Abstract
Introduction:
Fatigue is a frequent, debilitating symptom of inflammatory bowel disease (IBD). Despite this, studies report dissatisfaction among IBD patients regarding how little attention is given to fatigue-related issues during consultations. We performed a pilot randomized controlled trial (RCT) to assess whether a brief, structured, multidisciplinary psychological support program improved fatigue, mood and quality of life indices in patients with quiescent IBD.
Methods:
The intervention consisted of three small-group psychoeducational sessions over 6 months. Primary outcomes were effect on fatigue severity and impact scores. Secondary outcomes included effect on depression, anxiety, somatization scores, generic and disease-specific quality of life.
Results:
Twenty-three patients were enrolled, 10 in the intervention arm and 13 controls. Mean fatigue severity and impact scores improved for patients in the intervention group (by 14.5–13.1 and 49.7–45.8, respectively), and worsened in controls (by 11.5–12.6 and 33.5–35 respectively). Mean Short Form 36 (SF-36) scores for role limitations due to physical health decreased from 44.4 to 38.9 in the intervention group, but increased from 44.2 to 51.9 among controls. Energy scores in the intervention group improved from 17.8 to 26.6, but only from 31.4 to 31.7 among controls. Short IBD questionnaire scores improved in both groups, from 46.2 to 45.2 in controls compared with 44.4–40 in the intervention group.
Discussion:
In this small pilot RCT, positive effects were demonstrated on fatigue, energy levels and other quality of life outcomes. Larger, adequately powered studies with longer follow up are required.
ClincialTrials.gov identifier: NCT02709434.
Keywords: Crohn’s disease, fatigue, inflammatory bowel disease, quality of life, ulcerative colitis
Key summary
What is already known about this subject?
• Fatigue is common in inflammatory bowel disease (IBD), but patients report considerable dissatisfaction as to how little attention is given to fatigue by their treating physicians.
• Randomized controlled trial (RCT) data on nonpharmacologic interventions for fatigue in IBD are sparse, and especially so among patients in clinical and biochemical remission.
• Psychological interventions resulting in improvements in quality of life have been shown to result in significant reductions in subsequent healthcare usage.
What are the new findings?
• We assessed the efficacy of a brief, structured, multidisciplinary program of psychological support in fatigue management.
• This study adds to a small body of literature of RCTs in IBD that use fatigue and energy scores as primary endpoints.
• There were positive effects on fatigue, energy levels and a range of other quality of life outcomes, in spite of disease parameters that did not improve.
• This is the first RCT to report improved disease-specific quality of life scores following treatment of fatigue in a cohort of patients in clinical and biochemical remission.
How might it impact on clinical practice in the near future?
• Larger, adequately powered studies with a longer duration of follow-up, and which recruit IBD patients with fatigue, regardless of disease activity, are required.
Key summary
Fatigue is common in IBD, but patients report considerable dissatisfaction as to how little attention is given to fatigue by their treating physicians. RCT data on nonpharmacologic interventions for fatigue in IBD are sparse, and especially so among patients in clinical and biochemical remission.
We assessed the efficacy of a brief, structured, multidisciplinary program of psychological support in fatigue management.
This is the first RCT to report improved fatigue and energy levels, and disease-specific quality of life scores following treatment of fatigue in a cohort of patients in clinical and biochemical remission.
Larger, adequately powered studies with a longer duration of follow up, and which recruit IBD patients with fatigue, regardless of disease activity, are required.
Introduction
Fatigue is a frequent and debilitating symptom in inflammatory bowel disease (IBD). More than 40% of patients with IBD, even when in remission, suffer from fatigue and this rises to 86% when the disease is active.1 In one study, mean Multidimensional Fatigue Inventory scores of these patients were comparable with the mean scores reported in cancer patients who had completed chemotherapy.2 In a survey of perceived health status, fatigue ranked third on a 25-item rating form of concerns among IBD patients, after uncertainties about the disease, and the effects of medication.3
Despite this, studies have reported considerable dissatisfaction among patients with IBD as to how little attention is given to fatigue-related issues in their consultations, as well as in the range of interventions available to enable them to manage these symptoms effectively.4–6 In spite of the efforts of both patients and their advocacy organizations to highlight fatigue as a major concern, published evidence for treatment strategies is sparse. A systematic review on the topic in 2013 criticised the inconsistent definitions used across the literature, and noted that none of the reviewed studies asked patients to describe the experience of fatigue in their own words.7 A further systematic review of 43 studies found that IBD fatigue was consistently associated with disease activity, depression, anxiety and sleep difficulties but was unable to conclude on causation and found an inconsistent relationship between biochemical factors, such as anaemia and inflammation, and fatigue.8 The same study identified solution-focused therapy, thiamine and exercise as potential targets to improve fatigue in IBD. Regarding these therapeutic interventions, three small randomized controlled trials (RCTs) reported a favourable effect of infliximab and adalimumab on fatigue in IBD.9–11
In other chronic disease areas such as cancer, rheumatoid arthritis and multiple sclerosis psychotherapeutic interventions have been shown clearly to be beneficial.12–14 However, data from the IBD literature are sparse, with only one study reporting a benefit from a stress management program,15 and two RCTs from the same group suggesting that a strategy known as solution-focused therapy may also be superior to treatment as usual.16,17 We therefore performed a pilot RCT, which aimed to assess whether or not fatigue, energy and quality of life indices could be improved in a group of patients with quiescent IBD, who reported fatigue, via the delivery of a brief and simple structured multidisciplinary program of psychological support.
Materials and methods
Patients and setting
We performed a pilot RCT in patients with IBD recruited from outpatient clinics at Leeds Teaching Hospitals NHS Trust, UK, between December 2016 and April 2017. All patients received information about the study and gave written informed consent. The study was approved by the local research ethics committee (Reference Number: 16/YH/0235). The study was registered on ClincialTrials.gov (identifier: NCT02709434). All recruited individuals provided blood for complete blood count, urea and electrolytes, liver function tests, thyroid function tests, ferritin, vitamin B12, folate, vitamin D, calcium, magnesium, phosphate and C-reactive protein (CRP), as well as stool for faecal calprotectin (FC) (Immundiagnostik, Bensheim, Germany).
Data collection and synthesis
Demographic and disease-specific data
Once informed consent was obtained, demographic data included gender, age, ethnicity, marital status, educational level, tobacco and alcohol use, weight (in kilograms) and height (in metres), which were used to calculate body mass index (BMI), were collected. Medication history, including current use of 5-aminosalicylates (5-ASAs), glucocorticosteroids, immunosuppressants or biologic therapies, and disease location and behaviour for Crohn’s disease (CD) or distribution for ulcerative colitis (UC), as defined by the Montreal classification,18 were also recorded.
IBD activity data
Assessment of IBD activity was performed by physician using the Harvey–Bradshaw index (HBI) for CD,19 and the Simple Clinical Colitis Activity Index (SCCAI) for UC,20 with a score <5 used to define clinical remission for both, as recommended previously.21,22 In addition, we used an FC cut off of <250 µg/g of stool to define no evidence of mucosal inflammation, in line with the European Crohn’s and Colitis Organisation consensus on the use of FC to measure disease activity.23
Fatigue data
Fatigue was assessed using the IBD fatigue self-assessment scale,24 consisting of two main sections. Section one assesses frequency and severity of fatigue, and section two the impact of fatigue on daily activities. Higher scores represent higher fatigue severity, and a greater impact on daily life.
Mood, somatization and quality of life data
Anxiety and depression data were collected using the Hospital Anxiety and Depression Scale (HADS),25 with severity categorized, according to total HADS score, into normal (total HADS depression or anxiety score 0–7), borderline normal8–10 and abnormal (⩾11), as previously validated.25 Somatization data were collected using the Patient Health Questionnaire-15 (PHQ-15), which is derived from the validated full PHQ,26,27 which enquires about the presence of 15 somatic symptoms (or symptom clusters) over the last 4 weeks, with severity categorized as recommended previously.28
Finally, generic quality of life data were collected via the medical outcomes study 36-item Short Form (SF-36) health survey, a validated questionnaire used to assess physical and mental health status,29 and disease-specific quality of life using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ).30
Inclusion and exclusion criteria
To be included patients had to have quiescent IBD, both according to clinical (HBI or SCCAI <5) and biochemical (CRP <5 mg/l and FC <250 μg/g) indices no more than 1 month prior to randomization, and report fatigue by virtue of scoring 1 or more on Section I of the Crohn’s and Colitis UK IBD fatigue self-assessment scale. Patients were excluded if they had any correctable electrolyte (sodium, potassium, magnesium, calcium), vitamin (B12 or D) or iron deficiency anaemia (defined as Hb <11.5 g/dl for a woman or <13 g/dl for a man with ferritin <20 μg/l), or active disease according to either clinical or biochemical criteria. Pregnancy, inability to conduct intervention in English language and severe psychological comorbidity were other contraindications.
Randomization
After baseline assessment, eligible patients were randomized by selection of sequentially numbered opaque sealed envelopes by the research fellow to either a standard of care group, which consisted of treatment as usual, or to participate in the multidisciplinary psychoeducational intervention group, using lists drawn from a computer-generated series of random numbers. Allocation of treatment was concealed by the use of opaque, sealed envelopes. Blinding was not possible, as patients were clearly aware of the treatment arm they were assigned to such was the nature of the intervention.
Intervention
The standard of care group received standard medical care, as per their responsible gastroenterologist, with no additional psychological interventions. This consisted of planned review every 6 or 12 months depending on disease characteristics with demand-led appointments on top of that for active symptoms. The active intervention consisted of a series of three small-group psychoeducational sessions, delivered every 8 weeks over a period of 6 months. Each session lasted 1 hour. There were five patients in each group. The programme ran twice in parallel and the groups stayed together and did not overlap. The sessions were structured around psychological and physical interventions, which were geared towards understanding fatigue, energy conservation, management strategies and improving relaxation techniques tailored to the specific needs of patients with IBD. Sessions were facilitated by a senior occupational therapist with experience in fatigue management and small group work in chronic disease management, as well as nurse specialists in IBD and psychological medicine. The interventions used methods which have long been used in other patient groups with chronic diseases such as fibromyalgia, multiple sclerosis and inflammatory arthritis and drawing on the experience of gastroenterologists and specialist nurses in IBD the therapeutic intervention was modified to focus on the specific aspects of fatigue management in patients with IBD. The first session focused on analysing activity diaries the patients had brought with them to identify their patterns of activity, exercise, work, sleep and recreation over the week prior to the intervention. Core skills for managing fatigue such as grading, pacing, purposeful rest, time management strategies, enjoyable activities, sleep hygiene and a relaxation practice (diaphragmatic breathing) were taught and emphasized. The session finished with the setting of goals for them to work on before the next session, with pro-forma sheets provided to document these. Participants were also asked to complete the same activity diary for another 1 week in the week prior to their next session. Patients were provided with written material germane to the learnings at the end of every session.
The second session started by reflection on both the activity diaries, and how people had been able to progress on the strategies and goals identified in the first workshop, as well as including problem solving for any barriers that arose. This session also focused on managing external expectations and relationships, and discussed assertiveness, linked with themes from the previous session. The session finished with a different relaxation practice (visualisation) and further goal setting. Participants were again asked to complete an activity diary for 1 week.
The third and final session started by reflecting on the goals set and activity diaries, and then focused on the recognition and management of relapse of fatigue. A relapse plan booklet was devised by each patient, focusing on the core skills that had been particularly helpful to them, and what they could do to maintain their progress. The session finished with a third relaxation practice (progressive muscular relaxation), and further goal setting for the future.
Outcomes
The primary outcomes were the effect of the active and control interventions on fatigue severity and impact scores. Secondary outcomes included the effect on IBD activity indices, depression, anxiety and somatization scores, and generic and disease-specific quality of life. Questionnaires were administered at study entry and repeated at 6 months.
Statistical analysis
All questionnaire data at baseline and 6-month follow-up, including fatigue severity and impact, HADS scores, PHQ-15 scores and generic and disease-specific quality of life indices were analysed using means. Owing to the small number of patients in each treatment arm, we did not compare these using statistical testing, in order not to make spurious claims about efficacy of the intervention.
Results
Patient and disease characteristics
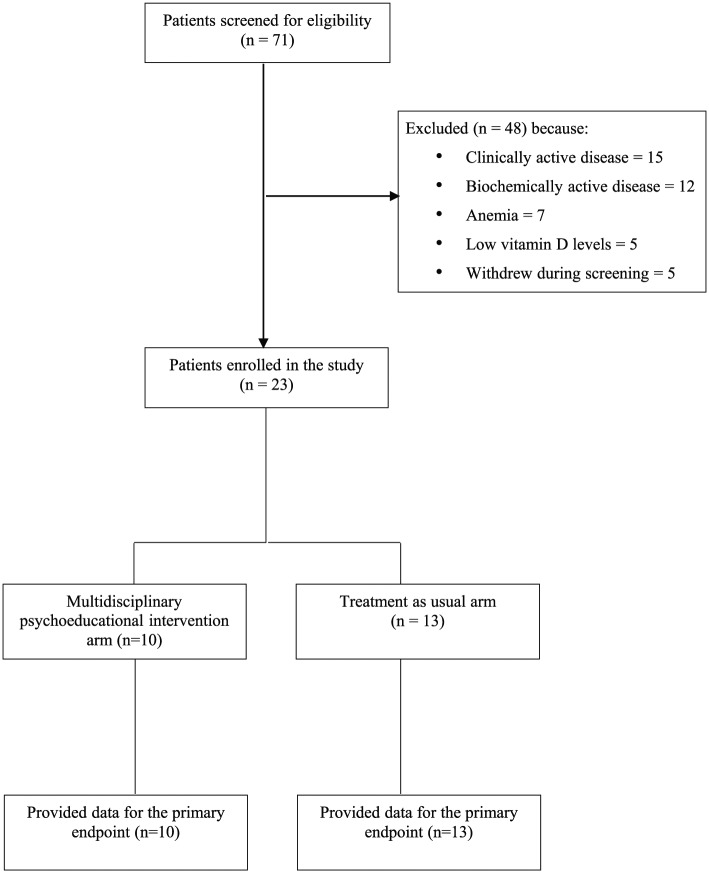
In total, 71 patients (31 male, 40 female) who reported fatigue were screened for entry into the trial. In total, 48 patients were screened but were not eligible for randomization (Figure 1). Reasons included abnormal clinical disease activity indices in 15, abnormal biochemical activity indices in 12, anaemia in seven, low vitamin D levels in five and failure to provide a stool sample in four. A further five patients withdrew during screening, one due to a family emergency, and the other four without giving a reason. Therefore, 23 patients were enrolled, with 10 in the active intervention arm and 13 in the control arm. The age range among those randomized was 19–61 years, and 6 men and 17 women were randomized. No significant differences were found between the treatment arms with respect to mean age, marital status, smoking status, educational attainment or disease activity, and distribution. Baseline characteristics of both groups are provided in Table 1. Attendance rates at the sessions were 86.7% overall. There were no patients lost to follow up.
Figure 1.
Flow of participants through the study.
Table 1.
Baseline characteristics of subjects in the active intervention and control arms.
| Control (n = 13) | Active Intervention (n = 10) | All patients (n = 23) | |||
|---|---|---|---|---|---|
| Mean age | 39.9 | 39.7 | 39.8 | ||
| Mean BMI | 23.8 | 24.9 | 24.3 | ||
| Gender (%) | Male | 30.8 | 20.0 | 26.1 | |
| Female | 69.2 | 80.0 | 73.9 | ||
| Marital status(%) | Married/cohabiting | 76.9 | 60 | 69.6 | |
| Divorced/separated | 7.7 | 20.0 | 13.0 | ||
| Never married | 15.4 | 20.0 | 17.4 | ||
| Smoker (%) | Yes | 46.2 | 10.0 | 30.4 | |
| No | 53.8 | 90.0 | 69.6 | ||
| Alcohol use (%) | Yes | 92.3 | 60.0 | 78.3 | |
| No | 7.7 | 40.0 | 21.7 | ||
| Educational level (%) | Secondary school | 23.1 | 40.0 | 30.4 | |
| Some technical school/college | 7.7 | 20.0 | 13.0 | ||
| Technical school/college graduate | 15.4 | 10.0 | 13.0 | ||
| Some university | 0 | 20.0 | 8.7 | ||
| University graduate | 30.8 | 10.0 | 21.7 | ||
| Postgraduate | 23.1 | 0 | 13.0 | ||
| Previous surgery (%) | Yes | 53.8 | 60.0 | 56.5 | |
| No | 46.2 | 40.0 | 43.5 | ||
| Type of IBD (%) | CD | 84.6 | 90.0 | 87.0 | |
| UC | 15.4 | 10.0 | 13.0 | ||
| Disease location or extent (%) | CD | Ileal | 15.4 | 40.0 | |
| Colonic | 15.4 | 10.0 | |||
| Ileocolonic | 46.2 | 40.0 | |||
| UC | Proctitis | 7.7 | 0 | ||
| Left-sided | 7.7 | 0 | |||
| Extensive colitis | 0 | 10.0 | |||
| Treatments (%) | 5-ASAs | 0 | 10.0 | 4.3 | |
| Immunosuppressants | 69.2 | 50.0 | 60.9 | ||
| Biologics | 84.6 | 70.0 | 78.3 | ||
| Glucocorticosteroids | 0 | 10.0 | 4.3 | ||
BMI, body mass index; CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Influence of the multidisciplinary psychoeducational intervention on fatigue severity and impact scores
Baseline fatigue scores for both fatigue severity and impact of fatigue on daily activities were worse at baseline in the active intervention group compared with the control arm. Following the intervention, both fatigue severity and impact scores improved for patients in the multidisciplinary psychoeducational intervention group (by 9.7% and 7.3%, respectively), whereas these worsened in controls (by 9.4% and 4.4% respectively). These data are provided in Table 2.
Table 2.
Fatigue, clinical disease activity, and quality of life scores at baseline and 6-month follow-up among subjects in the active intervention and control arms.
| Control (n = 13) | Active Intervention (n = 10) | Cohen’s D/ rYl | ||
|---|---|---|---|---|
| Mean fatigue severity score | Baseline | 11.5 | 14.5 | 0/0 |
| Follow up | 12.6 | 13.1 | ||
| Mean fatigue impact score | Baseline | 33.5 | 49.7 | 0.091/ 0.045 |
| Follow up | 35.0 | 45.8 | ||
| Mean HBI | Baseline | 2.8 | 2.4 | −0.119/ 0.059 |
| Follow up | 3.8 | 5.4 | ||
| Anxiety score | Baseline | 4.2 | 5.7 | 0.629/ 0.300 |
| Follow up | 5.3 | 6.9 | ||
| Depression score | Baseline | 7.7 | 6.6 | −0.847/ 0.390 |
| Follow up | 8.5 | 8.7 | ||
| Somatization score | Baseline | 10.5 | 11.8 | −0.716/ 0.337 |
| Follow up | 11.2 | 11.7 | ||
| SF-36 physical functioning score | Baseline | 73.6 | 77.4 | 0.490/ 0.238 |
| Follow up | 69.6 | 72.4 | ||
| SF-36 role limitations due to physical health score | Baseline | 44.2 | 44.4 | −0.135/ 0.067 |
| Follow up | 51.9 | 38.9 | ||
| SF-36 role limitations due to emotional problems score | Baseline | 59.0 | 37.0 | −0.224/ 0.111 |
| Follow up | 59.0 | 48.2 | ||
| SF-36 energy score | Baseline | 31.4 | 17.8 | −0.442/ 0.216 |
| Follow up | 31.7 | 26.6 | ||
| SF-36 emotional wellbeing score | Baseline | 70.5 | 58.8 | 0.040/ 0.020 |
| Follow up | 67.4 | 61.5 | ||
| SF-36 social functioning score | Baseline | 64.4 | 61.5 | −0.044/ 0.022 |
| Follow up | 64.7 | 62.6 | ||
| SF-36 pain score | Baseline | 65.6 | 57.0 | −0.274/ 0.136 |
| Follow up | 71.7 | 55.3 | ||
| SF-36 general health score | Baseline | 44.2 | 36.1 | 0.024/ 0.012 |
| Follow up | 41.5 | 39.4 | ||
| SIBDQ score | Baseline | 46.2 | 44.4 | 0.569/ 0.274 |
| Follow up | 45.2 | 40.0 |
HBI, Harvey–Bradshaw index; SF-36, 36-item Short Form health survey; SIBDQ, Short Inflammatory Bowel Disease Questionnaire.
Influence of the multidisciplinary psychoeducational intervention on clinical disease activity and psychological parameters
There were not enough patients with UC randomized to calculate any data on SCCAI, but for patients with CD HBI did not improve, and actually worsened among patients in the active intervention group (mean score 2.4 at baseline, compared with 5.4 at follow up). Among controls, mean HBI at baseline was 2.8, compared with 3.8 at follow up. Anxiety and depression scores worsened slightly from baseline to 6 months in both the control arm and the multidisciplinary psychoeducational intervention arm (4.2–5.3 and 5.7–6.9, respectively, for anxiety and 7.7–8.5 and 6.6–8.7, respectively, for depression). Somatisation scores were stable among both controls and the multidisciplinary psychoeducational intervention group between baseline and 6 months (10.5–11.2 and 11.8–11.7, respectively).
Influence of the multidisciplinary psychoeducational intervention on generic and disease-specific quality of life
The SF-36 questionnaire provided subcategory data on generic quality of life according to physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy, emotional wellbeing, social functioning, pain and perception of general health (Table 2). Physical functioning worsened in both arms, by 5.4% in controls and by 6.4% in the intervention arm. Role limitations due to physical health decreased by 12.5% in the intervention group and increased by 17.4% among controls. Role limitations due to emotional problems remained the same in the control arm, and increased by 30.3% in the intervention group. Energy scores in the intervention group were improved by 49.4%, but by only 0.9% among controls. Emotional wellbeing was improved by 4.6% in the intervention group, but worsened by 4.4% among controls. Social functioning was broadly unchanged in both groups, increasing by 0.4% in controls and 1.7% in the intervention group. Pain scores worsened by 9.3% in controls, but improved by 3% in the intervention arm. Finally, perception of general health worsened by 6.1% among controls, but improved by 9.2% in the intervention arm. In terms of disease-specific quality of life, SIBDQ scores improved in both groups, but by only 2.2% in controls compared with 9.9% in the intervention group.
Discussion
We present data from a pilot RCT, in which a multidisciplinary psychoeducational intervention focusing on fatigue management in patients with quiescent IBD, the majority of whom had CD, was compared with a standard of care control group. Although this was a small pilot study, we were able to demonstrate positive effects on fatigue, energy levels and a range of other quality of life outcomes. This was in spite of disease parameters that, if anything, appeared to worsen, with an increase in HBI scores among those with CD. This is the first RCT to report improved SIBDQ scores in a cohort of patients with IBD in clinical and biochemical remission, following a fatigue intervention.
The multidisciplinary psychoeducational intervention did not improve anxiety, depression or somatization scores, nor was it designed to. However, by improving energy and fatigue levels, such interventions may be of benefit to the unmet needs of the many patients with quiescent IBD who report debilitating levels of fatigue, with a consequent negative impact on quality of life. The observed natural history of previous longitudinal studies of patients with IBD does not suggest an improvement in fatigue scores over time, regardless of their disease course.31,32 Therefore, the improved outcome for fatigue levels in patients undergoing the intervention, albeit in a relatively small group, is encouraging.
Limitations of this study include its small sample size, and the fact that it was unblinded, and therefore patients were aware of the treatment arm they were assigned to. This may have led to a high placebo response rate among those randomized to the active treatment, and disappointment among those who received the control intervention, which may have led to a worsening of their fatigue symptoms. In addition, we were unable to repeat CRP and FC measurements at the end of the study. We had initially intended to randomise 40 patients but delays in the ethics process meant we were only able to randomize 23 suitable patients in the lifetime of the grant. A longer period of follow up after the end of the study would also have been desirable. Some previous studies of psychological therapies in patients with IBD have shown a lack of durability of their beneficial effects, with regression to the mean over time, once the intervention has been completed.15,33–35 Nonetheless, we believe that this study adds to a small body of literature of RCTs in IBD that use fatigue and energy scores as primary endpoints, and utilize validated questionnaires to define quality of life parameters.
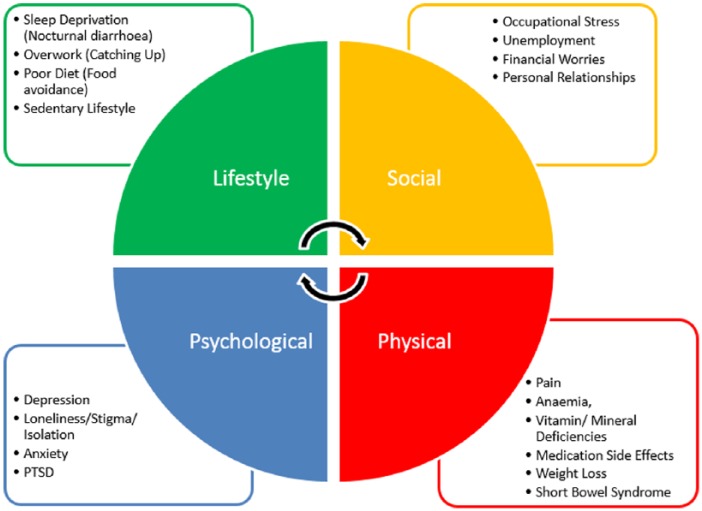
Fatigue in IBD is a complex, multidimensional problem for patients requiring an accessible, multidisciplinary response from providers. Whether fatigue represents true inflammatory activity, anaemia, mood disorder or symptom-reporting in general remains unclear.36,37 The many factors leading to fatigue in IBD probably reflect the ability of both CD and UC to interfere across many domains of life (Figure 2). We would therefore argue that, given the complexity of fatigue in IBD, the multidisciplinary approach piloted in this trial, encompassing gastroenterology, psychology and occupational therapy, may be more likely to provide comprehensive and durable benefits to patients with IBD. These issues are of significant concern to both patients and practitioners in IBD, because fatigue and impaired quality of life appear to be related to an increased risk for relapse or symptoms of the disease.38–42
Figure 2.
Factors contributing to fatigue in inflammatory bowel disease.
Although most of the available evidence on interventions to reduce fatigue comes from trials of biologic therapies, nonpharmacological interventions, such as physical activity and psychosocial interventions, have been applied in IBD populations, but these have mainly focused on mental health symptoms or overall quality of life.15,34,35,43,44 Many of these studies have used nonvalidated questionnaires and are therefore difficult to reliably interpret. One study reported that stress management techniques had a beneficial effect on tiredness, compared with treatment as usual in patients with CD.15 In another study, solution-focused therapy, directed at adequacy of existing coping abilities of patients with fatigue, had a positive effect on both fatigue and generic and disease-specific quality of life in patients with IBD, but the effect diminished during follow up.17 This study involved seven visits, making the strategy considerably more labour intensive than the one we report, which carries clear cost implications. The advantages of affordable strategies for fatigue management are self-evident, as psychological interventions resulting in improvements in quality of life have been shown, in some studies, to result in significant reductions in subsequent healthcare usage.45,46 Other studies have shown physical exercise and activity to be beneficial,47–49 but it may be more difficult in practice to tailor such interventions to a diverse group of patients with chronic IBD.
The results of this pilot RCT will hopefully lead to larger studies of fatigue management strategies, which investigate whether the signals we observed, in terms of improvements in fatigue severity, impact and quality of life, are replicated. These should be adequately powered, with a longer duration of follow up, and assess the cost-effectiveness of strategies for fatigue management. We selected patients with troublesome fatigue whose disease was in both clinical and biochemical remission not only because they are a group of patients with significant unmet needs, but also to reduce the confounding effect of disease activity in a small cohort. Larger studies that recruit patients with IBD with fatigue would be particularly welcome. Ultimately, an ideal future scenario may be one where a multidisciplinary team is able to tailor a bespoke suite of solutions for patients with IBD and fatigue, which may include exercise, pharmacologic, nutritional and psychoeducational measures to fit the patient’s profile and disease characteristics.
Footnotes
Author contributions: AOC, PJH, DP, AE and LW conceived and drafted the study. RR, LW, DJG and RCS collected all data. ACF and AOC analysed and interpreted the data. AOC and ACF drafted the manuscript. All authors commented on drafts of the manuscript. All authors have approved the final draft of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Crohn’s and Colitis UK (Reference Number: SP2015/3).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Anthony O’Connor  https://orcid.org/0000-0003-1722-4820
https://orcid.org/0000-0003-1722-4820
Contributor Information
Anthony O’Connor, Department of Gastroenterology, Tallaght University Hospital, Belgard Road, Tallaght, Dublin D24NR0A, Ireland Leeds Gastroenterology Institute, St. James’ University Hospital, Leeds, UK Tallaght Hospital/Trinity College Dublin, Dublin, Ireland.
Raguprakash Ratnakumaran, Leeds Gastroenterology Institute, St. James’ University Hospital, Leeds, UK; Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, UK.
Lisa Warren, Leeds Gastroenterology Institute, St. James’ University Hospital, Leeds, UK.
Debbie Pullen, Leeds and York Partnership NHS Foundation Trust, Leeds General Infirmary, Leeds, UK.
Anna Errington, Leeds and York Partnership NHS Foundation Trust, Leeds General Infirmary, Leeds, UK.
David J. Gracie, Leeds Gastroenterology Institute, St. James’ University Hospital, Leeds, UK Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, UK.
Rebecca C. Sagar, Leeds Gastroenterology Institute, St. James’ University Hospital, Leeds, UK Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, UK.
P. John Hamlin, Leeds Gastroenterology Institute, St. James’ University Hospital, Leeds, UK.
Alexander C. Ford, Leeds Gastroenterology Institute, St. James’ University Hospital, Leeds, UK; Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, UK.
References
- 1. van Langenberg DR, Gibson PR. Systematic review: fatigue in inflammatory bowel disease. Aliment Pharmacol Ther 2010; 32: 131–143. [DOI] [PubMed] [Google Scholar]
- 2. Minderhoud IM, Oldenburg B, van Dam PS, et al. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. Am J Gastroenterol 2003; 98: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 3. Drossman DA, Leserman J, Li ZM, et al. The rating form of IBD patient concerns: a new measure of health status. Psychosom Med 1991; 53: 701–712. [DOI] [PubMed] [Google Scholar]
- 4. de Rooy EC, Toner BB, Maunder RG, et al. Concerns of patients with inflammatory bowel disease: Results from a clinical population. Am J Gastroenterol 2001; 96: 1816–1821. [DOI] [PubMed] [Google Scholar]
- 5. Czuber-Dochan W, Dibley LB, Terry H, et al. The experience of fatigue in people with inflammatory bowel disease: an exploratory study. J Adv Nurs 2013; 69: 1987–1999. [DOI] [PubMed] [Google Scholar]
- 6. Stjernman H, Tysk C, Almer S, et al. Worries and concerns in a large unselected cohort of patients with Crohn’s disease. Scand J Gastroenterol 2010; 45: 696–706. [DOI] [PubMed] [Google Scholar]
- 7. Czuber-Dochan W, Ream E, Norton C. Review article: description and management of fatigue in inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37: 505–516. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein GR, Bala M, Han C, et al. Infliximab improves quality of life in patients with Crohn’s disease. Inflamm Bowel Dis 2002; 8: 237–243. [DOI] [PubMed] [Google Scholar]
- 9. Artom M, Czuber-Dochan W, Sturt J, et al. Targets for health interventions for inflammatory bowel disease-fatigue. J Crohns Colitis 2016; 10: 860–869. [DOI] [PubMed] [Google Scholar]
- 10. Minderhoud IM, Samsom M, Oldenburg B. Crohn’s disease, fatigue, and infliximab: Is there a role for cytokines in the pathogenesis of fatigue? World J Gastroenterol 2007; 13: 2089–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loftus EV, Feagan BG, Colombel JF, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol 2008; 103: 3132–3141. [DOI] [PubMed] [Google Scholar]
- 12. Gielissen MF, Verhagen S, Witjes F, et al. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol 2006; 24: 4882–4887. [DOI] [PubMed] [Google Scholar]
- 13. van Kessel K, Moss-Morris R, Willoughby E, et al. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med 2008; 70: 205–213. [DOI] [PubMed] [Google Scholar]
- 14. Hammond A, Bryan J, Hardy A. Effects of a modular behavioural arthritis education programme: a pragmatic parallel-group randomized controlled trial. Rheumatology 2008; 47: 1712–1718. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Vega E, Fernandez-Rodriguez C. A stress management programme for Crohn’s disease. Behav Res Ther 2004; 42: 367–383. [DOI] [PubMed] [Google Scholar]
- 16. Vogelaar L, Van’t Spijker A, Vogelaar T, et al. Solution focused therapy: a promising new tool in the management of fatigue in Crohn’s disease patients psychological interventions for the management of fatigue in Crohn’s disease. J Crohns Colitis 2011; 5: 585–591. [DOI] [PubMed] [Google Scholar]
- 17. Vogelaar L, Van’t Spijker A, Timman R, et al. Fatigue management in patients with IBD: a randomised controlled trial. Gut 2014; 63: 911–918. [DOI] [PubMed] [Google Scholar]
- 18. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl. A): 5–36. [DOI] [PubMed] [Google Scholar]
- 19. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980; 1: 514. [DOI] [PubMed] [Google Scholar]
- 20. Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998; 43: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol 2010; 8: 357–363. [DOI] [PubMed] [Google Scholar]
- 22. Jowett SL, Seal CJ, Phillips E, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol 2003; 38: 164–171. [DOI] [PubMed] [Google Scholar]
- 23. Rogler G, Aldeguer X, Kruis W, et al. Concept for a rapid point-of-care calprotectin diagnostic test for diagnosis and disease activity monitoring in patients with inflammatory bowel disease: expert clinical opinion. J Crohns Colitis 2013; 7: 670–677. [DOI] [PubMed] [Google Scholar]
- 24. Czuber-Dochan W, Norton C, Bassett P, et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis 2014; 8: 1398–1406. [DOI] [PubMed] [Google Scholar]
- 25. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 26. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 27. Spitzer RL, Williams JB, Kroenke K, et al. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol 2000; 183: 759–769. [DOI] [PubMed] [Google Scholar]
- 28. Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002; 64: 258–266. [DOI] [PubMed] [Google Scholar]
- 29. McHorney CA, Ware JE, Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 30. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996; 91: 1571–1578. [PubMed] [Google Scholar]
- 31. Lix LM, Graff LA, Walker JR, et al. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1575–1584. [DOI] [PubMed] [Google Scholar]
- 32. Nijrolder I, van der Windt DA, van der Horst HE. Prognosis of fatigue and functioning in primary care: a 1-year follow-up study. Ann Fam Med 2008; 6: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Milne B, Joachim G, Niedhardt J. A stress management programme for inflammatory bowel disease patients. J Adv Nurs 1986; 11: 561–567. [DOI] [PubMed] [Google Scholar]
- 34. Oxelmark L, Magnusson A, Lofberg R, et al. Group-based intervention program in inflammatory bowel disease patients: effects on quality of life. Inflamm Bowel Dis 2007; 13: 182–190. [DOI] [PubMed] [Google Scholar]
- 35. Mussell M, Bocker U, Nagel N, et al. Reducing psychological distress in patients with inflammatory bowel disease by cognitive-behavioural treatment: exploratory study of effectiveness. Scand J Gastroenterol 2003; 38: 755–762. [DOI] [PubMed] [Google Scholar]
- 36. Ratnakumaran R, Warren L, Gracie DJ, et al. Fatigue in inflammatory bowel disease reflects mood and symptom-reporting behavior rather than biochemical activity or anemia. Clin Gastroenterol Hepatol. Epub ahead of print 21 November 2017. DOI: 10.1016/j.cgh.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 37. Jonefjall B, Simren M, Lasson A, et al. Psychological distress, iron deficiency, active disease and female gender are independent risk factors for fatigue in patients with ulcerative colitis. United European Gastroenterol J 2018; 6: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reinisch W, Sandborn WJ, Bala M, et al. Response and remission are associated with improved quality of life, employment and disability status, hours worked, and productivity of patients with ulcerative colitis. Inflamm Bowel Dis 2007; 13: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 39. Bernstein CN, Singh S, Graff LA, et al. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol 2010; 105: 1994–2002. [DOI] [PubMed] [Google Scholar]
- 40. Hoivik ML, Moum B, Solberg IC, et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut 2013; 62: 368–375. [DOI] [PubMed] [Google Scholar]
- 41. Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol 2003; 98: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 42. Nurmi E, Haapamaki J, Paavilainen E, et al. The burden of inflammatory bowel disease on health care utilization and quality of life. Scand J Gastroenterol 2013; 48: 51–57. [DOI] [PubMed] [Google Scholar]
- 43. Diaz Sibaja MA, Comeche Moreno MI, Mas Hesse B. Protocolized cognitive-behavioural group therapy for inflammatory bowel disease. Rev Esp Enferm Dig 2007; 99: 593–598. [DOI] [PubMed] [Google Scholar]
- 44. Gracie DJ, Irvine AJ, Sood R, et al. Effect of psychological therapy on disease activity, psychological comorbidity, and quality of life in inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017; 2: 189–199. [DOI] [PubMed] [Google Scholar]
- 45. Deter HC, Keller W, von Wietersheim J, et al. Psychological treatment may reduce the need for healthcare in patients with Crohn’s disease. Inflamm Bowel Dis 2007; 13: 745–752. [DOI] [PubMed] [Google Scholar]
- 46. de Boer AG, Sprangers MA, Bartelsman JF, et al. Predictors of health care utilization in patients with inflammatory bowel disease: a longitudinal study. Eur J Gastroenterol Hepatol 1998; 10: 783–789. [DOI] [PubMed] [Google Scholar]
- 47. Nathan I, Norton C, Czuber-Dochan W, et al. Exercise in individuals with inflammatory bowel disease. Gastroenterol Nurs 2013; 36: 437–442. [DOI] [PubMed] [Google Scholar]
- 48. Loudon CP, Corroll V, Butcher J, et al. The effects of physical exercise on patients with Crohn’s disease. Am J Gastroenterol 1999; 94: 697–703. [DOI] [PubMed] [Google Scholar]
- 49. Ng V, Millard W, Lebrun C, et al. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin J Sport Med 2007; 17: 384–388. [DOI] [PubMed] [Google Scholar]