Abstract
Background:
A few young patients were brought in with subacute combined spinal cord degeneration at the Department of Neurology in our hospital. They all have used laughing gas for recreational purposes.
Case:
A 30-year-old woman, known with alcohol abuse, was presented to our Department of Neurology for having paresthesia and unstable movements of arms, legs, and trunk for 9 days. She has used 50 laughing gas patterns per day. The diagnosis of laughing gas–induced combined spinal cord degeneration was evident by the low count of vitamin B12 combined with lesions shown on magnetic resonance imaging (MRI). Abstaining from the laughing gas, weekly intramuscular injections of hydroxocobalamin and revalidation, she was fully recovered in 8 weeks.
Conclusions:
Recreational use of laughing gas seems to be more used in our society, however, without having any knowledge of the neurological consequences. The right diagnosis and treatment can provide full recovery in these patients. Furthermore, attention for this diagnosis can help increase social awareness.
Keywords: Cobalamin, combined spinal cord degeneration, laughing gas, nitrous oxide, inverted V-sign
Introduction
It is a well-known sight: empty laughing gas whippets laying on the streets, on playing grounds, and on parking places.1 The use of laughing gas, also known as nitrous oxide (N2O), is emerging and the gas is easily available; however, users are not aware of its adverse effects following large dose or long-term use of N2O.2 To intensify the public debate on this manner, and to demonstrate the variety of symptoms one may encounter,3 we present a patient with combined spinal cord degeneration as a result of frequent laughing gas use. In addition, we provide an overview of the cases recently discussed, to illustrate that the diagnosis and treatment of neurological effects of laughing gas use are not yet coherent. Furthermore, we demonstrate that laughing gas–induced combined spinal cord degeneration can be diagnosed using both magnetic resonance imaging (MRI) and laboratory findings.
Pathophysiology
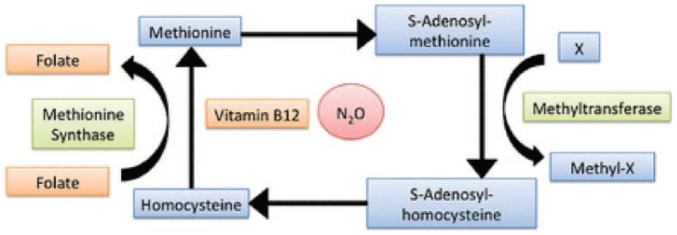
Laughing gas reacts with cobalamin (vitamin B12) and oxidizes the cobalt ion, making the entire vitamin B12-complex inactive.3 Vitamin B12 is a cofactor of methionine synthase, which converts homocysteine to methionine (Figure 1). Inadequate conversion to methionine generates insufficient S-adenosyl methionine, which is required for myelin production and maintenance. Eventually, this will lead to demyelination, especially in the dorsal column of the spinal cord. Because of this, combined spinal cord degeneration will arise: not only the corticospinal tract but also the posterior funiculus is affected, thus clarifying the sensitive and motoric deficits.4
Figure 1.
The role of vitamin B12 and laughing gas in the homocysteine/methionine pathway.4
Case
Patient A, a 30-year-old-woman, was presented to our Neurology Department with loss of power and sensory disorders in both arms and legs. The patient suffered from signs of asthma and regularly abused alcohol, however, she never experienced sensory disorders before. Her symptoms have begun 9 days ago and started with tingling sensations from the abdomen to the toes. Five days later, she had coordination problems in both hands, combined with tingling sensations continuing to her neck. She also showed Lhermitte sign while flexing her neck.
Physical examination showed a well-oriented, moderately cared woman in a wheelchair, from which she could stand up only with the help of others. The cranial nerves were intact, power in both her arms was normal, but power in both her legs was Medical Research Council (MRC) scale 4 out of 5.
Gnostic sensitivity was reduced in both arms and legs, with the right side being more affected than the left side. Vibration sensitivity was absent in both her legs. Her reflexes on the arms and legs were symmetrically elevated, and plantar reflexes were according to Babinski on both sides. Her movements were atactic and unstable.
The patient was recently institutionalized for alcohol abuse and was smoking a pack of cigarettes a day. She admitted using 50 laughing gas whippets a day for the last 2 months. Unfortunately, the patient has not given her motive considering the use of this amount of laughing gas whippets. She was hospitalized and received further treatment at the Neurology Department.
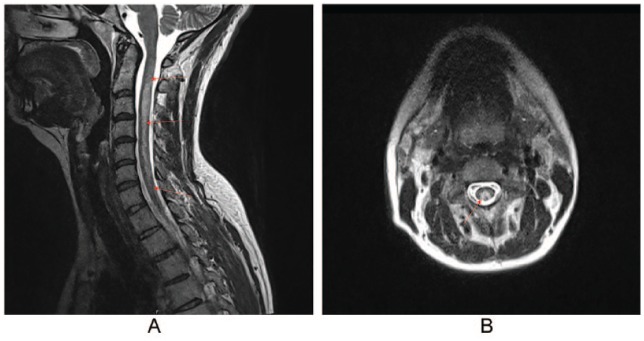
Given her clinical state, our patient had both deficits in her corticospinal tract and the posterior funiculus on both sides, mostly localized in the cervical part of the spinal cord. A cervicothoracic MRI was performed, which showed a hyperintensity in the cervical spinal cord on T2-weighted pictures (Figure 2A and B). These anomalies, combined with the frequent use of laughing gas, indicated a vitamin B12 deficiency–induced myelopathy. Laboratory findings showed a low serum vitamin B12, alongside both raised homocysteine and methylmalonic acid values, demonstrating the symptomatic vitamin B12 deficiency (Table 1).
Figure 2.
(A) T2-weighted sagittal image of the cervical spinal cord. From C2 to C7, a hyperintensity is shown. (B) T2-weighted axial image of cervical spinal cord. An “inverted V-sign” is shown.
Table 1.
Laboratory findings.
| Reference | ||
|---|---|---|
| Hemoglobin | 8.3 | 7.0-9.3 mmol/L |
| Hematocrit | 0.40 | 0.32-0.44 L/L |
| MCV | 96 | 82-98 fL |
| Vitamin B12 | 116 | 140-490 pmol/L |
| Folic acid | 14.8 | 8.8-60.8 nmol/L |
| Homocysteine | 48.3 | <11.4 μmol/L |
| Methyl malonic acid | 1.43 | 0.0-0.35 μmol/L |
Abbreviation: MCV, mean corpuscular volume.
The diagnosis of laughing gas–induced combined spinal cord degeneration was evident by these findings; intramuscular injections with 1000 µg vitamin B12 twice per week were started. The laughing gas use was discussed with the patient, alongside the necessity to stop at once.
During hospitalization, her symptoms were gradually improving, but her walking was still quite atactic. After 4 weeks, she was referred to medical rehabilitation; after 1 month, she was fully recovered.
Discussion
A systematic search for comparable cases was conducted in PubMed, to identify the variety of symptoms and treatments. Keywords in the search were vitamin B12, combined spinal cord degeneration, laughing gas, and their synonyms. In total, 75 articles were identified, from which 48 were excluded after reading titles. Then, 8 articles were excluded based on their abstract. Furthermore, only the case reports were included: a total of 12 cases (Table 2).
Table 2.
Laughing gas–induced myelopathy, case studies.
| Reference | Gender, age | Neurologic examination | MRI | Treatment: intramuscular hydroxocobalamin injections |
|---|---|---|---|---|
| Massey et al4 | Male, 36 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity C1-T11 | 6× 1000 μg/day for 2 weeks, then 1000 μg/week + physiotherapy |
| Hsu et al5 | Male, 19 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity cervical in dorsal and lateral column | 1000 μg/day for 5 days, then 2 months 1000 μg/week |
| Yuan et al6 | Female, 20 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity C1-Th12, inverted V-sign | 1000 μg/day for 3 months |
| Chen et al7 | Female, 20 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity C1-C6 dorsal column | 2000 μg/day first 3 days, then orally |
| Mancke et al8 | Male, 35 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity posterior column, inverted V-sign | 1000 μg/day + methionine for 7 days, then orally 400 μg/day for 6 months |
| Morris et al9 | Male, 22 | Posterior funiculus disorder; corticospinal tract disorder | No lesions | 1000 μg for 7 days, afterwards 1000 μg/week orally + multivitamins daily |
| Thompson et al10 (3×) | Male, 22 | Posterior funiculus disorder; corticospinal tract disorder | No lesions | IVIG 0.4 g/kg/day for 5 days, then 1000 μg/day |
| Male, 27 | Posterior funiculus disorder; corticospinal tract disorder | Not conducted | IVIG 0.4 g/kg/day for 5 days, then 1000 μg/day | |
| Female, 23 | Posterior funiculus disorder | Not conducted | 1000 μg | |
| Rheinboldt et al11 | Male, 35 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity C2-C3 and C6-C7 | One injection of 1000 μg; supportive care |
| Cheng et al12 | Female, 22 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity dorsal column C2-C4 | 1000 μg/day + physiotherapy |
| Wijesekera et al13 | Female, 19 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity central and dorsal column C2-Th11 | 6× 1000 μg injections, then symptomatic therapy |
| Singer et al14 | Female, 27 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity dorsal cervical column | Monthly 1000μ μg injections for 10 months |
| Butzkueven et al15 | Male, 23 | Posterior funiculus disorder; corticospinal tract disorder | T2 hyperintensity dorsal cervical column | 1000 μg/day and methionine orally 1 g/day for 3 months |
Abbreviations: IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging.
Diagnostics
The underlying principle of diagnosing laughing gas–induced myelopathy is knowing the nitrous oxide drug use and history of the patient. A subacute start of sensory and motoric symptoms combined with laughing gas use calls for an extensive neurological examination.10
Most case studies report a loss of proprioception in the lower extremities as the most important finding indicating dorsal column pathology.5–7,9,10,12–15 Ataxia is also found frequently; falling and being unable to walk are extreme symptoms of loss of proprioception.9 Lost coordination and a positive Romberg are found frequently.5–7,9,12,15 Further sensory examination can point out diminished vibration and touch senses, mostly bilateral.
Lateral dorsal pathology is characterized by hyperreflexia in upper or isolated in the lower extremities; one case has described finding a clonus.8 Muscle weakness and fine motoric abilities may decline; even a positive bilateral Babinski can be found.6,11
Active or passive bending of the neck can indicate a positive Lhermitte sign.5,9,10,12
Through laboratory research, a functional vitamin B12 deficiency can be demonstrated. Complete blood count will mostly show a macrocytic anemia; however, abundant folic acid consumed through diet will not result in the absence of megaloblasts in the blood count.16 Low count of vitamin B12 can be the next step, but symptoms are not always accompanied by decreased values,6,10,11,14 for instance after vitamin B12 supplementation.7
To be certain that vitamin B12 is pathologically deficient, both homocysteine and methylmalonic acid must be measured. Vitamin B12 is a cofactor of both,17 so a functional shortage can result in raised homocysteine and methylmalonic acid values.4,8–10,12,14
Magnetic resonance imaging can demonstrate hyperintensity in the dorsal and lateral columns of the spinal cord. However, MRI is not always conducted, or no lesions can be seen.9,10 It can give an important insight on the diagnosis: symptoms depended on the location of the lesions. Hyperintensity can cover the entire cervical spinal cord.6,13 The axial images can show an “inverted V-sign” at the height of the lesion (Figure 2B)6,8,18: this demonstrates the involvement of both dorsal and lateral columns of the cervical spinal cord.
Treatment
Abstinence of laughing gas will be the first step in treating this combined spinal cord degeneration. Alongside, vitamin B12 must be supplemented, as the nitrous oxide has exhausted the cobalamin stock, thus leading to the symptoms.
In the case study, no exact answer is given considering the treatment of laughing gas–induced combined spinal cord degeneration. Most patients are treated with intramuscular hydroxocobalamin injections of 1000 µg; however, both duration and interval of these injections vary greatly. Treatment of only one injection13 or daily injections for a period of 2 months15 are described. Also, considerable variation in frequency is described: from daily to weekly injections4,5,9 or monthly injections of hydroxocobalamin are provided.14
Besides these variations, it is not clearly described whether supportive therapy must be provided, such as physiotherapy,4,13–15 or whether methionine must be supplemented next to cobalamin.8,15
The National Health Care Institute advises weekly intramuscular injections for a longer period of time (2 years) when severe neurological symptoms are present.19
Conclusions
As presented in our case, it is essential to know about the use of laughing gas when a patient is presented with subacute spinal cord degeneration. This can be the first step in diagnosing laughing gas–induced myelopathy, which is straightforward to treat by supplementing vitamin B12. MRI is important and essential for the diagnosis.
Although no consensus is reached considering the exact treatment, more research is required regarding laughing gas–induced subacute combined spinal cord degeneration.
Attitude of society relating to the use of laughing gas is liberalizing, which can lead to more and more severe cases being presented to the ER. N2O may be a relatively safe drug in recreational use, but certainly not in high daily dose, when used very frequently or at very young age. Therefore, the knowledge of pathophysiology, diagnosis, and treatment is essential, and social awareness is of great interest.
Acknowledgments
The corresponding author takes full responsibility for the article. Neither the case nor images have been published before. Furthermore, all authors have agreed on the conditions noted on the Authorship Agreement Form. Considering the case discussed and MRI images used, written consent was obtained from the represented patient.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MRO carried out the online case study, collected the data and drafted the manuscript. STF made substantial contribution to the concept of the case, by critical reading of the article with critical revision and approved the version to be published.
Informed Consent: Written consent was obtained from the patient.
ORCID iD: Marthe R Onrust  https://orcid.org/0000-0001-7813-2917
https://orcid.org/0000-0001-7813-2917
References
- 1. Kuiper M., Youth is. “doing” laughing gas more often. NRC Handelsblad. December 17, 2017. Available at: https://www.nrc.nl/nieuws/2017/12/17/jeugd-doet-steeds-vaker-een-ballonnetje-a1585307. [Google Scholar]
- 2. van Amsterdam JJ, Nabben T, van den Brink W. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. 2015;73:790–796. [DOI] [PubMed] [Google Scholar]
- 3. Conjaerts S, Bruijnes A, Beerhorsten K, Beekman R. Laughing gas induced polyneuropathy. Ned Tijdschr Geneeskd. 2017;161:D2044. [PubMed] [Google Scholar]
- 4. Massey TH, Pickersgill TT, Peall KJ. Nitrous oxide misuse and vitamin B12 deficiency. BMJ Case Rep. 2016;2016:bcr2016215728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu CKCK, Chen YQ, Lung VZ, His SC, Lo HC, Shyu HY. Myelopathy and polyneuropathy caused by nitrous oxide toxicity: a case report. Am J Emerg Med. 2012;30:1016.e3-e6. [DOI] [PubMed] [Google Scholar]
- 6. Yuan JLJL, Wang SK, Jiang T, Hu WL. Nitrous oxide induced subacute combined degeneration with longitudinally extensive myelopathy with inverted V-sign on spinal MRI: a case report and literature review. BMC Neurol. 2017;17:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen HJ, Huang CS. Nitrous oxide-induced subacute combined presenting with dystonia and pseudoathetosis: a case report degeneration. Acta Neurol Taiwan. 2016;25:50–55. [PubMed] [Google Scholar]
- 8. Mancke F, Kaklauskaite G, Kollmer J, Weiler M. Psychiatric comorbidities in a young man with subacute myelopathy induced by abusive nitrous oxide consumption: a case report. Subst Abuse Rehabil. 2016;7:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris N, Lynch K, Greenberg SA. Severe motor neuropathy or neuronopathy due to nitrous oxide toxicity after correction of vitamin B12 deficiency. Muscle Nerve. 2015;4:614–616. [DOI] [PubMed] [Google Scholar]
- 10. Thompson AAG, Leite MI, Lunn MP, Bennett DL. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15:207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rheinboldt M, Harper D, Parrish D, Francis K, Blasé J. Nitrous oxide induced myeloneuropathy: a case report. Emerg Radiol. 2013;21:85–88. [DOI] [PubMed] [Google Scholar]
- 12. Cheng HM, Park JH, Hernstadt D. Subacute combined degeneration of the spinal cord following recreational nitrous oxide use. BMJ Case Rep. 2013;2013:bcr2012008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wijesekera NT, Davagnanam I, Miszkiel K. Subacute combined cord degeneration: a rare complication of nitrous oxide misuse. Neuroradiol J. 2009;22:194–197. [DOI] [PubMed] [Google Scholar]
- 14. Singer M, Lazardis C, Nations S, Wolfe G. Reversible nitrous oxide-induced myeloneuropathy with pernicious anemia: case report and literature review. Muscle Nerve. 2008;37:125–129. [DOI] [PubMed] [Google Scholar]
- 15. Butzkueven H, King JO. Nitrous oxide myelopathy in an abuser of whipped cream bulbs. J Clin Neurosci. 2000;7:73–75. [DOI] [PubMed] [Google Scholar]
- 16. Senthilkumaran S, Balamurugan N, Menezes RG, Thirumalaikolundusubramanian P. Nitrous oxide toxicity: technical and therapeutic aspects. Am J Emerg Med. 2013;31:406. [DOI] [PubMed] [Google Scholar]
- 17. Buizert A, Sharma RR, Koppen H. When the laughing stops: subacute combined spinal cord degeneration caused by laughing gas use. J Addict Med. 2017;11:235–236. [DOI] [PubMed] [Google Scholar]
- 18. Briani C, Dalla Torre CC, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5:4521–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Health Care Institute. Hydroxocobalamine (Vitamin B12). Pharmacotherapeutic Compass. Dutch Pharmacological Institute 2018. Available at: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/h/hydroxocobalamine__vitamine_b12_ [Google Scholar]