Abstract
Background
The Strategy to Reduce the Incidence of Postoperative Delirium in the Elderly trial tested the hypothesis that limiting sedation during spinal anaesthesia decreases in-hospital postoperative delirium after hip fracture repair. This manuscript reports the secondary outcomes of this trial, including mortality and function.
Methods
Two hundred patients (≥65 yr) undergoing hip fracture repair with spinal anaesthesia were randomised to heavier [modified Observer's Assessment of Alertness/Sedation score (OAA/S) 0–2] or lighter (OAA/S 3–5) sedation, and were assessed for postoperative delirium. Secondary outcomes included mortality and return to pre-fracture ambulation level at 1 yr. Kaplan–Meier analysis, multivariable Cox proportional hazard model, and logistic regression were used to evaluate intervention effects on mortality and odds of ambulation return.
Results
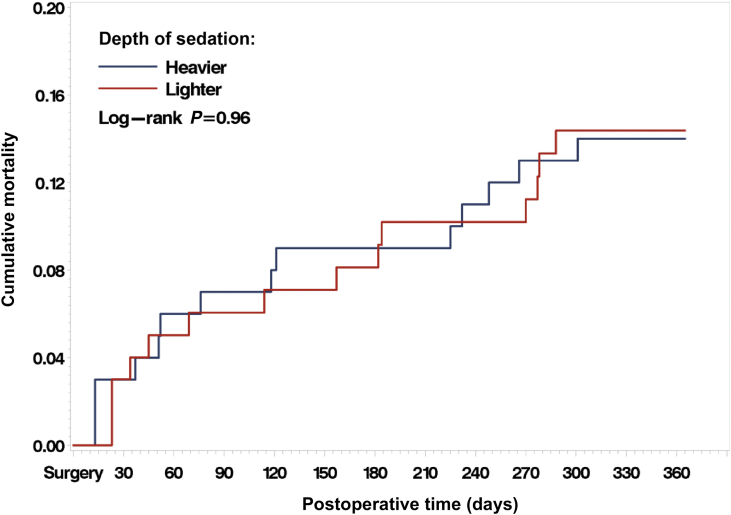
One-year mortality was 14% in both groups (log rank P=0.96). Independent risk factors for 1-yr mortality included: Charlson comorbidity index [hazard ratio (HR)=1.23, 95% confidence interval (CI), 1.02–1.49; P=0.03], instrumental activities of daily living [HR=0.74, 95% CI, 0.60–0.91; P=0.005], BMI [HR=0.91, 95% CI 0.84–0.998; P=0.04], and delirium severity [HR=1.20, 95% CI, 1.03–1.41; P=0.02]. Ambulation returned to pre-fracture levels, worsened, or was not obtained in 64%, 30%, and 6% of 1 yr survivors, respectively. Lighter sedation did not improve odds of ambulation return at 1 yr [odds ratio (OR)=0.76, 95% CI, 0.24–2.4; P=0.63]. Independent risk factors for ambulation return included Charlson comorbidity index [OR=0.71, 95% CI, 0.53–0.97; P=0.03] and delirium [OR=0.32, 95% CI, 0.10–0.97; P=0.04].
Conclusions
This study found that in elderly patients having hip fracture surgery with spinal anaesthesia supplemented with propofol sedation, heavier intraoperative sedation was not associated with significant differences in mortality or return to pre-fracture ambulation up to 1 yr after surgery.
Clinical trial registration
ClinicalTrials.gov NCT00590707.
Keywords: activities of daily living; anaesthesia, spinal; delirium; hip fractures; mortality
Editor's key points.
-
•
There is some evidence suggesting that deeper sedation or deeper hypnosis during general anaesthesia is causally linked to worse intermediate-term postoperative outcomes, including death.
-
•
This 200-patient clinical trial, conducted in older adults undergoing surgery for hip fracture, addressed, as secondary outcomes, whether ‘deeper’ propofol sedation during spinal anaesthesia was associated with worse survival and ambulation ability at 1 yr after the operation.
-
•
There was no significant difference between the ‘lighter’ vs ‘deeper’ sedation groups in 1-yr mortality rates (14% in both groups; adjusted hazard ratio ‘lighter’/‘deeper’ = 1.40; 95% confidence interval, 0.64–3.03; P=0.40), or in the odds for return to preoperative ambulation ability (adjusted odds ratio ‘lighter’/‘deeper’=0.77; 95% confidence interval, 0.31–1.96; P=0.59).
-
•
These findings do not support targeting ‘lighter’ vs ‘deeper’ sedation during hip fracture surgery under spinal anaesthesia for the prevention of 1-yr mortality or for improved 1-yr ambulation ability.
Postoperative delirium (POD) is one of the most common complications after hip fracture repair.1 POD after hip fracture repair is associated with an increased likelihood of dependency in activities of daily living (ADLs) and instrumental activities of daily living (IADLs),2, 3 and a higher risk of not returning to pre-fracture level of mobility.4 Furthermore, both duration of POD5 and POD superimposed on dementia6 are risk factors for mortality after surgery. Given the above, interventions which decrease POD have the potential to influence both functional and mortality outcomes.
One anaesthetic approach reported to decrease incident delirium is limitation of sedation depth during spinal anaesthesia. In a small clinical trial, patients undergoing spinal anaesthesia for hip fracture repair were randomly assigned to receive heavier or lighter sedation, based on a processed EEG index. Heavier sedation was found to be a risk factor for POD on the 2nd postoperative day7 and was associated with higher mortality in patients with greater medical comorbidity.8 These results were hypothesis generating regarding the relationship between depth of sedation and POD. The study also raised the question whether depth of sedation influenced the intermediate-term outcomes of mortality and day-to-day function.
STRIDE (A Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients) was a randomised, two-group, parallel, superiority trial whose principal objective was to assess heavier vs lighter sedation during spinal anaesthesia in elderly patients undergoing hip fracture repair. The primary outcome of the STRIDE trial was the incidence of delirium from postoperative day 1–5, or until hospital discharge if this occurred before day 5. These results were previously reported.9 In short, no statistically significant difference in the incidence of postoperative in-hospital delirium was found between the groups, but a significant effect modification by level of comorbidity was observed, where lighter sedation was associated with lower in-hospital delirium incidence in patients with low preoperative comorbidity.9 In this study, we report the following secondary pre-specified outcomes from the STRIDE trial: persistent delirium at 1 month; change in functional outcomes from preoperative baseline at 1 month and 1 yr follow-up; and mortality at 1 yr after surgery. In addition, exploratory analysis was performed to determine the independent risk factors for 1 yr mortality and return to pre-fracture ambulation level.
Methods
Study design and participants
Institutional review board approval was obtained for the prospective STRIDE trial on 9/27/2010 (NA_00041873). The trial was first registered at ClinicalTrials.gov under registration number NCT00590707 in January 2008. All participants provided their written informed consent. STRIDE was conducted at a single clinical centre. A detailed description of the entire trial protocol was published previously in the supplemental material of Li and colleagues.10
Briefly, patients ≥65 yr old who were undergoing hip fracture repair with spinal anaesthesia and propofol sedation, and who did not have preoperative delirium or severe dementia, were randomised to receive either heavier [modified Observer's Assessment of Alertness/Sedation score (OAA/S) 0–2] or lighter (OAA/S 3–5) intraoperative sedation.11 The inclusion criteria were: 1) admission to Johns Hopkins Bayview Medical Center, Baltimore, MD, USA for surgical repair of traumatic hip fracture; 2) 65 yr or older; 3) a preoperative Mini-Mental State Examination (MMSE) score of 15 or higher12; and 4) receiving spinal anaesthesia. The exclusion criteria included: 1) receiving general anaesthesia; 2) inability to speak or understand English; 3) severe chronic obstructive pulmonary disease or congestive heart failure; 4) refusal to give informed consent; 5) non-participating attending surgeon; 6) hip fractures in both hips on same admission; 7) repair of another fracture concurrently with the hip fracture; 8) prior hip surgery on the same hip to be repaired in the current surgery; and 9) preoperative delirium. Randomisation was stratified according to age and MMSE scores assessed before surgery.
Data collection at baseline before surgery
Before surgery, baseline ADL/IADL, MMSE, and geriatric depression score (GDS)13 were obtained, in additional to demographic information. Evaluations using Confusion Assessment Method (CAM),14 Delirium Rating Scale-R-98 (DRS-R-98),15 and abbreviated digit span test (DST) were also collected at baseline.
Intervention
After enrolment and satisfactory administration of spinal anaesthesia, the patient was randomly assigned to one of two groups in blocks with equal allocation, stratified by age and MMSE at baseline. During the intraoperative period, one group had the depth of sedation [as measured by the use of the modified Observer's Assessment of Alertness/Sedation Scale (OAA/S)] maintained at an OAA/S score of 0–2. This was the heavier sedation group. Patients in the other group had the depth of sedation maintained at an OAA/S score of 3–5. This was the lighter sedation group. The propofol was titrated individually for each participant to achieve and maintain the depth of sedation required by that participant's assigned treatment group (lighter or heavier sedation). The depth of sedation for all participants was measured by the OAA/S, administered every 15 min during the surgery, and the bispectral index (BIS) was also recorded. The BIS monitor readout was covered throughout the surgery so that the study anaesthesiologist/anaesthetist remained masked to the BIS values while administering propofol. The BIS readings served as an independent measure of the level of adherence to the trial interventions. BIS monitoring was not used as the primary means of determining depth of sedation because of its known limitations in terms of signal quality in the sedated state.16
Outcomes at 1 month and 1-yr follow-up
Both 1 month and 1-yr post-surgery assessments were done in person. At both 1 month and 1 yr follow-up, GDS, ADL/IADL, hand grip strength, 3-m walking speed, and timed chair rise were documented. Patients and outcome assessors were masked to the randomisation assignment.
Patients were also assessed for delirium and delirium severity at 1 month after operation. Delirium was evaluated using validated instruments including the CAM, MMSE, Abbreviated DST, and DRS-R-98, followed by case-by-case diagnostic adjudication by the Delirium Consensus Panel. Delirium was defined in this study by criteria for delirium presented in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)17 as assessed on the CAM (see protocol in supplemental material of Li and colleagues10 for details). Members of the consensus panel and research personnel carrying out the data collections and diagnosis adjudications were all masked to the randomisation assignment.
Mortality outcome assessment was done through regular phone contact with family members and search of the National Death Index, Social Security Death index, and obituaries, and exact date of death was ascertained. Time to death from the surgery date was calculated based on date of death.
Statistical power
STRIDE was powered to detect hypothesised intervention effect on the primary outcome of postoperative delirium from POD 1 to 5 or discharge, whichever occurred first, where 200 patients were randomised with equal allocation to the two intervention groups. In a prior, smaller randomised controlled trial, the Kaplan–Meier estimate for 1 yr mortality was 31.5% for the deep sedation group and 17.3% for the light sedation group.7 With the larger sample size of 200 patients in the STRIDE trial, and the assumptions of 31.5% mortality by 1 yr in the heavier sedation group and proportional hazards between groups, the current trial had 80% power to detect a 15.5% or more absolute reduction in 1 yr mortality, the main secondary outcome, in the lighter sedation group using a two-sided log rank test with alpha of 0.05. We anticipated complete follow-up on mortality outcome at 1 yr after randomisation.
Statistical analysis
All analyses on intervention effects were conducted with the intention-to-treat principle. The statistical analysis plan for these secondary analyses was finalised and approved by the steering committee before the main trial results were known. Exploratory analyses were also conducted to evaluate potential impact of in hospital POD on mortality and functional outcomes over 1 yr after surgery, and to explore for other predictors of outcomes in this patient population.
Mortality at 1 yr
To examine the difference in cumulative mortality between the intervention groups, Kaplan–Meier analysis was used to estimate the non-parametric 1 yr survival curves for both intervention groups. The difference between the two survival curves was tested using the log-rank test. The relative risk of 1 yr mortality comparing the heavier sedation with the lighter sedation intervention group was evaluated through estimated hazard ratios from the semi-parametric Cox proportional hazards model, with corresponding confidence intervals. The main model used intervention group assignment as the primary predictor and included the stratification variables of age and MMSE at baseline as covariates. Additional models were constructed to explore for the potential impact of interaction between intervention and baseline Charlson co-morbidity index (CCI), and baseline and in-hospital POD-related (e.g. incident cases, number of days with POD, mean DRS-R-98 severity score) variables as potential independent predictors, on the mortality outcome during the 1 yr follow-up. The proportional hazards assumption was evaluated by examining the intervention group by survival time interaction term in the proportional hazards model and by examining the Schoenfeld residuals.
Functional outcomes over time
Return to pre-fracture ambulation level was used as the primary functional outcome of interest based on the ability to obtain measurements at all three time points (baseline, 1 month, and 1 yr) and its importance in patient-centred outcomes. Ambulation level was derived from the ADL and was coded as follows: 1) goes about grounds or city; 2) ambulates within residence or about one block distant; 3) ambulates with assistance of another person, railing, cane, walker, wheelchair; 4) sits unsupported in chair or wheelchair, but cannot propel self without help; and 5) bedridden more than half the time.
Separate logistic regression models were constructed for regaining pre-fracture ambulation at 1 month and at 1 yr using the intervention group assignment as the primary predictor variable. The models included the stratification variables of age and MMSE at baseline as covariates. Variables collected before operation, during operation, or during the 1 month follow-up assessments were used in multivariable modelling as appropriate. For example, ambulation level at baseline was adjusted for in the model to account for the varying degrees of difficulty to regain ambulation associated to the levels at baseline [e.g. it would be a lot more difficult for patients at level 1 (goes about grounds or city) at baseline to return to that level of ambulation at 12 months than patients at level 5 (bedridden more than half the time) at baseline to regain the same level of function 1 yr later]. The adjusted odds ratio estimates were derived from the models, and adjusted relative risk estimates and 95% confidence intervals (CI) for the corresponding estimates computed.
Results
Baseline characteristics and clinical course up to 30 days after surgery
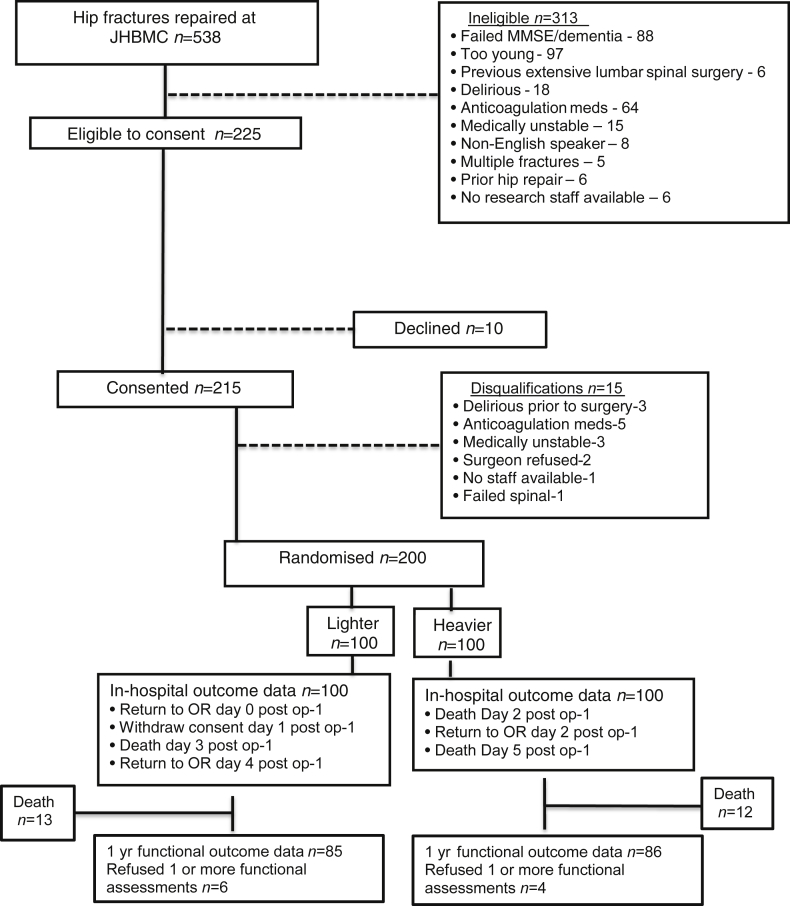
Figure 1 outlines the CONSORT diagram for STRIDE. Of 538 patients with hip fracture screened from 18 November 2011 through 19 May 2016, 200 patients were randomised to either lighter or heavier sedation. The interventional groups were well matched (Table 1), and there were no significant baseline differences between groups. Briefly, the study cohort consisted of 200 participants characterised by mean age of 82 [standard deviation (sd), 8] yr, 73% female, 97% white, CCI score of 1.5 (1.8), and baseline MMSE of 24 (4). Most participants had ASA physical status of 3 or higher. The individual components of the CCI having a 10% or greater incidence included: diabetes without end organ damage, chronic obstructive pulmonary disease, cerebrovascular disease, myocardial infarction, congestive heart failure, and dementia. Twelve percent of patients were admitted to ICU after surgery. In the first 30 days after surgery, there was a 23% readmission rate with renal and cardiovascular complications being most common. Six patients, with even distribution across the heavier and lighter groups, died within 30 days after surgery. There was good separation of interventional groups intraoperatively by both modified OAA/S [4.1 (0.9) vs 0.2 (0.9); lighter vs heavier, respectively; P<0.001] and BIS [82.3 (9.4) vs 57.0 (14.8); lighter vs heavier, respectively; P<0.001]. The overall in-hospital delirium incidence was 36.5%; 39% vs 34% in heavier and lighter sedation groups, respectively. The incidence of persistent delirium at 1 month was low (2% overall) and not significantly different between intervention groups [one/96 (1%) and three/97 (3%) in lighter and heavier groups, respectively]. Additional study patient characteristics and their breakdown by interventional group have been previously reported.9
Fig 1.
CONSORT diagram for STRIDE study. Patients were recruited between November 18, 2011 and May 19, 2016. MMSE, Mini-Mental State Examination; OR, operating room; STRIDE, Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients; JHBMC, Johns Hopkins Bayview Medical Center.
Table 1.
Baseline characteristics and clinical course through 30 days after hip fracture repair surgery. sd, standard deviation
| Total (n=200) | Lighter (n=100) | Heavier (n=100) | |
|---|---|---|---|
| Baseline variables | n (%) | ||
| ASA physical status classification | |||
| Mild systemic disease (2) | 61 (30.5) | 37 (37.0) | 24 (24.0) |
| Severe systemic disease (3) | 128 (64.0) | 59 (59.0) | 69 (69.0) |
| Systemic disease that is a constant threat to life (4) | 11 (5.5) | 4 (4.0) | 7 (7.0) |
| Charlson comorbidity items | |||
| Myocardial infarction | 23 (11.5) | 12 (12.0) | 11 (11.0) |
| Congestive heart failure | 23 (11.5) | 11 (11.0) | 12 (12.0) |
| Peripheral vascular disease | 12 (6.0) | 7 (7.0) | 5 (5.0) |
| Chronic obstructive pulmonary disease | 32 (16.0) | 17 (17.0) | 15 (15.0) |
| Cerebrovascular disease | 32 (16.0) | 17 (17.0) | 15 (15.0) |
| Cancer | |||
| Any solid tumour | 18 (9.0) | 8 (8.0) | 10 (10.0) |
| Leukaemia | 2 (1.0) | 0 (0.0) | 2 (2.0) |
| Lymphoma | 4 (2.0) | 1 (1.0) | 3 (3.0) |
| Metastatic solid tumour | 3 (1.5) | 1 (1.0) | 2 (2.0) |
| Diabetes mellitus | |||
| Without end organ damage | 33 (16.5) | 15 (15.0) | 18 (18.0) |
| With end organ damage | 9 (4.5) | 4 (4.0) | 5 (5.0) |
| Chronic liver disease | |||
| Mild | 2 (1.0) | 1 (1.0) | 1 (1.0) |
| Moderate or severe | 1 (0.5) | 0 (0.0) | 1 (1.0) |
| Chronic kidney disease | 4 (2.0) | 1 (1.0) | 3 (3.0) |
| Dementia (n=199/99/100) | 22 (11.1) | 10 (10.0) | 12 (12.1) |
| In-hospital variables | |||
| Day from surgery until discharge Mean (sd) |
3.9 (2.5) | 4.1 (2.6) | 3.7 (2.5) |
| Admission to ICU from surgery until discharge | 24 (12.0) | 11 (11.0) | 13 (13.0) |
| Complications within 30 days after surgery | |||
| Wound complications | 13 (6.5) | 7 (7.0) | 6 (6.0) |
| Pulmonary complications | 17 (8.5) | 8 (8.0) | 9 (9.0) |
| CNS complications | 6 (3.0) | 3 (3.0) | 3 (3.0) |
| Cardiovascular complications | 28 (14.0) | 16 (16.0) | 12 (12.0) |
| Renal complications | 43 (21.5) | 24 (24.0) | 19 (19.0) |
| Orthopaedic complications | 6 (3.0) | 5 (5.0) | 1 (1.0) |
| Hospital readmission | 46 (23.0) | 24 (24.0) | 22 (22.0) |
| Death | 6 (3.0) | 3 (3.0) | 3 (3.0) |
Follow-up
There were 28 deaths (14 each arm), one refusal (lighter), and 10 (n=6 in lighter sedation and n=4 in heavier sedation) incomplete ambulation data at 1 yr. Ability to perform individual functional tests (grip strength, timed 10-m walk, timed chair rise) varied by test (Table 2).
Table 2.
Functional outcomes at preoperative baseline, 1 month (30 days), and 12 month (1 yr) after operation. *Three out of 100 in each intervention group were deceased by the time of testing. †Fourteen out of 100 in each intervention group were deceased by the time of testing
| Measure | Baseline |
1 month* |
12 month† |
|||
|---|---|---|---|---|---|---|
| Lighter | Heavier | Lighter | Heavier | Lighter | Heavier | |
| Activities of daily living | 4.7 (1.5), n=100 | 4.6 (1.6), n=100 | 3.2 (1.5), n=94 | 3.1 (1.5), n=95 | 4.2 (1.5), n=79 | 4.1 (1.6), n=82 |
| Instrumental activities of daily living | 5.8 (2.2), n=100 | 5.9 (2.3), n=100 | 2.6 (2.0), n=94 | 2.5 (1.8), n=95 | 4.8 (2.6), n=79 | 5.0 (2.5), n=82 |
| Grip strength, dominant hand | ||||||
| Tested, mean (sd) | 17 (7), n=91 | 17 (8), n=91 | 17 (8), n=72 | 17 (8), n=74 | ||
| Timed chair rise (s) | ||||||
| Unsafe/unable to perform | n=26 (28%) | n=35 (37%) | n=8 (10%) | n=9 (11%) | ||
| Tested, mean (sd) | 2.7 (3.1), n=68 | 3.2 (6.2), n=59 | 1.8 (1.1), n=69 | 1.9 (1.5), n=72 | ||
| Timed 3 m walk (s) | ||||||
| Unsafe/unable to perform | n=39 (41%) | n=48 (51%) | n=9 (11%) | n=11 (13%) | ||
| Tested, mean (sd) | 10.6 (6.7), n=55 | 9.7 (5.8), n=46 | 6.7 (3.2), n=68 | 6.6 (3.0), n=70 | ||
1-yr mortality
Intention-to-treat analysis showed no significant difference between intervention groups in mortality up to 1 yr (Figure 2, log rank P=0.96). Analysis using Cox proportional hazard model estimated the hazard ratio of 1 yr mortality for lighter vs heavier sedation being 0.85 (95% CI, 0.44–1.97) after accounting for age and MMSE scores, the variables used for stratified randomisation. There was no statistically significant effect modification of intervention effect on 1-yr mortality by baseline CCI (P-interaction=0.44).
Fig 2.
Kaplan–Meier curves showing intention to treat analysis for postoperative mortality between heavier vs lighter sedation intervention groups.
Factors associated with survival at 1 yr after surgery (Supplementary Table S1) included higher BMI, MMSE, and ADLs/IADLs, and lower baseline CCI. In addition, differences in baseline place of residence and postoperative ICU status were apparent between survivors and non-survivors. Neither the occurrence of in-hospital POD, complications within the 1 month postoperative period, nor any intraoperative variable studied was associated with 1-yr mortality. Regression analysis showed that the independent risk factors for 1 yr mortality in this hip fracture cohort included low baseline BMI, and incremental increases in CCI and incremental decreases in IADL. In-hospital delirium severity was a mortality risk factor (Table 3), but not incidence. The mean BIS value during surgery was not a significant 1-yr predictor of mortality in multivariable modelling (P=0.70). No significant interaction was found between baseline CCI and intraoperative BIS in determining 1-yr mortality (P=0.68 for interaction) (see Supplementary Table S2).
Table 3.
Cox proportional hazard regression analysis examining independent risk factors for 1-yr mortality after surgery in geriatric patients after hip fracture repair surgery
| Analysis of maximum likelihood estimates | ||||
|---|---|---|---|---|
| Parameter | Hazard ratio | 95% hazard ratio confidence limits | P-value | |
| Age at baseline, per year order | 1.000 | 0.949 | 1.054 | 0.996 |
| Mini-mental state examination at baseline, per point higher | 1.059 | 0.920 | 1.218 | 0.424 |
| Lighter vs heavier sedation | 1.393 | 0.641 | 3.027 | 0.402 |
| BMI at baseline, per kg m−2 higher | 0.914 | 0.838 | 0.998 | 0.044 |
| Charlson comorbidity index score, per point higher | 1.234 | 1.021 | 1.491 | 0.030 |
| Instrumental activities of daily living at baseline, per point higher | 0.741 | 0.600 | 0.914 | 0.005 |
| Postoperative delirium in hospital, yes vs no | 0.341 | 0.110 | 1.052 | 0.061 |
| Mean delirium rating scale severity score in hospital, per point higher | 1.203 | 1.025 | 1.412 | 0.024 |
Functional outcome
Among the survivors, patients' ambulation level at 1 yr was improved/unchanged, worse, or not obtained in 64%, 30%, and 6% of participants, respectively, compared with their pre-fracture levels. Ambulation status at 1-yr follow-up (Supplementary Table S3) was associated with baseline IADL, BMI, CCI, and level of education. The odds of regaining pre-fracture ambulation level was not significantly different between intervention groups. No intraoperative variables were associated with ambulatory status at 1 yr. Given that the likelihood of regaining pre-fracture ambulation level is greatly influenced by the patients' ambulatory level at baseline, multivariable logistic regression analysis on odds of regaining vs worsening of pre-fracture was adjusted for the level of pre-fracture ambulation. After multiple adjustment, odds of regaining pre-fracture ambulation level was still not different between intervention groups (Table 4). Independent predictors of returning to pre-fracture ambulatory status at 1 yr in this patient population included baseline CCI and in-hospital delirium. As expected, poorer level of pre-fracture ambulation was associated with a greater likelihood of regaining or improving ambulation from pre-fracture function at 1 yr.
Table 4.
Logistic regression analysis examining independent risk factors for the odds ratio of returned to vs declined from pre-fracture level of ambulation at 12 months after operation
| Effect | Odds ratio estimate | 95% wald confidence limits | P-value | |
|---|---|---|---|---|
| Age at baseline | 0.941 | 0.874 | 1.014 | 0.111 |
| Mini-mental state examination at baseline | 0.971 | 0.820 | 1.151 | 0.737 |
| Instrumental activities of daily living at baseline | 1.414 | 0.988 | 2.023 | 0.058 |
| BMI at baseline | 0.978 | 0.883 | 1.084 | 0.673 |
| Charlson comorbidity index at baseline | 0.714 | 0.526 | 0.969 | 0.031 |
| Education | ||||
| Non-high school graduate vs college graduate or higher | 0.486 | 0.087 | 2.705 | 0.091 |
| High school graduate or equivalent vs college graduate or higher | 1.560 | 0.355 | 6.847 | 0.257 |
| Some college vs college graduate or higher | 1.501 | 0.267 | 8.449 | 0.419 |
| Lighter vs heavier sedation | 0.774 | 0.306 | 1.957 | 0.589 |
| Delirium in hospital, yes/no | 0.318 | 0.104 | 0.966 | 0.043 |
| Ambulation at baseline | 13.783 | 5.196 | 36.561 | <0.001 |
No statistically significant difference between intervention groups in any other functional outcome measured at either 1 or 12 months after surgery was observed (Table 2). At 1 month after surgery, patients' ADL and IADL levels had not returned to their corresponding baseline levels. Although 94% of the patients were able to complete the grip strength test for the dominant hand at 1 month, about 33% and 45% of all patients evaluated could not perform chair rise and 3-m walk, respectively, with higher proportions observed in the heavier compared with the lighter sedation group (37% vs 28% for chair rise, and 51% vs 41% for walking). On average, improvement in functional outcomes occurred from 1 to 12 months in all measurements except grip strength. However, patients whose ambulation had worsened from pre-fracture status at 12 months had lower ADL/IADL scores and slower timed chair rise/3-m walk in comparison with those participants demonstrating return to pre-fracture ambulatory status at 12 months.
Discussion
This study reports the secondary outcomes of the STRIDE trial. When comparing heavier vs lighter sedation during spinal anaesthesia for hip fracture repair, there was no significant difference between study groups in delirium incidence at 30 days, functional outcomes at 12 month follow-up, and 1 yr mortality. These results are reassuring in that level of intraoperative sedation comparable with those studied in STRIDE is probably not associated with worsened functional outcomes or increased mortality up to 1 yr after surgery.
Hip fracture repair is associated with high postoperative mortality. Large case series report a 5.1% 30-day mortality18 and 25% 1 yr mortality,19 with a mean survival of 3.75 yr (95% CI, 3.13–4.54).20 The 14% 1-yr mortality in the current study is comparable with other prospective hip fracture series,21 but lower than the smaller randomised trial conducted by members of the same research team as the STRIDE study.8 Exclusion criteria (severe dementia and medications which prevent the safe administration of spinal anaesthesia) were similar between these studies. However, anaesthetic management in STRIDE was performed exclusively by four senior anaesthesiologists and may have contributed to the different findings in the two studies. In addition, as discussed below, ongoing changes in management of hip fractures occurred at our institution over the time period of the two trials. The baseline risk factors of low BMI,22 incremental IADL decreases,23 and incremental CCI increases24 for 1 yr mortality observed in STRIDE are consistent with the literature. However, the previously reported higher mortality with male sex21 was not observed, possibly owing to our predominantly female population. As the use of intraoperative EEG-based depth of anaesthesia monitoring has become commonplace, several observational case series report an association between deeper anaesthesia levels and mortality.25 However, it is unclear whether this link represents an epiphenomenon, where deeper anaesthesia is merely a marker of poor prognosis, particularly as these studies have not assessed for individual sensitivity to anaesthetics. We found that neither interventional group nor individual BIS values were risk factors for 1 yr mortality. Furthermore, it is unlikely that anaesthetic depth management alone would influence 1-yr mortality given that patients in this age group and undergoing this type of surgery would likely have other events after the index surgery that may influence longer term survival. Our bias is that if any differences were to be found, they might assert themselves in the first 30 days after surgery. We report 30-day complications in Table 1 and show no difference between intervention groups. These results are in keeping with a recent meta-analysis showing no difference in 30-day mortality comparing regional and general anaesthesia techniques following hip fracture repair.26 For this reason, we further investigated other predictors of 1-yr mortality and reported them in the manuscript. Of note, we found, as others have, that severity of POD in hospital was predictive of mortality at 1 yr, after multivariable adjustment. Heavier vs lighter sedation levels during hip fracture repair under spinal anaesthesia have been associated with higher mortality in patients with CCI score >4.8 STRIDE found no interaction between interventional group and CCI for 1-yr mortality. The reasons behind study outcome differences are complicated, but could be explained by ongoing changes in practice as evidenced by the shorter time to surgery, shorter hospitalisation after surgery, and fewer ICU days in the STRIDE trial compared with the previous, smaller trial. In addition, the incidence of pulmonary and cardiovascular complications was higher in subjects with CCI>4 comparing heavier vs lighter sedation in the previous trial,8 whereas no difference was found between treatment arms in STRIDE.
Neither the occurrence of POD nor days of POD in hospital were predictors of 1-yr mortality in STRIDE. Similarly, previous studies have found that, when accounting for confounders, incident delirium was not an independent risk factor for intermediate-term mortality.20, 27 However, several delirium-associated characteristics do appear to be associated with mortality.28 Specific to surgical patients, trend-level associations between delirium severity and 6 month mortality after hip fracture repair29 have been reported, but STRIDE is the first study to demonstrate statistically significant relationships between POD severity and 1 yr mortality.
Participants with lower pre-fracture ambulation level had greater odds of maintaining level of function by 1 yr compared with participants with a higher pre-fracture ambulation level. This indicates a floor effect in terms of return to function with poorer pre-fracture ambulation status and is consistent with previous reports documenting decreased odds of change in ambulatory status as pre-fracture ambulatory function decreases.4, 30 Other studies have reported an association between poor recovery of ambulation after hip fracture repair and CCI24, 31 and delirium episodes,4 similar to the STRIDE results.
Limitations
1) The sample size of STRIDE was based on the hypothesised intervention effect on the primary outcome of delirium incidence during postoperative day 1–5, or to hospital discharge. Because mortality was the secondary outcome of greatest interest, the minimal size of intervention effect on mortality that could be detected after sample size determination was evaluated. However, there was no formal determination of sample size requirements in relation to any secondary outcomes. In addition, most of the modelling with adjustment was done for exploratory purposes, so there were also no power calculations for these. 2) A single site trial limits generalisability. 3) Exclusion of participants with MMSE <15 may have limited the sample to a more cognitively intact cohort than the usual hip fracture population. 4) Exclusion of anticoagulant users may have biased towards a population with fewer cardiovascular risk factors. 5) Pre-fracture walking ability was assessed by patient and family interview, and may be subject to bias. 6) Averaging BIS values over the intraoperative period can conceal potentially important short periods of low BIS and cumulative duration of low BIS, both of which have been associated with worse intermediate-term survival.25
Strengths
1) The study was a randomised, double-blinded design with excellent intermediate-term follow-up to 1 yr. 2) There was a high rate of enrolment. 3) Assessments of the delirium outcome were rigorously conducted by multidisciplinary consensus panel using all available data. 4) STRIDE was a large enough study to detect a clinically important 15% difference in mortality between treatment groups.
Conclusions
In planning the STRIDE trial, the mechanisms studied focused on intermediate-term effects of delirium and its relationship to both mortality and function. The intervention did not affect overall delirium incidence in hospital, nor did it affect the intermediate-term outcomes studied. Yet, the importance of POD was still apparent when analysing factors associated with mortality and function. In addition, the same theme emerges throughout both the short- and intermediate-term study results, and that is the role of underlying comorbidity in determining not only incident delirium in hospital, but intermediate-term outcomes as well. Although the intervention did not produce an overall decrease in delirium incidence or intermediate-term outcomes in the elderly hip fracture population, it is hoped that reporting the other covariates important in determining intermediate-term outcomes will help to stimulate further work focusing on perioperative management based on comorbidity as a possible means of preventing POD or reducing delirium severity, and thus improving surgical outcomes in the elderly.
Nonetheless, the STRIDE results provide reassurance for the anaesthesiologist managing hip fracture patients. Particularly for 1-yr mortality, given the ongoing controversy surrounding the association between anaesthetic depth and mortality, the STRIDE interventional groups had similar outcomes. Furthermore, the heavier levels of sedation studied in STRIDE were not extreme and are consistent with current practice in the USA.32
In conclusion, the results from this analysis show that there is no difference in mortality or return to ambulation 1 yr after hip fracture surgery in elderly patients receiving heavier and lighter intraoperative sedation.
Authors' contributions
Concept and design: FS, KN, AG, PR, SM, KS, NW.
Acquisition, analysis, or interpretation of data: all authors.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: FS, NW, GB, EO.
Obtained funding: FS, AG, NW.
Administrative, technical, or material support: FS, KN, AG, GB, EO, SM, KS, JO.
Supervision: FS, KN, GB, NW.
Classification interpretation of all included hip fractures: EH, SM.
Data management: KS.
Full data access: FS. and NW.
Declarations of interest
FS, NW, KS, EO, JO, MJ, AG, and GEB. declare that they have no conflicts of interest. SCM has board membership of Fragility Fracture Network; is past president of the International Geriatric Fracture Society; is an editorial board member of the Journal of American Geriatrics Society; is deputy editor of the Geriatric Surgery and Rehabilitation; and has stock options with Delta Orthopedics. KN has done functional near infrared spectroscopy (fNIRS) research for Hitachi and consulting work for Merck-Schering Pharmaceuticals.
Funding
National Institute on Aging grant RO1 AG033615. DePuy Synthes for Fellowship grant and research grants to E.H. Lilly (research funding) and Otsuka (travel funding) to PR.
Acknowledgements
We thank the assessment team (Kori Kindbom, Rachel Burns, and Michael Sklar, Johns Hopkins University School of Medicine) for their follow-up of patients. These individuals were part of the study assessment and follow-up team and were compensated for their services through the study's National Institutes of Health grant. We also thank the data safety and monitoring board members (Jeffrey Carson, Rutgers University, Lee Fleisher, University of Pennsylvania, Steve Epstein, Georgetown University, Jay Magaziner, University of Maryland, Anne Lindblad, the Emmes Corporation and Sarang Kim, Rutgers University) for their time, effort, and oversight (see protocol), and Linda Sevier, Johns Hopkins Bayview Medical Center, for her preparation of the manuscript.
Handling editor: M. Avidan
Editorial decision date: 21 December 2018
Footnotes
This article is accompanied by a Special Article: Hypnotic depth and postoperative death: a Bayesian perspective and an independent Discussion of a clinical trial by Vlisides et al., Br J Anaesth 2019:122:421–427, doi: https://doi.org/10.1016/j.bja.2019.01.012.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.12.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee H.B., Mears S.C., Rosenberg P.B., Leoutsakos J.M., Gottschalk A., Sieber F.E. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59:2306–2313. doi: 10.1111/j.1532-5415.2011.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolan M.M., Hawkes W.G., Zimmerman S.I. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M527–M534. doi: 10.1093/gerona/55.9.m527. [DOI] [PubMed] [Google Scholar]
- 3.Marcantonio E.R., Flacker J.M., Michaels M., Resnick N.M. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 4.Vochteloo A.J., Moerman S., Tuinebreijer W.E. More than half of hip fracture patients do not regain mobility in the first postoperative year. Geriatr Gerontol Int. 2013;13:334–341. doi: 10.1111/j.1447-0594.2012.00904.x. [DOI] [PubMed] [Google Scholar]
- 5.Bellelli G., Mazzola P., Morandi A. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014;62:1335–1340. doi: 10.1111/jgs.12885. [DOI] [PubMed] [Google Scholar]
- 6.Lee H.B., Oldham M.A., Sieber F.E., Oh E.S. Impact of delirium after hip fracture surgery on one-year mortality in patients with or without dementia: a case of effect modification. Am J Geriatr Psychiatr. 2017;25:308–315. doi: 10.1016/j.jagp.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieber F.E., Zakriya K.J., Gottschalk A. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown C.H., 4th, Azman A.S., Gottschalk A., Mears S.C., Sieber F.E. Sedation depth during spinal anesthesia and survival in elderly patients undergoing hip fracture repair. Anesth Analg. 2014;118(5):977–980. doi: 10.1213/ANE.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieber F.E., Neufeld K.J., Gottschalk A. Effect of depth of sedation in older patients undergoing hip fracture repair on postoperative delirium: the STRIDE randomized clinical trial. JAMA Surg. 2018;153:987–995. doi: 10.1001/jamasurg.2018.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T., Wieland L.S., Oh E. Design considerations of a randomized controlled trial of sedation level during hip fracture repair surgery: a strategy to reduce the incidence of postoperative delirium in elderly patients. Clin Trial. 2017;14:299–307. doi: 10.1177/1740774516687253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass P.S., Bloom M., Kearse L., Rosow C., Sebel P., Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Folstein M.F.F.S., McHugh P.R. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage J.A., Sheikh J.I. 9/Geriatric Depression Scale (GDS) Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 14.Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 15.Trzepacz P.T., Mittal D., Torres R., Kanary K., Norton J., Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 16.Johansen J.W. Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol. 2006;20:81–99. doi: 10.1016/j.bpa.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association Task Force on DSM-IV . American Psychiatric Association; Washington, D.C.: 2000. Diagnostic and statistical manual of mental Disorders:DSM-IV-TR. [Google Scholar]
- 18.White S.M., Moppett I.K., Griffiths R. Secondary analysis of outcomes after 11,085 hip fracture operations from the prospective UK Anaesthesia Sprint Audit of Practice (ASAP-2) Anaesthesia. 2016;71:506–514. doi: 10.1111/anae.13415. [DOI] [PubMed] [Google Scholar]
- 19.Uchida K., Aoki T., Ishizuka B. Postoperative delirium and plasma melatonin. Med Hypotheses. 1999;53:103–106. doi: 10.1054/mehy.1998.0724. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk A., Hubbs J., Vikani A.R., Gottschalk L.B., Sieber F.E. The impact of incident postoperative delirium on survival of elderly patients after surgery for hip fracture repair. Anesth Analg. 2015;121:1336–1343. doi: 10.1213/ANE.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magaziner J., Chiles N., Orwig D. Recovery after hip fracture: interventions and their timing to address deficits and desired outcomes—evidence from the Baltimore hip studies. Nestle Nutr Inst Workshop Ser. 2015;83:71–81. doi: 10.1159/000382064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solbakken S.M., Meyer H.E., Stigum H. Excess mortality following hip fracture: impact of self-perceived health, smoking, and body mass index. A NOREPOS study. Osteoporos Int. 2017;28:881–887. doi: 10.1007/s00198-016-3795-0. [DOI] [PubMed] [Google Scholar]
- 23.Pioli G., Lauretani F., Davoli M.L. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67:1272–1277. doi: 10.1093/gerona/gls097. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Zabaleta J., Pita-Fernandez S., Seoane-Pillado T., Lopez-Calvino B., Gonzalez-Zabaleta J.L. Comorbidity as a predictor of mortality and mobility after hip fracture. Geriatr Gerontol Int. 2016;16:561–569. doi: 10.1111/ggi.12510. [DOI] [PubMed] [Google Scholar]
- 25.Leslie K., Short T.G. Sedation depth and mortality: a large randomized trial is required. Anesth Analg. 2014;118:903–905. doi: 10.1213/ANE.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell C.M., McLoughlin L., Patterson C.C., Clarke M., McCourt K.C., McBrien M.E., McAuley D.F., Shields M.O. Perioperative outcomes in the context of mode of anaesthesia for patients undergoing hip fracturesurgery: systematic review and meta-analysis. Br J Anaesth. 2018;120:37–50. doi: 10.1016/j.bja.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton G.M., Wheeler K., Di Michele J., Lalu M.M., McIsaac D.I. A systematic review and meta-analysis examining the impact of incident postoperative delirium on mortality. Anesthesiology. 2017;127:78–88. doi: 10.1097/ALN.0000000000001660. [DOI] [PubMed] [Google Scholar]
- 28.Jackson T.A., Wilson D., Richardson S., Lord J.M. Predicting outcome in older hospital patients with delirium: a systematic literature review. Int J Geriatr Psychiatr. 2016;31:392–399. doi: 10.1002/gps.4344. [DOI] [PubMed] [Google Scholar]
- 29.Marcantonio E., Ta T., Duthie E., Resnick N.M. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 30.Pajulammi H.M., Pihlajamaki H.K., Luukkaala T.H., Nuotio M.S. Pre- and perioperative predictors of changes in mobility and living arrangements after hip fracture—a population-based study. Arch Gerontol Geriatr. 2015;61:182–189. doi: 10.1016/j.archger.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Burgos E., Gomez-Arnau J.I., Diez R., Munoz L., Fernandez-Guisasola J., Garcia del Valle S. Predictive value of six risk scores for outcome after surgical repair of hip fracture in elderly patients. Acta Anaesthesiol Scand. 2008;52:125–131. doi: 10.1111/j.1399-6576.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- 32.Sieber F.E., Gottshalk A., Zakriya K.J., Mears S.C., Lee H. General anesthesia occurs frequently in elderly patients during propofol-based sedation and spinal anesthesia. J Clin Anesth. 2010;22:179–183. doi: 10.1016/j.jclinane.2009.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.