Summary
Propofol infusion syndrome is a rare, potentially fatal condition first described in children in the 1990s and later reported in adults. We provide a narrative review of what is currently known about propofol infusion syndrome, including a structured analysis of all published case reports; child and adult cases were analysed separately as propofol is no longer used for long-term sedation in children. The review contains an update on current knowledge of the pathophysiology of this condition along with recommendations for its diagnosis, prevention, and management. We reviewed 108 publications documenting 168 cases of propofol infusion syndrome. We evaluated clinical features and analysed factors influencing mortality in child and adult cases using separate multivariate analysis models. We used separate multiple linear regression models to analyse relationships between cumulative dose of propofol and the number of features seen and organ systems involved. Lipidaemia, fever, and hepatomegaly occurred more frequently in children than in adults, whilst rhabdomyolysis and hyperkalaemia were more frequent in adults. Mortality from propofol infusion syndrome is independently associated with fever and hepatomegaly in children, and electrocardiogram changes, hypotension, hyperkalaemia, traumatic brain injury, and a mean propofol infusion rate >5 mg kg−1 h−1 in adults. The cumulative dose of propofol was associated with an increased number of clinical features and the number of organ systems involved in adult cases only. Clinicians should consider propofol infusion syndrome in cases of unexplained metabolic acidosis, ECG changes, and rhabdomyolysis. We recommend early consideration of continuous haemofiltration in the management of propofol infusion syndrome.
Keywords: adult; child; critical care; haemofiltration; propofol; propofol infusion syndrome; rhabdomyolysis, sedation
Editor's key points.
-
•
Propofol infusion syndrome is a rare, potentially fatal condition. Although long-term propofol infusions are no longer used in children because of its risk, much of the literature is based on these cases.
-
•
Propofol infusion syndrome should be considered in unexplained metabolic acidosis, ECG changes, and rhabdomyolysis, and continuous haemofiltration should be considered early in its management.
Because of favourable pharmacokinetics and rapidly reversible sedation, propofol has become one of the most commonly used drugs in anaesthesia and intensive care internationally.1 The World Health Organization has included it in the ‘model list of essential medicines’.2 Propofol infusion syndrome is a rare and potentially fatal condition first reported in children in 19903 and more recently in adults receiving long-term (>48 h) high-dose (>5 mg kg−1 h−1) propofol infusions.4, 5 The condition was known as propofol-related infusion syndrome because of the historical uncertainty by the manufacturer and others over a causal relationship. The first paediatric patients described were characterised by profound metabolic acidosis and bradycardia leading to asystole (cardiac arrest),6 and the presence of these features is included in some definitions of propofol infusion syndrome.7 Clearly, reliance on the development of preterminal features to make a diagnosis is of limited practical clinical value. Other commonly reported biochemical and clinical features associated include rhabdomyolysis and acute kidney injury (AKI).8 Table 1 summarises the reported clinical features associated with propofol infusion syndrome.
Table 1.
Reported clinical features of propofol infusion syndrome, based on the MedDRA system organ class detailed on the Summary of Product Characteristics for Diprivan 1%.
| Clinical features of propofol infusion syndrome | |
|---|---|
| Cardiac disorders | Cardiac failure including pulmonary oedema; widening of the QRS complex; bradycardia; ventricular tachycardia or fibrillation; asystole |
| Vascular disorders | Hypotension |
| Renal and urinary disorders | Acute kidney injury; change in urine colour |
| Musculoskeletal and connective tissue disorders | Rhabdomyolysis |
| Metabolism and nutrition disorders | Metabolic acidosis; hyperkalaemia; lipidaemia |
| Hepatobilliary disorders | Hepatomegaly; elevated liver transaminases |
Krajčová and colleagues9 analysed all case reports published up to 2014. They highlighted how the typical manifestation of propofol infusion syndrome has changed from a condition observed only in children receiving high doses of propofol to more recent cases in older patients receiving infusions within recommended dose limits. We suspected that the analysis by Krajčová and colleagues, in which they analysed all cases collectively, may not be relevant to modern practice where only adult cases are likely to occur.
There is no widely accepted definition of propofol infusion syndrome, and this may have contributed to continued scepticism as to whether or not it is a phenomenon related to propofol or whether reported cases are simply manifestations of the critical illness itself. Using a structured literature review process to identify relevant reports, our aim was to produce a narrative review of propofol infusion syndrome, combined with separate analyses of reported cases in adults and children. Specifically, we were interested in the range and frequency of clinical features and organ systems affected along with predictors of mortality.
Methods
Article selection
A literature search was conducted between January 2, 2018 and April 1, 2018 using the following search terms: ‘propofol’, OR ‘propofol.mp’ AND ‘infusion*’ OR ‘infusion.mp.’ AND ‘Syndrome*’ in the search engines Ovid MEDLINE® (1989–2018; http://ovidsp.uk.ovid.com/sp-3.20.0b/ovidweb.cgi), Web of Knowledge (1989–2018; https://webofknowledge.com), and Google Scholar (http://scholar.google.co.uk/), to identify peer-reviewed articles written in English and likely to describe human cases of propofol infusion syndrome in both adults and children. For reports not written in English, translation software (http://translate.google.co.uk/) was used. The reference sections of the articles identified in the electronic search were also scanned for additional references. We limited the search period to the past 30 yr, as propofol infusion syndrome was not reported before 1990.6 The process by which the articles were selected was based upon the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol.10 The methodology for article selection is illustrated in Figure 1. The titles and abstracts of all articles found using the search engines were screened by two reviewers (LM and SH) as follows: (i) human case reports, and (ii) cases reported where propofol was the primary drug infused.
Fig 1.
Flow chart of study selection process for reported paediatric and adult cases of propofol infusion syndrome.
Data collection and handling
The selected articles were collected and reviewed in their portable document format, and data from each case report were collated and entered onto a Microsoft Excel (Microsoft, city) spreadsheet. The data included patient characteristics, propofol mean infusion rate and duration, clinical features, biochemical data, and outcome. No attempt was made to contact the authors of case reports that provided limited data or information. Where the mean infusion rate of propofol was not quoted, we calculated or estimated it from available data. Where values were absent, data were coded as ‘missing’. We grouped the clinical features and biochemical data together based on the organ system(s) they affected. This was based on the MedDRA system organ classes as used in the summary product characteristics for Diprivan (manufacturer; location, URL) 1% (Table 1).
Statistical analysis
Data from each case report were entered onto a Microsoft Excel spreadsheet, and then exported to Stata (version 14.1; Stata Corp. Ltd., College Station, TX, USA) for coding and analysis. Descriptive statistics are presented using medians and range. Proportions were compared using the ‘N – 1’ χ2 test (MedCalc® statistical software, https://www.medcalc.org/calc/comparison_of_proportions.php; last accessed October 1, 2018). For logistic regression analyses, model generation used a forward stepwise multivariate regression approach with children and adults analysed using separate models; the univariate effect on mortality was calculated for variables highlighted from previous studies. Variables showing a significant influence on mortality (P<0.05) were then analysed for correlation between each other, and if significant, the stronger predictor of mortality in the univariate analysis was taken forward to the multiple regression model. A multiple logistical regression was then calculated along with receiver operating characteristic (ROC) curve area under the curve. Values are reported as odds ratios with 95% confidence intervals.
We developed predictive regression models for the effect of mean infusion rate and cumulative dose of propofol on mortality of children and adults. For multivariate linear regression, we used the regress function to identify any relationship between the number of clinical features and organ systems involved and cumulative dose of propofol in children and adults. A P-value <0.05 was considered significant: corrections for multiple comparisons were not made.
Results
From our literature search, we identified 108 papers (Fig. 1) containing 168 reported cases of propofol infusion syndrome in adults and children whose characteristics are summarised in Table 2. To date, 44 paediatric cases and 124 adult cases have been reported (Supplementary Tables S1–S4). Of these cases, 21 paediatric and 65 adult patients survived, whilst 23 paediatric and 59 adult patients died. The median ages of children and adults were 3.9 and 33 yr, respectively, with similar numbers of males and females in both groups. The BMI data for adults indicated that patients with a range of body habitus were affected. BMI data for children were not available, but the body weights on average were comparable with age-norm values. The median mean infusion rate of propofol was higher in children (7.75 mg kg−1 h−1) than in adults (5.1 mg kg−1 h−1), as was the median cumulative dose of propofol received (494 vs 380 mg kg−1, respectively).
Table 2.
Characteristics of paediatric and adult patients reported with propofol infusion syndrome
| Children (n=44) | Adults (n=124) | |
|---|---|---|
| Males/females | 18/16 (missing in 10 cases) | 62/41 (missing in 21 cases) |
| Age (yr), median (range) | 3.92 (0.08–15) (missing in 2 cases) | 33 (16–73) (missing in 23 cases) |
| Body weight (kg), median (range) | 15 (1.38–44) (missing in 15 cases) | 70 (33–192) (missing in 105 cases) |
| Duration of propofol infusion (h), median (range) | 66 (0.67–144) (missing in 1 case) | 72 (2–229) (missing in 4 cases) |
| Mean infusion rate (mg kg−1 h−1), median (range) | 7.8 (3–70) (missing in 6 cases) | 5.1 (1.5–13.68) (missing in 29 cases) |
| Cumulative propofol dose (mg kg−1), median (range) | 493.8 (4.09–1697) (missing in 6 cases) | 380.4 (12–1368) (missing in 30 cases) |
| Number of deaths | 23 (52%) | 59 (48%) |
There was no single clinical feature common to all reported cases in either children or adults. The most common feature, affecting almost 80% of both children and adults, was metabolic acidosis, with ECG changes the second most common feature (75% of children and almost 63% of adults) (Table 3). Lipidaemia, fever, and hepatomegaly occurred more frequently in children than in adults, whilst rhabdomyolysis and hyperkalaemia were more frequent in adults compared with children (Table 3). The overall mortality was 52% in children and 48% in adults.
Table 3.
Frequency of involvement of clinical features in published cases of propofol infusion syndrome in adults and children with associated univariate effect on mortality. CI, confidence interval; OR, odds ratio. ∗Missing value for mean infusion rate of propofol in six children and 29 adults. †Missing value for duration of propofol infusion in one child and four adults. ‡Missing value for cumulative dose of propofol in six children and 30 adults. ¶Missing clinical and biochemical data in 11 adults
| Frequency, n (%) |
Comparison of incidence in children vs adults |
Unadjusted mortality risk |
||||||
|---|---|---|---|---|---|---|---|---|
| Child | Adult | Child |
Adult |
|||||
| 95% CI of difference in proportion (P-value) | OR (95% CI) | P-value | OR (95% CI) | P-value | ||||
| Propofol dose and duration of infusion | ||||||||
| Mean infusion rate (continuous data, mg kg−1 h−1)∗ | — | — | — | 1.0 (0.9–1.0) | 0.44 | 1.4 (1.2–1.8) | <0.05 | |
| Mean infusion rate >5 mg kg−1 h−1 | 32 (84.2) | 49 (51.6) | 15.0–45.6% (<0.05) | 1.0 (0.2–5.7) | 1.0 | 5.2 (2.2–12.4) | <0.05 | |
| Duration of infusion (continuous data, h)† | — | — | — | — | 1.0 (1.0–1.0) | 0.96 | 1.0 (1.0–1.0) | 0.06 |
| Duration of infusion >48 h | 28 (65.1) | 84 (70.0) | –10.3 to 21.6% (0.55) | 2.3 (0.6–8.4) | 0.20 | 2.6 (1.1–6.0) | <0.05 | |
| Cumulative dose (continuous data, mg kg−1)‡ | — | — | — | — | 1.00 (1.0–1.0) | 0.12 | 1.0 (1.0–1.0) | <0.05 |
| Cumulative propofol dose >240 mg kg−1 | 27 (71.1) | 77 (81.9) | –4.1 to 27.9% (0.17) | 7.7 (1.4–42.7) | <0.05 | 4.6 (1.4–15.3) | <0.05 | |
| Clinical and biochemical features¶ | ||||||||
| Metabolic acidosis | 35 (79.6) | 87 (77.0) | –13.0 to 15.2% (0.73) | 2.7 (0.6–12.4) | 0.21 | 2.1 (0.8–5.1) | 0.11 | |
| ECG changes | 33 (75.0) | 71 (62.8) | –4.4 to 26.0% (0.15) | 1.4 (0.4–5.7) | 0.60 | 5.9 (2.5–13.7) | <0.05 | |
| Rhabdomyolysis | 17 (38.6) | 70 (62.0) | 6.1–38.8% (<0.05) | 0.3 (0.09–1.1) | 0.08 | 0.9 (0.4–2.0) | 0.83 | |
| Acute kidney injury | 18 (40.9) | 57 (50.4) | –7.7 to 25.5% (0.28) | 0.4 (0.1–1.4) | 0.14 | 0.5 (0.2–1.1) | 0.07 | |
| Urine colour change | 4 (9.1) | 12 (10.6) | –11.3 to 10.4 (0.78) | 0.3 (0.03–2.9) | 0.28 | 2.0 (0.6–6.9) | 0.30 | |
| Hypotension | 14 (31.8) | 35 (31.0) | –14.0 to 17.4% (0.92) | 0.9 (0.2–3.1) | 0.84 | 3.9 (1.6–9.5) | <0.05 | |
| Raised lactate | 11 (25.0) | 35 (31.0) | –10.4 to 19.7% (0.46) | 0.4 (0.1–1.7) | 0.23 | 1.1 (0.5–2.5) | 0.77 | |
| Lipidaemia | 19 (43.2) | 25 (22.1) | 5.2–37.1% (<0.05) | 1.0 (0.3–3.4) | 0.97 | 1.5 (0.6–3.7) | 0.38 | |
| Hyperkalaemia | 5 (11.4) | 38 (33.6) | 7.3–33.3% (<0.05) | 0.6 (0.09–3.8) | 0.56 | 3.3 (1.4–7.6) | <0.05 | |
| Cardiac failure | 11 (25.0) | 31 (27.4) | –13.8 to 16.0% (0.76) | 3.2 (0.7–14.2) | 0.13 | 0.8 (0.4–1.8) | 0.62 | |
| Fever | 16 (36.4) | 14 (12.4) | 9.5–39.6% (<0.05) | 7.8 (1.8–34.1) | <0.05 | 3.9 (1.0–14.8) | <0.05 | |
| Elevated liver enzymes | 5 (11.4) | 14 (12.4) | –12.5 to 10.7% (0.86) | 0.6 (0.09–3.8) | 0.56 | 1.25 (0.4–2.7) | 0.97 | |
| Hepatomegaly | 10 (22.7) | 3 (2.7) | 9.0–34.4% (<0.05) | 12.9 (1.5–113) | <0.05 | 0.4 (0.04–5.1) | 0.52 | |
| Traumatic brain injury | 5 (11.4) | 44 (35.0) | 8.6–34.7% (<0.05) | 1.4 (0.2–9.5) | 0.714 | 6.2 (2.7–14.3) | <0.05 | |
| Number of organ systems involved | ||||||||
| 1 | 7 (15.9) | 9 (8.0) | –2.3 to 21.9% (0.14) | — | — | — | — | |
| 2 | 8 (18.2) | 23 (20.4) | –13.0 to 14.2% (0.76) | 0.8 (0.1–5.8) | 0.78 | 6.6 (1.1–39) | <0.05 | |
| 3 | 12 (27.3) | 33 (29.2) | –14.5 to 16.0% (0.81) | 0.8 (0.1–4.9) | 0.76 | 2.3 (0.4–12.7) | 0.35 | |
| 4 | 9 (20.5) | 27 (23.9) | –12.2 to 16.0% (0.65) | 0.9 (0.1–6.9) | 0.95 | 4.4 (0.8–25.1) | 0.10 | |
| 5 | 6 (13.6) | 15 (13.3) | –10.0 to 14.3% (0.96) | 0.8 (0.08–6.7) | 0.80 | 7.0 (1.0–46.9) | <0.05 | |
| 6 | 2 (4.6) | 6 (5.3) | –10.3 to 7.3% (0.86) | 0.8 (0.03–17.5) | 0.86 | 7.0 (0.7–70.7) | 0.1 | |
Upon univariate analysis, fever, hepatomegaly, and cumulative dose of propofol >240 mg kg−1 were associated with an increased risk of mortality in children. Fever was also associated with an increased risk of death in adults, as were hyperkalaemia (but not rhabdomyolysis), hypotension, ECG changes, traumatic brain injury, mean infusion rate >5 mg kg−1 h−1, duration of infusion >48 h, and cumulative dose >240 mg kg−1. When we grouped clinical features into organ systems, we found no association between the involvement of any particular organ system and mortality in children or adults (Table 3). In the multivariate analysis of 38 child cases with sufficient data, we found that only fever and hepatomegaly were predictive of mortality (area under ROC curve=0.89). In the analysis of 83 adult cases with sufficient data, we found ECG changes, hypotension, hyperkalaemia, traumatic brain injury, and mean propofol infusion rate >5 mg kg−1 h−1 to be associated with an increased mortality (area under ROC curve=0.90).
Using logistic regression analysis, we assessed the relationship between dose of propofol and death. We used separate models to examine the effect of cumulative dose of propofol on mortality in adults and children. We found a statistically significant association between cumulative dose of propofol and predicted mortality in adults (Fig. 2b), but not in children (Fig. 2a).
Fig 2.
(a) Predicted mortality of children with diagnosed propofol infusion syndrome against cumulative dose of propofol infused (data missing in eight). (b) Predicted mortality of adults with diagnosed propofol infusion syndrome against cumulative dose of propofol infused (data missing in 28). Data were calculated from logistic regression analysis of 168 published case reports. CI, confidence interval.
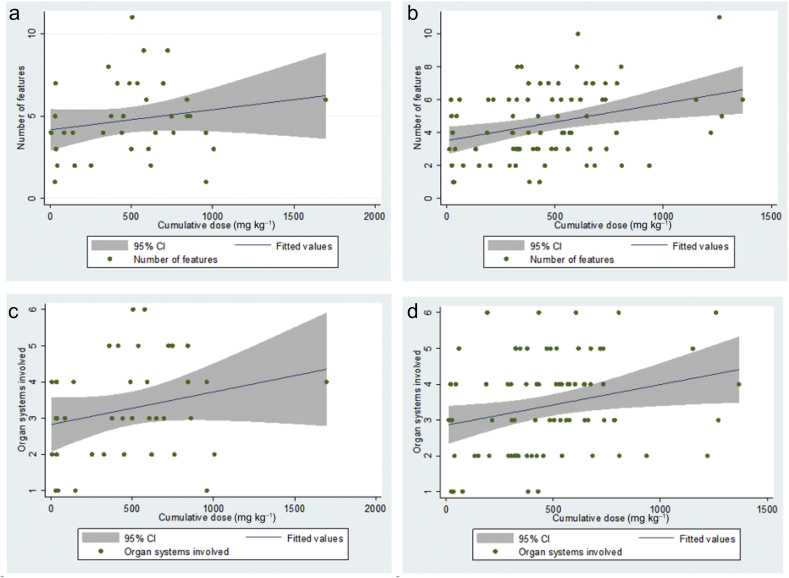
There was a statistically significant, albeit modest, association between cumulative dose of propofol and the number of features of propofol infusion syndrome in adults [F(1,81)=9.07; P=0.003], with cumulative dose accounting for 10.1% of the variability in the number of features (Fig. 3b). A similar predictive effect was seen between cumulative dose of propofol and the number of organ systems involved in adults [F(1,81)=5.56; P=0.021], with cumulative dose accounting for 6.42% of the variability in the number of organ systems involved (Fig. 3d). Conversely, in children, there was no significant association between the cumulative dose of propofol and either the number of features seen or the number of organ systems involved (Fig. 3a and c).
Fig 3.
(a) Linear regression model showing that cumulative dose of propofol infused did not statistically significantly predict the number of features of propofol infusion syndrome reported in published child cases [F(1,36)=2.21; P=0.146], with cumulative dose accounting for 5.78% of the variability in the number of features. (b) Linear regression model showing that cumulative dose of propofol infused statistically significantly predicts the number of features of propofol infusion syndrome reported in published adult cases [F(1,81)=9.07; P=0.003], with cumulative dose accounting for 10.1% of the variability in the number of features. (c) Linear regression model showing that cumulative dose of propofol infused did not statistically significantly predict the number of organ systems involved in reported child cases of propofol infusion syndrome [F(1,36)=2.21; P=0.146], with cumulative dose accounting for 5.78% of the variability in the number of organ systems involved. (d) Linear regression model showing that cumulative dose of propofol infused statistically significantly predicts the number of organ systems involved in reported adult cases of propofol infusion syndrome [F(1,81)=5.56; P=0.021], with cumulative dose accounting for 6.42% of the variability in the number of organ systems involved. CI, confidence interval.
Discussion
Principal findings
We reviewed all 168 cases of propofol infusion syndrome reported in the medical literature. In contrast to Krajčová and colleagues,9 we analysed child and adult cases using separate multiple regression models, and our different findings highlight the importance of our approach. Our data reinforce the variability in the presenting features of propofol infusion syndrome, with cardiovascular and metabolic features being most commonly involved in both adults and children (Table 3). The significance of some of the metabolic features is difficult to interpret, especially that of lipidaemia, which is a recognised consequence of propofol administration. Interestingly, the number of organ systems involved was not related to mortality (Table 3).
Clinical and biochemical features
Range and variability
We found variability in the presenting features of propofol infusion syndrome. For this reason, we propose categorising primary and secondary features, with primary features defined as those that can be the only clinical feature of propofol infusion syndrome, and secondary features as those that only occur in combination with one or more other features. We did this by analysing published cases where propofol infusion syndrome was diagnosed with only one identified clinical feature:
-
(i)
Primary features: metabolic acidosis, ECG changes, and rhabdomyolysis
-
(ii)
Secondary features: AKI, hyperkalaemia, lipidaemia, cardiac failure, fever, elevated liver enzymes, and raised lactate
Differences in clinical features between children and adults
When first described, propofol infusion syndrome was typically seen in children receiving high doses of propofol. In contrast, the more recent cases are adult patients, often elderly, receiving propofol within the recommended dose limits who develop a varying combination of mild acidosis, elevated creatine kinase, and sometimes AKI and ECG changes.
Recognition of features associated with mortality
In our multivariate analysis, fever and hepatomegaly were the only features with an effect on mortality in children. The effect of hepatomegaly might be explained by its clinical diagnosis being missed in survivors, whereas this is unlikely post-mortem. Our modelling of adult cases showed that ECG changes, hypotension, hyperkalaemia, and traumatic brain injury were associated with mortality. In their multivariate analysis of published cases before 2014, Krajčová and colleagues9 found that only fever and traumatic brain injury were significant predictors of death.
Risk factors
Cumulative dose
Previous reviews have suggested that the cumulative dose of propofol is the main risk factor for the development of propofol infusion syndrome.11 We have demonstrated a linear relationship between the cumulative dose of propofol received and both the number of features of propofol infusion syndrome and the number of organ systems involved in adults (Fig. 3b and d). No such association was seen in children, and this may be related to the small number of published reports. However, we emphasise that there are reported cases of propofol infusion syndrome that have occurred at low cumulative doses with multisystem involvement.12, 13
Obesity
We were unable to identify any relationship between patient weight or BMI and the development of propofol infusion syndrome or mortality from it, with these data being unavailable in most reports. However, pharmacokinetic studies have suggested that the dose of propofol for sedation should be calculated based on lean, rather than actual, body weight.14, 15 For the average patient, these weights are similar, and thus, the dose is the same, but in obese patients, the difference becomes important. There was a patient with a BMI of 75 kg m−2 where the propofol dose was not calculated based on lean body weight and the patient received a higher dose of propofol for a prolonged period, which could be a reason for the development of propofol infusion syndrome.16
Vasopressors
In their review of neurological ICU patients, Smith and colleagues17 reported a relationship between the use of vasopressors and the development of propofol infusion syndrome. They suggested that this effect could be caused by the action of propofol and vasopressors on the heart.8, 17 A study in sheep found that, when infused concurrently, vasopressors produced a dose-dependent reversal of the anaesthetic effect of propofol by decreasing its blood concentration, attributing this to the increased clearance of propofol from increased cardiac output.18 This can be further explained by the negative inotropic effect on the heart caused by the antagonistic action of propofol on myocardial adrenoreceptors.19 Given the surge of catecholamines in neurological injury, Smith and colleagues17 suggested that this effect could lead to escalating propofol requirements in order to maintain sedation in these patients. It is yet unproved whether this relationship is caused by or is an effect of propofol infusion syndrome: the development of acidosis and mitochondrial dysfunction seen in propofol infusion syndrome will impair the vasomotor tone, and this could then lead to the increased requirement for vasopressors. We did not analyse the effect of vasopressors or steroids, as these data were missing in the majority of published cases.
Steroids
The administration of steroids in the ICU has also been linked to the development of propofol infusion syndrome.8 The development of rhabdomyolysis as part of propofol infusion syndrome might be through a similar mechanism to the action of steroids in the development of ICU-related myopathy.20 The proposed mechanism behind this is the triggering of the ubiquitin–proteasome system, which results in muscle damage through myofilament derangement.21 There is also evidence that steroids reduce mitochondrial energy production by affecting gene transcription.22, 23 Given the effects of propofol on the mitochondria and that propofol infusion syndrome has been seen in patients with mitochondrial disease,24 it is possible that the administration of corticosteroids acts as a priming factor for the development of propofol infusion syndrome.
Critical illness
Critical illness has been reported to be implicated in the development,25 whilst traumatic brain injury has been linked with death from propofol infusion syndrome.9 We had hoped to analyse the impact of the severity of illness, using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, on outcomes, but these data were rarely reported. Previous data have shown that patients with traumatic brain injury have an increased risk of developing propofol infusion syndrome, and the risk in these patients is doubled when receiving >5 mg kg−1 h−1 propofol compared with those receiving lower doses,11 and our findings are consistent with this. However, high cumulative doses of propofol in patients with traumatic brain injury may reflect attempts to control rising intracranial pressure in patients with more severe injury. It would be interesting to be able to evaluate the relationship between the APACHE II score (which incorporates the Glasgow Coma Scale score), cumulative propofol dose, and the development of propofol infusion syndrome in this patient group.
In addition, severe neurological injury causes an exaggerated stress response, in which there are high concentrations of circulating catecholamines and glucocorticoids, and these may be priming factors in the development of propofol infusion syndrome. The subsequent clinical use of vasopressors, steroids, and high-dose propofol might then trigger the condition. Further to this, critically ill patients switch from carbohydrate-based metabolism to lipolysis, leading to an increase in free fatty acid concentrations,26 which are implicated in the pathophysiology of propofol infusion syndrome.27, 28, 29 For these reasons, multimodal sedation regimens designed to limit propofol requirements should be used in patients with traumatic head injury, and clinicians should maintain a high index of suspicion for the development of propofol infusion syndrome. Vasile and colleagues8 suggested similar considerations in patients with other conditions likely to exhibit a marked physiological stress response, such as subarachnoid haemorrhage, status epilepticus, meningitis, encephalitis, and stroke, and in patients with severe burns, trauma, severe infections, pancreatitis, and acute exacerbation of asthma.
Contemporary definition of propofol infusion syndrome
The first reported case of propofol infusion syndrome occurred in children in 1990,30 and the phenomenon was further illustrated in a case series published by Parke and colleagues6 in 1992. The case series reported five cases occurring in children aged from 4 weeks to 6 yr.6 With the increasing incidence of propofol-related deaths, the US Food and Drug Administration (FDA) performed an investigation in 1992, which was unable to find a link between propofol and the deaths. However, the investigation did encourage a trial to be performed.31 In the UK, the use of propofol as a long-term sedation agent in paediatric patients was abandoned.32 In 1994, the manufacturers of propofol published a warning of the use of propofol in long-term sedation in children with respiratory tract infections.32 After reviewing all the reported cases of child death associated with propofol, Bray33 described the phenomenon as ‘propofol infusion syndrome’. An unpublished trial conducted by the manufacturers in 1999 led the FDA and the Canadian Health Board to request that the product label should recommend that propofol was not to be used for long-term sedation in paediatric patients.4 The first case reported in adults involved a patient with an exacerbation of asthma receiving long-term sedation for mechanical ventilation of the lungs.34 The first reported adult death attributed to propofol infusion syndrome occurred in 1998.35 In 2001, Cremer and colleagues11 published a review of adults who died in a neurointensive care unit after being admitted with a head injury: seven of their deaths were attributed to propofol infusion syndrome. Subsequently, the literature has been dominated by adult cases. Despite this, the features of paediatric cases receive prominence in recent literature. Our review and analyses emphasise the distinctive nature of propofol infusion syndrome affecting adults, both in terms of the clinical features and those factors associated with a poor outcome.
Through this review and analysis, we suggest an updated definition of propofol infusion syndrome: propofol infusion syndrome occurs in critically ill patients receiving propofol infusions, typically either high dose (>5 mg kg−1 h−1) or of long duration (>48 h), and is characterised by one or more of otherwise unexplained metabolic acidosis, rhabdomyolysis, or ECG changes, with or without AKI, hyperkalaemia, lipidaemia, cardiac failure, fever, elevated liver enzymes, or raised lactate.
Pathophysiology
The mechanism behind the development of propofol infusion syndrome is yet unclear. Previous theories suggested accumulation of inactive propofol metabolites,33 lipid microembolisation,34 and impaired hepatic lactate metabolism.6 It has been suggested that propofol infusion syndrome resembles some mitochondrial diseases,36 such as medium-chain acyl coenzyme A (CoA) dehydrogenase deficiency, when the defective mitochondria are placed under significant physiological stress, such as trauma, surgery, or sepsis.37
Initial theories and studies suggested that propofol acts as a mitochondrial uncoupler,38, 39 whilst others have hypothesised that it interferes with mitochondrial fatty acid oxidation.28, 29, 40 Further studies have suggested that propofol interacts with cytochrome C and cytochrome aa3,41 which argues against its role as an uncoupler.42 Cray and colleagues43 suggested that propofol has a disruptive effect on the respiratory chain, leading to reduced ATP production, cellular hypoxia, and, ultimately, metabolic acidosis. Kam and Cardone4 proposed that this was either via the inhibition of coenzyme Q of cytochrome C, or via the inhibition of the fatty acid transporters, carnitine palmitoyl transferase I and II (CPT I/II).
Wolf and colleagues27 postulated that propofol causes an increase in malonylcarnitine, an inhibitor of CPT I. Fatty acids are activated on the outer mitochondrial membrane, but are oxidised within the mitochondrial matrix. Short- and medium-chain fatty acids can freely diffuse across the mitochondrial membrane; however, longer-chain fatty acids, such as palmitoyl CoA, require CPT I, which acts as a shuttle system to move them into the matrix. Thus, the inhibition of CPT I by malonylcarnitine and propofol itself5 causes fatty acids to accumulate in the mitochondria, leading to dysfunction of the respiratory chain, and the cascade of reduced ATP production occurs.27, 44
Vanlander and colleagues45 tested this theory in rats, with their results indicating that propofol inhibits coenzyme Q, which transfers electrons from complex II to complex III on cytochrome C. They hypothesised that this effect could be caused by the similar structure of propofol and coenzyme Q.45 Recently, Vollmer and colleagues44 were able to provide the first microscopic evidence of mitochondrial involvement using electron microscopy showing electron dense inclusions in the mitochondria of cardiac muscle.
Our case report analysis suggests that the cumulative dose of propofol is important in the aetiology of propofol infusion syndrome, either through high infusion rates, prolonged duration of infusion, or both.9, 11 However, cases have occurred after low-dose short-duration infusions. In two such cases, the patients were found to have a genetic mitochondrial defect, which put them at a greater susceptibility to mitochondrial dysfunction.24, 46 When considering all of these studies, the evidence points to a defect in the production of ATP as the probable causative mechanism of propofol infusion syndrome. Whilst the exact pathophysiology is unclear, it is the mitochondria and, more specifically, the respiratory chain, that currently represents the most interesting pathway.
Prevention and recognition of propofol infusion syndrome
The only definitive way to prevent propofol infusion syndrome would be not to use propofol infusion for sedation of the critically ill. Such a decision would need to be taken with proportionate considerations of the risk of propofol infusion syndrome and the benefits of propofol. The benefits, in turn, may be absolute if there was no alternative to propofol, or relative if alternative agents were available, but were less cost-effective.
Use of propofol and the incidence of propofol infusion syndrome
In the early 1980s, propofol was introduced as an anaesthetic induction agent,47 and then later, both as an induction and maintenance anaesthetic.48 After approval from the FDA, the indications for propofol expanded to include long-term sedation in intensive care.49 Currently, 70% of propofol use is for sedation.49 Despite the common use of propofol for sedation of the critically ill, we found only 164 cases of propofol infusion syndrome reported in the literature since 1990. However, in a prospective study of critically ill patients, Roberts and colleagues25 reported an incidence of 1.1%, equating to three or four patients per year in an ICU admitting 300–400 patients.50 The contrast between the scarcity of published reports and the data of Roberts and colleagues25 might suggest overdiagnosis in the latter study, but may simply reflect increasing awareness of the condition and the lack of interest of journals in publishing further case reports of previously well-described conditions. In the same study, there was a mortality of 18% in patients who developed propofol infusion syndrome,25 but it is not clear that all of these died from the condition. Our finding of a mortality of 48% in adults amongst published reports may therefore reflect a publication bias for the most severe cases.
Whilst the incidence of fatal cases of propofol infusion syndrome may be less in adults than in children (considering the relative numbers of adults and children who receive intensive care treatment), the overall burden of the adult condition may be considerable. Indeed, the evidence for harm in adults may exceed that available for children when licensing authorities withdrew the indication in children. A large prospective epidemiological study of propofol infusion syndrome in adults is warranted.
Strategies to reduce the risk of propofol infusion syndrome
Increased dosage of propofol is associated with propofol infusion syndrome,11 with the risk also associated with infusion duration.5 Recommendations in the literature include the avoidance of infusion rates of more than 5 mg kg−1 h−1 for more than 48 h5, 51 to always use propofol in combination with other sedative agents (such as opioids), and to monitor pH, lactate, and creatine kinase when infusions are prolonged, especially if high doses cannot be avoided.52 The AstraZeneca Summary of Product Characteristics for Diprivan 1% and the German Medical Association recommend a lower maximum infusion rate of 4 mg kg−1 h−1.53
There is no clear inflection point from our predictive model for the mean infusion rate of propofol or the cumulative dose of propofol and mortality from propofol infusion syndrome (Fig. 3a and b). Setting maximum rates can result in a false sense of security as long as the maximum rates are not exceeded or, even worse, if a maximum rate is perceived as the standard rate. We recommend to always limit the infusion rate to the lowest possible by the use of multimodal sedation. The product characteristic summary recommends the consideration of alternative sedative agents if there are escalating propofol requirements with prolonged infusion, or if there is the onset of metabolic acidosis. As this review has shown, rhabdomyolysis is a prominent feature in adults, and we suggest that the daily measurement of creatine kinase concentrations after 48 h of propofol-based sedation may aide the recognition of propofol infusion syndrome. As creatine kinase concentrations take 12–24 h to peak after the onset of rhabdomyolysis,51 earlier measurement is likely to be of no clinical benefit.
Studies have implicated critical illness31 itself in the development of propofol infusion syndrome, but if this was to be a reason to avoid propofol infusions, then there seems little point in using propofol in intensive care, except perhaps for planned postoperative mechanical ventilation. We have already discussed strategies for limiting propofol infusions in patients with traumatic head injury. Considering the real possibility of a mitochondrial aetiology and reports in patients with inborn errors of mitochondrial function,54 it would seem prudent to avoid propofol infusions in patients with suspected mitochondrial impairment. The position with the use of vasopressors52 and steroids4 in conjunction with propofol infusions and their role in the pathophysiology of propofol infusion syndrome is less clear. There are few indications where there is evidential support for the use of steroids in critical care, and propofol is perhaps not the best choice of sedation agent in patients with cardiovascular compromise requiring escalating doses of vasopressors. Low-carbohydrate intake has also been implicated, with some evidence suggesting that the risk of propofol infusion syndrome could possibly be lowered by supplementary carbohydrate infusion. Although further research is required,8 it would seem pragmatic to provide a constant source of carbohydrate to patients receiving a prolonged infusion of propofol.
Management
There are no established guidelines for the treatment for propofol infusion syndrome. The success of treatment is likely to be dependent on early diagnosis. The best approach to early diagnosis is being aware that the condition exists, what its clinical features are, and maintaining a high index of suspicion should those clinical features develop in a patient receiving a propofol infusion. Once the diagnosis is made, there is the simultaneous imperative to eliminate propofol from the body and treat the effects of propofol infusion syndrome. There is no antidote, but commencing an infusion of dextrose is unlikely to do harm (as long as blood glucose is monitored and controlled with insulin if necessary) and may have some benefit if propofol infusion syndrome has a mitochondrial aetiology.
Treatment of the features of propofol infusion syndrome
We suggest that emergent treatment should focus on the clinical features shown to be associated with mortality: ECG changes, hyperkalaemia, hypotension, and fever. Various ECG changes have been reported, and their treatment should be along standard lines for the arrhythmia in question. Although acidosis itself was not a feature shown to be directly associated with mortality, it may be the cause of an arrhythmia and will obtund responses to catecholamines in the treatment of hypotension. Patients are likely to benefit from increased minute ventilation to compensate for metabolic acidosis.55 Extracorporeal membrane oxygenation has also been reported to be beneficial in some cases,56, 57 where response to vascular compartment filling and vasopressors/inotropes was inadequate.
Should hyperkalaemia, acidosis, or fever not respond adequately or in a sustained way to simpler conventional measures, we urge the early consideration of the application of haemofiltration58 before the development of hypotension severe enough to preclude it.
Elimination of propofol
Administration of propofol should cease, with sedation maintained, using an alternative hypnotic agent, such as dexmedetomidine or midazolam. The remaining propofol in the body is metabolised by the liver into water-soluble metabolites, which are then excreted rapidly by the kidneys. However, in the presence of AKI, as seen in 50.4% of adult cases, this excretion is likely to be hindered. Here, again, continuous haemofiltration could be beneficial. Although it cannot eliminate the highly lipophilic parent drug, continuous haemofiltration can eliminate the toxic water-soluble propofol metabolites.59 Honore and Spapen60 highlighted the need to monitor citrate concentrations through the ionised/total calcium ratio if citrate is used as an anticoagulant for haemofiltration. Citrate is metabolised in the mitochondria, and it is possible that the hepatic and skeletal muscle metabolism of citrate could be hindered in propofol infusion syndrome, resulting in citrate intoxication.60 If the ionised/total calcium ratio exceeds 2.25, citrate should be substituted with unfractionated heparin.61
Limitations of this review
Notably, we included all reported cases of paediatric and adult propofol infusion syndrome, but we have recognised through our analysis that, in many case reports, the data were incomplete. For example, the clinical features that the authors associated with propofol infusion syndrome were not always clearly defined. The mean infusion rate of propofol could not be calculated because of missing data in 36 cases, whilst 11 cases were excluded on the basis of incomplete clinical data. Statistical analysis was hampered by the relatively small volume of reported cases, making it difficult to either confidently confirm or refute that certain clinical features are associated with propofol infusion syndrome.
Furthermore, with the analysis of case reports, there is a risk of publication bias and that the published case reports may not be truly representative of propofol infusion syndrome. For example, since the introduction of safety recommendations, authors may be less likely to publish cases in which clinicians exceeded recommended doses. Furthermore, as propofol infusion syndrome consists of a number of features that overlap with common ICU conditions, its diagnosis is reliant on clinical interpretation, and it is possible that rather than being a manifestation of propofol infusion syndrome, a particular sign was related to another coexisting condition.
Finally, as APACHE II (or similar) illness severity scores were seldom published, we were unable to correct for severity of critical illness or pre-existing organ dysfunction. It is possible (but, we think unlikely) that data included in our analysis, such as cumulative propofol dose, may have been a surrogate for these. Consequently, we would urge authors publishing on ‘rare’ conditions or syndromes to include data of this nature wherever possible.
Conclusions
It is evident that the use of propofol for long-term sedation is associated with a number of cases of propofol infusion syndrome in both adults and children around the world. Propofol infusion syndrome presents in a number of ways from cardiovascular collapse to a metabolic response, and is a disease of multiple organ systems. We have been able to demonstrate associations between the cumulative dose of propofol and predicated mortality, and the number of clinical features and organ systems involved in adults. Our multivariate analysis highlights the variation in both the typical features of propofol infusion syndrome and those associated with mortality. As there is no diagnostic test, a high degree of clinical suspicion is required in all patients receiving high-dose short-term infusions and patients receiving long-duration infusions with a variable dose range. At present, treatment is mainly supportive, and we recommend that clinicians keep an open mind and consider propofol infusion syndrome in cases of unexplained metabolic acidosis, ECG changes, and rhabdomyolysis. We recommend early consideration of continuous renal replacement therapy in the management of propofol infusion syndrome. Future research is required into the epidemiology, prevention, diagnosis, and management of propofol infusion syndrome.
Authors' contributions
Study conception/design: all authors.
Literature search and data collection: SH, LM.
Data analysis and interpretation: all authors.
Drafting of manuscript: SH, LM.
All authors reviewed drafts of the manuscript and approved the final version.
Declaration of interest
PMH is an editorial board member of British Journal of Anaesthesia.
Handling editor: J.G. Hardman
Editorial decision date: 20 December 2018
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.12.025.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ho K.M., Ng J.Y. The use of propofol for medium and long-term sedation in critically ill adult patients: a meta-analysis. Intensive Care Med. 2008;34:1969–1979. doi: 10.1007/s00134-008-1186-5. [DOI] [PubMed] [Google Scholar]
- 2.WHO . March 2011. WHO model list of essential medicines: 17th list.http://www.who.int/medicines/publications/essentialmedicines/en/index.html [Google Scholar]
- 3.Hatch D.J. Propofol-infusion syndrome in children. Lancet. 1999;353:1117–1118. doi: 10.1016/S0140-6736(99)90018-1. [DOI] [PubMed] [Google Scholar]
- 4.Kam P.C., Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690–701. doi: 10.1111/j.1365-2044.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 5.Diedrich D.A., Brown D.R. Analytic reviews: propofol infusion syndrome in the ICU. J Intensive Care Med. 2011;26:59–72. doi: 10.1177/0885066610384195. [DOI] [PubMed] [Google Scholar]
- 6.Parke T.J., Stevens J.E., Rice A.S. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992;305:613–616. doi: 10.1136/bmj.305.6854.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray R.J. Propofol infusion syndrome in children. Pediatr Anesth. 1998;8:491–499. doi: 10.1046/j.1460-9592.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 8.Vasile B., Rasulo F., Candiani A., Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29:1417–1425. doi: 10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- 9.Krajčová A., Waldauf P., Anděl M., Duška F. Propofol infusion syndrome: a structured review of experimental studies and 153 published case reports. Crit Care. 2015;19:398. doi: 10.1186/s13054-015-1112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremer O.L., Moons K.G., Bouman E.A., Kruijswijk J.E., de Smet A.M., Kalkman C.J. Long-term propofol infusion and cardiac failure in adult head-injured patients. Lancet. 2001;357:117–118. doi: 10.1016/S0140-6736(00)03547-9. [DOI] [PubMed] [Google Scholar]
- 12.Merz T.M., Regli B., Rothen H.U., Felleiter P. Propofol infusion syndrome—a fatal case at a low infusion rate. Anesth Analg. 2006;103:1050. doi: 10.1213/01.ane.0000239080.82501.c7. [DOI] [PubMed] [Google Scholar]
- 13.Dengler B., Garvin R., Seifi A. Can therapeutic hypothermia trigger propofol-related infusion syndrome? J Crit Care. 2015;30:823–824. doi: 10.1016/j.jcrc.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Servin F., Farinotti R., Haberer J.P., Desmonts J.M. Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide. A clinical and pharmacokinetic study. Anesthesiology. 1993;78:657–665. doi: 10.1097/00000542-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Erstad B.L. Dosing of medications in morbidly obese patients in the intensive care unit setting. Intensive Care Med. 2004;30:18–32. doi: 10.1007/s00134-003-2059-6. [DOI] [PubMed] [Google Scholar]
- 16.Ramaiah R., Lollo L., Brannan D., Bhananker S.M. Propofol infusion syndrome in a super morbidly obese patient (BMI = 75) Int J Crit Illn Inj Sci. 2011;1:84–86. doi: 10.4103/2229-5151.79290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith H., Sinson G., Varelas P. Vasopressors and propofol infusion syndrome in severe head trauma. Neurocritical Care. 2009;10:166–172. doi: 10.1007/s12028-008-9163-y. [DOI] [PubMed] [Google Scholar]
- 18.Myburgh J.A., Upton R.N., Grant C., Martinez A. Epinephrine, norepinephrine and dopamine infusions decrease propofol concentrations during continuous propofol infusion in an ovine model. Intensive Care Med. 2001;27:276–282. doi: 10.1007/s001340000793. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W., Fontenot H.J., Wang S.N., Kennedy R.H. Propofol-induced alterations in myocardial beta-adrenoceptor binding and responsiveness. Anesth Analg. 1999;89:604–608. doi: 10.1097/00000539-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 20.De Jonghe B., Sharshar T., Lefaucheur J.-P. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 21.Mitch W.E., Goldberg A.L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 22.Scheller K., Sekeris C.E. The effects of steroid hormones on the transcription of genes encoding enzymes of oxidative phosphorylation. Exp Physiol. 2003;88:129–140. doi: 10.1113/eph8802507. [DOI] [PubMed] [Google Scholar]
- 23.Roussel D., Dumas J.-F., Simard G., Malthièry Y., Ritz P. Kinetics and control of oxidative phosphorylation in rat liver mitochondria after dexamethasone treatment. Biochem J. 2004;382:491–499. doi: 10.1042/BJ20040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savard M., Dupre N., Turgeon A.F., Desbiens R., Langevin S., Brunet D. Propofol-related infusion syndrome heralding a mitochondrial disease: case report. Neurology. 2013;81:770–771. doi: 10.1212/WNL.0b013e3182a1aa78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts R.J., Barletta J.F., Fong J.J. Incidence of propofol-related infusion syndrome in critically ill adults: a prospective, multicenter study. Crit Care. 2009;13:R169. doi: 10.1186/cc8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cree M.G., Wolfe R.R. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab. 2008;294:E1–E9. doi: 10.1152/ajpendo.00562.2007. [DOI] [PubMed] [Google Scholar]
- 27.Wolf A., Weir P., Segar P., Stone J., Shield J. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet. 2001;357:606–607. doi: 10.1016/S0140-6736(00)04064-2. [DOI] [PubMed] [Google Scholar]
- 28.Wolf A.R., Potter F. Propofol infusion in children: when does an anesthetic tool become an intensive care liability? Paediatr Anaesth. 2004;14:435–438. doi: 10.1111/j.1460-9592.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 29.Withington D.E., Decell M.K., Al Ayed T. A case of propofol toxicity: further evidence for a causal mechanism. Paediatr Anaesth. 2004;14:505–508. doi: 10.1111/j.1460-9592.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- 30.Notis fra Bivirkningsnaenet. Propofol (Diprivan) bivirkninger. Ugeskr Laeger. 1990;152:1176. [PubMed] [Google Scholar]
- 31.Ahlen K., Buckley C.J., Goodale D.B., Pulsford A.H. The ‘propofol infusion syndrome’: the facts, their interpretation and implications for patient care. Eur J Anaesthesiol. 2006;23:990–998. doi: 10.1017/S0265021506001281. [DOI] [PubMed] [Google Scholar]
- 32.Rosen D.J., Nicoara A., Koshy N., Wedderburn R.V. Too much of a good thing? Tracing the history of the propofol infusion syndrome. J Trauma. 2007;63:443–447. doi: 10.1097/TA.0b013e31809fe910. [DOI] [PubMed] [Google Scholar]
- 33.Bray R.J. Propofol-infusion syndrome in children. Lancet. 1999;353:2074–2075. doi: 10.1016/s0140-6736(05)77896-x. [DOI] [PubMed] [Google Scholar]
- 34.Marinella M.A. Lactic acidosis associated with propofol. Chest. 1996;109:292. doi: 10.1378/chest.109.1.292. [DOI] [PubMed] [Google Scholar]
- 35.Hanna J.P., Ramundo M.L. Rhabdomyolysis and hypoxia associated with prolonged propofol infusion in children. Neurology. 1998;50:301–303. doi: 10.1212/wnl.50.1.301. [DOI] [PubMed] [Google Scholar]
- 36.DiMauro S. Mitochondrial myopathies. Curr Opin Rheumatol. 2006;18:636–641. doi: 10.1097/01.bor.0000245729.17759.f2. [DOI] [PubMed] [Google Scholar]
- 37.Feillet F., Steinmann G., Vianey-Saban C. Adult presentation of MCAD deficiency revealed by coma and severe arrythmias. Intensive Care Med. 2003;29:1594–1597. doi: 10.1007/s00134-003-1871-3. [DOI] [PubMed] [Google Scholar]
- 38.Branca D., Roberti M.S., Vincenti E., Scutari G. Uncoupling effect of the general anesthetic 2,6-diisopropylphenol in isolated rat liver mitochondria. Arch Biochem Biophys. 1991;290:517–521. doi: 10.1016/0003-9861(91)90575-4. [DOI] [PubMed] [Google Scholar]
- 39.Rigoulet M., Devin A., Avéret N., Vandais B., Guérin B. Mechanisms of inhibition and uncoupling of respiration in isolated rat liver mitochondria by the general anesthetic 2,6-diisopropylphenol. Eur J Biochem. 1996;241:280–285. doi: 10.1111/j.1432-1033.1996.0280t.x. [DOI] [PubMed] [Google Scholar]
- 40.Baumeister F.A., Oberhoffer R., Liebhaber G.M. Fatal propofol infusion syndrome in association with ketogenic diet. Neuropediatrics. 2004;35:250–252. doi: 10.1055/s-2004-820992. [DOI] [PubMed] [Google Scholar]
- 41.Schenkman K.A., Yan S. Propofol impairment of mitochondrial respiration in isolated perfused guinea pig hearts determined by reflectance spectroscopy. Crit Care Med. 2000;28:172–177. doi: 10.1097/00003246-200001000-00028. [DOI] [PubMed] [Google Scholar]
- 42.Vanlander A.V., Jorens P.G., Smet J. Inborn oxidative phosphorylation defect as risk factor for propofol infusion syndrome. Acta Anaesthesiol Scand. 2012;56:520–525. doi: 10.1111/j.1399-6576.2011.02628.x. [DOI] [PubMed] [Google Scholar]
- 43.Cray S.H., Robinson B.H., Cox P.N. Lactic acidemia and bradyarrhythmia in a child sedated with propofol. Crit Care Med. 1998;26:2087–2092. doi: 10.1097/00003246-199812000-00046. [DOI] [PubMed] [Google Scholar]
- 44.Vollmer J.P., Haen S., Wolburg H. Propofol related infusion syndrome: ultrastructural evidence for a mitochondrial disorder. Crit Care Med. 2018;46:e91–e94. doi: 10.1097/CCM.0000000000002802. [DOI] [PubMed] [Google Scholar]
- 45.Vanlander A.V., Okun J.G., de Jaeger A. Possible pathogenic mechanism of propofol infusion syndrome involves coenzyme Q. Anesthesiology. 2015;122:343–352. doi: 10.1097/ALN.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 46.Mtaweh H., Bayır H., Kochanek P.M., Bell M.J. Effect of a single dose of propofol and lack of dextrose administration in a child with mitochondrial disease. J Child Neurol. 2014;29:1576–1577. doi: 10.1177/0883073813515301. [DOI] [PubMed] [Google Scholar]
- 47.Rutter D.V., Morgan M., Lumley J., Owen R. ICI 35868 (Diprivan): a new intravenous induction agent. A comparison with methohexitone. Anaesthesia. 1980;35:1188–1192. doi: 10.1111/j.1365-2044.1980.tb05076.x. [DOI] [PubMed] [Google Scholar]
- 48.Doze V.A., Westphal L.M., White P.F. Comparison of propofol with methohexital for outpatient anesthesia. Anesth Analg. 1986;65:1189–1195. [PubMed] [Google Scholar]
- 49.White P.F. Propofol Anesthesiology. 2008;109:1132–1136. doi: 10.1097/ALN.0b013e31818ddba8. [DOI] [PubMed] [Google Scholar]
- 50.Loh N.-H.W., Nair P. Propofol infusion syndrome. Contin Educ Anaesth Crit Care Pain. 2013;13:200–202. [Google Scholar]
- 51.Huerta-Alardín A.L., Varon J., Marik P.E. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otterspoor L.C., Kalkman C.J., Cremer O.L. Update on the propofol infusion syndrome in ICU management of patients with head injury. Curr Opin Anaesthesiol. 2008;21:544–551. doi: 10.1097/ACO.0b013e32830f44fb. [DOI] [PubMed] [Google Scholar]
- 53.Summary of product characteristics for Propofol 1%. Medicines & Healthcare Regulatory Agency; London: 2018. http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1538712747843.pdf [Google Scholar]
- 54.Parness J., Savard M., Turgeon A.F. Propofol-related infusion syndrome heralding a mitochondrial disease: case report. Neurology. 2014;82:461. doi: 10.1212/01.wnl.0000443933.18269.20. [DOI] [PubMed] [Google Scholar]
- 55.Takasu A., Iwamoto S., Ando S. Effects of various concentrations of inhaled oxygen on tissue dysoxia, oxidative stress, and survival in a rat hemorrhagic shock model. Resuscitation. 2009;80:826–831. doi: 10.1016/j.resuscitation.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Culp K.E., Augoustides J.G., Ochroch A.E., Milas B.L. Clinical management of cardiogenic shock associated with prolonged propofol infusion. Anesth Analg. 2004;99:221–226. doi: 10.1213/01.ANE.0000117285.12600.C1. [DOI] [PubMed] [Google Scholar]
- 57.Fudickar A., Bein B. Propofol infusion syndrome: update of clinical manifestation and pathophysiology. Minerva Anestesiol. 2009;75:339–344. [PubMed] [Google Scholar]
- 58.Casserly B., O’Mahony E., Timm E.G., Haqqie S., Eisele G., Urizar R. Propofol infusion syndrome: an unusual cause of renal failure. Am J Kidney Dis. 2004;44:e98–e101. doi: 10.1053/j.ajkd.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 59.Favetta P., Degoute C.S., Perdrix J.P., Dufresne C., Boulieu R., Guitton J. Propofol metabolites in man following propofol induction and maintenance. Br J Anaesth. 2002;88:653–658. doi: 10.1093/bja/88.5.653. [DOI] [PubMed] [Google Scholar]
- 60.Honore P.M., Spapen H.D. Propofol infusion syndrome: early blood purification to the rescue? Crit Care. 2016;20:197. doi: 10.1186/s13054-016-1364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oudemans-van Straaten H.M., Ostermann M. Bench-to-bedside review: citrate for continuous renal replacement therapy, from science to practice. Crit Care. 2012;16:249. doi: 10.1186/cc11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.