Abstract
To preserve residual hearing during cochlear implant (CI) surgery, it is desirable to use intraoperative monitoring of inner ear function (cochlear monitoring), especially during electrode insertion. A promising method is electrocochleography (ECochG). Within this project, the relations between ongoing responses (ORs), recorded extra- and intracochlearly (EC and IC), and preservation of residual hearing were investigated. Before, during, and after insertion of hearing preservation electrodes, intraoperative ECochG recordings were performed EC using a cotton wick electrode and after insertion also IC using the CI electrode (MED-EL) and a research software tool. The stimulation was delivered acoustically using low frequency tone bursts. The recordings were conducted in 10 adult CI recipients. The amplitudes of IC ORs were detected to be larger than EC ORs. Intraoperative EC thresholds correlated highly to preoperative audiometric thresholds at 1000 Hz, IC thresholds highly at 250 Hz and 500 Hz. The correlations of both intraoperative ECochG recordings to postoperative pure tone thresholds were low. When measured postoperatively at the same appointments, IC OR thresholds correlated highly to audiometric pure tone thresholds. For all patients, it was possible to record ORs during or directly after electrode insertion. Consequently, we conclude that we did not observe any cases with severe IC trauma. Delayed hearing loss could not be predicted with our method. Nevertheless, intraoperative ECochG recordings are a promising tool to gain further insight into mechanisms impacting residual hearing. Postoperatively recorded IC OR thresholds seem to be a reliable tool for frequency specific hearing threshold estimation.
Keywords: cochlear implant, hearing preservation, electrocochleography
Introduction
During the last years, the indication criteria for receiving a cochlear implant (CI) were extended toward patients with residual hearing, typically in the low frequency range. Accordingly, CI electrodes were designed that aim to preserve residual hearing. Several studies show a large benefit for the patient when electric hearing via CI is combined with acoustic hearing in the low frequency range. In an early multicenter study, James et al. (2005) showed benefits of ipsilateral residual hearing combined with a standard-length Nucleus Contour Advance perimodiolar electrode array. Lenarz et al. (2009) showed large benefits for combined electric acoustic hearing with the Nucleus Hybrid-L electrode, and Baumgartner et al. (2007) and Helbig et al. (2011) showed large benefits with the MED-EL FlexEAS electrode. Büchner et al. (2009) investigated the impact of acoustic hearing in the ipsilateral ear depending on the frequency range transmitted via the acoustic component. They detected large advantages for the speech perception in noise even with acoustic frequencies below 300 Hz, so even in this very low frequency range, it is beneficial to preserve residual hearing. In a recent study, Büchner, Illg, Majdani, and Lenarz (2017) found that among CI recipients with shorter electrodes (MED-EL Flex20 and Flex24), users of electric acoustic stimulation showed significantly better speech comprehension results than users of electric stimulation only. Reviews showed the large benefits of electric acoustic stimulation (Incerti, Ching, & Cowan, 2013; von Illberg, Baumann, Kiefer, Tillein, & Adunka, 2011).
The preservation of residual hearing is successful in many, but not all cases (Gstoettner et al., 2006; Suhling et al., 2016; von Illberg et al., 1999). Thus, hearing preservation still needs to be improved. Therefore, online monitoring of hearing is desired during the surgery. Furthermore, group data could help to identify critical steps of the CI electrode insertion as well as to separate whether a deterioration of residual hearing would be caused during the insertion itself or by postoperative processes. With general anesthesia, only objective measurement methods can be used. Currently, the recording of electrical potentials of the cochlea (electrocochleography [ECochG]) is commonly used, either extracochlearly (EC; Adunka, Roush, Grose, Macpherson, & Buchman, 2006; Choudhury et al., 2012; Harris, Cruise, Gibson, Bate, & Sanli, 2011; Mandalà, Colletti, Tonoli, & Colletti, 2012) or intracochlearly (IC; Calloway et al., 2014; Campbell, Kaicer, Briggs, & O’Leary, 2015; Koka, Saoji, & Litvak, 2017).
ECochG recorded signals have several components: compound action potential (CAP), summation potential, cochlear microphonics (CM), and auditory neurophonics (ANN; Forgues et al., 2014), but especially in the low frequency range CMs and ANNs are difficult to separate from each other and are often referred to as ongoing responses (ORs). Adunka et al. (2010) used a special recording electrode and placed it into the cochlea of nine normal hearing gerbils (10 ears). Helmstaedter, Lenarz, Erfurt, Kral, and Baumhoff (2018) used a CI electrode with additional recording contacts and inserted it into 10 guinea pigs. Both concluded that the CAP seemed to be a more sensitive indicator for hearing losses. Unfortunately, CAPs can be recorded more reliable in the higher frequency range where CI patients usually have a larger degree of hearing loss already. Also CMs are regarded to reflect the status of hair cells in the cochlea and therewith could be a measure of cochlear health. A deterioration of CMs during the CI electrode insertion could indicate a deterioration of residual hearing. Thus, in case of such a deterioration, the surgeon could immediately adapt the insertion. Therewith, the preservation of the residual hearing could be improved.
For stimulation in ECochG usually tone bursts or clicks are presented by insert earphones. The recordings can be conducted at different sites. For EC recordings, an electrode is placed at the outer side of the cochlea, commonly at the round window. These recordings can be obtained independent of a CI, which means that they can be taken before, during, and after electrode insertion, regardless of the CI brand or even without implanting a CI. Also the recording site remains stable during the surgery. Data acquisition can be conducted with a quick and reliable clinical device. Usually the reference and ground electrode are placed at the forehead or vertex or mastoid using adhesive or subdermal electrodes.
Especially with stimulation levels of 80 dB normal hearing level (nHL) and more there is a certain possibility for false positive responses which was investigated, for example, by Teschner, Lenarz, and Battmer (2012) using a transtympanic needle electrode. Here ECochG recordings were performed on a wet flannel and a pumpkin with a stiff needle electrode. The oscillations of the needle electrode due to the stimulus could lead to a fluctuating contact between electrode and tissue which leads to impedance fluctuations in the rhythm of the stimulus and therewith generates a false positive response. To remedy this drawback in EC recordings, for example, a cotton wick (CW) electrode could be used where the tip of the electrode is wrapped by wet medical cotton (Calloway et al., 2014; Mandalà et al., 2012). This should lead to a constant contact between electrode and tissue without oscillations of a stiff electrode and therewith reduce the risk of false positive responses.
For IC recordings, it is convenient to use the CI electrode. The substantial advantage of this method is that no additional invasive measurement electrode is necessary, thus recordings can be repeated anytime during and after implantation. The hardware and software of the CI are used to record potentials at the electrode contacts inside the cochlea. Due to signal processing in all implants and transmission time between coil and implant, these telemetric recordings are slower than recordings by clinical amplifiers. Another point is the change of recording site during insertion, and no recoding before insertion can be conducted for comparison.
Several studies confirm that especially intraoperative recordings yield the opportunity to monitor severe trauma to cochlear structures during implantation (Adunka et al., 2016; Dalbert, Sim, et al., 2015; Radeloff et al., 2012). However, correlations to postoperative residual hearing still differ strongly, and the relationship remains unclear. Adunka et al. (2016), Dalbert, Sim, et al. (2015), and Radeloff et al. (2012), for example, did not find strong correlations with the postoperative residual hearing, whereas other studies detected significant correlations to residual hearing (Fitzpatrick et al., 2014) or speech perception (Abbas, Tejani, Scheperle, & Brown, 2017). Kim, Tejani, Abbas, and Brown (2017) combined ECochG data with ECAP data and therewith found better correlations to speech perception than with ECochG data alone.
The study presented here strives to gain further insights into the ECochG recordings and their predictive force of hearing preservation. Also long-term effects of cochlear health, which might be detectable in ECochG as well as in audiometric thresholds, are to be investigated. For this MED-EL, implant recipients are measured EC and IC and followed for a period of 1 year postimplantation. As the study is still ongoing, we concentrate on the data up to the time of the first fitting (FF) which is scheduled 5 weeks after surgery.
Methods and Materials
In this study, IC ECochG was performed intraoperatively. The recordings were repeated postoperatively at the test switch-on (TSO) (1–3 days after surgery) and at the FF appointment (5 weeks after surgery). Intraoperative EC ECochG is performed routinely in our clinic for CI users with residual hearing. The recordings were compared with pre- and postoperatively obtained behavioral thresholds. The study was approved by the ethical standards of the institutional review board. Informed consent was obtained for all subjects participating in this study.
Preoperative Evaluation
The preoperative CI candidate evaluation is standardized at our clinic (Haumann et al., 2012). This standard evaluation spans subjective and objective audiometric evaluation including pure tone audiometry and ECochG as well as vestibular testing, neuroimaging, medical evaluations, and counseling interviews with the clinic’s engineers and therapists. The individual patient chooses an implant system according to his or her personal preferences, and the length of the electrode is determined together with the surgeon depending on residual hearing and length of the cochlea (Würfel, Lanfermann, Lenarz, & Majdani, 2014).
The ECochG in the preexamination is recorded transtympanically using the Nicolet Synergy EDX system (Natus Medical Incorporated, Pleasanton, CA, USA). For CAP recordings, a click is used (100 μs, alternating polarity), for CM recordings, a high frequency burst (2 kHz, rise, plateau, and fall time each 1 ms, rarefaction polarity). The stimuli are calibrated to nHL and presented via a 1 m sound tube. The pure tone audiometry is measured using an audiometer type AD2117 (earlier AD17 and AD2017) by Audio-DATA GmbH, Duvensee, Germany. These stimuli are calibrated to hearing level (HL) and presented using headphones (HDA300, earlier HDA200, Sennheiser electronic GmbH, Wedemark, Germany). The audiometer limits for each frequency are given in Table 1.
Table 1.
Details of the Stimulation and the Recording Parameters According to This Study’s Protocol.
| Frequency (Hz) | 250 | 500 | 1000 |
|---|---|---|---|
| Rise time (ms) | 4 | 2 | 1 |
| Plateau time (ms) | 4 | 4 | 4 |
| Fall time (ms) | 4 | 2 | 1 |
| Lower filter (Hz) | 100 | 200 | 300 |
| Upper filter (Hz) | 2000 | 2000 | 3000 |
| Maximum stimulation level of ECochG (dB nHL) | 95 | 99 | 105 |
| Maximum stimulation level of audiometer (dB HL; air conduction) | 100 | 110 | 110 |
ECochG = electrocochleography.
Intraoperative Recordings
During the CI surgery, EC and IC recordings were performed as described in this section.
EC recordings using a CW electrode at the promontory
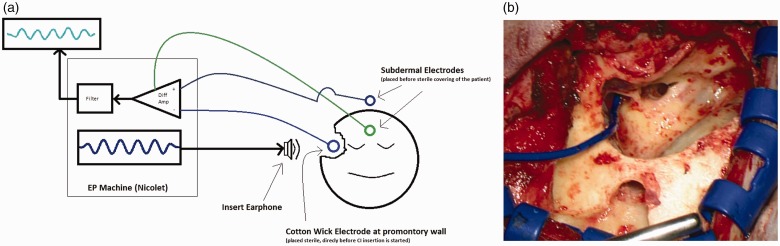
For EC recordings, a sterile foam insert transducer plug was placed in the outer ear canal. A sterile disposable steel wire (Medtronic, MI, USA) served as recording electrode for the EC recordings. The end was deinsulated and twisted into a small piece of medical cotton with the aim of increasing the recording surface and for reducing impedance fluctuations which could pretend a false positive response. This CW electrode was placed onto the promontory close to the round window and was kept moist throughout the recording process with physiologic saline. For artifact reduction, contact of the recording electrode with the ossicular chain was avoided. The reference needle electrode was placed onto the vertex and the ground needle electrode onto the forehead. The Nicolet Viking EDX system (Natus Medical Incorporated, Pleasanton, CA, USA) was used to generate acoustic stimuli and to record electrical potentials. The stimulation was delivered using insert earphones (Nicolet TIP300, Natus Medical Incorporated, Pleasanton, CA, USA) placed in the outer ear canal. A schematic drawing of the setup as well as an intraoperative view is shown in Figure 1.
Figure 1.
Extracochlear recordings. (a) Schematic drawing. (b) Intraoperative view. In Figure 1(b), the blue line emerging from the left edge is the cable belonging to the cotton wick electrode.
The stimulus level was calibrated to nHL. Typically, the series of frequencies were first tested at 70 dB nHL. When ORs were detected, stimulus intensity was gradually decreased by steps of 10 dB to determine the threshold at the tested frequency, otherwise the intensity was increased in 10 dB steps until clear responses were detected or the maximum stimulation level was reached. The stimuli were tone bursts of different frequencies. The specific parameters as well as the filters for the recording protocol are shown in Table 1. The repetition rate was 19.1 Hz, the recording window length was 15 ms, the polarity was set to rarefaction, and the number of stimulation averages was set at 10. These parameters were chosen in order to run a quick and clinically applicable protocol with keeping the anesthesia time as short as possible.
After preparing the intraoperative situs and after initiating the measurement setup, we first performed preinsertion recordings at different stimulation intensities at all measured frequencies. This aimed to identify the thresholds. The frequency yielding the best results (clear responses with low degree of artifacts) was chosen for the recordings being performed during the CI electrode insertion process. Most often a stimulation with 500 Hz or 1000 Hz was found to give best responses and therewith was used. Stimulation intensity was chosen where clear responses could be seen. After having inserted the complete electrode, threshold recordings were repeated at all frequencies using the same procedure.
IC recordings using the CI electrode
For IC recordings, the regular CI electrode was used together with the clinical CI measurement setup for MED-EL implants (MAX-Box, coil, and laptop) and a research software tool (EP Tool, MED-EL). The recording can be performed on every electrode contact, for our study, we chose Contact 1 which is the most apical contact. The research EP software allowed for recording windows to be increased up to 19.4 ms and to be adopted for the stimuli used. For our measurements, we used the 19.4 ms recording window to get a clear view of the complete wave for better signal processing analysis. The number of averages was set to 50, yielding a resulting recording time per wave of about 30 s.
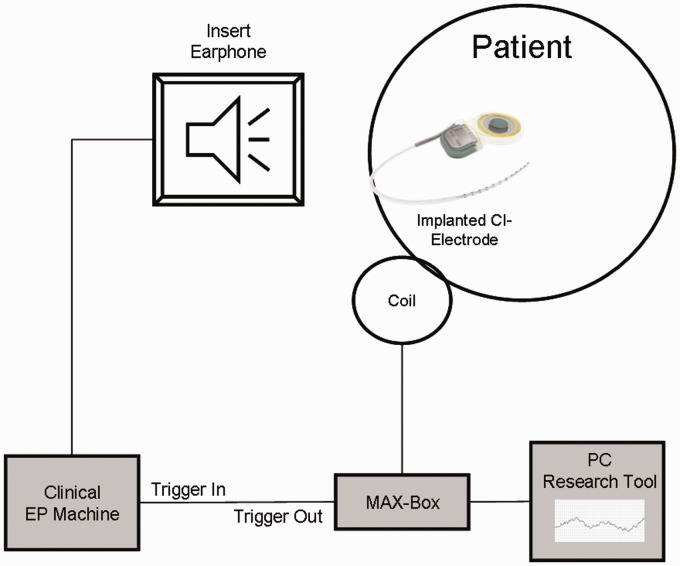
These IC recordings were conducted after the full insertion of the CI electrode, thus after completing the EC recordings. A simultaneous recording of IC and EC during the CI electrode insertion is unfortunately not possible due to communication artifacts when the IC recording is switched on, so the IC recordings were only done after insertion. The stimuli were again generated by the Nicolet Viking EDX system using the insert earphone already placed for the EC recordings. The two devices were connected via a trigger cable, and the trigger was generated by the recording software tool. A schematic drawing of the setup is shown in Figure 2.
Figure 2.
Schematic drawing of the intracochlear recordings.
As in the majority of cases the residual hearing lies in the low frequency range, recordings were performed with tone bursts at 250 Hz, 500 Hz, and 1 kHz. Therefore, the same stimuli were used as for EC recordings. As the signal quality of the CI amplifier is usually not as good as the quality of the clinical amplifier more averages had to be used (50 averages). Stimulation pattern and threshold detection were performed as described for the EC ECochG recordings.
Postoperative Recordings
Postoperative IC recordings with the CI electrode were repeated postoperatively at the TSO (TSO, 1–3 days after surgery) and the FF appointment (5 weeks after surgery). The setting was very similar to the intraoperative setting. In the postoperative situation, 100 averages were recorded, as measurement time was not as critical as during surgery. Acoustic stimulation was delivered by the Nicolet Synergy EDX system (Natus Medical Incorporated, Pleasanton, CA, USA). Measurements were stopped if the subject reported the signal as being too loud. Pure tone audiometry was performed at all appointments.
Subject Demographics
Ten subjects were included in this study (34–83 years, Ø 60.5 years, 4 males, 6 females) who currently have passed several appointments. All received a MED-EL Synchrony implant with different electrode lengths and inserted electrode depths (IEDs). The details of the subjects and implanted electrodes are given in Table 2.
Table 2.
Details of the Subjects and the Implanted Electrodes.
| ID | Electrode | Inserted electrode depth (mm) | Age at surgery (years) | Sex | Side | Duration of hearing impair (years) | Etiology |
|---|---|---|---|---|---|---|---|
| S01 | Flex28 | 20 | 38.1 | F | L | 26.4 | Sudden hearing losses, progressive |
| S02 | Flex24 | 24 | 65.3 | F | L | 49.2 | Menière’s disease, progressive |
| S03 | Flex28 | 28 | 57.1 | M | R | 1.2 | Traumatic(?), progressive |
| S04 | Flex28 | 28 | 60.4 | F | L | 20.3 | Unknown |
| S05 | Flex24 | 24 | 80.9 | M | L | 13.3 | Unknown, progressive |
| S06 | Flex28 | 20 | 83.3 | F | R | 12.3 | Unknown, progressive |
| S07 | Flex24 | 24 | 45.7 | F | R | 45.7 | Perinatal hypoxia |
| S08 | Flex24 | 20 | 63.3 | M | L | 44.1 | Traumatic, acute |
| S09 | Flex20 | 20 | 33.9 | F | L | 33.9 | Senear-Usher Syndrome Type II |
| S10 | Flex28 | 28 | 77.4 | M | L | 48.1 | Genetic, progressive |
Note. F = female; M = male; L = left; R = right.
Data Analysis
The OR recordings were analyzed using Fast Fourier Transform (FFT). For the analysis, custom written tools were implemented into MATLAB R2017a (The Mathworks, Inc., Natick, Massachusetts, USA). The raw data were exported from the recording system and imported into MATLAB. The processing took place in the following steps: At first, the signal was resampled to a convenient frequency in respect to the analysis (118.75 kHz for 250 Hz stimulation frequency and 119 kHz for 500 Hz and 1 kHz stimulation frequency). This was done in order to meet the exact stimulus frequency bin for the stimulation frequency in the FFT. Afterwards, the signal part comprising the response was extracted from the recording. For this, the resampled signal was scanned for presence and amplitude of the stimulus frequency using a sliding window and the MATLAB function fft. The starting point was set to the time where 50% of the maximum amplitude of the FFT scan was reached. The end point was set according to the length of the applied stimulus (3 periods or 12 ms after the starting point for the 250 Hz stimulus frequency, 4 periods or 8 ms for the 500 Hz, and 6 periods or 6 ms for the 1000 Hz). This segment was detrended linearly using the respective MATLAB function which sets the mean of the signal to zero. After that the FFT was calculated on the processed data again using the MATLAB function fft. For the noise floor estimation, the recording with 0 dB stimulation level was used for each stimulation frequency. Here the amplitudes of all bins from the FFT were averaged that lay within the filter limits as given in Table 1.
The OR threshold was determined as follows for each frequency: The FFT was calculated as described for all loudness levels in descending order of the stimulation level. Presence or absence of ORs was decided by an experienced audiologist visually regarding the time and frequency domain. If the amplitude at the stimulus frequency bin clearly exceeded the amplitudes at the neighboring bins and a wave form could be recognized in the time signal, the response was accepted. The OR threshold was set to the lowest stimulation level where the response was continuously accepted. In case of no stimulus response at the highest level, the response was set to 10 dB above the stimulation limit (see Table 1). This approach was also used for pure tone audiometry. In one case, no stimulus response could be seen in the EC ECochG before insertion, so in this case the postinsertion recording was skipped and assumed to yield no response.
The amplitude course of the EC recorded ORs during insertion was classified into four behavior groups. Therefore, the amplitude at the stimulus frequency bin was calculated for each trial, and with the MATLAB function polyfit, a regression line was performed. The difference between first and last point of the regression line was calculated. If the amplitude at the end was more than 0.1 μV higher than at the beginning, the amplitude behavior of the OR was classified to rise (↗). If the difference was less than 0.1 μV, the amplitude behavior of the OR was classified to remain stable (→). If the amplitude at the end was more than 0.1 μV smaller than at the beginning, but ORs were still present, the amplitude behavior of the OR was classified to drop (↘). If the amplitude dropped and no ORs were visible any more, the amplitude behavior was classified to get lost (↓).
The differences in the course of the thresholds during the different appointments were tested for significance on a 5% level with a paired-sample T-test, and the correlation coefficients were calculated using the according MATLAB functions. The IEDs were classified for hearing preservation similar to Suhling et al. (2016). The audiometric pure tone low frequency hearing threshold (PTAlow) was calculated as mean of the audiometric thresholds at 250 Hz, 500 Hz, and 1000 Hz. The postoperative hearing loss was classified to yield a PTAlow shift between pre- and postoperative data up to 15 dB, a shift between 15 dB and 30 dB, and a shift of more than 30 dB. Similar, the mean low EC OR shift EClow was calculated as mean of the EC OR thresholds at the three low frequencies 250 Hz, 500 Hz, and 1000 Hz.
Results
Hearing Preservation as Measured by Pure Tone Audiometry
The hearing preservation as measured by pure tone audiometry is classified in Table 3. The individual audiometric threshold shifts together with the EC recorded OR threshold shifts are given in Table 4. The audiometric threshold shift in the low frequency range was significant between pre- and both postoperative threshold data, but not significant between TSO and first fit. The correlation coefficient between preoperative data and TSO was r = 0.82** and r = 0.61 between preoperative data and FF data (shown in Figure 3).
Table 3.
Hearing Preservation Classified by Audiometric Threshold Shifts Before and After Surgery (TSO As Well As First Fitting Appointment).
| ΔPTAlow,post–ΔPTAlow,pre |
ΔPTAlow,firstfit–ΔPTAlow,pre |
|||||
|---|---|---|---|---|---|---|
| ΔPTAlow ≤15 dB | 15 dB< ΔPTAlow ≤30 dB | ΔPTAlow >30 dB | ΔPTAlow ≤15 dB | 15 dB< ΔPTAlow ≤30 dB | ΔPTAlow >30 dB | |
| IED 20 n = 4 | 1 | 1 | 2 | 1 | 2 | 1 |
| 25% | 25% | 50% | 25% | 50% | 25% | |
| IED 24 n = 3 | 0 | 2 | 1 | 0 | 2 | 1 |
| 0% | 66.7% | 33.3% | 0% | 66.7% | 33.3% | |
| IED 28 n = 3 | 0 | 0 | 3 | 0 | 1 | 2 |
| 0% | 0% | 100% | 0% | 33.3% | 66.7% | |
IED = inserted electrode depth; PTA = pure tone average.
Table 4.
Thresholds As Well As Threshold Shifts and Behavior Groups as Measured with Different Methods.
| Preoperative transtymp ECochG threshold (dB nHL) |
TSO |
First fit |
EC OR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | IED (mm) | PTAlow (dB HL) before surgery | CM (2 kHz) | CAP (click) | PTAlow (dB HL) 0.25–1 kHz | Hearing preservation group | PTAlow (dB HL) 0.25–1 kHz | Hearing preservation group | Threshold shift (dB) 0.25–1 kHz | Amplitude behavior group |
| S01 | 20 | 51.7 | 90 | NR | 60.0 | 0–15 dB | 85.0 | >30 dB | 6.7 | → |
| S02 | 24 | 93.3 | 80 | 95 | 116.7 | 15–30 dB | 116.7 | 15–30 dB | 0.0 | ↘ |
| S03 | 28 | 60.0 | 80 | NR | 103.3 | >30 dB | 116.7 | >30 dB | −3.3 | ↗ |
| S04 | 28 | 75.0 | 80 | 90 | 111.7 | >30 dB | 98.3 | 15–30 dB | 15.0 | ↘ |
| S05 | 24 | 55.0 | 80 | NR | 90 | >30 dB | 116.7 | >30 dB | 3.3 | → |
| S06 | 20 | 61.7 | 90 | NR | 96.7 | >30 dB | 81.7 | 15–30 dB | NR | |
| S07 | 24 | 43.3 | 90 | NR | 70.0 | 15–30 dB | 61.7 | 15–30 dB | 21.7 | ↘ |
| S08 | 20 | 48.3 | 80 | NR | 81.7 | >30 dB | 71.7 | 15–30 dB | 0.0 | → |
| S09 | 20 | 58.3 | 80 | NR | 88.3 | 15–30 dB | 65.0 | 0–15 dB | 2.0 | → |
| S10 | 28 | 68.3 | 80 | NR | 116.7 | >30 dB | 116.7 | >30 dB | 0.0 | → |
Note. IED = inserted electrode depth; NR = no response; CAP = compound action potential; CM = cochlear microphonics; PTA = pure tone average; OR = ongoing response; EC = extracochlear; ECochG = Electrocochleography; TSO = test switch-on.
Figure 3.
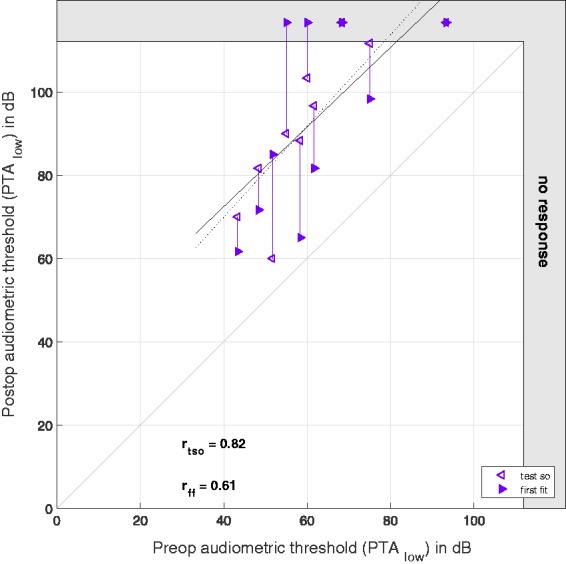
Pure tone audiometry as PTAlow before and after surgery. On the x axis, the preoperative hearing thresholds are plotted, and on the y axis, the postoperative hearing thresholds as measured at the (TSO) (hollow markers) and the first fitting week (full markers). The data points representing the same subjects are connected by vertical lines. The gray area at the right and upper edge represents the area above the possible stimulation levels. The dashed regression line refers to the (TSO) data and the full line to the first fitting data. The correlation coefficients are given for the preoperative thresholds compared with the postoperative thresholds at the (TSO) and the first fitting, respectively. The gray bisecting line indicates the ideal relationship between pre- and postoperative data.
EC Recorded ORs
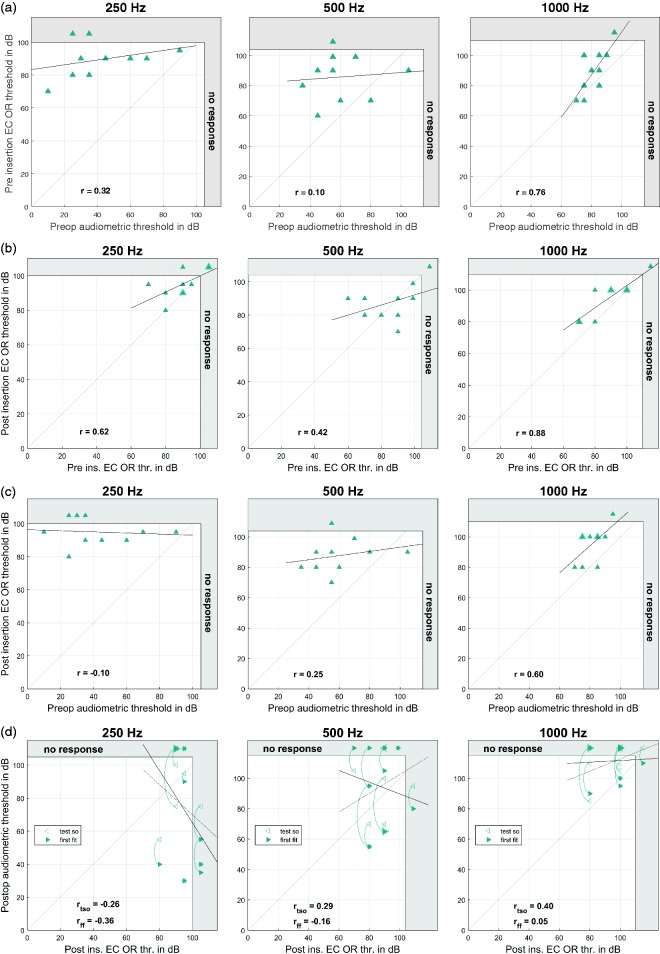
In 9 of the 10 cases, stimulus responses could be detected in EC OR recordings, in Subject 6, no responses could be detected. The differences between audiometric pure tone thresholds and EC OR thresholds at different time points are given in Table 5, and the individual data are shown in Figure 4.
Table 5.
Differences Between Audiometric Pure Tone Thresholds and EC OR Thresholds at Different Time Points (TSO—1–3 days After Surgery; FF—5 Weeks After Surgery).
| 250 Hz |
500 Hz |
1000 Hz |
|
|---|---|---|---|
| Mean ± standard deviation in dB, correlation | |||
| Preoperative audiogram vs. preinsertion OR | 47.0 ± 23.1 .32 | 25.2 ± 24.2 .10 | 8.0 ± 10.1 .76* |
| EC OR before vs. after insertion | 5.5 ± 8.6 .62 | 2.1 ± 14.7 .42 | 6.0 ± 7.0 .88*** |
| Preoperative audiogram vs. postinsertion EC OR | 52.5 ± 26.7 −.10 | 27.3 ± 20.4 .25 | 14.0 ± 9.4 .60 |
| Postoperative audiogram (TSO) vs. postinsertion EC OR | 20.5 ± 32.0 −.26 | −7.2 ± 22.6 .29 | −15.5 ± 12.8 .40 |
| Postoperative audiogram (FF) vs. postinsertion. EC OR | 22.0 ± 39.5 −.36 | −6.7 ± 30.1 −.16 | −16.0 ± 16.3 .05 |
Note. OR = ongoing response; EC = extracochlear; FF = first fit; TSO = test switch-on.
p < .05 (significant). ***p < .001 (highly significant).
Figure 4.
Intraoperative extracochlearly (EC) recorded OR thresholds. (a) EC OR thresholds before insertion versus pure tone audiometry before surgery. (b) Intraoperative EC OR thresholds before and after insertion. (c) EC OR thresholds after insertion versus pure tone audiometry before surgery. (d) Intraoperative EC OR thresholds after insertion versus pure tone audiometry after surgery as measured at the TSO (hollow markers) and the first fitting appointment (full markers). Thicker markers represent multiple data points. The data points representing the same subjects are connected by curved lines. The dotted regression line refers to the TSO data and the full line to the first fitting data. The gray area at the right and upper edge represents the area above the possible stimulation levels. From the left to the right panels, the different frequencies are shown.
EC = extracochlear; OR = ongoing response; TSO = test switch-on.
IC Recorded ORs
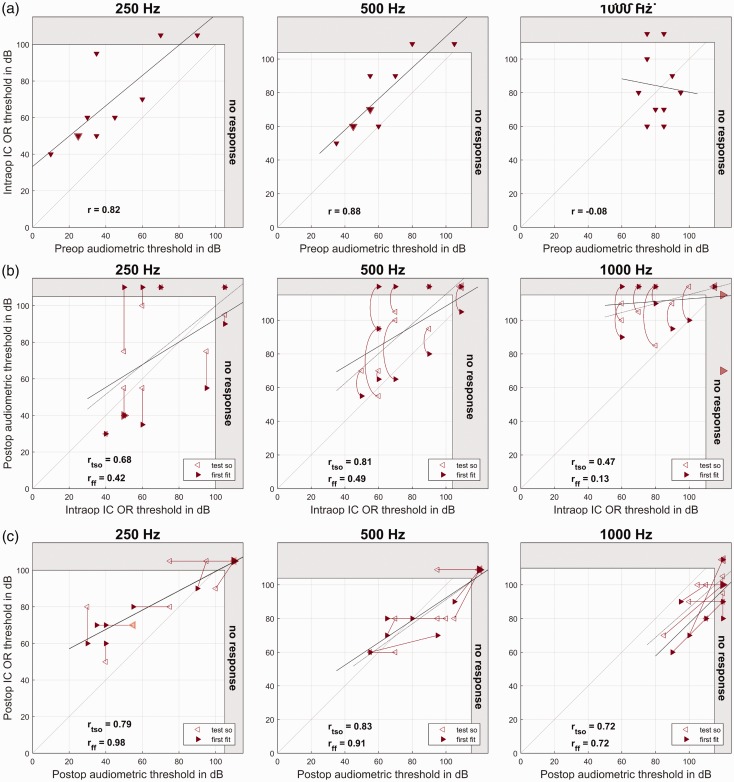
In 9 of the 10 cases, intraoperative IC OR responses could be detected for at least one frequency, in Subject 2, there was no detectable stimulus response. At the TSO, responses to acoustic stimulation could be detected in all subjects except Subjects 4 and 10 for at least one frequency. At the FF appointment, a stimulus response was detected for at least one frequency in all subjects except Subject 10. The differences between audiometric pure tone thresholds and EC OR thresholds at different time points are given in Table 6, and the individual data are shown in Figure 5.
Table 6.
Differences Between Audiometric Pure Tone Thresholds and IC OR Thresholds at Different Time Points (TSO—1–3 Days After Surgery; FF—5 Weeks After Surgery).
| 250 Hz |
500 Hz |
1000 Hz |
|
|---|---|---|---|
| Mean ± standard deviation in dB, correlation | |||
| Preoperative audiogram vs. postinsertion IC OR | 26.0 ± 14.5 .82** | 16.3 ± 10.3 .88*** | 2.5 ± 22.5 −.08 |
| Postoperative audiogram (TSO) vs. postinsertion IC OR | −6.0 ± 21.7 .68* | −18.2 ± 13.9 .81** | −27.0 ± 18.3 .47 |
| Postoperative audiogram (FF) vs. postinsertion IC OR | −4.5 ± 33.8 .42 | −17.7 ± 24.5 .49 | −27.5 ± 22.4 .13 |
| Postoperative audiogram (TSO) vs. postoperative IC OR (TSO) | 11.5 ± 18.0 .79** | −7.4 ± 12.9 .83** | −15.0 ± 10.0 .72* |
| Postoperative audiogram (FF) vs. postinsertion IC OR (FF) | 12.0 ± 17.5 .98*** | −5.9 ± 11.8 .91*** | −23.0 ± 11.4 .72* |
Note. OR = ongoing response; IC = intracochlear; FF = first fit; TSO = test switch-on.
p < .05 (significant), **p < .01 (high significant), ***p < .001 (highly significant).
Figure 5.
Intracochlearly (IC) recorded OR thresholds. (a) Intraoperative IC OR recordings compared with pure tone audiometry before surgery. On the x axis, the IC OR thresholds are plotted, and on the y axis, the preoperative hearing thresholds. (b) Intraoperative IC OR recordings after insertion compared with pure tone audiometry after surgery. On the x axis, the postinsertion OR thresholds are plotted, and on the y axis, the postoperative hearing thresholds as measured at the TSO (hollow markers) and the first fitting week (full markers). (c) Postoperative IC OR recordings versus pure tone audiometry during the follow up. On the x axis, the IC OR thresholds are plotted, and on the y axis, the hearing thresholds. Both are measured at the TSO (hollow markers) and the first fitting week (full markers). Larger markers represent multiple data points. The data points representing the same subjects are connected by straight or curved lines. The dotted regression line refers to the TSO data and the full line to the first fitting data. The gray area at the right and upper edge represents the area above the possible stimulation levels. From the left to the right panels, the different frequencies are shown.
IC = intracochlear; OR = ongoing response.
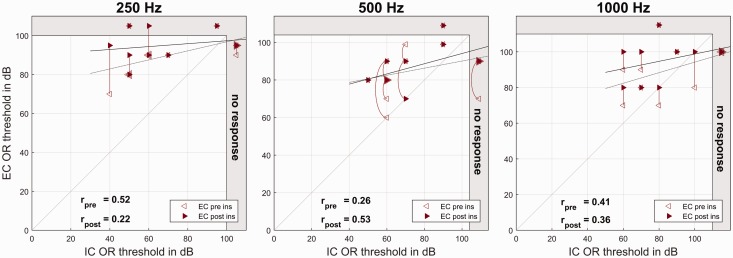
Relation Between EC and IC OR Recordings
The differences between intraoperative IC and EC OR thresholds for the group data are given in Table 7, and the individual data are shown in Figure 6.
Table 7.
Differences Between Intraoperative Extra- and Intracochlearly Recorded OR Thresholds.
| 250 Hz |
500 Hz |
1000 Hz |
|
|---|---|---|---|
| Mean ± standard deviation in dB, correlation | |||
| Preinsertion EC vs. postinsertion IC OR | 21.0 ± 20.9 .52 | 8.9 ± 22.8 .26 | 5.5 ± 19.6 .41 |
| Postinsertion EC vs. postinsertion IC OR | 26.5 ± 23.9 .22 | 11.4 ± 18.1 .53 | 11.5 ± 19.6 .36 |
OR = ongoing response; EC = extracochlear; IC = intracochlear.
Figure 6.
Intraoperative ECochG recordings. On the x axis, the (postinsertion) IC ECochG thresholds are plotted, and on the y axis, the EC ECochG thresholds before (hollow markers) and after CI insertion (full markers). Larger markers represent multiple data points. The data points representing the same subjects are connected by vertical or curved lines. The dotted regression line refers to the preinsertion data and the full line to the postinsertion data. The gray area at the right and upper edge represents the area above the possible stimulation levels. From the left to the right panels, the different frequencies are shown.
IC = intracochlear; OR = ongoing response; EC = extracochlear.
Example
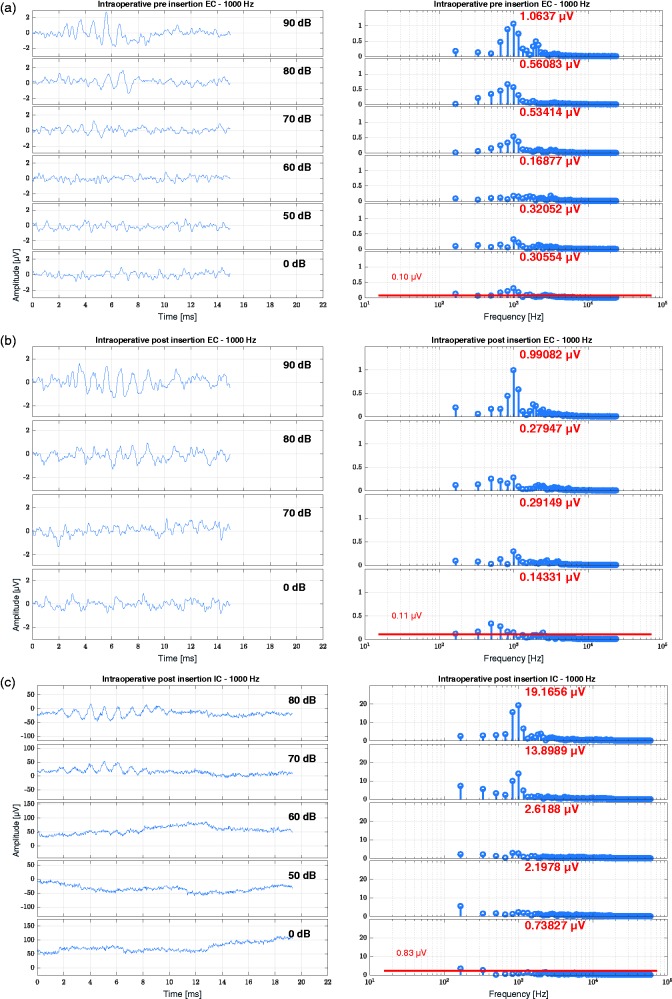
In Figure 7, an intraoperative recording is shown for a subject with 24 mm IED for the 1 kHz stimulation. The amplitudes and noise floor estimations for this subject are given in Table 8.
Figure 7.
Results of an intraoperative OR recording (Subject S07, age 45.7 years, 24-mm inserted electrode depth on the right side). (a) EC recording before insertion, (b) EC recording after insertion, and (c) IC recording after insertion. Here the data are shown for a 1 kHz tone burst. The pure tone threshold at 1 kHz was before surgery at 75 dB HL, at the TSO at 110 dB HL and at the first fitting at 90 dB HL. From top to bottom, the stimulation level is decreasing. On the left panels, the time signal is shown, and on the right panels, the frequency analysis by FFT. The red number at each FFT plot states the amplitude at the frequency bin of the stimulus level. In the lowest FFT plot of each panel representing the recording at 0 dB, the noise floor estimation is plotted and numeralized.
IC = intracochlear; EC = extracochlear.
Table 8.
Amplitude and Noise Floor Estimation for the Recordings in Subject S07.
| EC preinsertion |
EC postinsertion |
IC intraoperative |
IC TSO |
IC first fit |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NF in μV | Amp in μV | NF in μV | Amp in μV | NF in μV | Amp in μV | NF in μV | Amp in μV | NF in μV | Amp in μV | |
| 250 Hz | 0.08 | 0.34 | 0.07 | 0.14 | 1.29 | 11.16 | 0.53 | 11.24 | 0.81 | 14.85 |
| 500 Hz | 0.07 | 0.84 | 0.08 | 0.27 | 1.46 | 12.50 | 0.78 | 4.86 | 0.87 | 24.07 |
| 1000 Hz | 0.10 | 1.06 | 0.11 | 0.99 | 0.83 | 19.17 | 0.70 | 8.99 | 0.87 | 13.29 |
The amplitudes are given at maximum stimulated level, and the noise floor was calculated from the recordings at 0 dB. NF = Noise floor; Amp = amplitude at stimulus frequency; EC = extracochlear; IC = intracochlear.
Course of the Recorded Thresholds
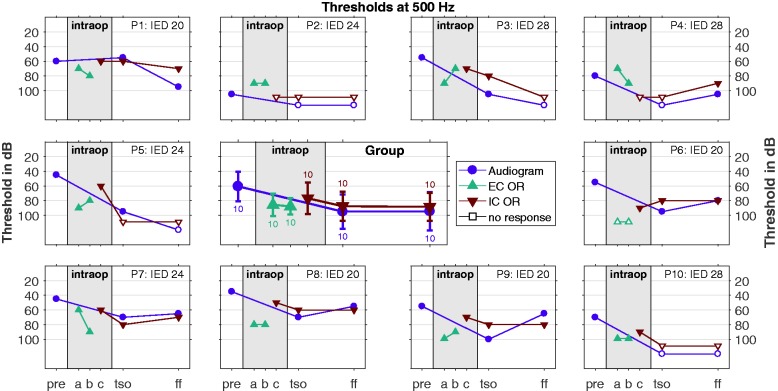
In Figure 8, the time course of the recorded thresholds at 500 Hz is presented for all subjects up to the FF appointment. Here the averaged group data are shown as well as the individual data for the different recording methods (pure tone audiometry, EC OR and IC OR). The group data show some deterioration in the intraoperative EC ECochG threholds as well as in the audiogram before and after surgery, but in the individual data, there is some variation.
Figure 8.
Course of the thresholds (OR and audiogram) over time up to the first fitting appointment. Here the data are shown for the 500 Hz stimulation, the data for 250 Hz and 1 kHz can be found in the Supplementary Files which are available online at the homepage of the journal. In the middle panel, the group data for all subjects are shown (mean and standard deviation as well as number of cases), and in the outer panels the individual data for all subjects. The stimulus response thresholds as well as the audiometric thresholds are represented by filled markers, whereas cases with no responses are represented by hollow markers 10 dB above the stimulation limit. On the x axis, the time course is plotted with the following appointments: “pre”—before surgery, “a”— EC recording before insertion, “b”—EC recording after insertion, “c”—IC recording after insertion, “TSO”—test switch-on, typically 2 days after surgery, and “FF”—first fitting, 5 weeks after surgery.
IC = intracochlear; OR = ongoing response; EC = extracochlear.
More results as well as all figures in color print can be found in the Supplementary Files which are available online at the homepage of the journal.
Discussion
In this study, different methods of assessing the status of the cochlea were applied. Measurements of ECochG were conducted at different recording sites. The EC ECochG recording using a CW electrode is already part of our clinical routine. The status of the cochlea is monitored before, during, and directly after CI electrode insertion. In this study, the IC recording of ECochG was conducted using the MED-EL system. This method of measuring ECochG can be performed intraoperatively as well as postoperatively during the clinical follow up. When combining both methods, the status of the inner ear of the patient can be monitored objectively in a tight time schedule. The focus of this investigation lies on evaluation of different methods for cochlear monitoring with respect to their relation to residual hearing.
Still, one of the aims of this study as well as similar studies conducted by several clinics and research groups is the development of an online biofeedback system for the surgeon during CI electrode insertion. It has to be taken into account that ECochG and pure tone audiometry measure different entities. Pure tone audiometry measures hearing, but the OR in the ECochG signals is composed largely from hair cells (the CM), and there may or may not be neural responses present (the ANN). Fitzpatrick et al. (2014) as well as Campbell et al. (2015), for example, hypothesized effects of surviving hair cells that react to the stimuli, but deteriorated neural structures that prevent the subject from hearing the sound. Thus, the OR does not necessarily reflect the hearing of the patient. Also the uncertainty remains that the pure tone audiometry is conducted before surgery and 1 to 3 days after surgery, whereas the ECochG recordings are conducted intraoperatively before, during, and after CI electrode insertion. Thus, if the intraoperative postinsertion recordings are still good and the audiometric threshold after surgery deteriorates, it is difficult to separate whether the postinsertion recording yielded a false positive response or the residual hearing deteriorated due to postoperative processes.
Our results concerning EC OR recordings show that there were good correlations between preoperative audiogram and preinsertion EC OR thresholds especially for the 1000 Hz stimulus. Here the EC OR thresholds were close to the audiometric thresholds with a low standard deviation. The detected EC OR thresholds for 250 Hz were much higher than the audiometric thresholds especially in cases of good residual hearing. In two cases, no ECochG responses to 250 Hz stimulus could be detected at all, but for the eight other cases, the correlation was good. For 500 Hz, no correlation was found. In most of the cases, the EC OR thresholds were higher than the audiometric thresholds for all three frequencies.
In the one case, where no ORs were seen in the EC ECochG for all frequencies in the preinsertion recording the postinsertion EC ECochG recording was skipped. Later it turned out that the ear canal of this subject was very wide, so the standard earplug from the insert earphone could have been too small and maybe some sound energy was lost. With IC ECochG, we could detect stimulus responses, but only at high stimulation levels. For the postoperative appointments, we used larger earplugs. Monitoring measurements were not always successful in individual patients and appointments. Other studies also show that there are cases where no responses can be detected despite some residual hearing (Dalbert, Pfiffner, et al., 2015; Fitzpatrick et al., 2014). The conclusions drawn from the corresponding incomplete data are still meaningful, as most subjects could be measured successfully. Another aim of ECochG recordings was to identify critical steps and processes for the group in order to test options for further directions of achieving hearing preservation, for example, improving operation techniques or addressing postoperative deterioration processes. The group results are representative of the presently still increasing group of CI recipients with residual hearing and will help to improve hearing preservation.
Between pre- and postinsertion, the EC OR thresholds slightly deteriorated, but there were good correlations detected for 1000 Hz, some for 250 Hz but again no good correlations for 500 Hz. Consistently, there were also good correlations between preoperative audiogram and postinsertion EC OR thresholds for 1000 Hz stimuli. However, the correlations between postinsertion EC OR thresholds and postoperative audiometry were low, only for 1000 Hz stimulation, there was a correlation of 0.40 to the TSO data, but it was not significant and it vanished in the later course as measured at the FF appointment. Especially for 1000 Hz, the strong correlations before and during surgery indicate that the EC ORs in principle could reflect the status of the cochlea, but not necessarily the status of the hearing itself. Also the evoked potential thresholds depend on the noise floor. With more repetitions, the ECochG thresholds would presumably be even lower. Nevertheless, the in general preserved responses also indicate that in our investigated cases, no severe trauma occurred during the insertion, thus suggesting that the structures were preserved.
The behavior of the OR amplitudes during the insertion was classified to four groups. In all cases, the OR amplitude varied between the recorded trials, but here the global trend was regarded. In one case, the amplitude rose during the insertion, and in five cases, the amplitude remained stable. In three cases, the amplitude deteriorated, but ORs were still present, and in no case, the ORs were lost. This also supports the assumption that in our investigated cases, no severe trauma occurred during electrode insertion and the structures were preserved. In some cases, there was a relationship to the later residual hearing detectable, but in some cases not.
However, the weak relations compared with the postoperative pure tone audiometry indicate that there were postoperative processes leading to a deterioration of the residual hearing which could be due to, for example, inflammatory or fibromatous processes. These delayed hearing losses could not be predicted by the intraoperative EC recordings. This finding is in line with studies of other research groups, for example, Adunka et al. (2016), Dalbert, Sim, et al. (2015), and Radeloff et al. (2012). Nevertheless this finding could indicate that intraoperative monitoring using EC ECochG recordings could help in reducing trauma by inserting the electrode. However, in many cases, the residual hearing seems to deteriorate afterwards due to postoperative processes, which should be addressed more deeply in further research.
The research tool from MED-EL allows recording IC ECochG via the implant electronics. As the CI electrode lies in the perilymph, the risk of false positive responses should be very low. All of our subjects had measurable residual hearing in all three frequencies before surgery, so in one case, an anecdotic recording with 2000 Hz was conducted where the subject was deaf and indeed no response could be seen. In our study, the intraoperative IC ECochG recordings took place after the CI electrode insertion. The recorded amplitudes were by far larger than with EC ECochG recordings, which was also described by other research groups (Calloway et al., 2014; Dalbert, Pfiffner, et al., 2015), and the response thresholds were lower. No significant correlations between EC and IC ECochG thresholds were detected.
Especially for 250 Hz stimulation, the intraoperative IC OR thresholds were by far lower and therewith by far closer to the preoperative audiometric thresholds than with EC recordings, but still above the audiometric thresholds. Also for 500 Hz, the IC OR thresholds were above the audiometric thresholds. Nevertheless, good correlations between preoperative audiometric thresholds and intraoperative IC OR thresholds were detected for 250 Hz and 500 Hz. For 1000 Hz stimulation, IC OR thresholds were above the audiometric thresholds in five of the cases and in the other five cases they were below, but the correlation was weak. When comparing the intraoperative IC OR thresholds to the postoperative audiometric thresholds at the time of the TSO there were again good correlations detected for 250 Hz and 500 Hz, but only a low correlation for 1000 Hz. In the later time course as measured at the FF week, the correlations degraded for all three frequencies. This finding also supports the hypothesis that the residual hearing may still be undisturbed after the CI electrode insertion but deteriorates in the time course after the surgery.
For an intraoperative real-time monitoring, the IC ECochG recordings currently have got some drawbacks. The main drawback lies in the change of the recording site during the insertion which was already reported by Calloway et al. (2014). No preinsertion reference recording can be conducted, and changes during the insertion could result from cochlear trauma as well as the change of the recording site when the distance to the generator is changed. Nevertheless, sudden drops in the amplitude should correspond to a cochlear trauma. Acharya, Tavora-Vieira, and Rajan (2016) successfully applied a preliminary MED-EL recording tool in two children during CI insertion, but another drawback of telemetric IC recording systems is a long recording time due to the signal processing in the implant. Also, the MED-EL research tool currently does not allow for a simultaneous recording of alternate polarities which limits the possibilities for analysis. Thus, in order to achieve a real-time application, the recording tool still needs to be improved.
Postoperatively, the IC OR thresholds and the audiometric thresholds were recorded without any intervention in between. Nevertheless at the TSO appointment, there were difficulties in the contact between coil and implant in some cases due to the swellings which may impact the quality of the recordings, but at the FF appointment, all recordings went on very smoothly. When conducted at the same appointment, the correlation coefficients between IC OR thresholds and audiometric thresholds were very high for all frequencies, especially at the FF appointment, so the IC ORs seem to be a reliable tool for a frequency-specific hearing objective threshold estimation which is in line with the findings of other research groups (Campbell et al., 2015; Dalbert, Pfiffner, et al., 2015; Koka et al., 2017). This would mean that the intraoperative IC OR thresholds after insertion could reflect the status of the cochlea at that time quite reliably, but there are some delayed processes of hearing deteriorations.
It is noticeable that for 250 Hz, the IC OR thresholds were worse than the audiometric thresholds, for 500 Hz, they were equal, and for 1000 Hz, they were better than the audiometric thresholds. A possible explanation could be that the distance from the generator of the stimulus response induces a difference in the detection threshold. A recent work of Timm et al. (2018) shows large variations of the coverage of the cochlea by the CI electrode. This was due to different inserted electrode lengths but also highly dependent on individual cochlea lengths. By average, the coverage of the cochlea with 28-mm inserted electrode length was 76.4% (n = 165) which corresponds to a frequency of 372.21 Hz for the first electrode contact according to Greenwood (1990). The average cochlear coverage with 24-mm inserted electrode length was 67.9% (n = 52) corresponding to 635.42 Hz for the first contact, and for 20-mm inserted electrode length, the average coverage was 56% (n = 46) corresponding to 1.242.91 Hz for the first contact.
In 70% of our subjects, the electrode was inserted to a maximum of 24 mm, thus still being away from the apical generators of the lowest frequencies. In this study, the recording was conducted on electrode Contact 1 which is the most apical contact. The further apical the stimulation occurs, the more the recorded IC OR threshold could be away from the stimulus response generator and the threshold difference seemed to get worse. This is an effect which has to be investigated in further studies with larger subgroups of different electrode insertion lengths or with long electrodes when recording on different CI electrode contacts.
It is commonly known that the objective estimation of low frequency hearing (especially at 250 Hz) is more difficult than the estimation of higher frequencies. Also the shift between the estimation and the hearing threshold is larger. This is in part because the noise in biological systems typically shows a relationship (Szendro, Vincze, & Szasz, 2001). In this respect, it is also remarkable that with IC ECochG recordings, the 250 Hz recording lies by far closer to the audiometric hearing threshold than with EC recordings. According to Stakhovskaya, Sridhar, Bonham, and Leake (2007), the hair cells for 1 kHz stimulation and therewith the generators of the stimulus response are located at an rotation angle from round window of nearly 360°, which means that they are located at the second turn of the cochlear quite close to the EC recording electrode. The generators for 500 Hz and especially 250 Hz are deeper in the bone and therewith the EC recording is further away. On the contrary, the CI electrode for IC recordings is by far closer to the generators. Maybe also this effect should be taken into account when interpreting objective audiometry in the low frequency range.
Since behavior will certainly be more sensitive than an evoked potential recording, thresholds better than behavior suggest the correlation is not due to a cause and effect relationship. Also it has to be taken into account that the thresholds measured in this study could be improved by using more repetitions which would lower the noise levels and therewith improve the detectability of responses closer to the thresholds. For IC recordings, we used intraoperatively 50 averages. Postoperatively, we used 100 averages which clearly improved the noise floor. With EC recordings, the noise floor is lower even with 10 averages, but here also the amplitudes of the responses are by far lower than with IC recordings.
Other research groups also detected shifts between ECochG thresholds and audiometric thresholds. Fitzpatrick et al. (2014) reported about intraoperative EC recordings at the round windows with which they were able to detect responses to stimuli presented at 90 dB from almost all (80 of 84) subjects, even those with PTA thresholds greater than 100 dB, but they did not differentiate between different stimulation frequencies.
Campbell et al. (2015) also described lower IC thresholds compared with audiometric thresholds with Nucleus CI422 electrodes, both recorded postoperatively. They found these lower thresholds for all investigated frequencies (500 Hz, 1000 Hz, and 1500 Hz with a larger shift in the higher frequencies). They hypothesized effects of surviving hair cells that react to the stimuli, but deteriorated neural structures that prevent the subject from hearing the sound. However, they reported that due to the small group in their pilot study (n = 5), they did not perform a statistical analysis, but stated that their IC ECochG course over the frequencies was in line with the course of the audiometric thresholds.
In Dalbert, Pfiffner, et al. (2015), the IC OR thresholds recorded by the Advanced Bionics system and the HiFocus Midscale electrode were lower or equal to the audiometric thresholds. They did not give the exact relations, but similar to our findings, this effect was more pronounced for 1000 Hz and less pronounced for 250 Hz with quite good relations, whereas the relation for 500 Hz altogether seemed weaker.
Koka et al. (2017) compared postoperative IC OR thresholds recorded with the Advanced Bionics system to subjective thresholds where the stimulation was delivered with the same device that was used for the ECochG recordings. They conducted the IC ECochG recordings with alternating polarity and itemized the data for summation and difference responses. In their study, the relation between IC OR and behavioral thresholds explained more than 80% of the variance. The IC OR thresholds were lower than or equal to the behavioral thresholds which was more pronounced for the summation potentials than for the difference potentials. The effect seemed to be more pronounced for the lower frequencies below 1000 Hz, but no case was reported where the IC ECochG response was clearly above the behavioral threshold. In the present study and the others cited above, the comparison was conducted with pure tone audiometry, so maybe there are some effects between subjective sensation and objective responses to short stimuli, calibrated to dB nHL, and pure tones which are calibrated to dB SPL or dB HL.
Nevertheless, when identifying correction factors, the IC ECochG recordings could be used as frequency-specific objective estimation of the hearing threshold. In the new clinical MED-EL software (MAESTRO 7.0) which was recently released, this recording tool is included, so now these recordings can be conducted routinely. A possible application is the fitting of electric acoustic stimulation where the cut-off frequency as well as the amplification of the acoustic component could be adjusted in accordance to corrected IC OR thresholds. Another application could be the objective long-term monitoring of the residual hearing. These applications are very promising especially for children where no pure tone audiometry can be conducted, but of course it has to be kept in mind that the ear canals of children are smaller and therewith the transfer of the results from adults of children has to be conducted with care.
The correlations between intraoperative OR thresholds and pre- and postoperative data were weaker than the data obtained at the same appointment. This might very well be an effect of the interventions and the elapsed time allowing for deterioration processes between the appointments. However, also the intraoperative recording protocol was kept short in order to save anesthesia time. With EC ECochG recordings, only 10 averages were performed per stimulation level, as we are aiming for a real-time clinical tool for quickly realizing an upcoming damage of the cochlea. With IC recordings, the number of averages was set to 50 compared with 100 averages postoperatively where time was less critical. With less averages, the stimulation level has to be higher in order for the response to lie significantly above noise. Mainly intraoperatively, there was some low frequency noise, as the operation theatre is not electrically shielded. For visual threshold detection and a fast detection of deteriorations, our protocol is convenient, but for automated threshold detections and a thorough analysis and cancellation of this low frequency noise, more averages and longer stimulation bursts have to be used. There were also some suspected latency shifts which could also be investigated more thoroughly when using more averages. This would improve the clarity of the signal in terms of the signal-to-noise-ratio and therewith improve the detection of the point when the response starts.
Conclusion
In this study, EC and IC ECochG recordings were conducted intra- and postoperatively and compared with the pure tone audiometry. The experience showed that there is no all-in-one device suitable for every purpose. Both ECochG methods and parameters in the study protocol have strengths and disadvantages, so the setup has to be chosen according to the desired purposes. We were looking for a fast and clinically applicable tool for online biofeedback during CI surgery. EC ECochG recordings could be such a tool. As the signal quality of the CI amplifier is usually not as good as the quality of the clinical amplifier, more averages are needed in IC recordings; therefore, EC recordings are much faster than IC recordings. For all patients, it was possible to record ORs during or directly after electrode insertion. Consequently, we conclude that we did not observe any cases with severe IC trauma. Delayed hearing loss could not be predicted with our method. Nevertheless such a tool could help to reduce immediate trauma and give valuable hints for further research in hearing preservation which should also address postoperative deterioration processes.
IC ECochG recordings can give similar hints, but during insertion, the recording site is moving. Their main advantage is a very high correlation to the pure tone audiometry threshold when conducted postoperatively at the same appointment. Thus, for wide spread intraoperative applications, more research is necessary, but in the postoperative follow up and CI fitting, IC ECochG recordings show a large potential.
Supplemental Material
Supplemental Material for Monitoring of the Inner Ear Function During and After Cochlear Implant Insertion Using Electrocochleography by Sabine Haumann, Marina Imsiecke, Günther Bauernfeind, Andreas Büchner, Victor Helmstaedter, Thomas Lenarz and Rolf B. Salcher in Trends in Hearing
Acknowledgments
We would like to thank Marek Polak, Max Fröhlich, Daniel Schurzig, and Cornelia Batsoulis from MED-EL for their technical contribution and great support throughout this project. We would also like to thank our surgeons, our nurses and technical staff from the operation theatre, our staff for function diagnosis, and our CI engineers and therapists for their support. Last but not least, we want to thank our patients who participated in this study.
Author Note
Informed consent has been obtained from all individuals included in this study. The research related to human use has been complied with all the relevant national regulations, institutional policies, and in accordance the tenets of the Helsinki Declaration and has been approved by the authors’ institutional review board or equivalent committee.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Cluster of Excellence “Hearing4all” (EXC 1077/1) of the German Research Foundation.
References
- Abbas P., Tejani V., Scheperle R., Brown C. (2017) Using neural response telemetry to monitor physiological responses to acoustic stimulation in hybrid cochlear implant users. Ear & Hearing 38(4): 409–425. doi: 10.1097/AUD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya A., Tavora-Vieira D., Rajan G. (2016) Using the implant electrode array to conduct real-time intraoperative hearing monitoring during pediatric cochlear implantation: Preliminary experiences. Otology & Neurotology 37: e148–e153. doi:10.1097/MAO.0000000000000950. [DOI] [PubMed] [Google Scholar]
- Adunka O., Giardina C., Formeister E., Choudhury B., Buchman C., Fitzpatrick D. (2016) Round window electrocochleography before and after cochlear implant electrode insertion. The Laryngoscope 126(5): 1193–1200. doi:10.1002/lary.25602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adunka O., Roush P., Grose J., Macpherson C., Buchman C. A. (2006) Monitoring of cochlear function during cochlear implantation. The Laryngoscope 116(6): 1017–1020. doi:10.1097/01.mlg.0000217224.94804.bb. [DOI] [PubMed] [Google Scholar]
- Adunka O. F., Mlot S., Suberman T. A., Campbell A. P., Surowitz J., Buchmann C. A., Fitzpatrick D. C. (2010) Intracochlear recordings of electrophysiological parameters indicating cochlear damage. Otology & Neurotology 31(8): 1233–1241. doi:10.1097/MAO.0b013e3181f1ffdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner W. D., Jappel A., Morera C., Gstöttner W., Müller J., Kiefer J., Nielsen S. (2007) Outcomes in adults implanted with the FLEXsoft electrode. Acta Oto-Laryngologica 127: 579–586. doi:10.1080/00016480600987784. [DOI] [PubMed] [Google Scholar]
- Büchner A., Illg A., Majdani O., Lenarz T. (2017) Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One 12(5): e0174900 doi:10.1371/journal.pone.0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchner A., Schüssler M., Battmer R. D., Stöver T., Lesinski-Schiedat A., Lenarz T. (2009) Impact of low-frequency hearing. Audiology & Neurotology 14(Suppl. 1): 8–13. doi:10.1159/000206490. [DOI] [PubMed] [Google Scholar]
- Calloway N., Fitzpatrick D., Campbell A., Iseli C., Pulver S., Buchman C., Adunka O. (2014) Intracochlear electrocochleography during cochlear implantation. Otology & Neurotology 35: 1451–57. doi:10.1097/MAO.0000000000000451. [DOI] [PubMed] [Google Scholar]
- Campbell L., Kaicer A., Briggs R., O’Leary S. (2015) Cochlear response telemetry: Intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otology & Neurotology 36: 399–405. doi:10.1097/MAO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- Choudhury B., Fitzpatrick D., Buchman C., Wei B., Dillon M., He S., Adunka O. (2012) Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otology & Neurotology 33(9): 1507–1515. doi:10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbert A., Pfiffner F., Röösli C., Thoele K., Sim J., Gerig R., Huber A. (2015) Extra- and intracochlear electrocochleography in cochlear implant recipients. Audiology& Neurotology 20: 339–348. doi:10.1159/000438742. [DOI] [PubMed] [Google Scholar]
- Dalbert A., Sim J., Gerig R., Pfiffner F., Röösli C., Huber A. (2015) Correlation of electrophysiological properties and hearing preservation in cochlear implant patients. Otology & Neurotology 36: 1172–1180. doi:10.1097/MAO.0000000000000768. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D., Campbell A., Choudhury B., Dillon M., Forgues M., Buchman C., Adunka O. (2014) Round window electrocochleography just prior to cochlear implantation: Relationship to word recognition outcomes in adults. Otology & Neurotology 35(1): 64–71. doi:10.1097/MAO.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgues M., Koehn H., Dunnon A., Pulver S., Buchman C., Adunka O., Fitzpatrick D. (2014) Distinguishing hair cell from neural potentials recorded at the round window. Journal of Neurophysiology 111: 580–593. doi:10.1152/jn.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. (1990) A cochlear frequency-position function for several species-29 years later. The Journal of the Acoustical Society of America 87(6): 2592–2605. doi:10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Gstoettner W., Helbig S., Maier N., Kiefer J., Radeloff A., Adunka O. (2006) Ipsilateral electric acoustic stimulation of the auditory system: Results of long-term hearing preservation. Audiology & Neurotology 11(Suppl 1): 49–56. doi:10.1159/000095614. [DOI] [PubMed] [Google Scholar]
- Harris R., Cruise A., Gibson W., Bate K., Sanli H. (2011) Preliminary results and technique for electrophysiological intra-operative monitoring of residual hearing during cochlear implantation. Cochlear Implants International 12(4): 209–215. doi:10.1179/146701011X12950038111657. [DOI] [PubMed] [Google Scholar]
- Haumann S., Hohmann V., Meis M., Herzke T., Lenarz T., Büchner A. (2012) Indication criteria for cochlear implants and hearing aids: Impact of audiological and non-audiological findings. Audiology Research 2(1): 55–64, e12. doi:10.4081/audiores.2012.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig S., de Heyning P. V., Kiefer J., Baumann U., Kleine-Punte A., Brockmeier H., Gstoettner W. (2011) Combined electric acoustic stimulation with the PULSARCI(100) implant system using the FLEX(EAS) electrode array. Acta Oto-Laryngologica 131(6): 585–595. doi:10.3109/00016489.2010.544327. [DOI] [PubMed] [Google Scholar]
- Helmstaedter V., Lenarz T., Erfurt P., Kral A., Baumhoff P. (2018) The summating potential is a reliable marker of electrode position in electrocochleography: Cochlear implant as theragnostic probe. Ear & Hearing 39(4): 687–700. doi:10.1097/AUD.0000000000000526. [DOI] [PubMed] [Google Scholar]
- Incerti P., Ching T., Cowan R. (2013) A systematic review of electric-acoustic stimulation: Device fitting ranges, outcomes, and clinical fitting practices. Trends in Amplification 17(1): 3–26. doi:10.1177/1084713813480857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C., Albegger K., Battmer R., Burdo S., Deggouj N., Deguine O., Fraysse B. (2005) Preservation of residual hearing with cochlear implantation: How and why. Acta Oto-Laryngologica 125(5): 481–491. doi:10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- Kim J. R., Tejani V., Abbas P., Brown C. (2017) Intracochlear recordings of acoustically and electrically evoked potentials in nucleus hybrid L24 cochlear implant users and their relationship to speech perception. Frontiers in Neuroscience 11: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K., Saoji A., Litvak L. (2017) Electrocochleography in cochlear implant recipients with residual hearing: Comparison with audiometric thresholds. Ear & Hearing 38(3): e161–e167. doi:10.1097/AUD.0000000000000385. [DOI] [PubMed] [Google Scholar]
- Lenarz T., Stöver T., Büchner A., Lesinski-Schiedat A., Patrick J., Pesch J. (2009) Hearing conservation surgery using the hybrid-L electrode: Results from the first clinical trial at the Medical University of Hannover. Audiology & Neurotology 14(Suppl 1): 22–31. doi:10.1159/000206492. [DOI] [PubMed] [Google Scholar]
- Mandalà M., Colletti L., Tonoli G., Colletti V. (2012) Electrocochleography during cochlear implantation for hearing preservation. Otolaryngology—Head and Neck Surgery 146(5): 774–781. doi:10.1177/0194599811435895. [DOI] [PubMed] [Google Scholar]
- Radeloff A., Shehata-Dieler W., Scherzed A., Rak K., Harnisch W., Hagen R., Mlynski R. (2012) Intraoperative monitoring using cochlear microphonics in cochlear implant patients with residual hearing. Otology & Neurotology 33: 348–354. doi:10.1097/MAO.0b013e318248ea86. [DOI] [PubMed] [Google Scholar]
- Stakhovskaya O., Sridhar D., Bonham B., Leake P. (2007) Frequency map for the human cochlear spiral ganglion: Implications for cochlear implants. Journal of the Association for Research in Otolaryngology 8: 220–233. doi:10.1007/s10162-007-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhling M., Majdani O., Salcher R., Leifholz M., Büchner A., Lesinski-Schiedat A., Lenarz T. (2016) The impact of electrode array length on hearing preservation in cochlear implantation. Otology & Neurotology 37(8): 1006–1015. doi:10.1097/MAO.0000000000001110. [DOI] [PubMed] [Google Scholar]
- Szendro P., Vincze G., Szasz A. (2001) Pink-noise behaviour of biosystems. European Biophysics Journal 30(3): 227–231. doi:10.1007/s002490100143. [DOI] [PubMed] [Google Scholar]
- Teschner M., Lenarz T., Battmer R. (2012) Validity of cochlear microphonics at high sound pressure levels as an important clinical aspect. ORL—Journal for Oto-Rhino-Laryngology, Head & Neck Surgery 74(1): 38–41. doi:10.1159/000334948. [DOI] [PubMed] [Google Scholar]
- Timm M., Majdani O., Weller T., Windeler M., Lenarz T., Büchner A., Salcher R. B. (2018) Patient specific selection of lateral wall cochlear implant electrodes based on anatomical indication ranges. PLoS One 13(10): e0206435 doi:10.1371/journal.pone.0206435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Illberg C., Baumann U., Kiefer J., Tillein J., Adunka O. (2011) Electric-acoustic stimulation of the auditory system: A review of the first decade. Audiology & Neurotology 16(Suppl 2): 1–30. doi:10.1159/000327765. [DOI] [PubMed] [Google Scholar]
- von Illberg C., Kiefer J., Tillein J., Pfenningdorff T., Hartmann R., Stürzebecher E., Klinke R. (1999) Electro-acoustic stimulation of the auditory system: New technology for severe hearing loss. ORL—Journal for Oto-Rhino-Laryngology, Head & Neck Surgery 61(6): 334–340. doi:10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- Würfel W., Lanfermann H., Lenarz T., Majdani O. (2014) Cochlear length determination using Cone Beam Computed Tomography in a clinical setting. Hearing Research 316: 65–72. doi:10.1016/j.heares.2014.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Monitoring of the Inner Ear Function During and After Cochlear Implant Insertion Using Electrocochleography by Sabine Haumann, Marina Imsiecke, Günther Bauernfeind, Andreas Büchner, Victor Helmstaedter, Thomas Lenarz and Rolf B. Salcher in Trends in Hearing