Summary
Caudal epidural blockade in children is one of the most widely administered techniques of regional anaesthesia. Recent clinical studies have answered major pharmacodynamic and pharmacokinetic questions, thus providing the scientific background for safe and effective blocks in daily clinical practice and demonstrating that patient selection can be expanded to range from extreme preterm births up to 50 kg of body weight. This narrative review discusses the main findings in the current literature with regard to patient selection (sub-umbilical vs mid-abdominal indications, contraindications, low-risk patients with spinal anomalies); anatomical considerations (access problems, age and body positioning, palpation for needle insertion); technical considerations (verification of needle position by ultrasound vs landmarks vs ‘whoosh’ or ‘swoosh’ testing); training and equipment requirements (learning curve, needle types, risk of tissue spreading); complications and safety (paediatric regional anaesthesia, caudal blocks); local anaesthetics (bupivacaine vs ropivacaine, risk of toxicity in children, management of toxic events); adjuvant drugs (clonidine, dexmedetomidine, opioids, ketamine); volume dosing (dermatomal reach, cranial rebound); caudally accessed lumbar or thoracic anaesthesia (contamination risk, verifying catheter placement); and postoperative pain. Caudal blocks are an efficient way to offer perioperative analgesia for painful sub-umbilical interventions. Performed on sedated children, they enable not only early ambulation, but also periprocedural haemodynamic stability and spontaneous breathing in patient groups at maximum risk of a difficult airway. These are important advantages over general anaesthesia, notably in preterm babies and in children with cardiopulmonary co-morbidities. Compared with other techniques of regional anaesthesia, a case for caudal blocks can still be made.
Keywords: regional anaesthesia, caudal block, paediatric, perioperative care, postoperative pain, ultrasound-guided
Editor's key points.
-
•
Caudal blockade is one of the most frequently performed regional anaesthetic techniques in children.
-
•
Recent findings and developments could increase success rates and safety.
Regional anaesthesia in children has become increasingly popular over the past few decades. A variety of peripheral and central nerve blocks have been developed to ensure that perioperative pain can be effectively controlled. The same developments have made it possible to reduce the dose concentrations of systemic drugs, thus setting the stage for periprocedural spontaneous breathing and early ambulation. Even more importantly, these reduced dose concentrations have improved haemodynamic stability in a population of potentially high-risk patients. Notable examples of these specific risks would include cardiopulmonary failure, respiratory depression, or prematurity.
Whether any long-term outcome parameters may be affected by regional anaesthesia per se continues to remain unclear. Yet, given ongoing debates about the neurotoxicity of general anaesthesia, especially in younger patient populations, it is still reasonable to assume that regional anaesthesia may offer some advantages.1 The present review article will focus on caudal blockade, one of the most widely administered techniques of regional anaesthesia in paediatric patients who undergo sub-umbilical interventions. The authors have made an effort to highlight the practical aspects of caudal procedures, to provide an overview of the current literature, and to discuss ongoing controversies.
Historical considerations
The first author to describe caudal anaesthesia as applied to children (here in connection with urologic surgical procedures) was Meredith Campbell in 1933.2 Over time, this idea has developed into a technique of great interest, especially for use in premature infants and in newborns, considering that these paediatric subgroups are, as a result of an immature state of the CNS, at high risk of perioperative respiratory depression. The first major experience from a single centre, including a cohort of 1100 children and confirming the reliability of the method, was reported by Veyckemans and colleagues3 in 1992. They were also the first to relate complication rates to anaesthetists' experience, concluding that the technique was easy to perform even for beginners.
Nearly one-quarter of anaesthetic procedures which are today performed on children involve regional anaesthesia.4 Chief among them are single-injection caudal blocks, accounting for 34–40% of patients in paediatric regional anaesthesia.4, 5 Based on central blocks, their share ranges from 80% in European centres up to 97% in the USA.4, 5 According to data from the two largest multicentre studies available on the incidence and morbidity of regional anaesthesia in paediatric patients, caudal blocks are most commonly administered to children in the age range of 12 months up to 3 yr.4, 5
Indications, contraindications, pitfalls
Sub-umbilical vs mid-abdominal indications
Caudal anaesthesia is indicated for surgical and non-surgical painful interventions in body areas from the sub-umbilical region downwards. Examples include procedures such as inguinal hernia repair, cystoscopy/transurethral manipulation, circumcision, anal atresia, treatment of limb ischemia, treatment of intussusception, or cast application to immobilise newborns with hip dysplasia. Daily clinical experience has shown that the success rate is limited, and the prospect of success basically unpredictable, when caudal anaesthesia is used for mid-abdominal surgical interventions such as umbilical hernia repair. Reasons for this shortcoming might be age-dependent differences in the levels of sensory analgesia achievable by caudal blockade, and unpredictable secondary spread of local anesthetics.6, 7 Hence, interventions of this type are better managed by rectus sheath blockade8, 9 or lumbar/thoracic epidural anaesthesia.
Contraindications vs low-risk patients with spinal anomalies
Contraindications to caudal anaesthesia in children would include local site infection, pilonidal cyst, or spinal dysraphism such as tethered cord syndrome. In the presence of other spinal/meningeal anomalies, we suggest conducting a preoperative anatomical investigation by ultrasound or MRI. Performing a careful risk-benefit analysis on this basis can help to identify patients at low risk of inadvertent nerve lesions, who might benefit from regional instead of general anaesthesia despite their anomaly (e.g. children with a difficult airway or preterm infants with a history of respiratory depression episodes). Any caudal blocks in these specific patients should be performed with ultrasound guidance and only by anaesthetists highly experienced with this technique. On a related note, five children with spinal dysraphism have recently been reported as successfully managed by transversus abdominis plane blocks for major abdominal surgery.10
Other preoperative assessments
A thorough presurgical case history is required to rule out any congenital coagulation disorders or therapeutic anticoagulation. Preoperative laboratory testing is indicated only if the patient or any of his or her family members have a positive bleeding history.11
Anatomical considerations
Access for caudal anaesthesia
A profound understanding of anatomical characteristics is key to the success of caudal blockade, and infants do differ from adults with regard to their sacral anatomy and fat distribution.12 As a result of the individual nature of the developmental fusion processes which the sacral vertebrae and ligaments undergo during childhood, the anatomy of the sacrum is highly variable. The epidural space can be entered through the sacral hiatus. Palpating structures for identification may be difficult if the cornua are of substantial thickness, and accessing the epidural space may be difficult in older children if the sacrococcygeal membrane cannot be penetrated because of an advanced stage of ossification. Other than that, and pharmacologically speaking, caudal blocks are both feasible and can be safely applied in children up to 50 kg of body weight.13
Age and body positioning
At what segmental level the spinal cord and dural sac terminate in a given patient will vary with both age and body positioning. Regarding age, the pubertal growth spurt has been found to involve cranial movement of the spinal cord termination from the L3 to a L1−L2 level within only 12 months.14 In contrast, Shin and colleagues15 observed in a study population of children that the dural sac ended lower than S2—S3 in 8% of patients. This lower position of the spinal cord and dura increases the risk of inadvertent dural puncture in newborns and toddlers. As to body positioning, Koo and colleagues16 demonstrated that lateral placement of patients with their neck, hips, and knees maximally flexed was associated with significant cephalad shifting of the dural sac. In other words, finding the right position for a patient can help to avoid complications.
Palpation for needle insertion
With the patient in the left lateral decubitus position and the hips and knees flexed, the sacral hiatus can be identified using either the conventional landmark technique or ultrasound (Fig. 1). First of all, the posterior superior iliac spines are palpated via anatomical landmarks, the line between both spines (Tuffier's line) representing the base of an equilateral triangle the tip of which indicates the position of the sacral hiatus.17 The sacrococcygeal ligament can be palpated between the two sacral cornua, which is where the needle should penetrate the skin at an approximate 45° angle. Once the ligament has been passed, a flatter angle is adjusted by descending the needle before it can be advanced to the correct final position.18 It should be noted that Tuffier's line does not seem to be an adequate reference point in neonates placed in a lateral flexed position, as it will shift to a significantly more caudal position in this scenario.19 Before the local anaesthetic can be applied, cautious aspiration or passive drainage is required to rule out an inadvertent intravascular or spinal needle location.
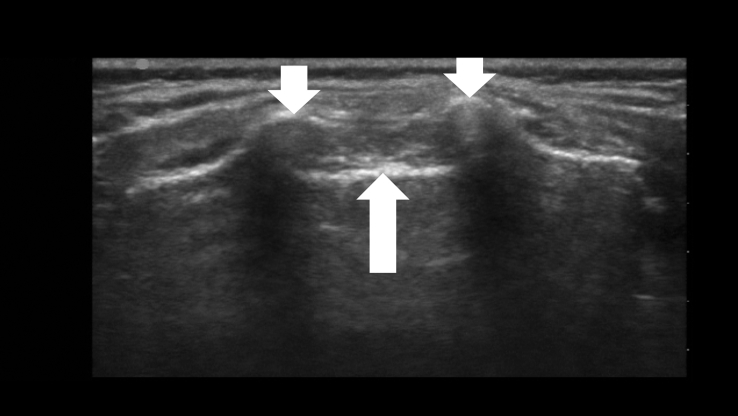
Fig 1.
A transverse ultrasound view illustrating the sacrococcygeal ligament (upward arrow) and the two sacral cornua (two downward arrows).
Technical considerations
Ultrasound vs landmarks
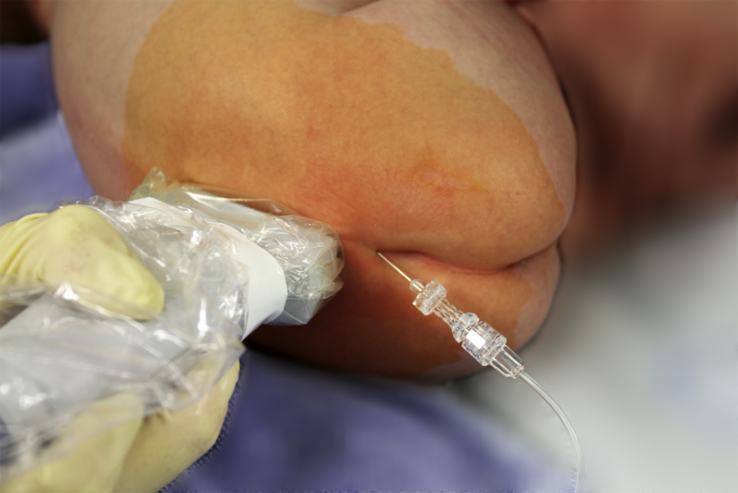
While the landmark-based approach to caudal anaesthesia does yield convincing success rates, it is also, as a well-known complication, prone to block failure with the result of inadequate anaesthesia.5, 17 Ultrasound guidance, by comparison, offers two key advantages: it helps to identify small anatomical structures, and allows the spread of the local anaesthetic to be seen (Fig 2, Fig 3).1, 17, 20, 21 Nor does ultrasound guidance impose any special requirements on patient positioning or an additional aseptic technique. A sterile preparation of the ultrasound probe is obligatory. Fig 2, Fig 3, Fig 4 illustrate how a high-frequency linear-array transducer with a sterile cover is placed longitudinally in a position slightly paramedian to the lumbar spine and how the spread of local anaesthetic can be seen with this technique.1 The superiority of ultrasound-guided puncture is so obvious as to remain unchallenged, especially in preterm babies and in infants whose sacral anatomy is not well understood.15, 19 Yet no data from large-scale prospective studies are currently available to confirm that ultrasound offers better morbidity and long-term outcomes in children of any age group managed by caudal anaesthesia.17, 20
Fig 2.
The longitudinal paramedian position of the linear high-frequent ultrasound probe for observation of the administration of local anaesthetic for caudal blockade.
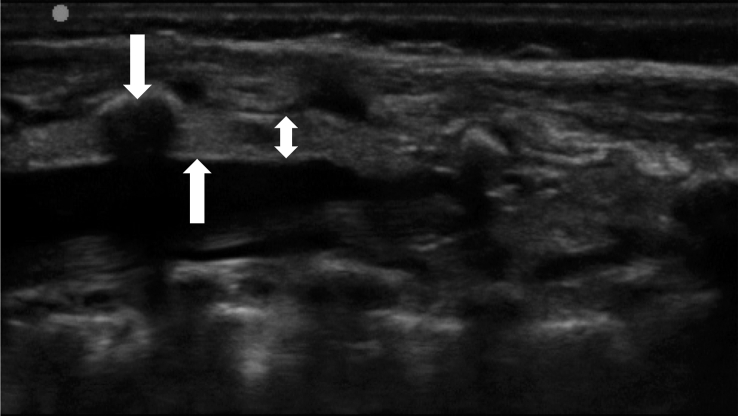
Fig 3.
Ultrasound image of the epidural space in a baby weighing 3 kg. The upward arrow indicates the dura mater, the double-ended arrow the epidural space, and the downward arrow the L5 spinous process. (Left to right=cranial to caudal.)
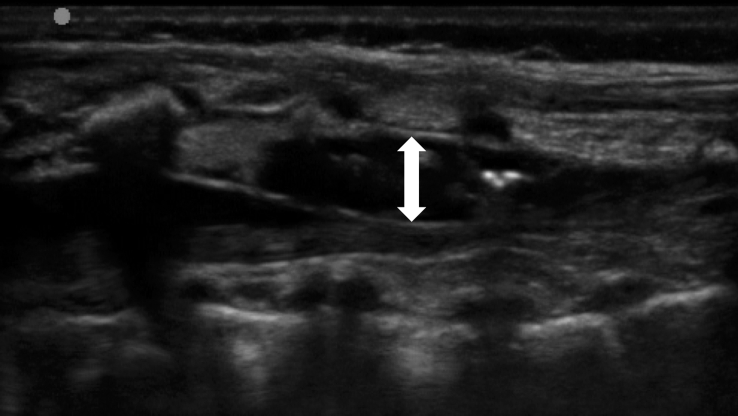
Fig 4.
Ultrasound visualisation of how the local anaesthetic is spreading inside the epidural space. The double-ended arrow indicates the dimensional increase of the epidural space in the anteroposterior plane. (Left to right=cranial to caudal.)
The ‘whoosh’ and ‘swoosh’ tests
In the USA, most single-injection caudal blocks in children take place without any technical aids or imaging.5 Periprocedural ultrasound guidance, although devoid of side-effects, was used in only 3% of patients. This raises the question of what other methods could ensure puncture/injection into the right space and how safe they are. Landmark-based palpation of the sacral hiatus cannot guarantee that a needle is correctly inserted to its target position. Known risks in this regard include, as has been noted above, accidental puncture of the dura or intravascular access. The ‘whoosh’ test was first described by Lewis and colleagues22—in a 1992 report involving adult patients—as a specific and sensitive method to confirm needle placement in caudal anaesthesia. Injecting 2 ml of air into the epidural space resulted in a ‘whoosh’ sound, which was verified through a stethoscope held over the thoracolumbar spine. After reports of neurological and haemodynamic side-effects from this air injection,23, 24 the method was refined by injecting not air, but local anaesthetics, accordingly dubbed the ‘swoosh’ test.25
Ultrasound vs swoosh/whoosh testing
Both the ‘whoosh’ and the ‘swoosh’ test proved to be similarly reliable when properly used, with current recommendations supporting the use of either air or saline for the loss-of-resistance technique.26, 27, 28 What has remained a major limitation of both tests is the subjectivity of examiners in judging the correct noise. Having observed in 2006 that even the introduction of Doppler ultrasound failed to compensate for this limitation,26 Raghunathan and colleagues29 presented data 2 yr later affirming their 2006 hypothesis that ultrasound was the superior method. They found that the best single indicator of successful epidural injection was to visualise by ultrasound in real time the turbulence generated by the local anaesthetic within the caudal space. More evidence supporting the benefit of ultrasound guidance in neuraxial blocks has been gathered since. A recent Cochrane Review has found that the use of ultrasound improves the success rate of blocks and increases their duration, especially in young children.30
Training and equipment requirements
Learning curve
Caudal blocks in children are easy to learn. In a study investigating the learning curve, residents who had no prior experience in paediatric anaesthesia or in performing caudal blocks were found to require only 32 blocks for an 80% success rate.31 The learning curve started out in a steep increase, followed by a slight flattening once 15 blocks had been performed. The eventual success rates of these untrained residents were comparable with those of experienced paediatric anaesthetists. The evaluation of learning curves in this context is also relevant when ultrasound is used as a method of confirmation of a correct spread of the local anaesthetic, because the puncture for caudal blockade is based on landmarks.
Needle types
Selecting the right equipment is essential to caudal anaesthesia. A large-scale study showed that wrong tools led to preventable neurological complications.32 It is not appropriate to use the same materials in children as in adults. Cannulae for single-injection caudal blocks are today available in different types and sizes, including a range of narrow-gauge (22- up to 25-gauge) short-bevel Tuohy and Crawford needles with or without a connected injection line.1, 33 Tissue trauma decreases with the calibre of the needle.12 While stylet needles have become an established part of standard operating procedures in spinal and thoracic epidural anaesthesia, paediatric caudal blocks are most commonly performed with needles that do not feature a stylet.34
Spreading of epidermoid tissue?
It has been hypothesised that hollow needles might increase the risk of epidermoid tumours after lumbar puncture because of tissue coring into the spinal space.35, 36 Yet the incidence of acquired epidermoid tumours is so low that sporadic case reports are the only source of available data.37, 38, 39, 40 A 2008 study suggests that cell transportation by caudal needles of any type is confined to non-nucleated epithelial cells.36 Add to this the lower risk of accidental spinal puncture in caudal than in thoracic epidural procedures, and the risk of epidermoid tumours developing in the wake of caudal anaesthesia should be infinitesimal. In any event, as most centres have a policy of applying transdermal local anaesthetics before blocks, prepuncture with a hypodermic needle might be an adequately benign and humane strategy of avoiding the spread of epidermal tissue.
Complications and safety
Paediatric regional anaesthesia
Regional anaesthesia in children provides a good balance between safety and risks during the perioperative period.27, 41 Data from a large European multicentre study (Ecoffey and colleagues4) suggest that regional anaesthesia is remarkably safe and has a very low overall complication rate of 0.12% in paediatrics.4 Data from a large Stateside multicentre study (Polaner and colleagues5) draw an even stronger picture to the same effect. Factors apparently increasing the risk in a significant way include an age of <6 months, central nerve blocks, and use of a catheter.4, 5
Caudal epidural blockade
Caudal blocks are known to involve haemodynamic/systemic or local adverse events. Examples include arrhythmia, hypotension when combined with general anaesthesia, respiratory depression resulting from inadvertent expansion of anaesthetics, toxicity-related seizures, infection/inflammation of the puncture site, sacral osteomyelitis, or local nerve injury. Yet, the morbidity associated with any of these events is low. Ecoffey and colleagues4 analysed data of 31 132 regional anaesthetic procedures and identified only eight patients with complications related to caudal blocks: six dural taps (without postdural puncture headache), one nerve injury, and one case of cardiac toxicity. Polaner and colleagues5 even reported ‘no complications in the caudal group’, pointing out that the main periprocedural problems were inability to place the block and block failure.5 While central regional techniques in adults are preferably conducted with the patient awake to get instant feedback on paraesthesias, pain, or symptoms of local anaesthetic systemic toxicity, children are generally sedated, so as to ensure immobility during the puncture.41, 42, 43, 44 The available literature confirms that regional anaesthesia is safe during deep sedation or general anaesthesia.27, 32, 41, 42
Choice of local anaesthetics
Bupivacaine vs ropivacaine
Common drugs for caudal blockade are (levo)bupivacaine 0.125–0.25% and ropivacaine 0.1–0.375%, used at a volume of 0.5–1.5 ml kg−1 depending on the desired dermatomal level.12, 27, 41 Current guidelines recommend that doses should not exceed 2 mg ml−1 for ropivacaine and 2.5 mg ml−1 for bupivacaine, and the recommended volumes are 0.5 ml kg−1 when sacral dermatomes, 1.0 ml kg−1 when lumbar dermatomes, or 1.25 ml kg−1 when lower thoracic dermatomes are achieved.45 That said, evidence has recently been provided that caudal anaesthesia is safe and effective with ropivacaine used at 3.1 mg ml−1 for a volume of 1 ml kg−1 in children up to 50 kg of body weight.13 Ropivacaine is known to cause less postoperative motor blockade than bupivacaine.18, 45 Its systemic absorption from the caudal epidural space is prolonged, but can be further extended by addition of epinephrine,46 to be diluted at a 1:200 000 ratio.41
Higher risk of toxicity in children
Two factors contribute to the higher risk of toxicity from local anaesthetics in children: alterations in plasma concentrations of alpha-1 acid glycoprotein (AGP) and immaturity of the cytochrome P450 (CYP) system. Both factors are age-dependent. Local anaesthetics get bound to AGP on administration, so that a low AGP concentration results in a higher free (non-protein-bound) circulating fraction of local anaesthetics. Recent literature shows that AGP concentrations are comparably low at birth and increase during the 1st year of life.45, 47 The CYP system matures during adolescence. Bupivacaine is metabolised by the CYP3A4 subtype and reaches maximum clearance at 12 months of age, but ropivacaine is metabolised by CYP1A2 and may not reach maximum clearance before 6–8 yr.12, 45 Fortunately, a greater volume of distribution compared with adults mitigates the risk of toxicity in children.12, 41 While this greater volume will reduce peak concentrations in plasma after a single bolus injection, it cannot—because of the aforementioned immaturity of the metabolism—prevent accumulation of the drug when applied continuously.45
Toxic events and their management
Systemic toxic events from local anaesthetics may involve cardio- or neurotoxicity. Current guidelines recommend that any haemodynamic deterioration be treated by Intralipid® 20% as first-line therapy along with epinephrine/adrenaline for cardiopulmonary resuscitation until circulation is restored or extracorporeal membrane oxygenation has been installed.27, 41 The Intralipid® should be administered in these situations i.v. as a rapid bolus injection of 1–1.5 ml kg−1 followed by continuous infusion (0.25 mg−1 kg−1 min−1) and repeated boli every 3–5 min up to 2–5 (−10) ml kg−1. Some regimens make the administration of a maintenance fluid (0.25 ml kg−1 min−1) seem useful.41 Neurotoxic seizures need to be treated with propofol, benzodiazepines, or barbiturates.41 Recent discussions about cardiac output affecting the vascular absorption of drugs from tissue have suggested that it may be useful to make allowances for the higher HR in under 2-yr-olds by reducing the doses of local anaesthetics, thus decreasing the risk of systemic toxicity further.45
Use of adjuvant drugs
Recommendations and rationale
Preservative-free morphine and clonidine are registered drugs for epidural use.45 Any of the other drugs listed below are administered widely, but off label in this context. The very latest recommendations issued by the European/American Society of Regional Anaesthesia endorse the use of alpha-2 agonists (clonidine, dexmedetomidine), preservative-free morphine, and ketamine as adjuvants in caudal blocks.45 All of these agents effectively prolong the duration of a settled block, thus helping to reduce doses for systemic sedoanalgesia during surgery and—a point that is mainly of interest in day surgery—to maintain pain relief in the postoperative course.45
Applicable drugs
Clonidine is the most common adjunctive drug for single-injection caudal blocks. Various mechanisms have been proposed to account for its favourable effect.48, 49 Chief among them is presumably that clonidine binds to alpha-2 receptors in the dorsal horn of the spinal cord.50, 51 Dosages of 1–2 μg kg−1 are recommended as effective. The use of clonidine in preterm babies and in infants <3 months old is being debated because of a hypothesised risk of apnoea in this group of children.50, 51
Dexmedetomidine has a shorter half-life time than clonidine. European guidelines do not indicate specific dosages, but several authors have suggested 1–2 μg kg−1 as effective.45, 51, 52, 53 According to an up-to-date review, caudal anaesthesia will last longer with dexmedetomidine than with morphine as adjuvant while remaining on a par with clonidine in quality.54 Haemodynamic effects, notably bradycardia, were uncommon and mostly related to the higher (2 μg kg−1) dose concentration.54
Opioids have a long tradition as adjuvant drugs in caudal anaesthesia.55 Current guidelines recommend 10–30 μg kg−1 for morphine, but advise against fentanyl or sufentanil.45 These two, being lipophilic opioids, provide up to 4 h of effective anaesthesia, whereas morphine as a water-soluble drug is effective for up to 24 h.56 Caudal epidural morphine has side-effects of reduced gastrointestinal mobility and postoperative nausea/vomiting. Pruritus is another well-known and common problem, but the true risk is respiratory depression, sometimes with a delayed onset.50 Thus, morphine use should be confined to strictly selected patients.
Ketamine binds to spinal opioid and N-methyl-D-aspartate receptors and has no respiratory side-effects.49 In a preservative-free form, both racemic ketamine and esketamine can be safely administered at 0.5–1 mg kg−1 into the epidural space.57, 58 However, as animal models have revealed neuronal apoptosis upon intrathecal application,59 current European guidelines recommend a conservative dose concentrations of 0.5 mg kg−1 to minimise side-effects.45
Volume dosing and cranial spread
Dermatomal reach
Both to ensure an adequate level of analgesia during caudal blockade and to avoid side-effects, it is essential to calculate the proper amount of local anaesthetic. A number of confounders are discussed in the current literature as affecting the cranial spread of local anaesthetics. They include body weight, body height, age, and injection speed. Weight-based formulas have a long tradition in paediatric regional anaesthesia, but calculating the dose of a local anaesthetic takes more than the patient's weight. One also has to consider the desired reach of a block in terms of dermatomal level. Epidural space volume is known to increase continuously from caudal to cranial. A recent study has revealed median volumes of 1.30, 1.57, and 1.78 ml kg−1 at the L1, T10, and T6 levels, respectively.60 Thus it stands to reason that current guidelines continue to recommend the well-established formula—introduced by Armitage61 in 1979—whereby 0.5 ml kg−1 may be expected to reach sacral, 1.0 ml kg−1 lumbar, and 1.25 ml kg−1 mid-thoracic dermatomes.45
Cranial rebound
Several studies have relied on ultrasound to investigate the cranial spread of local anaesthetics in paediatric caudal blocks.6, 7, 62, 63 The reach of high-volume blocks was found to be inversely related to age.64 Radiography and ultrasound revealed that, no matter how much of a local anaesthetic was used, its cranial spread never seemed to reach past the T-10 level immediately upon injection.62, 64, 65 In contrast, skin testing revealed T-4 dermatomal levels within several minutes of carrying out the injection. Lundblad and colleagues7 discovered a rebound mechanism of CSF behind this phenomenon. In phase I of this mechanism, CSF recedes in the direction of the cranium as epidural pressure is rapidly building up during injection of the local anaesthetic. Doppler measurements have, indeed, demonstrated resultant increases in intracerebral pressure and significant reductions in cerebral blood flow on administering high volume (1.5 ml kg−1) blocks.7, 66
In phase II, physiological interactions between intracranial and spinal pressure gradients cause the CSF to return in a caudal direction, thus forcing in its stead the epidural bulk of local anaesthetic to flow in the direction of the cranium. This secondary spread of the drug covers a distance of approximately two more spinal-cord segments within 15 min of injection.7 In contrast to relative pressure changes, the speed of injection does not affect the cranial spread of local anaesthetics.63 Despite the available evidence, the mechanisms of this cranial spread are not fully understood. Weight-based formulas may hold a make-believe promise of ensuring intraoperative analgesia, but, in daily clinical practice, caudal blocks are occasionally inadequate even at dose concentrations high enough to affect the intracerebral oxygen delivery, with unknown long-term implications.62, 66 There is an urgent need to investigate these issues further.
Caudal approach for lumbar and thoracic anaesthesia
Rationale
While the term ‘caudal block’ is mainly used of single-injection procedures, a caudal approach can also be taken for lumbar and thoracic epidural catheterisation. In clinical practice, however, this variant is so uncommon that only 1% of children were managed by caudally inserted catheters in the aforementioned large-scale multicentre study of European provenance.4 The first authors to report on thoracic epidural anaesthesia via the caudal route in children were Bosenberg and colleagues67 in 1988. Their idea was to enable postoperative continuous administration of analgesic drugs in small children without having to perform a thoracic epidural puncture, given the difficult anatomic background and the associated risk of peri-interventional complications.
Contamination risk
Two major issues arise, the first one concerning the high risk of caudal catheters getting bacterially contaminated in this environment of nearby excretory organs. Rates of 25% and 16% have been reported for Gram-positive and -negative colonisation, respectively, despite aseptic insertion.68 Fortunately, however, severe complications such as meningitis, epidural abscess, or systemic sepsis are rare,68 and subcutaneous tunnelling of the catheter or a slightly higher insertion point (L-5/S-1 in a ‘midline modified Taylor approach’) can help to control the risk of infection.69, 70
Verification of catheter placement
The second issue concerns the requirement to verify correct placement of the catheter, with regard to both an adequate spinal-cord level and the epidural position. Although failure rates of 20–30% in epidural catheter tip placement have been reported, only half of epidural catheters that are caudally placed but threaded to a thoracic level are verified by the use of imaging techniques to lie at the correct spinal level.5, 71, 72 All the rest are checked exclusively by clinical examination and tactile feedback. Misplacement of such catheters may lead not only to clinical side-effects during surgery, but also to overdosing of local anaesthetics because of inadequate postoperative analgesia.71 Valid approaches to verify placement would include radiological methods such as epidurograms or fluoroscopy, electric stimulation, and ultrasound.71, 73 A brief rundown follows.
Verification by epidurogram is the most popular approach in the USA.5 Taenzer and colleagues71 reported catheter placements not within the epidural space in 1.6% of patients, even though none of the findings by clinical examination and by tactile feedback (using loss-of-resistance techniques) had been out of the ordinary. In 0.4%, the epidurograms even disclosed abdominal positioning of catheters. The main limitations of this otherwise very reliable method, namely radiation exposure and contrast,71 prompted efforts to develop a valid alternative.
Electric epidural stimulation via a specially designed catheter was first described by Tsui and colleagues73—and by Tsui and colleagues74—in 2001 and 2002, respectively. A low electrical current generated at its tip elicited motor responses from intercostal muscles, indicating that the tip was inside the epidural space.73 As the catheter was being inserted, muscle twitches were seen moving from the lower limb to thoracic levels. Because this original method did not work after neuromuscular block, it was developed further by rendering the epidural catheter capable of monitoring ECG signals. As the tip of this catheter was being cranially advanced, the QRS complexes captured along the way would increasingly match the thoracic ECG tracings monitored from the surface.74 This method has never been popular in daily clinical practice, given that it is unable to distinguish between epidural, intrathecal, and intravascular positioning.71 Moreover, electric stimulation catheters used in a porcine model led to severe complications such as spinal-cord injury or subdural bleeding.75
Ultrasound guidance continues to be debated as far as this specific scenario (i.e. caudal access for lumbar or thoracic anaesthesia) is concerned. Taenzer and colleagues71 spoke out against its use because of its suboptimal image quality in older children and its inability to display the spread of contrast from the epidural to the intrathecal space. Simpao and colleagues76 have, in a recent study, specifically recommended the use of ultrasound to verify after operation where the catheter is located, given a high documented incidence of perioperative dislocations after initially precise positioning in neonates and infants.71, 76 Other reports have emphasised that young infants present with excellent conditions for ultrasound imaging, not least because vertebral ossification is still incomplete at this age.1,77
Gold standard vs considerations of postoperative pain
Persistent postoperative pain?
Caudal blocks are described as a safe and efficient way to offer perioperative analgesia in paediatric patients, including neonates. Evidence is still lacking as to whether they contribute to persistent postoperative pain. In a late-breaking study, 4% of paediatric patients had persistent neuropathic pain 6 months after outpatient surgical procedures (inguinal hernia repair and orchidopexy) which had been managed by combinations of single-injection caudal blockade and general anaesthesia.78 While the authors of that study, in the absence of a control group, were not in a position to discuss the contribution of the caudal blocks to the incidence of this persistent pain, they nevertheless did argue in favour of regional anaesthesia. Further studies on this issue are urgently needed.
Concluding remarks
A systematic review of caudal blocks for inguinal hernia repair in children did not yield significant differences in postoperative pain scores or in the need for rescue analgesia compared with surgical wound infiltration or nerve blocks.79 That said, what matters is that all of these techniques protect the children from perioperative pain and, combined with periprocedural sedation, enable surgical interventions with spontaneous breathing in patient groups at maximum risk of a difficult airway. We consider this specific point to be the main advantage of regional anaesthesia in paediatric patients. Judging from many years of experience, and despite the growing popularity of abdominal wall blocks in recent years, a case can still be made for favouring caudal blocks.
Authors' contributions
Contributed equally to the concept, design, drafting and final approval of the manuscript: all authors.
Declaration of interest
PM is a board member of the British Journal of Anaesthesia. All other authors declare no conflicts of interest.
Funding
Departmental funding.
Acknowledgements
We wish to thank Wilfried Preinfalk for his invaluable contribution in editing the manuscript for language (www.medword.at).
Handling editor: J.G. Hardman
Editorial decision date: 30 November 2018
References
- 1.Marhofer P., Lönnqvist P.A. The use of ultrasound-guided regional anaesthetic techniques in neonates and young infants. Acta Anaesthesiol Scand. 2014;58:1049–1060. doi: 10.1111/aas.12372. [DOI] [PubMed] [Google Scholar]
- 2.Campbell M.F. Caudal anesthesia in children. J Urol. 1933;30:245–250. [Google Scholar]
- 3.Veyckemans F., Van Obbergh L.J., Gouverneur J.M. Lessons from 1100 paediatric caudal blocks in a teaching hospital. Reg Anesth. 1992;17:119–125. [PubMed] [Google Scholar]
- 4.Ecoffey C., Lacroix F., Giaufré E., Orliaguet G., Courrèges P. Association des Anesthésistes Réanimateurs Pédiatriques d'Expression Française (ADARPEF). Epidemiology and morbidity of regional anesthesia in children: a follow-up one-year prospective survey of the French-Language Society of Paediatric Anaesthesiologists (ADARPEF) Paediatr Anaesth. 2010;20:1061–1069. doi: 10.1111/j.1460-9592.2010.03448.x. [DOI] [PubMed] [Google Scholar]
- 5.Polaner D.M., Taenzer A.H., Walker B.J. Paediatric Regional Anesthesia Network (PRAN): a multi-institutional study of the use and incidence of complications of pediatric regional anesthesia. Anesth Analg. 2012;115:1353–1364. doi: 10.1213/ANE.0b013e31825d9f4b. [DOI] [PubMed] [Google Scholar]
- 6.Lundblad M., Lönnqvist P.A., Eksborg S., Marhofer P. Segmental distribution of high-volume caudal anesthesia in neonates, infants, and toddlers as assessed by ultrasonography. Paediatr Anesth. 2011;21:121–127. doi: 10.1111/j.1460-9592.2010.03485.x. [DOI] [PubMed] [Google Scholar]
- 7.Lundblad M., Eksborg S., Lönnqvist P.A. Secondary spread of caudal block as assessed by ultrasonography. Br J Anaesth. 2012;108:675–681. doi: 10.1093/bja/aer513. [DOI] [PubMed] [Google Scholar]
- 8.Willschke H., Bosenberg A., Marhofer P. Ultrasonography-guided rectus sheath block in paediatric anaesthesia – a new approach to an old technique. Br J Anaesth. 2006;97:244–249. doi: 10.1093/bja/ael143. [DOI] [PubMed] [Google Scholar]
- 9.Gurnaney H.G., Maxwell L.G., Kraemer F.W., Goebel T., Nance M.L., Ganesh A. Prospective randomized observer-blinded study comparing the analgesic efficiacy of ultrasound-guided rectus sheath block and local anaesthetic infiltration for umbilical hernia repair. Br J Anaesth. 2011;107:790–795. doi: 10.1093/bja/aer263. [DOI] [PubMed] [Google Scholar]
- 10.Çevikkalp E., Erbüyün K., Erbüyün S.C., Ok G. Ultrasound guided transversus abdominis plane block. Saudi Med J. 2018;39:92–96. doi: 10.15537/smj.2018.1.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfanner G., Koscielny J., Pernerstorfer T. Preoperative evaluation of the bleeding history. Recommendations of the working group on perioperative coagulation of the Austrian Society for Anaesthesia, Resuscitation and Intensive Care. Anaesthesist. 2007;56:604–611. doi: 10.1007/s00101-007-1182-0. [DOI] [PubMed] [Google Scholar]
- 12.Jöhr M. Regional anaesthesia in neonates, infants and children: an educational review. Eur J Anaesthesiol. 2015;32:289–297. doi: 10.1097/EJA.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 13.Keplinger M., Marhofer P., Klug W. Feasibility and pharmacokinetics of caudal blockade in children and adolescents with 30-50 kg of body weight. Paediatr Anaesth. 2016;26:1053–1059. doi: 10.1111/pan.12972. [DOI] [PubMed] [Google Scholar]
- 14.van Schoor A.N., Bosman M.C., Bosenberg A.T. Descriptive study of the differences in the level of the conus medullaris in four different age groups. Clin Anat. 2015;28:638–644. doi: 10.1002/ca.22505. [DOI] [PubMed] [Google Scholar]
- 15.Shin S.K., Hong J.Y., Kim W.O., Koo B.N., Kim J.E., Kil H.K. Ultrasound evaluation of the sacral area and comparison of sacral interspinous and hiatal approach for caudal block in children. Anesthesiology. 2009;111:1135–1140. doi: 10.1097/ALN.0b013e3181bc6dd4. [DOI] [PubMed] [Google Scholar]
- 16.Koo B.N., Hong J.Y., Kim J.E., Kil H.K. The effect of flexion on the level of termination of the dural sac in paediatric patients. Anesthesia. 2009;64:1072–1076. doi: 10.1111/j.1365-2044.2009.06031.x. [DOI] [PubMed] [Google Scholar]
- 17.Mirjalili S.A., Taghavi K., Frawley G., Craw S. Should we abandon landmark-based technique for caudal anesthesia in neonates and infants. Paediatr Anaesth. 2015;25:511–516. doi: 10.1111/pan.12576. [DOI] [PubMed] [Google Scholar]
- 18.Jöhr M., Berger T. Caudal blocks. Paediatr Anaesth. 2012;22:44–50. doi: 10.1111/j.1460-9592.2011.03669.x. [DOI] [PubMed] [Google Scholar]
- 19.van Schoor A., Bosman M.C., Bosenberg A.T. The value of Tuffier’s line for neonatal neuraxial procedures. Clin Anat. 2014;27:370–375. doi: 10.1002/ca.22218. [DOI] [PubMed] [Google Scholar]
- 20.Lönnqvist P.A. Is ultrasound guidance mandatory when performing paediatric regional anaesthesia? Curr Opin Anaesthesiol. 2010;23:337–341. doi: 10.1097/ACO.0b013e328339276f. [DOI] [PubMed] [Google Scholar]
- 21.Liu J.Z., Wu X.Q., Li R., Zhang Y.J. A comparison of ultrasonography versus traditional approach for caudal block in children. Zhonghua Yi Xue Za Zhi. 2012;92:882–885. [PubMed] [Google Scholar]
- 22.Lewis M.P., Thomas P., Wilson L.F., Mulholland R.C. The ‘whoosh’ test. A clinical test to confirm correct needle placement in caudal epidural injections. Anaesthesia. 1992;47:57–58. doi: 10.1111/j.1365-2044.1992.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 23.Flandin-Blety C., Barrier G. Accidents following extradural analgesia in children. The results of a retrospective study. Paediatr Anaesth. 1995;5:41–46. doi: 10.1111/j.1460-9592.1995.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 24.Guinard J.P., Borboen M. Probable venous air embolism during caudal anesthesia in a child. Anesth Analg. 1993;76:1134–1135. doi: 10.1213/00000539-199305000-00036. [DOI] [PubMed] [Google Scholar]
- 25.Orme R.M., Berg S.J. The ‘swoosh’ test—an evaluation of a modified ‘whoosh’ test in children. Br J Anaesth. 2003;90:62–65. [PubMed] [Google Scholar]
- 26.Talwar V., Tyagi R., Mullick P., Gogia A.R. Comparison of ‘whoosh’ and modified ‘swoosh’ test for identification of the caudal epidural space in children. Paediatr Anaesth. 2006;16:134–139. doi: 10.1111/j.1460-9592.2005.01729.x. [DOI] [PubMed] [Google Scholar]
- 27.Lönnqvist P.A., Ecoffey C., Bosenberg A., Suresh S., Ivani G. The European society of regional anesthesia and pain therapy and the American Society of Regional Anesthesia and Pain Medicine joint committee practice advisory on controversial topics in paediatric regional anesthesia I and II: what do they tell us? Curr Opin Anaesthesiol. 2017;30:613–620. doi: 10.1097/ACO.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz D., Raghunathan K., Han D. The ‘Doppler-swoosh’ test: a further modification to the ‘swoosh’ test. Paediatr Anaesth. 2007;17:600–601. doi: 10.1111/j.1460-9592.2006.02163.x. [DOI] [PubMed] [Google Scholar]
- 29.Raghunathan K., Schwartz D., Connelly N.R. Determining the accuracy of caudal needle placement in children: a comparison of the swoosh test and ultrasonography. Paediatr Anaesth. 2008;18:606–612. doi: 10.1111/j.1460-9592.2008.02529.x. [DOI] [PubMed] [Google Scholar]
- 30.Guay J., Suresh S., Kopp S. The use of ultrasound guidance for perioperative neuraxial and peripheral nerve blocks in children: a Cochrane Review. Anesth Analg. 2017;124:948–958. doi: 10.1213/ANE.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 31.Schuepfer G., Konrad C., Schmeck J., Poortmans G., Staffelbach B., Jöhr M. Generating a learning curve for pediatric caudal epidural blocks: an empirical evaluation of technical skills in novice and experienced anesthetists. Reg Anesth Pain Med. 2000;25:385–388. doi: 10.1053/rapm.2000.7590. [DOI] [PubMed] [Google Scholar]
- 32.Giaufré E., Dalens B., Gombert A. Epidemiology and morbidity of regional anesthesia in children: a one-year prospective survey of the French-Language Society of Pediatric Anesthesiologists. Anesth Analg. 1996;83:904–912. doi: 10.1097/00000539-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 33.The New York School of Regional Anesthesia. Available from http://www.nysora.com/paediatric-epidural-spinal-anesthesia-analgesia (accessed 9 September 2018).
- 34.Fahy C.J., Costi D.A., Cyna A.M. A survey of aseptic precautions and needle type for paediatric caudal block in Australia and New Zealand. Anaesth Intensive Care. 2013;41:102–107. doi: 10.1177/0310057X1304100117. [DOI] [PubMed] [Google Scholar]
- 35.Campbell D.C., Douglas M.J., Tavlor G. Incidence of tissue coring with the 25-gauge Quincke and Whitacre spinal needles. Reg Anesth. 1996;21:582–585. [PubMed] [Google Scholar]
- 36.Guldogus F., Baris Y.S., Baris S., Karakaya D., Kelsaka E. Comparing tissue coring potentials of hollow needles without stylet and caudal needles with stylet: an experimental study. Eur J Anaesthesiol. 2008;25:498–501. doi: 10.1017/S0265021508003906. [DOI] [PubMed] [Google Scholar]
- 37.Morita M., Miyauchi A., Okuda S., Oda T., Aono H., Iwasaki M. Intraspinal epidermoid tumor of the cauda equina region: seven cases and a review of the literature. J Spinal Disord Tech. 2012;25:292–298. doi: 10.1097/BSD.0b013e31821e2464. [DOI] [PubMed] [Google Scholar]
- 38.Issaivanan M., Cohen S., Mittler M., Johnson A., Edelman M., Redner A. Iatrogenic spinal epidermoid cyst after lumbar puncture using needles with stylet. Paediatr Hematol Oncol. 2007;28:600–603. doi: 10.3109/08880018.2011.613093. [DOI] [PubMed] [Google Scholar]
- 39.Per H., Kumandas S., Gümüs H., Yikilmaz A., Kurtsoy A. Iatrogenic epidermoid tumor: late complication of lumbar puncture. J Child Neurol. 2007;22:332–336. doi: 10.1177/0883073807300531. [DOI] [PubMed] [Google Scholar]
- 40.Potgieter S., Dimin S., Lagae L. Epidermoid tumours associated with lumbar punctures performed in early neonatal life. Dev Med Child Neurol. 1998;40:266–269. doi: 10.1111/j.1469-8749.1998.tb15460.x. [DOI] [PubMed] [Google Scholar]
- 41.Shah R.D., Suresh S. Applications of regional anaesthesia in paediatrics. Br J Anaesth. 2013;111(Suppl 1):i114−24. doi: 10.1093/bja/aet379. [DOI] [PubMed] [Google Scholar]
- 42.Taenzer A.H., Walker B.J., Bosenberg A.T. Asleep versus awake: does it matter? Paediatric regional block complications by patient state: a report from the Paediatric Regional Anesthesia Network. Reg Anesth Pain Med. 2014;39:279–283. doi: 10.1097/AAP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 43.Neal J.M., Bernards C.M., Hadzic A. ASRA practice advisory on neurologic complications in regional anesthesia and pain medicine. Reg Anesth Pain Med. 2008;33:404–415. doi: 10.1016/j.rapm.2008.07.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernards C.M., Hadzic A., Suresh S., Neal J.M. Regional anesthesia in anesthetized or heavily sedated kids. Reg Anesth Pain Med. 2008;33:449–460. doi: 10.1016/j.rapm.2008.07.529. [DOI] [PubMed] [Google Scholar]
- 45.Suresh S., Ecoffey C., Bosenberg A. The European society of regional anaesthesia and pain therapy/American society of regional anesthesia and pain medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg Anesth Pain Med. 2018;43:211–216. doi: 10.1097/AAP.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 46.Van Obbergh L.J., Roelants F.A., Veyckemans F., Verbeeck R.K. In children, the addition of epinephrine modifies the pharmacokinetics of ropivacaine injected caudally. Can J Anesth. 2003;50:593–598. doi: 10.1007/BF03018647. [DOI] [PubMed] [Google Scholar]
- 47.Anell-Olofsson M., Ahmadi S., Lönnqvist P.A., Eksborg S., von Horn H., Bartocci M. Plasma concentrations of alpha-1-acid glycoprotein in preterm and term newborns: influence of mode of delivery and implications for plasma protein binding of local anaesthetics. Br J Anaesth. 2018;121:427–431. doi: 10.1016/j.bja.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 48.Wolff M., Heugel P., Hempelmann G., Scholz A., Mühling J., Olschewski A. Clonidine reduces the excitability of spinal dorsal horn neurons. Br J Anaesth. 2007;98:353–361. doi: 10.1093/bja/ael379. [DOI] [PubMed] [Google Scholar]
- 49.Mossetti V., Vicchio N., Ivani G. Local anesthetics and adjuvants in pediatric regional anesthesia. Curr Drug Targets. 2012;13:952–960. doi: 10.2174/138945012800675713. [DOI] [PubMed] [Google Scholar]
- 50.Lundblad M., Lönnqvist P.A. Adjunct analgesic drugs to local anaesthetics for neuroaxial blocks in children. Curr Opin Anaesthesiol. 2016;29:626–631. doi: 10.1097/ACO.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 51.Bosenberg A. Adjuvants in pediatric regional anesthesia. Pain Manag. 2012;2:479–486. doi: 10.2217/pmt.12.51. [DOI] [PubMed] [Google Scholar]
- 52.Marhofer P., Brummett C.M. Safety and efficiency of dexmedetomidine as adjuvant to local anesthetics. Curr Opin Anaesthesiol. 2016;29:632–637. doi: 10.1097/ACO.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 53.Tong Y., Ren H., Ding X., Jin S., Chen Z., Li Q. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: a meta-analysis. Paediatr Anaesth. 2014;24:1224–1230. doi: 10.1111/pan.12519. [DOI] [PubMed] [Google Scholar]
- 54.Trifa M., Tumin D., Tobias J.D. Dexmedetomidine as an adjunct for caudal anesthesia and analgesia in children. Minerva Anestesiol. 2018;84:836–847. doi: 10.23736/S0375-9393.18.12523-5. [DOI] [PubMed] [Google Scholar]
- 55.Krane E.J., Tyler D.C., Jacobson L.E. The dose response of caudal morphine in children. Anesthesiology. 1989;71:48–52. doi: 10.1097/00000542-198907000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Bujedo B.M., Santos S.G., Azpiazu A.U. A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag. 2012;8:177–192. doi: 10.5055/jom.2012.0114. [DOI] [PubMed] [Google Scholar]
- 57.Marhofer P., Krenn C.G., Plöchl W. (+)-ketamine for caudal block in paediatric anaesthesia. Br J Anaesth. 2000;84:341–345. doi: 10.1093/oxfordjournals.bja.a013436. [DOI] [PubMed] [Google Scholar]
- 58.Lönnqvist P.A., Walker S.M. Ketamine as an adjunct to caudal block in neonates and infants: is it time to re-evaluate? Br J Anaesth. 2012;109:138–140. doi: 10.1093/bja/aes228. [DOI] [PubMed] [Google Scholar]
- 59.Stratmann G. Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 60.Forestier J., Castillo P., Finnbogason T., Lundblad M., Eksborg S., Lönnqvist P.A. Volumes of the spinal canal and caudal space in children zero to three years of age assessed by magnetic resonance imaging: implications for volume dosage of caudal blockade. Br J Anaesth. 2017;119:972–978. doi: 10.1093/bja/aex280. [DOI] [PubMed] [Google Scholar]
- 61.Armitage E.N. Caudal block in children. Anaesthesia. 1979;34:396. [Google Scholar]
- 62.Brenner L., Marhofer P., Kettner S.C. Ultrasound assessment of cranial spread during caudal blockade in children: the effect of different volumes of local anaesthetics. Br J Anaesth. 2011;107:229–235. doi: 10.1093/bja/aer128. [DOI] [PubMed] [Google Scholar]
- 63.Triffterer L., Machata A.M., Latzke D. Ultrasound assessment of cranial spread during caudal blockade in children: effect of the speed of injection of local anaesthetics. Br J Anaesth. 2012;108:670–674. doi: 10.1093/bja/aer502. [DOI] [PubMed] [Google Scholar]
- 64.Koo B.N., Hong J.Y., Kil H.K. Spread of ropivacaine by a weight-based formula in a pediatric caudal block: a fluoroscopic examination. Acta Anaesthesiol Scand. 2010;54:562–565. doi: 10.1111/j.1399-6576.2010.02224.x. [DOI] [PubMed] [Google Scholar]
- 65.Thomas M.L., Roebuck D., Yule C., Howard R.F. The effect of volume of local anesthetic on the anatomic spread of caudal block in children aged 1–7 years. Paediatr Anaesth. 2010;20:1017–1021. doi: 10.1111/j.1460-9592.2010.03422.x. [DOI] [PubMed] [Google Scholar]
- 66.Lundblad M., Forestier J., Marhofer D., Eksborg S., Winberg P., Lönnqvist P.A. Reduction of cerebral mean blood flow velocity and oxygenation after high-volume (1.5 ml kg−1) caudal block in infants. Br J Anaesth. 2014;113:688–694. doi: 10.1093/bja/aeu161. [DOI] [PubMed] [Google Scholar]
- 67.Bosenberg A.T., Bland B.A., Schulte-Steinberg O., Downing J.W. Thoracic epidural anesthesia via caudal route in infants. Anesthesiology. 1988;69:265–269. doi: 10.1097/00000542-198808000-00020. [DOI] [PubMed] [Google Scholar]
- 68.Kost-Byerly S., Tobin J.R., Greenberg R.S., Billett C., Zahurak M., Yaster M. Bacterial colonization and infection rate of continuous epidural catheters in children. Anesth Analg. 1998;86:712–716. doi: 10.1097/00000539-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Gunter J.B. Thoracic epidural anesthesia via the modified Taylor approach in infants. Reg Anesth Pain Med. 2000;25:561–565. doi: 10.1053/rapm.2000.7585. [DOI] [PubMed] [Google Scholar]
- 70.Bubeck J., Boos K., Krause H., Thies K.C. Subcutaneous tunneling of caudal catheters reduces the rate of bacterial colonization to that of lumbar epidural catheters. Anesth Analg. 2004;99:689–693. doi: 10.1213/01.ANE.0000130023.48259.FB. [DOI] [PubMed] [Google Scholar]
- 71.Taenzer A.H., 5th Clark C., Kovarik W.D. Experience with 724 epidurograms for epidural catheter placement in pediatric anesthesia. Reg Anesth Pain Med. 2010;35:432–435. doi: 10.1097/AAP.0b013e3181ef4b76. [DOI] [PubMed] [Google Scholar]
- 72.Valairucha S., Seefelder C., Houck C.S. Thoracic epidural catheters placed by the caudal route in infants: the importance of radiographic confirmation. Paediatr Anaesth. 2002;12:424–428. doi: 10.1046/j.1460-9592.2002.00884.x. [DOI] [PubMed] [Google Scholar]
- 73.Tsui B.C., Seal R., Koller J., Entwistle L., Haugen R., Kearney R. Thoracic epidural analgesia via the caudal approach in pediatric patients undergoing fundoplication using nerve stimulation guidance. Anesth Analg. 2001;93:1152–1155. doi: 10.1097/00000539-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 74.Tsui B.C., Seal R., Koller J. Thoracic epidural catheter placement via the caudal approach in infants by using electrocardiographic guidance. Anesth Analg. 2002;95:326–330. doi: 10.1097/00000539-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 75.Gamble J.J., Ambros B., Séguin P., Benmansour P., Simko E. Stimulating thoracic epidural placement via a lumbar approach causes significant spinal cord damage in a porcine model. Can J Anesth. 2014;61:306–311. doi: 10.1007/s12630-014-0117-x. [DOI] [PubMed] [Google Scholar]
- 76.Simpao A.F., Gálvez J.A., Wartman E.C. The migration of caudally threaded thoracic epidural catheters in neonates and infants. Anesth Analg Adv Access Published February. 2018;23 doi: 10.1213/ANE.0000000000003311. [DOI] [PubMed] [Google Scholar]
- 77.Willschke H., Bosenberg A., Marhofer P. Epidural catheter placement in neonates: sonoanatomy and feasibility of ultrasonographic guidance in term and preterm neonates. Reg Anesth Pain Med. 2007;32:34–40. doi: 10.1016/j.rapm.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 78.Mossetti V., Boretsky K., Astuto M. Persistent pain following common outpatient surgeries in children: a multicenter study in Italy. Paediatr Anaesth. 2018;28:231–236. doi: 10.1111/pan.13321. [DOI] [PubMed] [Google Scholar]
- 79.Baird R., Guilbault M.P., Tessier R., Ansermino J.M. A systematic review and meta-analysis of caudal blockade versus alternative analgesic strategies for pediatric inguinal hernia repair. J Pediatr Surg. 2013;48:1077–1085. doi: 10.1016/j.jpedsurg.2013.02.030. [DOI] [PubMed] [Google Scholar]