Abstract
Monitoring HIV population viral load (PVL) has been advocated as an important means of inferring HIV transmission potential and predicting the future rate of new HIV infections (HIV incidence) in a particular community. However, the relationship between PVL measures and directly measured HIV incidence has not been quantified in any setting and, most importantly, in a hyperendemic sub-Saharan African setting. We assessed this relationship using one of Africa’s largest population-based prospective population cohorts in rural KwaZulu-Natal, South Africa in which we followed 8732 HIV-uninfected participants between 2011 and 2015. Despite clear evidence of spatial clustering of high viral loads in some communities, our results demonstrate that PVL metrics derived from aggregation of viral load data only from the HIV-positive members of a particular community did not predict HIV incidence in this typical hyperendemic, rural African population. Only once we used modified PVL measures, which combined viral load information with the underlying spatial variation in the proportion of the population infected (HIV prevalence), did we find a consistently strong relationship with future risk of HIV acquisition. For example, every 1% increase in the overall proportion of a population having detectable virus (PDVP) was independently associated with a 6.3% increase in an individual’s risk of HIV acquisition (P = 0.001). In hyperendemic African populations, these modified PVL indices could play a key role in targeting and monitoring interventions in the most vulnerable communities where the future rate of new HIV infections is likely to be highest.
INTRODUCTION
The rapid scale-up of combination antiretroviral therapy (ART) to more than 19 million people with HIV (1) has resulted in substantial population-level reductions in HIV-related mortality and evidence of reductions in the rate of new HIV infections in some developed (2) and developing (3) country contexts. Notwithstanding this impact, the rate of new HIV infections remains unacceptably high, with 70% of new HIV infections continuing to occur in sub-Saharan Africa (4). Some mathematical models suggest that under certain conditions, the HIV epidemic could be reversed by 2050 if high levels of ART coverage are achieved (in combination with other effective interventions such as medical male circumcision) (5). Scaling up ART therefore remains a key global priority. In response, the Joint United Nations Programme on HIV/AIDS (UNAIDS) has set “the ambitious but achievable” targets of diagnosing 90% of all people living with HIV to initiating 90% of all diagnosed people on ART and achieving virologic suppression for 90% of people on ART by 2020, with the aim of increasing all of these targets to 95% by 2030 (6). As the world ramps up to meet and exceed these targets, it becomes critical to not only monitor progress but also provide empirical evidence for any resultant impact of expanding levels of viral suppression on life expectancy and the rate of new HIV infections at a population level.
One of the ways in which the impact of moving toward the UNAIDS 90–90–90 treatment targets could be monitored is through measuring the trends in HIV population viral load (PVL) (7, 8). The HIV viral load level in semen or blood is the single most important biological determinant of transmission between an HIV-positive and an HIV-negative individual (9, 10). It follows that an aggregation of individual HIV viral RNA concentrations for a particular geography or community over a given time period (viz, PVL) could constitute a sensitive biological index of treatment program success and potentially estimate the “transmission potential” of a particular geography. Population/community viral load has been endorsed as a key group of measures by the Centers for Disease Control and Prevention (CDC) (11) and has been used to infer both the effectiveness of a treatment program and a proxy for HIV incidence (2,12–14).
Notwithstanding the above, the concept as a measure of both transmission potential and ART program effectiveness has been critiqued (15). One key issue raised by the authors of the article is the degree to which the in-care or facility-based viral load measures are reflective of the true underlying PVL. Other issues raised include the interpretation of various PVL metrics and their relationship to ongoing HIV transmissions, ecologic bias due to aggregation over large geographic regions or heterogeneous populations, and the failure of some PVL measures to account for the underlying HIV prevalence within the community (15). To date, no study has tested the relationship between these PVL measures and directly measured HIV incidence in a hyperendemic sub-Saharan African setting.
Here, we make use of one of Africa’s largest population-based prospective cohorts, which is located in the rural KwaZulu-Natal province of South Africa, to empirically quantify the relationship between the true PVL (among all HIV-infected participants irrespective of whether these participants have accessed care or know their HIV status) and the prospective risk of HIV acquisition for more than 8700 participants who were HIV-uninfected at baseline (HIV incidence). We use novel geospatial techniques to analyze the micro-level spatial variation in three key PVL measures—called the geometric mean viral load (MVL), prevalence of detectable viremia (PDV), and community transmission index (CTI)—derived from all HIV-positive participants. We then extend these three measures not only to consider HIV-positive participants but also to evaluate the entire population (irrespective of HIV status).
RESULTS
Population-based viral load survey
This study uses data from one of the most comprehensive demographic and HIV surveillance sites in Africa—the Africa Centre Demographic Information System (16). The site has collected sociodemographic information on a population of 87,000 participants within a circumscribed geographic area (438 km2) for more than a decade. Within the study site, ongoing population-based HIV surveillance and sexual behavior surveys take place annually among all adults ≥15 years of age. Of those contacted during the 2011 survey, 78.6% agreed to be tested for HIV in the survey and provide a dried blood spot (DBS) sample (3). We performed viral load measurements on the blood spots of all 2456 participants who tested HIV-positive in the population-based HIV surveillance round of 2011. Using the Generic HIV Viral Load kit (Biocentric), we successfully obtained 2420 (98%) viral load measurements after nucleic acid extraction. The extraction method has a lower detection limit of 1550 copies/ml and is described in greater detail elsewhere (17).
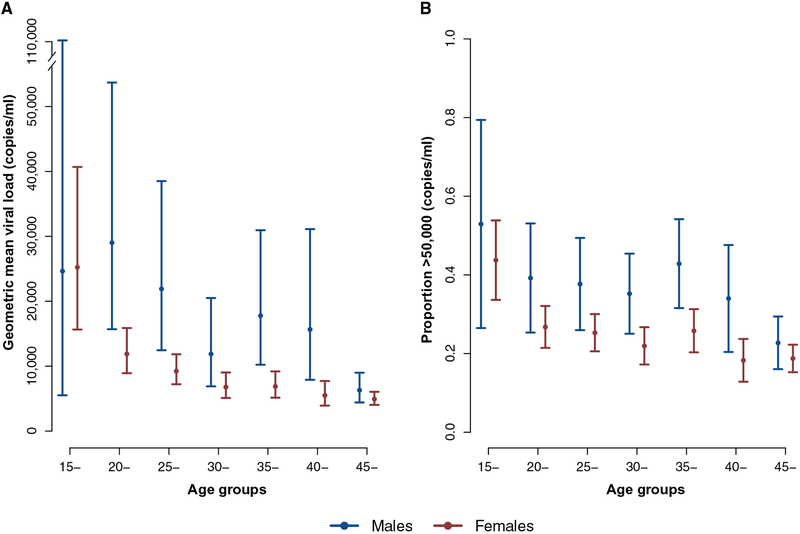
The geometric mean population-based viral load [95% confidence interval (CI)] among HIV-infected people was 8259 copies/ml (7507 to 9087 copies/ml) (Table 1). Of the 2420 HIV-positive participants in the population-based cohort, 30% (n = 726) had undetectable viral loads, and 26% (n = 629) had a viral load of >50,000 copies/ml. Males had overall higher geometric mean viral loads and a higher proportion of males were characterized by viral loads of >50,000 copies/ml (Fig. 1, A and B).
Table 1. Summary statistics: Viral load measurements by sex and age for the population-based survey data (2011).
CI, confidence interval.
| Samples | Geometric mean | >50,000 copies/ml | ||||
|---|---|---|---|---|---|---|
| N | (%) | Copies/ml | (95% CI) | Proportion | (95% CI) | |
| Overall | 2,420 | 8,259 | (7,507–9,087) | 0.26 | (0.24–0.27) | |
| Male | 506 (20.91) | 12,802 | (10,352–15,832) | 0.34 | (0.3–0.38) | |
| Female | 1,914 (79.09) | 7,356 | (6,614–8,181) | 0.24 | (0.22–0.26) | |
| Age group | ||||||
| 15− | 98 | (4.05) | 25,139 | (15,934–39,660) | 0.46 | (0.36–0.56) |
| 20− | 295 | (1219) | 13,508 | (10,376–17,586) | 0.29 | (0.24–0.35) |
| 25− | 405 | (16.74) | 10,815 | (8,606–13,592) | 0.28 | (0.24–0.33) |
| 30− | 360 | (14.88) | 7,766 | (6,026–10,008) | 0.26 | (0.22–0.31) |
| 35− | 353 | (14.59) | 8,460 | (6,529–10,963) | 0.28 | (0.23–0.32) |
| 40− | 243 | (10.04) | 6,823 | (5,026–9,262) | 0.23 | (0.18–0.29) |
| 45+ | 666 | (27.52) | 5,238 | (4,397–6,240) | 0.19 | (0.16–0.22) |
Fig. 1. Age-sex differences in viral load patterns in the 2011 population-based survey.
Geometric mean viral load (A) and proportion of viral loads (>50,000 copies/ml) (B). Estimates are shown with 95% confidence intervals.
Spatial variation in PVL indices
We used the viral load measurements of HIV-positive participants (n = 2420) from the 2011 survey to construct the following PVL indices across the study area: (i) the MVL, which is the geometric mean of the viral loads among all HIV-positive participants in the population-based survey; (ii) the PDV, which represents the proportion of the HIV-positive population that has a detectable viral load (>1550 copies/ml) (17); and (iii) the CTI, which is a potentially more sensitive biological measure than (i) and (ii) and represents the relation between the viral load and the risk of HIV transmission per unprotected sexual contact (17). To derive this measure, we used the result from Quinn et al. (9), who showed that each log10 increment in viral load was associated with a 2.45-fold increase in the rate ratio for HIV transmission risk. Following the notation of Wilson et al. (18), the risk of HIV transmission per sexual act is therefore given by β1 = 2.45log10(V1/V0)β0, where V1 is the viral load level associated with the participant, V0 = log10(150) is a baseline viral load level, and β0 = 0.003 is the probability of HIV transmission from a person with the baseline viral load level (V0) (18). The β0 value is based on a prior research undertaken in low-income settings (19). Using the standard binomial formula, we then calculated CTI = (1 − [1 – β1]100) × 100 to obtain the estimated number of transmission events that occur in 100 sexual contacts between persons at risk of infection and HIV-positive members of a particular community. We standardized all of the PVL measures against the age (10-year bands) and sex characteristics of the eligible population in 2011.
Next, we constructed population-based versions of the three PVL indices described above on the basis of the population in each community irrespective of HIV status. We use “P” (in subscript) to denote the fact that these modified versions of the PVL indices are based on information from the full population (irrespective of HIV status). In effect, all HIV-negative participants in a particular community are included by assigning a viral load value of zero (to construct the MVLP and PDVP indices) or a transmission probability of zero (to construct the CTIP index). Thus, for these analyses, we included 7919 individuals who tested HIV-negative in 2011 (total tested, 10,375). Hence, the denominator of these modified indices becomes the entire population rather than only those who have tested HIV-positive. By including information on the number of HIV-negative participants in a particular community, the modified PVL measures inherently account for the underlying spatial variation in HIV prevalence.
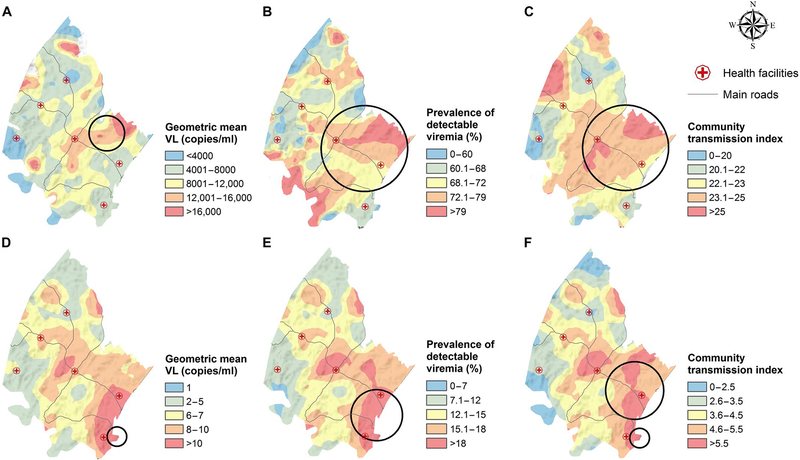
Marked spatial heterogeneity in all of the PVL measures (MVL, PDV, and CTI) was observed across the surveillance area. There was clear evidence of spatial clustering of participants with high geometric MVL (Fig. 2A) and proportion of participants with detectable viral loads (Fig. 2B) as well as the estimated number of transmission events per 100 sexual contacts between individuals at risk of infection and HIV-infected members of a particular community (Fig. 2C). Likewise, clear evidence for spatial clustering of the modified PVL measures (MVLp, PDVP, and CTIP) was observed when we considered the entire adult population (Fig. 2, D to F) in the calculation. Remarkably, in some of the high-incidence communities, although 65% of HIV-positive individuals had unsuppressed viral loads (Fig. 2B), >20% of the entire population (irrespective of HIV status, that is, PDVP) was viremic for HIV (Fig. 2E), underscoring the scale of the epidemic in these areas.
Fig. 2. Geographical variations in the population viral load (PVL) indices derived from the 2011 population-based viral load survey.
The PVL indices are derived from HIV-positive participants only (A to C) and both HIV-positive and HIV-negative participants (D to F). The PVL maps were obtained using a moving two-dimensional standard Gaussian kernel of a 3-km radius. The Kulldorff spatial clusters of high viral loads are shown as black circles. Further details of the Kulldorff spatial clustering results are given in table S9. VL, viral load.
Relationship between the PVL measures and the risk of HIV acquisition
We followed up all 8732 repeat-testers (males aged 15 to 54 and females aged 15 to 49) who were resident within the surveillance area between January 2011 and December 2015. During this period, we observed 859 seroconversions and 26,219 person-years of observation in the 8732 repeat-testers who were HIV-uninfected at baseline (crude incidence, 3.28 events per 100 person-years; Table 2). Our aim was to quantify the relationship between the PVL measures, which are derived from the HIV-positive population, and the prospective risk of HIV acquisition using a Cox proportional hazard model. We then compared these results against the set of PVL indices derived from the entire adult population (irrespective of HIV status).
Table 2.
Seroincidence rates per 100 person-years for the HIV cohort of repeat-testers who were HIV-uninfected at baseline (2011).
| Person-years | Events | Incidence per 100 person-years | ||
|---|---|---|---|---|
| Rate | 95% CI | |||
| Population HIV prevalence | ||||
| 15–24.9% | 15,693 | 480 | 3.06 | (2.8–3.34) |
| 25+% | 9,590 | 361 | 3.76 | (3.4–4.17) |
| Sex | ||||
| Female | 16,276 | 665 | 4.09 | (3.79–4.41) |
| Age strata | ||||
| 20− | 4,998 | 287 | 5.74 | (5.11–6.45) |
| 25− | 2,838 | 157 | 5.53 | (4.73–6.47) |
| 30− | 1,727 | 66 | 3.82 | (3–4.86) |
| 35− | 1,735 | 29 | 1.67 | (1.16–2.4) |
| 40− | 2,015 | 30 | 1.49 | (1.04–2.13) |
| 45+ | 3,675 | 33 | 0.9 | (0.64–1.26) |
| Area of residence | ||||
| Peri-urban | 8,381 | 312 | 3.72 | (3.33–4.16) |
| Urban | 1,019 | 37 | 3.63 | (2.63–5.01) |
| Marital status | ||||
| Married monogamous | 5,898 | 196 | 3.32 | (2.89–3.82) |
| Married polygamous | 5,242 | 215 | 4.1 | (3.59–4.69) |
| Number of partners in the last 12 months | ||||
| One | 10,291 | 368 | 3.58 | (3.23–3.96) |
| More than one | 4,100 | 163 | 3.98 | (3.41–4.64) |
| Household wealth quintile | ||||
| 2nd poorest | 5,428 | 163 | 3.00 | (2.58–3.5) |
| 3rd poorest | 5,932 | 218 | 3.67 | (3.22–4.2) |
| 4th poorest | 5,363 | 172 | 3.21 | (2.76–3.72) |
| Wealthiest | 4,146 | 105 | 2.53 | (2.09–3.07) |
| N = 8,732 | ||||
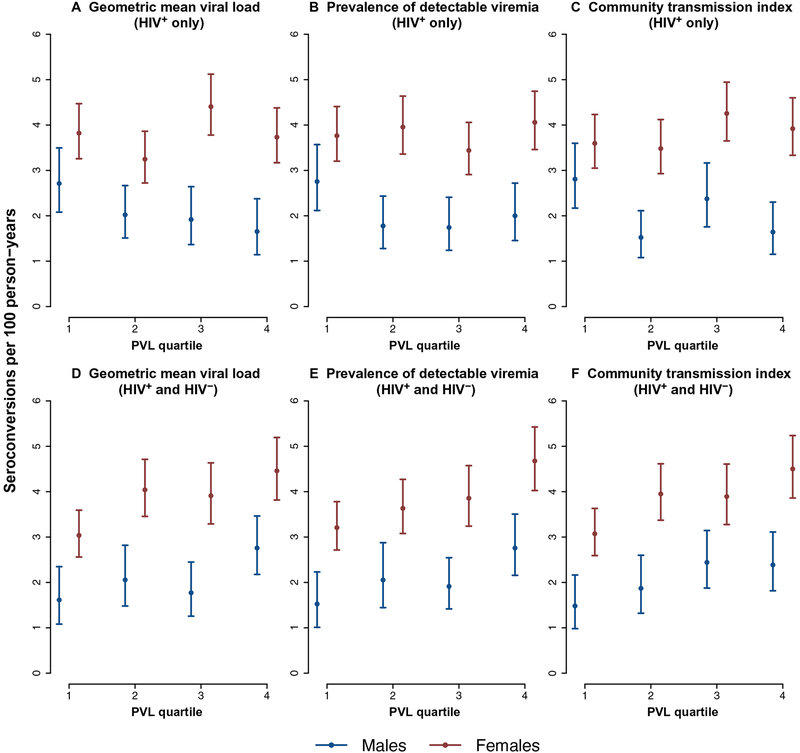
At an ecological level, communities with the overall highest viral loads and highest levels of unsuppressed viral loads were not characterized by the highest prospective crude HIV incidence (Fig. 3, A to C). However, with respect to the measures constructed on the basis of those who were HIV-infected and those who were HIV-uninfected, there was a clear dose-response pattern evident with communities with the highest overall viral loads across the whole population (irrespective of HIV status) having the highest crude HIV incidence (Fig. 3, D to F).
Fig. 3. Graphs showing the ecological relationship between HIV incidence (2011 to 2015) and PVL quartiles derived from the 2011 population-based viral load survey (quartile 1, communities with lowest PVL values).
The PVL indices are derived from HIV-positive participants only (A to C) and HIV-positive and HIV-negative participants (D to F). Incidence estimates are shown with 95% confidence intervals.
Results for the individual-level Cox proportional hazard models demonstrated that none of the PVL measures derived from the HIV-positive population predicted future individual HIV acquisition risk, both before and after controlling for key socioeconomic and behavioral factors, as well as HIV prevalence in the surrounding local community [adjusted hazard ratio (aHR), 1.000 to 1.048; P values ranged from 0.057 to 0.966], as shown in Table 3 (models A1 to A3). Addition of the PVL indices to the base model did not improve the model fit, as determined by the Akaike information criterion.
Table 3. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition and three PVL measures.
The PVL measures were derived from the HIV-positive cases (models A1 to A3) and the HIV-positive and HIV-negative cases (models B1 to B3) of a population-based survey. The full output is given in tables S1 and S2. Model 1 shows the unadjusted hazard ratios (HRs) for the PVL measures; model 2 shows these HRs after adjusting for age, sex, urban status, marital status, number of sexual partners in the last year, and household wealth; model 3 shows these HRs after adjusting for the model 2 covariates as well as HIV prevalence.
| Geometric mean viral load* | Prevalence detectable viremia† |
Community transmission index‡ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | HR | (95% CI) | P | |
| Population-based: HIV-positive cases only | |||||||||
| Model A2: Adjusted HR without HIV prevalence | 1.000 | (1.000–1.000) | 0.817 | 1.005 | (0.994–1.015) | 0.401 | 1.037 | (0.990–1.086) | 0.130 |
| Model A3: Adjusted HR with HIV prevalence | 1.000 | (1.000–1.000) | 0.475 | 1.008 | (0.996–1.019) | 0.190 | 1.048 | (0.999–1.100) | 0.057 |
| Population-based: HIV-positive and HIV-negative cases | |||||||||
| Model B2: Adjusted HR without HIV prevalence | 1.079 | (1.046–1.113) | <0.001 | 1.070 | (1.039–1.103) | <0.001 | 1.224 | (1.121–1.337) | <0.001 |
| Model B3: Adjusted HR with HIV prevalence | 1.091 | (1.045–1.138) | <0.001 | 1.063 | (1.025–1.103) | 0.001 | 1.193 | (1.079–1.320) | 0.001 |
| N | 8,732 | 8,732 | 8,732 | ||||||
For a one unit increase in geometric mean viral load.
For a 1% increase in the prevalence of detectable viremia.
For a predicted one transmission event increase per 100 sexual contacts.
By contrast, our results show that all of the modified PVL indices based on the entire adult population (irrespective of HIV status) were highly predictive of future HIV acquisition risk both before and after controlling for key socioeconomic and behavioral factors (Table 3, models B1 and B2). In addition, these indices were robust to the addition of HIV prevalence in the model (Table 3, model B3). A one-unit increase in the MVLP was independently associated with a 9.1% increase in the risk of HIV acquisition (aHR, 1.091; P < 0.001; Table 3, model B3). Every 1% increase in the PDVP was independently associated with a 6.3% increase in the risk of HIV acquisition (aHR, 1.063; P = 0.001; Table 3, model B3). Finally, every additional unit increase in the CTIp was independently associated with a 19.3% increase in the risk of HIV acquisition (aHR, 1.193; P = 0.001; Table 3, model B3).
HIV prevalence in the surrounding local community was a strong predictor of risk of HIV acquisition before and after controlling for other multiple independent risk factors (table S1). However, as expected, this strong HIV prevalence effect was rendered insignificant by the addition of any of the modified PVL metrics that were calculated on the entire population irrespective of HIV status (table S2). This is because these modified PVL indices already inherently account for variations in HIV prevalence, as they incorporate information on the spatial distribution of both HIV-negative and HIV-positive cases. In line with our previous work, across all the models, being older (>35 years), reporting no sexual partners in the last 12 months (versus one or more), and living in a household with higher wealth were protective against HIV acquisition (3).
Sex-specific PVL patterns and risk of acquisition of infection in the opposite sex
As a further robustness check of our findings, we constructed sex-specific PVL indices in the same manner. We then conducted a set of parallel analyses to ascertain the degree to which a female’s HIV acquisition hazard was related to viral load patterns (and HIV prevalence) of men in the surrounding local community and vice versa. The ability to detect any PVL effect on HIV acquisition risk is markedly attenuated in these analyses (because of smaller overall observation time and numbers of events) and the fact that the PVL measures are subject to more random noise, given the smaller number of observations used in their construction. Nevertheless, this suite of analyses also confirmed the same pattern described in the mixed sex analyses above. In these models, the PVL measures of the HIV-positive population was similarly not associated with HIV acquisition risk in the opposite sex both before and after adjustment for key socioeconomic, behavioral risk factors, and HIV prevalence in the surrounding local community (P values ranged from 0.18 to 0.99; see Tables 4 and 5 and tables S3 and S4). By contrast, all of the modified PVL measures that included information on the HIV-negative population were strongly predictive of acquisition risk in the opposite sex (P values ranged from <0.001 to 0.048; see also tables S5 and S6).
Table 4. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for females and the three male PVL measures.
The PVL measures were derived from the HIV-positive males (models A1 to A3) and the HIV-positive and HIV-negative males (models B1 to B3) of a population-based survey. The full output is given in tables S3 and S5. Model 1 shows the unadjusted HRs for the PVL measures; model 2 shows these HRs after adjusting for age, sex, urban status, marital status, number of sexual partners in the last year, and household wealth; model 3 shows these HRs after adjusting for the model 2 covariates as well as HIV prevalence.
| HIV acquisition risk for females | Geometric mean viral load* | Prevalence detectable viremia† | Community transmission index‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | HR | (95% CI) | P | |
| Population-based: HIV-positive males only | |||||||||
| Model A2: Adjusted HR without HIV prevalence | 1.000 | (1.000–1.000) | 0.836 | 1.001 | (0.995–1.008) | 0.662 | 1.003 | (0.991–1.016) | 0.599 |
| Model A3: Adjusted HR with HIV prevalence | 1.000 | (1.000–1.000) | 0.487 | 1.003 | (0.996–1.011) | 0.393 | 1.004 | (0.990–1.018) | 0.563 |
| Population-based: HIV-positive and HIV-negative males | |||||||||
| Model B2: Adjusted HR without HIV prevalence | 1.056 | (1.006–1.108) | 0.027 | 1.039 | (1.013–1.066) | 0.003 | 1.095 | (1.025–1.171) | 0.007 |
| Model B3: Adjusted HR with HIV prevalence | 1.160 | (1.058–1.271) | 0.001 | 1.061 | (1.020–1.104) | 0.004 | 1.110 | (1.015–1.214) | 0.022 |
| N | 5,188 | 5,188 | 5,188 | ||||||
For a one unit increase in geometric mean viral load.
For a 1% increase in the prevalence of detectable viremia.
For a predicted one transmission event increase per 100 sexual contacts.
Table 5. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for males and the three female PVL measures.
The PVL measures were derived from the HIV-positive females (models A1 to A3) and the HIV-positive and HIV-negative females (models B1 to B3) of a population-based survey. The full output is given in tables S4 and S6. Model 1 shows the unadjusted HRs for the PVL measures; model 2 shows these HRs after adjusting for age, sex, urban status, marital status, number of sexual partners in the last year, and household wealth; model 3 shows these HRs after adjusting for the model 2 covariates as well as HIV prevalence.
| HIV acquisition risk for males | Geometric mean viral load* | Prevalence detectable viremia† |
Community transmission index‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | HR | (95% CI) | P | |
| Population-based: HIV-positive females only | |||||||||
| Model A2: Adjusted HR without HIV prevalence | 1.000 | (1.000–1.000) | 0.989 | 0.997 | (0.972–1.021) | 0.782 | 1.010 | (0.943–1.082) | 0.773 |
| Model A3: Adjusted HR with HIV prevalence | 1.000 | (1.000–1.000) | 0.985 | 0.997 | (0.971–1.024) | 0.809 | 1.008 | (0.933–1.088) | 0.845 |
| Population-based: HIV-positive and HIV-negative females | |||||||||
| Model B2: Adjusted HR without HIV prevalence | 1.053 | (1.009–1.099) | 0.018 | 1.077 | (1.004–1.155) | 0.039 | 1.270 | (1.020–1.581) | 0.032 |
| Model B3: Adjusted HR with HIV prevalence | 1.081 | (1.009–1.158) | 0.028 | 1.106 | (1.001–1.221) | 0.048 | 1.452 | (1.057–1.997) | 0.021 |
| N | 3,544 | 3,544 | 3,544 | ||||||
For a one unit increase in geometric mean viral load.
For a 1% increase in the prevalence of detectable viremia.
For a predicted one transmission event increase per 100 sexual contacts.
PVL measures derived from routine data collected at health facilities
Because facility-based viral load data are typically used to derive community viral load metrics in some settings, we also wanted to establish the degree to which facility-based viral load measurements corresponded with their population-based counterparts and whether such data could be harnessed to infer transmission potential. We used the viral load measurements from routine clinical data collected at the facility level for 3196 patients living in the surveillance population who visited 1 of the 17 health care clinics in 2011 to compute the facility-based viral load versions of the measures described above (20). We were able to link these patients to the population database. All participants from the population- and facility-based viral load surveys were geolocated to their exact homestead of residence (3).
Results show that the overall geometric mean (95% CI) viral load for the facility-based data was 819 copies/ml (771 to 870 copies/ml) (table S7). Of the 3196 HIV-positive participants in the facility-based cohort, 87% (n = 2789) had undetectable viral loads, and 4% (n = 133) had a viral load of >50,000 copies/ml. Males had overall higher geometric mean viral loads and a higher proportion of males were characterized by viral loads of >50,000 copies/ml (fig. S1).
Use of routine facility-based viral load data (20) to construct the viral load indices demonstrated that there was little or no correlation between these indices and those derived using the equivalent population-based viral load data (correlation coefficient, −0.089, −0.32, and −0.28 for MVL, PDV, and CTI, respectively). Empirically, there was no relationship found between any of the facility-based viral load measures and future risk of acquisition of HIV infection both before and after adjustment for key risk factors, as well as HIV prevalence in the surrounding local community (table S8).
DISCUSSION
Our study has empirically tested the relationship between various PVL metrics and the prospective risk of HIV acquisition in a typical hyperendemic rural African setting. Despite identifying remarkable spatial variation in viral load patterns across the study area, with clear evidence of clustering of high viral loads in some communities, we did not find a relationship between any of the PVL indices derived solely from HIV-infected participants and prospective HIV incidence. Our findings occurred in the context of using population-based viral load measurements that were largely free from the biases typically associated with health facility-based viral load data. Only when we evaluated the viral load information as a function of the entire population (both HIV-positive and HIV-negative participants) was the resulting measures highly predictive of risk of new HIV infection. This pattern also held in a series of parallel analyses in which we tested the relationship between the sex-specific PVL patterns and the HIV acquisition risk in the opposite sex. Furthermore, our results demonstrate that in this rural African setting, facility-based viral load measures do not correlate well with their population-based counterparts and were not predictive of HIV incidence. On the basis of these findings, we would therefore caution against using such data to infer transmission potential in similar settings.
Another important finding to emerge from the analyses is the large differences in HIV viral load by sex at a population level. Overall, males had a substantially higher proportion with viral loads of >50,000 copies/ml (34% versus 24%) and higher geometric mean viral load (12,802 copies/ml versus 7,356 copies/ml) in comparison to females. This finding likely reflects the fact that men are less likely to be tested for HIV and successfully link to HIV care, as well as being less likely to successfully adhere to treatment, supporting previous findings in this setting and others (21–23).
Community viral load has been used as a proxy for HIV transmission potential and/or program effectiveness in many developed (2,12,24,25) and developing (14) country contexts. Previous work has been based on ecological associations between group-level variables and group-level outcomes and, hence, do not provide a strong basis for causal inference (26). Most recently, Solomon and colleagues conducted a cross-sectional study in India, which estimated the site-level correlations between four community viral load measures and an estimate of HIV incidence (derived from a multiassay algorithm) in people who inject drugs and men who have sex with men (14). In this high-risk population grouping, the authors demonstrate site-level correlations between three community viral load measures and the assay-derived estimate of HIV incidence. The “prevalence of viremia” measure (defined as the local prevalence of HIV-infected individuals with HIV viral loads of >150 copies/ml) showed the strongest correlation to the incidence estimate. Similar to the findings in our study, the “in-care viral load” measure was not correlated with the HIV incidence estimate among the high-risk groups, despite being a commonly used community viral load measure (2, 12).
Existing methods of calculating the community viral load have been critiqued for a number of reasons related to (i) the use of non-representative samples in facility-based (or in-care) data, (ii) the failure to incorporate information on population HIV prevalence, and (iii) improper aggregation across wide geographic regions or heterogeneous populations that make ecological biases unavoidable (15). We constructed PVL measures that address the first criticism by using information from all HIV-infected participants from a full population cohort (irrespective of their linkage to care or knowledge of HIV status) and address the second criticism by modifying the recommended PVL measures to incorporate information on the underlying variations in HIV prevalence (by including information on the distribution of HIV seronegative individuals in the derivation of the PVL indices). We have addressed the third criticism by creating a sensitive and relevant PVL estimate derived from the unique community around each HIV-uninfected member of the population-based, seroincidence cohort and directly measuring the time to HIV seroconversion in each individual in the cohort. Using this approach, we were able to adjust for a wide range of individual-level confounders in the analysis and avoid ecological fallacies in interpretation of the results.
Nevertheless, our work has some limitations. Most notably, the link between the PVL in the surrounding local population and HIV acquisition risk is dependent on a reasonable proportion of the population preferentially selecting partners from the surrounding local community. However, if this was not the case, we would not expect any of the community-level PVL indices used in the analyses to significantly influence the risk of HIV acquisition. As indicated above, we find strong independent relationships between all of the modified PVL measures that analyze the entire population (irrespective of HIV status) and risk of HIV acquisition. Moreover, in our previous work, we reported that partner choice is strongly affected by geography with 61% of women reporting at least one partnership with a man in the same immediate local Zulu community over a 5-year period (27). A potential second limitation is that we treat the PVL exposure as being time-invariant. Although unlikely, it is theoretically possible that there could be large systematic geographic shifts in viral load patterns over time that could bias the result (particularly in a more sensitive PVL measure such as the CTI). However, such a “measurement error” would likely bias the finding toward the null hypothesis and would not account for the strong positive associations identified between all of the modified PVL measures and future risk of HIV acquisition. In addition, there are difficulties common to any PVL measure in extrapolating the biological differences seen within-host to the population in a meaningful way. Such difficulties are particularly salient for sexually transmitted infections that are dependent on highly selective sexual partnership patterns including the phenomenon of HIV “serosorting” (28). Thus, large differences in viral load patterns between two populations would be relatively meaningless from a transmission potential perspective if there is a high degree of serosorting or if high viral loads occurred mainly in the segment of the population that was no longer sexually active as a result of, for example, HIV-related illness.
In our previous work, we demonstrated substantial space-time differences in ART coverage in this population, as the ART program had scaled up over 8 years (2004 to 2011). These large longitudinal differences in ART coverage independently predicted individual HIV acquisition risk (3). By 2011 (the year in which the viral load survey was conducted), the heterogeneity in ART coverage had decreased markedly (interquartile range, 31 to 41% in 2011 versus 0 to 27% for the whole study period) (3). With the reduced variation in ART coverage, unsurprisingly, we lack the power to see a significant effect between the cross-sectional estimate of ART coverage in 2011 and prospective risk of HIV acquisition (aHR, 1.00; P = 0.389, adjusting for HIV prevalence and other cofactors). PVL (especially as a function of the whole population irrespective of HIV status) is a more precise metric for deriving the proportion of the population capable of onward transmission of HIV. Gains in precision in the exposure measurement increase the power to detect significant effects. Hence, our analysis was able to detect the relationship between the modified PVL indices and the hazard of HIV acquisition.
There has been much discussion and debate about the utility of PVL measures to infer HIV transmission potential (14, 15, 29). Our results demonstrate that HIV PVL indices that explicitly account for variations in the relative size of the HIV-uninfected population could play a key role in targeting prevention interventions to vulnerable communities in hyperendemic African populations. Recent work has revealed the existence of subepidemics in many generalized epidemic settings, providing a clear rationale for targeting areas of high transmission (30). There has also been a recent shift toward a more locally tailored epidemic response based on differences in both the underlying epidemiology and characteristics of the population at risk (31–33). In this regard, our findings show that even in a severely affected rural African population with a well-established HIV treatment program, a PVL measure, such as the proportion of the overall population having HIV viremia (PDVp), could play a role in targeting and monitoring the effectiveness of interventions in the most vulnerable communities where future levels of HIV incidence are likely to be highest.
MATERIALS AND METHODS
Study design
Since 2004, the Africa Health Research Institute has conducted annual population-based HIV testing of all consenting adults aged 15 years or older (16). After obtaining written informed consent, field workers collect blood by finger prick and prepare DBSs for HIV testing according to the UNAIDS and World Health Organization’s Guidelines for Using HIV Testing Technologies in Surveillance. We conducted a population-based viral load survey in 2011 (described below) in the surveillance area and followed up participants known to be HIV-negative on 1 January 2011 until December 2015. We then used this information to quantify the relationship between PVL in the surrounding local community and future risk of acquisition of HIV infection. The analysis is described in detail below.
Setting
The study site has collected detailed sociodemographic and health-related information on a population of about 87,000 individuals within a circumscribed geographic area (438 km2 in area) in rural KwaZulu-Natal, South Africa (16). One of the strengths of the comprehensive surveillance platform is its longitudinal integrity and ability to record exact periods of time spent living at multiple locations by each individual under surveillance. In the study population, 29% of the adult population is infected with HIV (34). The rate of new infections remains high and relatively constant over time at about three new infections per 100 person-years (3, 35). The incidence of new HIV infection peaks in women at about 7.5 per 100 person-years (at age 25) and in men at 5 per 100 person-years (at age 30). The population is characterized by low levels of marriage, with only 31% of women and 23% of men ever having been married and 14% of those marriages being polygamous for men (36).
Construction of the PVL measures
We used the viral load measurements of the 2011 population-based survey (n = 2420) to obtain sensitive and realistic PVL measures in the unique virtual community surrounding each homestead in the study area. These PVL measures were computed by means of a moving two-dimensional Gaussian kernel of a 3-km search radius (37). The size of the kernel was determined from the results of a previous work (38). First, all participants were located to an exact homestead of residence, and the viral load measurements are superimposed on a geographic representation of the study area consisting of a grid of 30 × 30-m pixels. Next, the kernel moves systematically across the grid and calculates a Gaussian-weighted estimate of the PVL measure for the unique neighborhood around each and every pixel on the grid. The method is well suited to the scattered distribution of the population because it does not impose any static geographical boundaries on the data. Instead, it uses the precise location of each homestead to derive a PVL measure that is both responsive to local variations and robust to the effects of random noise.
We used exactly the same methods to quantify spatial variations in the viral load indices obtained from routine clinical data. These data were collected at the facility level for 3196 patients living in the surveillance population who visited 1 of the 17 health care clinics in the sub-district in 2011. Patients were geolocated to their exact homestead of residence through linkage to the population database (20).
Statistical analysis
We followed up all 8732 repeat-testers who were resident within the surveillance area between January 2011 and December 2015. A repeat-tester is a study participant who was known to be HIV-negative on 1 January 2011 and who was tested for HIV during the study period. Given the time to event structure of the data, we used Cox proportional hazard models to conduct the analyses. Because the data are interval-censored, we imputed a single random seroconversion date (using a uniform distribution) between the repeat-tester’s latest HIV-negative and earliest HIV-positive dates, as described in greater detail elsewhere (39). Participants seldom test every year, and the median interval of time between the last HIV-negative and first HIV-positive tests in this group of repeat-testers is 1.74 years.
We evaluated each of the PVL measures separately in the analysis. We adjusted for well-established determinants of HIV acquisition identified in our previous works (3, 35), that is, sex, age, area of residence (rural/urban), marital status, number of partners in the last 12 months, household socioeconomic status (based on household assets), and HIV prevalence in the unique community surrounding each HIV-negative individual. We computed community-level HIV prevalence using the Gaussian kernel methodology described above on the basis of the data from 10,375 participants who participated in population-based HIV testing in 2011 (3). We treated the PVL measures and the covariates as time-invariant. In other words, a repeat-tester was exposed to the PVL of his or her surrounding local community for the duration of the study (that is, until the right censoring date). As a further robustness check of our findings, we conducted a series of analyses to test whether the same patterns could be seen in the relationship between sex-specific PVL patterns and risk of acquisition of infection in the opposite sex. Last, we performed a further set of parallel analyses to ascertain whether there was any relationship between the viral load indices derived using the routine facility–based viral load data and future risk of HIV acquisition.
Supplementary Material
www.sciencetranslationalmedicine.org/cgi/content/full/9/420/eaam8012/DC1
Fig. S1. Proportion of facility-based viral loads of >50,000 copies/ml by sex for the year 2011.
Table S1. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between risk of HIV acquisition and three PVL measures constructed from the HIV-positive cases of a population-based survey.
Table S2. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between risk of HIV acquisition and three PVL measures constructed from the HIV-positive and HIV-negative cases of a population-based survey.
Table S3. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for females and the three PVL measures.
Table S4. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for males and the three PVL measures.
Table S5. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for females and the three PVL measures.
Table S6. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for males and the three PVL measures.
Table S7. Summary statistics: Viral load measurements by sex and age for the routine facility–based data.
Table S8. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between risk of HIV acquisition and three PVL measures constructed from the routine facility–based survey data.
Table S9. Kulldorff spatial clustering results for the PVL measures shown in Fig. 2.
Funding:
This research was supported by NIH grants (R01HD084233 and R01AI124389) from the National Institute of Child Health and Human Development. Funding for the Demographic Surveillance Information System and Population-based HIV survey was received from the Wellcome Trust, UK. F.T., T.d.O., A.V., and A.T. were supported by the South African Medical Research Council Flagship (MRC-RFA-UFSP-01–2013/UKZN HIVEPI). F.T. and T.d.O. were supported by Newton Advanced Fellowships from the U.K. Royal Society. T.B. was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professorship endowed by the German Federal Ministry of Education and Research.
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS), Ending AIDS. Progress towards the 90–90–90 targets, in Global AIDS Update (UNAIDS, 2017). [Google Scholar]
- 2.Montaner JSG, Lima VD, Barrios R, Yip B, Wood E, Kerr T, Shannon K, Harrigan PR, Hogg RS, Daly P, Kendall P, Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: A population-based study. Lancet 376, 532–539 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L, High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 339, 966–971 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS, Global AIDS response progress reporting 2013: Construction of core indicators for monitoring the 2011 UN political declaration on HIV/AIDS includes additional WHO/UNICEF universal access health sector indicators (UNAIDS, 2013). [Google Scholar]
- 5.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG, Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet 373, 48–57 (2009). [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS, 90–90–90: An ambitious treatment target to help end the AIDS epidemic (UNAIDS, 2014). [Google Scholar]
- 7.Jain V, Byonanebye DM, Liegler T, Kwarisiima D, Chamie G, Kabami J, Petersen ML, Balzer LB, Clark TD, Black D, Thirumurthy H, Geng EH, Charlebois ED, Amanyire G, Kamya MR, Havlir DV; SEARCH Collaboration, Changes in population HIV RNA levels in Mbarara, Uganda during scale-up of HIV antiretroviral therapy access. J. Acquir. Immune Defic. Syndr 65, 327–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain V, Liegler T, Kabami J, Chamie G, Clark TD, Black D, Geng EH, Kwarisiima D, Wong JK, Abdel-Mohsen M, Sonawane N, Aweeka FT, Thirumurthy H, Petersen ML, Charlebois ED, Kamya MR, Havlir DV, Assessment of population-based HIV RNA levels in a rural east African setting using a fingerprick-based blood collection method. Clin. Infect. Dis 56, 598–605 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH, Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med 342, 921–929 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med 365, 493–505 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC), Guidance on Community Viral Load: A Family of Measures, Definitions, and Method for Calculation (CDC, 2011). [Google Scholar]
- 12.Castel AD, Befus M, Willis S, Griffin A, West T, Hader S, Greenberg AE, Use of the community viral load as a population-based biomarker of HIV burden. AIDS 26, 345–353 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Das M, Chu PL, Santos G-M, Scheer S, Vittinghoff E, McFarland W, Colfax GN, Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLOS ONE 5, e11068 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon SS, Mehta SH, McFall AM, Srikrishnan AK, Saravanan S, Laeyendecker O, Balakrishnan P, Celentano DD, Solomon S, Lucas GM, Community viral load, antiretroviral therapy coverage, and HIV incidence in India: A cross-sectional, comparative study. Lancet HIV 3, e183–e190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WC, Powers KA, Smith MK, Cohen MS, Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect. Dis 13, 459–464 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanser F, Hosegood V, Bärnighausen T, Herbst K, Nyirenda M, Muhwava W, Newell C, Viljoen J, Mutevedzi T, Newell M-L, Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int. J. Epidemiol 37, 956–962 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viljoen J, Gampini S, Danaviah S, Valéa D, Pillay S, Kania D, Méda N, Newell M-L, Van de Perre P, Rouet F; World Health Organization/ANRS 1289 Kesho Bora Study Group, Dried blood spot HIV-1 RNA quantification using open real-time systems in South Africa and Burkina Faso. J. Acquir. Immune Defic. Syndr 55, 290–298 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM, Relation between HIV viral load and infectiousness: A model-based analysis. Lancet 372, 314–320 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Boily M-C, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M, Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. Lancet Infect. Dis 9, 118–129 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houlihan CF, Bland RM, Mutevedzi PC, Lessells RJ, Ndirangu J, Thulare H, Newell M-L, Cohort profile: Hlabisa HIV treatment and care programme. Int. J. Epidemiol 40, 318–326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MP, Larson B, Rosen S, Defining retention and attrition in pre-antiretroviral HIV care: Proposals based on experience in Africa. Trop. Med. Int. Health 17, 1235–1244 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen S, Fox MP, Retention in HIV care between testing and treatment in sub-Saharan Africa: A systematic review. PLOS Med. 8, e1001056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, Tanser F, Pillay D, Bärnighausen T, Mass HIV treatment and sex disparities in life expectancy: Demographic surveillance in rural south africa. PLOS Med. 12, e1001905 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood E, Kerr T, Marshall BDL, Li K, Zhang R, Hogg RS, Harrigan PR, Montaner JSG, Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: Prospective cohort study. BMJ 338, b1649 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stall R, Friedman M, Marshal M, Wisniewski S, What’s driving the U.S. epidemic in men who have sex with men?, paper presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 4 February 2008. [Google Scholar]
- 26.Susser M, Causal Thinking in the Health Sciences: Concepts and Strategies of Epidemiology (Oxford Univ. Press, 1973). [Google Scholar]
- 27.Tanser F, Bärnighausen T, Hund L, Garnett GP, McGrath N, Newell M-L, Effect of concurrent sexual partnerships on rate of new HIV infections in a high-prevalence, rural South African population: A cohort study. Lancet 378, 247–255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delva W, Helleringer S, Beyond risk compensation: Clusters of antiretroviral treatment (ART) users in sexual networks can modify the impact of ART on HIV incidence. PLOS ONE 11, e0163159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbeck J, Tanser F, Community viral load as an index of HIV transmission potential. Lancet HIV 3, e152–e154 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Tanser F, de Oliveira T, Maheu-Giroux M, Bärnighausen T, Concentrated HIV subepidemics in generalized epidemic settings. Curr. Opin. HIV AIDS 9, 115–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coburn BJ, Okano JT, Blower S, Using geospatial mapping to design HIV elimination strategies for sub-Saharan Africa. Sci. Transl. Med 9, eaag0019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson S-J, Cherutich P, Kilonzo N, Cremin I, Fecht D, Kimanga D, Harper M, Masha RL, Ngongo PB, Maina W, Dybul M, Hallett TB, Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: A modelling study. Lancet 384, 249–256 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Tanser F, Bärnighausen T, Dobra A, Sartorius B, Identifying ‘corridors of HIV transmission’ in a severely affected rural South African population: A case for a shift toward targeted prevention strategies. Int. J. Epidemiol dyx257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaidi J, Grapsa E, Tanser F, Newell M-L, Bärnighausen T, Dramatic increases in HIV prevalence after scale-up of antiretroviral treatment: A longitudinal population-based HIV surveillance study in rural kwazulu-natal. AIDS 27, 2301–2305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandormael A, Newell M-L, Bärnighausen T, Tanser F, Use of antiretroviral therapy in households and risk of HIV acquisition in rural KwaZulu-Natal, South Africa, 2004–12: A prospective cohort study. Lancet Glob. Health 2, e209–e215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosegood V, McGrath N, Moultrie T, Dispensing with marriage: Marital and partnership trends in rural KwaZulu-Natal, South Africa 2000–2006. Demogr. Res 20, 279–312 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waller LA, Gotway CA, Applied Spatial Statistics for Public Health Data (John Wiley & Sons, 2004), vol. 368. [Google Scholar]
- 38.Tanser F, Bärnighausen T, Cooke GS, Newell M-L, Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int. J. Epidemiol 38, 1008–1016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandormael A, Dobra A, Bärnighausen T, de Oliveira T, Tanser F, Incidence rate estimation, periodic testing, and the limitations of the mid-point imputation approach. Int. J. Epidemiol 2017, dyx134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
www.sciencetranslationalmedicine.org/cgi/content/full/9/420/eaam8012/DC1
Fig. S1. Proportion of facility-based viral loads of >50,000 copies/ml by sex for the year 2011.
Table S1. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between risk of HIV acquisition and three PVL measures constructed from the HIV-positive cases of a population-based survey.
Table S2. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between risk of HIV acquisition and three PVL measures constructed from the HIV-positive and HIV-negative cases of a population-based survey.
Table S3. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for females and the three PVL measures.
Table S4. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for males and the three PVL measures.
Table S5. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for females and the three PVL measures.
Table S6. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between the risk of HIV acquisition for males and the three PVL measures.
Table S7. Summary statistics: Viral load measurements by sex and age for the routine facility–based data.
Table S8. Results of the multivariable analysis (Cox proportional hazard model) to examine the relationship between risk of HIV acquisition and three PVL measures constructed from the routine facility–based survey data.
Table S9. Kulldorff spatial clustering results for the PVL measures shown in Fig. 2.