Abstract
Background
The outcome of indwelling pleural catheter (IPC) use in hepatic hydrothorax (HH) is unclear. This study aimed to review the safety and feasibility of the IPC in patients with refractory HH.
Methods
A retrospective multicenter study of patients with HH from January 2010 to December 2016 was performed. Inclusion criteria were refractory HH treated with an IPC and an underlying diagnosis of cirrhosis. Records were reviewed for patient demographics, operative reports, and laboratory values. The Kaplan-Meier method was used to estimate catheter time to removal. The Cox proportional hazard model was used to evaluate for independent predictors of pleurodesis and death.
Results
Seventy-nine patients were identified from eight institutions. Indication for IPC placement was palliation in 58 patients (73%) and bridge to transplant in 21 patients (27%). The median in situ dwell time of all catheters was 156 days (range, 16-1,978 days). Eight patients (10%) were found to have pleural space infection, five of whom also had catheter-site cellulitis. Two patients (2.5%) died secondary to catheter-related sepsis. Catheter removal secondary to spontaneous pleurodesis was achieved in 22 patients (28%). Median time from catheter insertion to pleurodesis was 55 days (range, 10-370 days). Older age was an independent predictor of mortality on multivariate analysis (hazard ratio, 1.05; P = .01).
Conclusions
We present, to our knowledge, the first multicenter study examining outcomes related to IPC use in HH. Ten percent infection risk and 2.5% mortality were identified. IPC placement may be a reasonable clinical option for patients with refractory HH, but it is associated with significant adverse events in this morbid population.
Key Words: cirrhosis, hepatic hydrothorax, indwelling tunneled pleural catheter, pleural effusion
Abbreviations: HH, hepatic hydrothorax; IPC, indwelling tunneled pleural catheter; MPE, malignant pleural effusion; TIPS, transjugular intrahepatic portosystemic shunt
Hepatic hydrothorax (HH) is a complication of cirrhosis and is often associated with significant morbidity and diminished quality of life.1, 2 This is particularly important when patients are poor candidates for transjugular intrahepatic portosystemic shunt (TIPS) and/or are awaiting liver transplant evaluation.3, 4, 5, 6, 7 Chest tubes carry a high complication rate and can result in electrolyte imbalance, renal failure, infection, and death. In addition, talc pleurodesis is largely unsuccessful in this population.8, 9, 10 Therefore, patients with refractory HH suffer through multiple hospital visits for symptom management with repeated thoracenteses. Although advances in pleural effusion treatment have led to a relatively widespread use of the indwelling tunneled pleural catheter (IPC) as a tool for management of malignant pleural effusions (MPEs),11, 12, 13 palliation in the setting of HH remains limited.
Although primarily used in the management of MPE, a growing body of literature suggests the utility and safety of IPCs in benign pleural effusions.13, 14, 15 Patients with HH are relatively immunocompromised and have decreased coagulation factor synthesis and function, bone marrow suppression, and thrombocytopenia, suggesting they are at higher risk of infectious and bleeding complications after IPC placement. The existing literature on IPCs in HH includes small, single-center studies, and the safety of IPC use in HH remains imprecisely defined. The aim of this study was to evaluate the clinical characteristics, safety, and outcomes of patients with HH who undergo IPC placement.
Methods
A multi-institution retrospective study of all patients with cirrhosis with HH undergoing IPC placement was performed at the following medical centers: Beth Israel Deaconess Medical Center, Johns Hopkins Medical Center, Lahey Hospital and Medical Center, Mayo Clinic, Vanderbilt University Medical Center, Virginia Commonwealth University Health System, University of North Carolina, and Washington University from January 2010 to December 2016. The institutional review board of all centers approved this study. Each patient underwent standard procedural consent for IPC placement per institutional practices and guidelines.
Data Collection
IPC placement was identified from databases of patients, using chart-reported coding and detailed review of medical history, hepatology consultation review, and implemented treatment strategies.
Inclusion criteria were the placement of an IPC for refractory HH, defined as HH not responding to maximized sodium restriction and diuresis requiring repeated thoracenteses for symptom management; adult age (> 18 years of age); and an underlying diagnosis of cirrhosis in patients with partial or no response to TIPS or those with a contraindication to TIPS as judged by the treatment team in the corresponding centers. Records were reviewed for patient demographics, hepatology and operative reports, pathology, cytology, microbiology, and laboratory values at time of IPC insertion.
Patient records were subsequently reviewed for evidence of complications, pleural infection by queries regarding post-IPC placement pleural fluid cultures, clinic visits, hospital admissions, and/or death records. Radiologic and operative records were examined regarding pleural space physiology at the time of IPC insertion to document evidence of expandable or trapped lung. The following complications were collected: IPC-related pleural infection defined by the presence of pleural pus, positive pleural fluid Gram stain/culture requiring subsequent intervention (antibiotics, IPC removal, etc), cellulitis requiring systemic antibiotics, evidence of renal failure and electrolyte imbalance based on physician’s clinical judgment, catheter malfunction, leakage of fluid at the site of catheter insertion, malnutrition defined by reduced muscle mass and strength based on physician’s clinical judgment, and subcutaneous fluid collection (seroma).
Data regarding IPC removal, timing, and reason for removal were collected. In patients dying with the IPC in situ, the date of death was considered as the date of catheter removal.
If the date of death or catheter removal was not available, to calculate days of IPC use, data were censored on the date that records were received from the participating institution. Data were collected and managed using REDCap electronic database tools hosted at Virginia Commonwealth University Health System.
Date of diagnosis of HH was the time of clinical diagnosis per a clinician in the corresponding center, based on the presence of effusion seen for the first time on chest imaging along with pleural fluid analysis. Time from diagnosis to the study end date in the participating center or time to death was used to estimate survival after HH was diagnosed.
All centers offer multidisciplinary care in thoracic oncology and are considered to have expertise in the management of pleural effusion and IPC placement. Catheters were placed by the interventional pulmonology team in each corresponding center using standard technique. Catheters were drained at home, on an every other day basis, and never exceeded beyond 1 L per drainage.
Statistical Analysis
Simple descriptive statistics were used to describe patient demographics and outcomes. Binary and categorical variables were reported as percent frequencies. Continuous variables were displayed as mean and SD. P values for the continuous and categorical variables were calculated using a one-way analysis of variance and χ2 test, respectively. Time to IPC removal and potential clinical predictors were analyzed using Cox proportional hazard models. The Kaplan-Meier method was used to demonstrate overall catheter time from insertion to removal and overall survival from the time of diagnosis.
Catheter removal secondary to spontaneous pleurodesis was considered an outcome of interest. Catheter removal because of infection, catheter malfunction, and death was considered competing risk. Cumulative incidence for each outcome was plotted, and a competing risk time-to-event regression model was applied. To identify predictors that affect pleurodesis, variables were assessed in univariate analysis and were included in a multivariate model if P < .25.
P < .05 was considered to be significant. Statistical analyses were performed using R 3.4.3 (R Foundation for Statistical Computing).
Results
Patient Characteristics
A total of 79 patients were identified from eight institutions (Table 1). All patients were included in the analysis. The mean age ± SD was 60 ± 10.7 years, and 54% of the patients were men. History of failed TIPS was noted in 16 patients (20%). Indication for IPC placement was palliation in 58 patients (73%) and bridge to transplant in 21 patients (27%). Among those with IPC placement as a bridge to liver transplant, 15 patients went on to receive liver transplant. The most common cause of cirrhosis was alcoholic liver disease. IPC placement occurred in the right pleural space in 70 patients (88%). The median number of thoracenteses performed prior to IPC placement was 5.1 (interquartile range, 6.2) and ranged from one to 42 thoracenteses. The assessment of pleural space physiology (expandable vs nonexpandable lung) was reported in 65 patients (82%).
Table 1.
Demographic Data
| Characteristics | Value |
|---|---|
| Age, y | 60 ± 10.7 |
| Sex | |
| Male | 43 (54) |
| Female | 36 (46) |
| Relevant medical history | |
| Prior TIPS | 16 (20) |
| Liver transplant (post-IPC) | 15 (19) |
| Etiology of liver disease | |
| Hepatitis C cirrhosis | 19 (24) |
| Alcohol-induced cirrhosis | 39 (49) |
| NASH cirrhosis | 21 (27) |
| Indication for IPC placement | |
| Palliation | 58 (73) |
| Bridge to Transplant | 21 (27) |
Values are No. (%) or mean ± SD. IPC = indwelling tunneled pleural catheter; NASH = nonalcoholic steatohepatitis; TIPS = transjugular intrahepatic portosystemic shunt.
Patient and procedure characteristics are shown in Tables 1 and 2, respectively.
Table 2.
Laboratory Values Prior to IPC Placement and Thoracentesis Characteristics
| Characteristic | Value |
|---|---|
| Laboratory tests | |
| ALT | 51.49 ± 72.2 |
| Creatinine | 1.84 ± 1.7 |
| AST | 74.78 ± 73.8 |
| Total bilirubin | 5.02 ± 6.8 |
| Albumin | 2.96 ± 0.8 |
| WBC count | 8.13 ± 8.7 |
| Platelet count | 108 ± 97.1 |
| INR | 1.62 ± 0.4 |
| MELD score | 18.1 ± 5.1 |
| IPC site | |
| Left | 9 (12) |
| Right | 70 (88) |
| Pleural fluid drainage | |
| Thoracentesis | 5.13 ± 6.3 |
| Transudate | 66 (85.7) |
| Exudate | 5 (6.5) |
| Pleural space physiology | |
| Non re-expandable | 6 (7) |
| Re-expandable | 59 (75) |
| Not assessed | 14 (18) |
Values are mean ± SD or No. (%). ALT = alanine transaminase; AST = aspartate transaminase; INR = international normalized ratio; MELD = model for end-stage liver disease. See Table 1 legend for expansion of other abbreviation.
IPC Removal
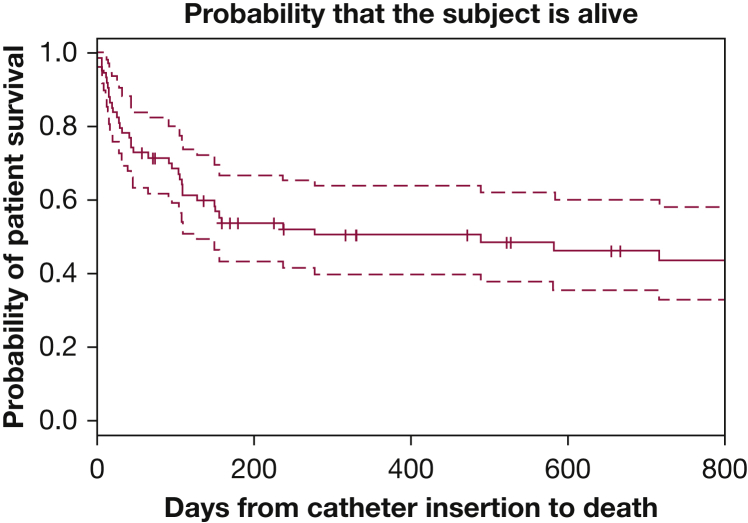
The median in situ dwell time of all catheters within the cohort was 156 days (range, 15-1,978 days), with a total number of 15,025 catheter days (Fig 1). The most common reason for IPC removal was patient death (n = 33). In the remaining patients, the catheter was removed because of spontaneous pleurodesis, infection, or catheter malfunction because of catheter tear or accidental removal.
Figure 1.
Kaplan-Meier curve depicting time from indwelling tunneled pleural catheter insertion to death, with a median of 156 d (range, 15-1,978 d) in the entire cohort.
Spontaneous pleurodesis occurred in 22 patients (28%) and was the second most common indication for IPC removal. In patients with spontaneous pleurodesis, median time to removal was 55 days (range, 10-370 days). Twenty-one of the 22 patients (96%) had documented expandable lung prior to catheter insertion. Among the 22 patients, IPC was placed as a bridge to transplant in 11 patients. Six of the 22 patients had died by the end of the study, and their median time from catheter removal to death was 106 days (range, 17-347 days).
Six IPCs were removed secondary to pleural space infection, with the median time to IPC removal of 61.5 days (range, 35-92 days). Additionally, two IPCs were removed because of catheter malfunction (median time to removal, 62.5 days; range, 30-95 days).
Univariate regression analysis did not identify any predictors of spontaneous pleurodesis that reached significance at P < .25; therefore, no multivariate model was considered. However, three significant predictors of time from IPC insertion to death were identified (P < .25). These factors were age (hazard ratio, 1.051; P < .001), WBC count (hazard ratio, 1.022; P = .06), and international normalized ratio (hazard ratio, 0.63; P = .24). On multivariate analysis, age was the only significant predictor with respect to time from catheter insertion to death, after adjusting for WBC count and international normalized ratio (hazard ratio, 1.047; P = .01).
Catheter-Related Complications
No immediate complications were reported after IPC placement, and no patient required additional thoracentesis postcatheter placement.
Pleural space infection was noted in eight patients (10%) and was the most common complication. Among these patients, five also developed cellulitis at the site of catheter insertion. Patients were treated with broad-spectrum antimicrobials, and six of the eight catheters (7.5%) were removed at the time of diagnosis, followed by continuous fluid drainage via a new chest tube.
Two patients did not undergo IPC removal and were managed successfully with continuous fluid drainage and antibiotics. Two of eight patients (2.5%) died because of catheter-related infection resulting in septic shock. Renal failure was noted in two patients, and one patient had severe electrolyte imbalance secondary to IPC drainage. These complications were based on the clinical judgment of the patients’ physician.
Among those with IPC placement as a bridge to transplant (n = 21), seven patients experienced IPC-related complications. Four patients went on to receive liver transplant (catheter site fluid leakage: n = 1, renal failure: n = 1, pleural space infection: n = 2). Three patients did not receive liver transplant (seroma: n = 1, pleural space infection successfully treated: n = 1, death because of catheter-related sepsis: n = 1). IPC prevented liver transplant in one patient because of IPC-related sepsis and death.
Details of less common or minor complications are shown in Table 3.
Table 3.
Indwelling Tunneled Pleural Catheter-Related Complications
| Complication | No. |
|---|---|
| Renal failure | 2 |
| Severe electrolyte imbalance | 1 |
| Severe malnutrition | 0 |
| Subcutaneous fluid collection (seroma) | 3 |
| Catheter site fluid leakage | 4 |
| Cellulitis | 5 |
| Parapneumonic effusion/empyema | 8 |
| Catheter-related sepsis leading to death | 2 |
Survival Data
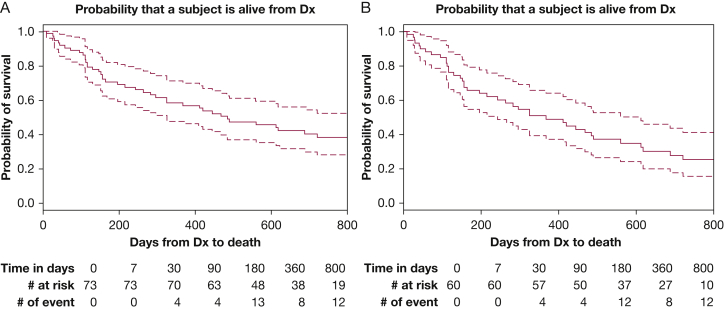
Date of HH diagnosis was available in 73 patients (92%). Median survival from the time of HH diagnosis was 485 days (95% CI, 325-722) in 73 patients. When excluding all transplant recipients, median survival decreased to 368 days (95% CI, 243-613) (Fig 2). Of the 15 patients who received liver transplant, only two patients had died at the end of the study in their corresponding centers. As a result, no survival curve was plotted for this population.
Figure 2.
A, Survival curve from the time of hepatic hydrothorax Dx in 73 patients with a documented Dx date in the cohort. B, Survival curve excluding transplant recipients. Dx = diagnosis.
Discussion
We present, to our knowledge, the first and only multicenter study to examine clinical outcomes related to the placement of IPC for refractory HH in patients with underlying cirrhosis and end-stage liver disease.
The median time to IPC removal within the cohort was 156 days; however, in patients achieving spontaneous pleurodesis, the median time to removal was 55 days. Although the spontaneous pleurodesis rate in our cohort is lower than that seen in the setting of MPE, it is important to note that despite large-volume effusions, 28% of patients with HH and IPC achieved this desirable outcome, whereas the reminder of the cohort had an acceptable complication rate and similar or more favorable survival compared with data published in existing literature.1
A 10% overall infection rate and 2.5% mortality rate because of IPC-related empyema and sepsis were identified within this cohort. When compared with the largest study of chest tubes in the management of HH,8 our complication rates are significantly lower than traditional chest tubes. In a study of 59 patients with cirrhosis and HH by Liu et al,8 pleural space infection was reported in 48% of patients. Eighty percent of the patients suffered from infection, renal failure, or electrolyte imbalance after chest tube placement and continuous drainage. Ultimately, 27% of these patients died, with infection being the most common cause of death. Although it is not possible to prove causality with a retrospective study design, we attribute the cause of low renal failure and electrolyte imbalance in our cohort to a regimented fluid drainage schedule of no more than 1 L on an every other day basis. The purpose of fluid drainage in our patient population was symptom improvement and not maximal drainage.
Refractory HH often requires repeat thoracentesis, which carries a higher complication rate in this population.16 TIPS has been used in the management of refractory HH in appropriate candidates; however, complete response is reported in 55% of patients. The reminder of patients will continue to require repeat thoracentesis for palliation or until liver transplant becomes an option.17 The aforementioned challenges call for evaluation of other management strategies, such as IPC use, and emphasize the importance of this study and future prospective trials.
Previous literature on IPCs is primarily focused on patients with MPE, where infection remains a common complication of IPC placement with reported rates of 4% to 25%.11, 12, 18, 19 Most prior studies of IPCs in HH are limited to case reports and case series or have involved patients with non-MPE of different etiologies, confusing the interpretation of risk estimates for specific patient groups because of disease heterogeneity.20, 21 In a single-center retrospective study of 62 patients with HH, IPC-related pleural space infection was reported in 16% of the patients, but other common complications of conventional chest tubes such as renal failure and electrolyte imbalance were not assessed.22 In contrast, our study represents a population of patients with HH managed by IPCs across eight different institutions and provides additional external validity, despite unmeasured variations among centers. Additionally, this study provides a detailed outline of minor and major complications and is the only study reporting on the effect of IPC drainage on renal function and electrolyte imbalance. Furthermore, we assess for predictors of pleurodesis and death after IPC placement, and report on IPC removal rate with relation to other competing risks of catheter removal and survival data after the diagnosis of HH.
To date, no literature on quality of life and palliation assessment is reported in patients with HH. IPCs are used as a palliative mode of therapy in managing large pleural effusions, but because of the lack of clear guidelines regarding IPCs in HH, there seems to be a selection and referral bias in our cohort. Although most institutions reported similar audit times, the number of patients undergoing IPC placement varied significantly among centers (3-25 patients). The lack of published randomized controlled trials in the management of refractory HH compounds the complex decision-making process of a multidisciplinary liver transplant team providing care to this patient population. Therefore, the management of refractory HH at this point is primarily provider and institution dependent. This study, and future studies in this area, can help providers of a multidisciplinary team to manage patients with HH-complicated cirrhosis.
There are several limitations in the current study. Because of its retrospective nature, this study has the common disadvantages inherent in such a study design. Particularly, lack of long-term follow-up data may affect the estimates of successful catheter removal. No specific predictors of spontaneous pleurodesis were identified on univariate analysis. This may be secondary to an overall small number of patients and low power. Additionally, in 11 of 22 patients with spontaneous pleurodesis, IPC was placed as a bridge to transplant. Most patients who received liver transplant underwent IPC removal after successful transplant. It is possible that other factors such as resolution of portal hypertension and cessation of fluid accumulation are the cause of catheter removal in these patients. This speculation cannot be proven in this retrospective study because ultrasound examination of the pleura prior to IPC removal is not available. This may result in inflation of the true spontaneous pleurodesis rate.
The participating institutions in this study are tertiary care hospitals, academic institutions, and referral centers for complex diseases. These institutions may care for patients that do not represent the general population. To better understand our patient population, we estimated patient survival from the time of HH diagnosis and demonstrated that median survival in our population was 368 days among the nontransplant candidates. In a retrospective analysis, Badillo and Rockey1 showed a mean survival ± SD of 321 ± 463 days in 64 patients receiving medical therapy defined by diuresis and repeat thoracentesis. In their study, patients with liver transplant (n = 5) had a higher survival rate as also noted in our population. Although the patients in the Badillo and Rockey study1 did not undergo IPC placement, this study indicates the overall low survival rate in HH as noted in our cohort.
In our study, the role of albumin replacement after IPC drainage was not evaluated. Albumin replacement, after large-volume paracentesis in patients with HH, is shown to reduce paracentesis-induced circulatory dysfunction.23 Currently, there are no guidelines regarding albumin supplementation with pleural fluid drainage, and its use is largely provider and patient dependent. The result of this study is reflective of real-world practice in the absence of evidence or guidelines. Future trials should evaluate the utility of albumin replacement in pleural fluid drainage using IPCs.
Additionally, we were unable to account for selection bias in our study. Data regarding the number of patients who were not referred or refused to undergo IPC placement were not available. Moreover, provider and institution bias could play a role. Exclusion of these potential IPC candidates could artificially lower our complication rate.
Finally, our study lacks a control group, and other, perhaps more relevant, end points such as symptomatic relief and quality of life assessment were not measured in this study.
Prospective randomized trials are needed to fully evaluate safety and assess quality of life, using IPCs compared with other HH management modalities. Patient-centric outcomes such as mean daily dyspnea and discomfort, mean days spent in the hospital, and overall quality of life measures using standard measurement techniques should be the focus of future studies.
Conclusions
Management of HH remains a challenging clinical scenario with suboptimal palliative options. Select patients may undergo liver transplant or respond to TIPS; however, a large portion of patients require repeat thoracentesis and hospital visits because of recurrent dyspnea and suffer from poor quality of life. The current study suggests that the use of an IPC in patients with HH may be an overall safe procedure and results in spontaneous pleurodesis in about 30% of patients. Because of the lack of randomized trials in this patient population, additional studies are warranted.
Acknowledgments
Author contributions: S. S. is the guarantor of the paper. S. S. participated in study design, data collection, data analysis, manuscript writing, and manuscript review. N. R. participated in study design, data analysis, and manuscript review. K. H. participated in data collection and manuscript review. R. K. participated in data collection and manuscript review. M. L. participated in data collection and manuscript review. M. A. participated in data collection and manuscript review. C. L. participated in data collection and manuscript review. A. M. participated in data collection and manuscript review. J. A. participated in data collection and manuscript review. F. M. participated in data collection and manuscript review. H. L. participated in data collection and manuscript review. M. K. participated in data collection and manuscript review. T. S. participated in data collection and manuscript review. L. K. participated in data analysis and manuscript review. A. C. participated in data collection and manuscript review.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: N. R. has received an unrestricted educational grant from Rocket Medical and provides clinical advice to them. None declared (S. S., K. H., R. K., M. L., M. A., C. L., A. M., J. A., F. M., H. L., M. K., T. S., L. K., A. C.).
Role of sponsors: The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCRR. All work and data analysis were performed and confirmed by each institution. All authors had access to the final data and manuscript submission.
Other contributions: We thank Trinidad Sanchez, MD, and Daniel Alape, MD, for their assistance in data collection.
Footnotes
FUNDING/SUPPORT: The use of the REDCap Database was supported by the Virginia Commonwealth UniversityCenter for Clinical and Translational Research, Richmond, Virginia [Grant NIH/NCRR Grant UL 1TR002649].
References
- 1.Badillo R., Rockey D.C. Hepatic hydrothorax: clinical features, management, and outcomes in 77 patients and review of the literature. Medicine (Baltimore) 2014;93(3):135–142. doi: 10.1097/MD.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinasewitz G.T., Keddissi J.I. Hepatic hydrothorax. Curr Opin Pulm Med. 2003;9(4):261–265. doi: 10.1097/00063198-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dhanasekaran R., West J.K., Gonzales P.C. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105(3):635–641. doi: 10.1038/ajg.2009.634. [DOI] [PubMed] [Google Scholar]
- 4.Siegerstetter V., Deibert P., Ochs A., Olschewski M., Blum H.E., Rossle M. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long-term results in 40 patients. Eur J Gastroenterol Hepatol. 2001;13(5):529–534. doi: 10.1097/00042737-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Spencer E.B., Cohen D.T., Darcy M.D. Safety and efficacy of transjugular intrahepatic portosystemic shunt creation for the treatment of hepatic hydrothorax. J Vasc Interv Radiol. 2002;13(4):385–390. doi: 10.1016/s1051-0443(07)61741-2. [DOI] [PubMed] [Google Scholar]
- 6.Everhart J.E., Lombardero M., Detre K.M. Increased waiting time for liver transplantation results in higher mortality. Transplantation. 1997;64(9):1300–1306. doi: 10.1097/00007890-199711150-00012. [DOI] [PubMed] [Google Scholar]
- 7.Lucey M.R., Brown K.A., Everson G.T. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Transplantation. 1998;66(7):956–962. doi: 10.1097/00007890-199810150-00034. [DOI] [PubMed] [Google Scholar]
- 8.Liu L.U., Haddadin H.A., Bodian C.A. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest. 2004;126(1):142–148. doi: 10.1378/chest.126.1.142. [DOI] [PubMed] [Google Scholar]
- 9.Runyon B.A., Greenblatt M., Ming R.H. Hepatic hydrothorax is a relative contraindication to chest tube insertion. Am J Gastroenterol. 1986;81(7):566–567. [PubMed] [Google Scholar]
- 10.Orman E.S., Lok A.S. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3(4):582–586. doi: 10.1007/s12072-009-9136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies H.E., Mishra E.K., Kahan B.C. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383–2389. doi: 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay A., Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129(2):362–368. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 13.Majid A., Kheir F., Fashjian M. Tunneled pleural catheter placement with and without talc poudrage for treatment of pleural effusions due to congestive heart failure. Ann Am Thorac Soc. 2016;13(2):212–216. doi: 10.1513/AnnalsATS.201507-471BC. [DOI] [PubMed] [Google Scholar]
- 14.Chen A., Massoni J., Jung D., Crippin J. Indwelling tunneled pleural catheters for the management of hepatic hydrothorax. A pilot study. Ann Am Thorac Soc. 2016;13(6):862–866. doi: 10.1513/AnnalsATS.201510-688BC. [DOI] [PubMed] [Google Scholar]
- 15.Mercky P., Sakr L., Heyries L., Lagrange X., Sahel J., Dutau H. Use of a tunnelled pleural catheter for the management of refractory hepatic hydrothorax: a new therapeutic option. Respiration. 2010;80(4):348–352. doi: 10.1159/000282493. [DOI] [PubMed] [Google Scholar]
- 16.Shojaee S., Khalid M., Kallingal G.J., Kang L., Rahman N.M. Repeat thoracentesis in hepatic hydrothorax and non-hepatic hydrothorax effusions: a case control study [published online ahead of print July 10, 2018] Respiration. 2018 doi: 10.1159/000490001. [DOI] [PubMed] [Google Scholar]
- 17.Singh A., Bajwa A., Shujaat A. Evidence-based review of the management of hepatic hydrothorax. Respiration. 2013;86(2):155–173. doi: 10.1159/000346996. [DOI] [PubMed] [Google Scholar]
- 18.Fysh E.T., Tremblay A., Feller-Kopman D. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144(5):1597–1602. doi: 10.1378/chest.12-3103. [DOI] [PubMed] [Google Scholar]
- 19.Mekhaiel E., Kashyap R., Mullon J.J., Maldonado F. Infections associated with tunnelled indwelling pleural catheters in patients undergoing chemotherapy. J Bronchology Interv Pulmonol. 2013;20(4):299–303. doi: 10.1097/LBR.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 20.Bhatnagar R., Reid E.D., Corcoran J.P. Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax. 2014;69(10):959–961. doi: 10.1136/thoraxjnl-2013-204563. [DOI] [PubMed] [Google Scholar]
- 21.Patil M., Dhillon S.S., Attwood K., Saoud M., Alraiyes A.H., Harris K. Management of benign pleural effusions using indwelling pleural catheters: a systematic review and meta-analysis. Chest. 2017;151(3):626–635. doi: 10.1016/j.chest.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 22.Kniese C., Diab K., Ghabril M., Bosslet G. Indwelling pleural catheters in hepatic hydrothorax: a single-center series of outcomes and complications. Chest. 2019;155(2):307–314. doi: 10.1016/j.chest.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwosk C.S., Krupa L., Mahtani A. Albumin reduces paracentesis-induced circulatory dysfunction and reduces death and renal impairment among patients with cirrhosis and infection: a systematic review and meta-analysis. Biomed Res Int. 2013;2013:295153. doi: 10.1155/2013/295153. [DOI] [PMC free article] [PubMed] [Google Scholar]