 0.19 | 0.16 | 1.21 (0.88–1.67) | 0.241 |
| Emergency procedure | ||||
| No | 1 (reference group) | – | ||
| Yes | 1.48 | 0.22 | 4.39 (2.81–6.2) | <0.001 |
| Preoperative ejection fraction | ||||
| >50% | 1 (reference group) | – | ||
| 30–50% | 0.56 | 0.35 | 1.75 (0.89–3.45) | 0.104 |
| <30% | 0.40 | 0.35 | 1.50 (0.75–2.98) | 0.250 |
| AO time* | 0.01 | 0.00 | 1.01 (1.00–1.01) | 0.006 |
| CBP time* | 0.01 | 0.00 | 1.01 (1.00–1.02) | 0.047 |
| Vasoactive-inotropic status | ||||
| VISmax<5 | 1 (reference group) | – | ||
| VISmax>5–15 | 0.72 | 0.19 | 2.06 (1.41–3.00) | <0.001 |
| VISmax>15–30 | 0.59 | 0.21 | 1.81 (1.19–2.74) | 0.005 |
| VISmax>30–45 | 1.63 | 0.26 | 5.09 (3.08–8.41) | <0.001 |
| VISmax>45 | 2.01 | 0.27 | 7.85 (4.63–13.32) | <0.001 |
| Model constant | −4.43 | 0.58 | 0.00 | <0.001 |
The discrimination power of VISmax was similar to the discrimination power of SOFA; AUC value 0.71 (95% CI, 0.67–0.74; P=0.32). VISmax had better discrimination power for unfavourable outcome than SAPS II and APACHE II (Fig. 2). The AUC values for these scores were lower than for VISmax: APACHE II, 0.67 (95% CI, 0.63–0.70; P=0.01); and SAPS II, 0.69 (95% CI, 0.65–0.72; P=0.048).
Patients with the highest cardiovascular SOFA score (n=541) had 24% probability for unfavourable outcome. However, incidence of unfavourable outcome in VISmax groups differed widely from 11% (group ‘>5–15’, n=78) to 41% (group ‘>45’, n=124) in this highest SOFA score group (P<0.05) (Supplementary Table S2). VISmax was able to discriminate patients with unfavourable outcome with AUC 0.68 (CI 95% 0.63–0.74, P<0.01) (Supplementary Table S2).
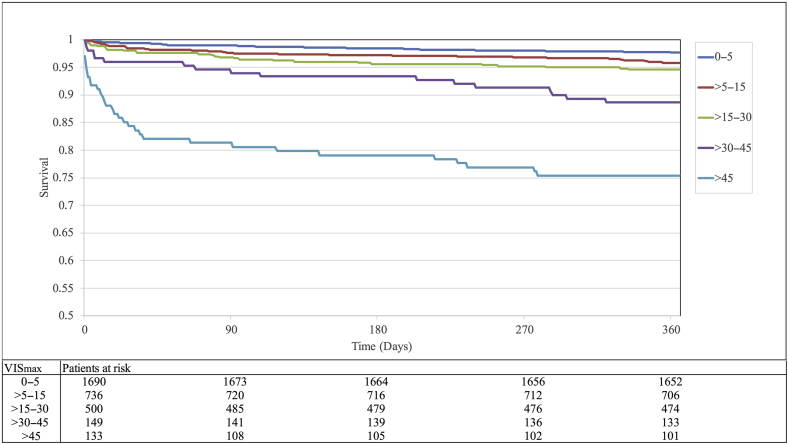
Higher VISmax predicted higher mortality rates, with the following AUC values: in-ICU, 0.87 (95% CI, 0.77–0.98); 30-day, 0.76 (95% CI, 0.69–0.83); 90-day, 0.73 (95% CI, 0.67–0.79); 180-day, 0.71 (95% CI, 0.65–0.76); and 1-yr, 0.70 (95% CI, 0.65–0.74). The first three VISmax groups (<5, >5–15, and >15–30) did not significantly differ from each other (Fig. 4). The VISmax groups >30–45 and >45 significantly differed between each other and with the first three groups (P<0.05) (Fig. 4). The two highest VISmax groups showed increasing all-cause mortality during the first 2 months, with a continued slow increase up to 1 yr (Fig. 4).
Fig 4.
Survival curves for each VISmax group. VISmax predicted cumulative mortality up to 1 yr. Mortality continuously increased within each group. There was no significant difference between the 0–5, >5–15, and >15–30 groups. The >30–45 and >45 groups significantly differed from each other and from the other groups (P=0.001). VISmax maximum vasoactive-inotropic score.
VISmax predicted the length of ICU stay according to the Cox regression analysis: hazard ratios for ICU discharge in the VISmax groups were 0.82 for group ‘>5–15’, 0.60 for group ‘>15–30’, 0.39 for group ‘>30–45’, and 0.37 for group ‘>45’ with P<0.01 (Supplementary Table S3). The length of ICU stay increased with increasing VISmax score. The median length of ICU stay for each VISmax group was: 0.93 days (0.86–0.98) for group ‘0–5’; 0.95 days (0.88–1.00) for group ‘>5–15’; 0.99 days (0.91–1.93) for group ‘>15–30’; 1.92 days (0.99–3.71) for group ‘>30–45’; and 2.97 days (1.73–6.33) for group ‘>45’. There was a significant difference between groups in pair-wise comparison of the medians (P<0.05).
The length of ICU stay increased with increasing VISmax score and also in the highest cardiovascular SOFA score group. The median length of ICU stay for each VISmax was 0.95 days (0.89–0.99) for group ‘>5–15’, 1.0 days (0.91–2.03) for group ‘>15–30’, 1.91 days (0.99–3.88) for group ‘>30–45’, and 2.97 days (1.72–5.94) for group ‘>45’. The groups ‘>5–15’ and ‘>15–30’ had a similar median length of stay at the ICU (P=0.852), but there was a significant difference between all other groups in pair-wise comparison of the medians (P<0.05).
Discussion
The main finding of this retrospective cohort study was that VISmax independently predicted unfavourable outcome after cardiac surgery in an unselected adult population. Moreover, VISmax predicted prolonged length of ICU stay. VISmax also showed good prediction accuracy and discrimination capability for mortality up to 1 yr after cardiac surgery in adults.
Previous studies showed that VISmax has good predictive accuracy and discrimination capability for infant and paediatric patients after cardiac surgery.13, 15, 16, 17, 18 Our study showed that VISmax has similar predictive accuracy and discrimination capability in an adult population after cardiac surgery. Furthermore, in this cardiac surgery population, VISmax showed better discrimination performance than the APACHE II and SAPS II scores, and similar to the SOFA score. Although VISmax performed comparably to the SOFA score, in the highest cardiovascular SOFA group, VISmax showed better discrimination capability for the composite outcome, and also predicted prolonged stay at the ICU.
VISmax might be superior to traditional scoring systems in this setting for several reasons. Traditional ICU scoring systems were initially developed and validated for general ICU patients,2 and then studied in cardiac surgery patients.1, 3, 6 Those scoring systems measure multiple organ dysfunction in critically ill patients, and only roughly quantitate pharmacological cardiovascular system support. In the early postoperative period after cardiac surgery, patients with a poor prognosis often do not manifest dysfunction of other organs to the same extent as they have cardiac dysfunction. Traditional scoring systems might better predict morbidity and mortality in cardiac surgery patients after the initial postoperative period, when multiorgan system dysfunction or failure is more likely to manifest.
Some previous studies have evaluated inotropic and vasoactive medication administration using a dichotomous approach, generating discrepant results regarding the association between the administration of these drugs and increased morbidity and mortality.27, 28, 29 A recent meta-analysis concluded that inotropes did not increase mortality in the overall population, but a Cochrane review highlighted the low quality of evidence concerning cardiogenic shock or low cardiac output states in many included studies.30, 31
We found that VISmax measured on the 1st postoperative day predicted mortality up to 1 yr, while most earlier studies reported only in-ICU, in-hospital, and 30-day mortality.1, 2, 8, 32, 33 The findings of the current study showed relatively low mortality in the lowest three VISmax groups for up to 1 yr.10, 34 Higher vasoactive and inotropic medication doses were associated with short-term and intermediate-term mortality after cardiac surgery (Fig. 4). In the two highest VISmax groups, mortality was relatively high in the first postoperative months, and then continued to increase slowly up to 1 yr. This finding may reflect intraoperative permanent myocardial damage or organ failure, our inadequate use of mechanical support devices (e.g. intra-aortic balloon pump and extracorporeal membrane oxygenator), instead of high doses of vasoactive drugs, or both. Alternatively, it is also possible that high doses of vasoactive drugs were merely reflective of cardiovascular morbidity, and that their administration did not themselves contribute to adverse outcomes.
Strengths and limitations
One strength of this study was the retrieval of data from an established ICU database. Second, we used a method of VISmax measurement in adult heart surgery, which was previously validated in paediatric cardiac surgery.13, 15, 16, 17 Third, we retrieved complete 1-yr mortality data, with no drop-outs, from a national register. Fourth, our composite outcome included mortality and adverse events, while some other studies evaluating scoring systems have focused on short-term mortality.1, 2, 8, 32, 33
One limitation was that this was a single-centre study; VISmax group adjustment may be required in other cardiac surgical units, which may differ regarding vasoactive and inotropic medication treatment regimens and availability of mechanical cardiovascular support devices. Second, predictive models have limitations related to prediction of infrequent events, and earlier studies show low mortality and morbidity in cardiac surgery.10, 20, 21, 22, 25 The occurrence of the composite outcome of morbidity and mortality was also infrequent in the current study (Table 1). Third, we did not compare VISmax to newer cardiac surgery-specific ICU scoring systems [e.g. cardiac surgery score (CASUS)] because information for determination of such scores was not available in our ICU database.8 Fourth, measuring single maximum values of inotropic and vasoactive support may involve confounding factors (e.g. momentary deeper sedation or procedures requiring temporarily increased pharmacological cardiovascular support). A potential alternative to the VISmax approach would be to assess total cumulative inotrope and vasoactive exposure.35, 36 Fifth, as a predictor of outcome, VISmax is calculated as a sum of the maximum dosing rate of all used inotropes and vasoconstrictors during the first 24 h, therefore VISmax cannot be used to evaluate the influence of a single inotrope or vasoconstrictor drug on the outcome.
Conclusions
VISmax independently predicted unfavourable outcomes after cardiac surgery in an adult population, including short- and intermediate-term morbidity and mortality. In addition, the length of ICU stay increased with increasing VISmax score. The discrimination capability of VISmax was better in this population than traditional ICU scoring systems APACHE II, SAPS II, and similar with SOFA. The five-level VISmax classification could potentially be included in ICU scoring systems for adults after cardiac surgery.
Authors' contributions
Study design: PL.
Data collection and analysis: all authors.
Writing paper: TK, PL.
Revising paper: all authors.
Acknowledgements
Special thanks to Tuomas Selander, Kuopio University Hospital, for help with statistical analyses.
Editorial decision: 04 December 2017
Handling editor: M. Avidan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.12.019.
Declaration of interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Chang C.H., Chen S., Fan P.C. Sequential organ failure assessment score predicts mortality after coronary artery bypass grafting. BMC Surg. 2017;17:22. doi: 10.1186/s12893-017-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doerr F., Badreldin A., Heldwein M.B. A comparative study of four intensive care outcome prediction models in cardiac surgery patients. J Cardiothorac Surg. 2011;6:21. doi: 10.1186/1749-8090-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doerr F., Badreldin A., Can F., Bayer O., Wahlers T., Hekmat K. SAPS 3 is not superior to SAPS 2 in cardiac surgery patients. Scand Cardiovasc J. 2014;48:111–119. doi: 10.3109/14017431.2014.890248. [DOI] [PubMed] [Google Scholar]
- 4.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Aorking Group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 5.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 6.Martinez J., Tuesta I.D., Plasencia E., Santana M., Mora M.L. Mortality prediction in cardiac surgery patients: comparative performance of Parsonnet and general severity systems. Circulation. 1999;99:2378–2382. doi: 10.1161/01.cir.99.18.2378. [DOI] [PubMed] [Google Scholar]
- 7.Nashef S.A.M., Roques F., Sharples L.D. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–744. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 8.Doerr F., Heldwein M.B., Bayer O. Combination of European System for Cardiac Operative Risk Evaluation (EuroSCORE) and Cardiac Surgery Score (CASUS) to improve outcome prediction in cardiac surgery. Med Sci Monit Basic Res. 2015;21:172–178. doi: 10.12659/MSMBR.895004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouchoukos N.T., Ebert P.A., Grover F.L., Lindesmith G.G. Report of the Ad Hoc Committee on risk factors for coronary artery bypass surgery. Ann Thorac Surg. 1988;45:348–349. doi: 10.1016/s0003-4975(10)62482-4. [DOI] [PubMed] [Google Scholar]
- 10.D’Agostino R.S., Jacobs J.P., Badhwar V. The society of thoracic surgeons adult cardiac surgery database: 2017 update on outcomes and quality. Ann Thorac Surg. 2017;103:18–24. doi: 10.1016/j.athoracsur.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Parsonnet V., Dean D., Bernstein A.D. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79:I3–I12. [PubMed] [Google Scholar]
- 12.Wynne-Jones K., Jackson M., Grotte G. Limitations of the Parsonnet score for measuring risk stratified mortality in the north west of England. Heart. 2000;84:71–78. doi: 10.1136/heart.84.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaies M.G., Gurney J.G., Yen A.H. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 14.Wernovsky G., Wypij D., Jonas R.A. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 15.Gaies M.G., Jeffries H.E., Niebler R.A. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the pediatric cardiac critical care consortium and virtual PICU System Registries. Pediatr Crit Care Med. 2014;15:529–537. doi: 10.1097/PCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favia I., Vitale V., Ricci Z. The vasoactive-inotropic score and levosimendan: time for VIS? J Cardiothorac Vasc Anesth. 2013;27:15–16. doi: 10.1053/j.jvca.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Davidson J., Tong S., Hancock H., Hauck A., da Cruz E., Kaufman J. Prospective validation of the vasoactive-inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med. 2012;38:1184–1190. doi: 10.1007/s00134-012-2544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia R.U., Walters H.L., Delius R.E., Aggarwal S. Vasoactive inotropic score (VIS) as biomarker of short-term outcomes in adolescents after cardiothoracic surgery. Pediatr Cardiol. 2016;37:271–277. doi: 10.1007/s00246-015-1273-7. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh A.M., Tong S., Deakyne S.J., Davidson J.A., Scott H.F. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med. 2017;18:750–757. doi: 10.1097/PCC.0000000000001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Omar Y., Kocher G.J., Bosco P. European Association for Cardio-Thoracic Surgery expert consensus statement on the prevention and management of mediastinitis. Eur J Cardiothorac Surg. 2017;51:10–29. doi: 10.1093/ejcts/ezw326. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Gu C., Gao M. Comparison of the incidence of postoperative neurologic complications after on-pump versus off-pump coronary artery bypass grafting in high-risk patients: a meta-analysis of 11 studies. Int J Cardiol. 2015;185:195–197. doi: 10.1016/j.ijcard.2015.03.115. [DOI] [PubMed] [Google Scholar]
- 22.Ryhammer P.K., Tang M., Hoffmann-Petersen J. Colloids in cardiac surgery-friend or foe? J Cardiothorac Vasc Anesth. 2017;31:1639–1648. doi: 10.1053/j.jvca.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 23.White H. Avatar of the universal definition of periprocedural myocardial infarction. J Am Coll Cardiol. 2013;62:1571–1574. doi: 10.1016/j.jacc.2013.08.721. [DOI] [PubMed] [Google Scholar]
- 24.Lahtinen P., Pitkänen O., Pölönen P., Turpeinen A., Kiviniemi V., Uusaro A. Levosimendan reduces heart failure after cardiac surgery: a prospective, randomized, placebo-controlled trial. Crit Care Med. 2011;39:2263–2270. doi: 10.1097/CCM.0b013e3182227b97. [DOI] [PubMed] [Google Scholar]
- 25.Ettema R.G., Peelen L.M., Kalkman C.J., Nierich A.P., Moons K.M., Schuurmans M.J. Predicting prolonged intensive care unit stays in older cardiac surgery patients: a validation study. Intensive Care Med. 2010;37:1480–1487. doi: 10.1007/s00134-011-2314-1. [DOI] [PubMed] [Google Scholar]
- 26.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 27.Shahin J., deVarennes B., Tse C.W., Amarica D.A., Dial S. The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit Care. 2011;15:R162. doi: 10.1186/cc10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen D.V., Hansen M.K., Johnsen S.P., Hansen M., Hindsholm K., Jakobsen C.J. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120:1098–1108. doi: 10.1097/ALN.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 29.Williams J.B., Hernandez A.F., Li S. Postoperative inotrope and vasopressor use following CABG: outcome data from the CAPS-care study. J Card Surg. 2011;26:572–578. doi: 10.1111/j.1540-8191.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belletti A., Castro M.L., Silvetti S. The Effect of inotropes and vasopressors on mortality: a meta-analysis of randomized clinical trials. Br J Anaesth. 2015;115:656–675. doi: 10.1093/bja/aev284. [DOI] [PubMed] [Google Scholar]
- 31.Schumann J., Henrich E.C., Strobl H. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;(1) doi: 10.1002/14651858.CD009669.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doerr F., Badreldin A., Bender E. Outcome prediction in cardiac surgery: the first logistic scoring model for cardiac surgical intensive care patients. Minerva Anestesiol. 2012;78:879–886. [PubMed] [Google Scholar]
- 33.Hekmat K., Doerr F., Kroener A. Prediction of mortality in intensive care unit cardiac surgical patients. Eur J Cardiothorac Surg. 2010;38:104–109. doi: 10.1016/j.ejcts.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 34.Siregar S., Groenwold R.H., de Mol B.A. Evaluation of cardiac surgery mortality rates: 30-day mortality or longer follow-up? Eur J Cardiothorac Surg. 2013;44:875–883. doi: 10.1093/ejcts/ezt119. [DOI] [PubMed] [Google Scholar]
- 35.Crow S.S., Robinson J.A., Bukhart H.M., Dearani J.A., Golden A.W. Duration and magnitude of vasopressor support predicts poor outcome after infant cardiac operations. Ann Thorac Surg. 2014;98:655–661. doi: 10.1016/j.athoracsur.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 36.Bangalore H., Gaies M., Ocampo E.C. The Total Inotrope Exposure Score: an extension of the Vasoactive Inotrope Score as a predictor of adverse outcomes after paediatric cardiac surgery. Cardiol Young. 2017;27:1146–1152. doi: 10.1017/S1047951116002602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.