Abstract
Background
Decisions to admit high-risk postoperative patients to critical care may be affected by resource availability. We aimed to quantify adult ICU/high-dependency unit (ICU/HDU) capacity in hospitals from the UK, Australia, and New Zealand (NZ), and to identify and describe additional ‘high-acuity’ beds capable of managing high-risk patients outside the ICU/HDU environment.
Methods
We used a modified Delphi consensus method to design a survey that was disseminated via investigator networks in the UK, Australia, and NZ. Hospital- and ward-level data were collected, including bed numbers, tertiary services offered, presence of an emergency department, ward staffing levels, and the availability of critical care facilities.
Results
We received responses from 257 UK (response rate: 97.7%), 35 Australian (response rate: 32.7%), and 17 NZ (response rate: 94.4%) hospitals (total 309). Of these hospitals, 91.6% reported on-site ICU or HDU facilities. UK hospitals reported fewer critical care beds per 100 hospital beds (median=2.7) compared with Australia (median=3.7) and NZ (median=3.5). Additionally, 31.1% of hospitals reported having high-acuity beds to which high-risk patients were admitted for postoperative management, in addition to standard ICU/HDU facilities. The estimated numbers of critical care beds per 100 000 population were 9.3, 14.1, and 9.1 in the UK, Australia, and NZ, respectively. The estimated per capita high-acuity bed capacities per 100 000 population were 1.2, 3.8, and 6.4 in the UK, Australia, and NZ, respectively.
Conclusions
Postoperative critical care resources differ in the UK, Australia, and NZ. High-acuity beds may have developed to augment the capacity to deliver postoperative critical care.
Keywords: critical care, health services research, patient safety, perioperative care, postoperative complications
Editor's key points.
-
•
Admission of high-risk postoperative patients to critical care is resource limited, with poorly defined guidelines for risk stratification.
-
•
A survey of critical care, high-dependency, and other ‘high-acuity’ bed availability in UK, Australian, and New Zealand hospitals was conducted following a modified Delphi approach to design the survey.
-
•
There are differences between countries in postoperative critical care capacity and staffing levels in general surgical wards, accounted for in part by adjusting for hospital size and tertiary care provision.
-
•
High-acuity care areas outside the ICU and high-dependency unit may have developed in order to meet the demands of high-risk postoperative patients.
Surgery is common and will become increasingly prevalent as populations grow and age.1, 2 Globally, the volume of surgery has been estimated at 313 million cases a year, with high-income countries conducting procedures at a mean rate of 11 168 per 100 000 population per year.3 Whilst surgery is usually a treatment for a disease, complications from surgery are associated with significant morbidity and mortality.4, 5 Critical care or protocolised pathways delivered in enhanced care areas are thought to mitigate against the risks of surgery by higher nurse-to-patient ratios, medical input from specialist intensivists, and availability of specific organ support therapies.6, 7 As the global burden of surgery increases, the number of patients at risk of perioperative complications increase correspondingly. Therefore, the capacity to admit prospectively high-risk patients to critical care after surgery becomes an increasing population concern.
In Australia and New Zealand (NZ), there are currently no national guidelines for risk stratifying postoperative critical care admissions. However, in the UK, the National Confidential Enquiry into Patient Outcome and Death recommends critical care admission when the preoperative estimated risk of mortality is ≥5%, whilst the Royal College of Surgeons of England and the Department of Health recommend that those with mortality risks ≥10% should be admitted.8, 9 Despite these guidelines, multiple observational studies report that critical care resources are not reliably allocated to patients at highest risk of death.10, 11, 12 In some countries, a lack of critical care capacity is thought to contribute to this phenomenon.13 A recent commentary suggests that alternative facilities are consequently being used to provide enhanced care to patients outside of the traditional ICU and high-dependency units (ICU/HDUs) in some hospitals in the UK.14 These ‘high-acuity’ beds may be able to provide a subset of the interventions and monitoring capabilities usually associated with critical care, and provide the necessary environment to manage postoperative recovery of high-risk surgical patients.
We therefore performed a survey to assess the available postoperative facilities for high-risk patients in UK, Australian, and NZ hospitals as part of the Second Sprint National Anaesthesia Project: Epidemiology of Critical Care Provision After Surgery (SNAP-2: EPICCS) study, an international observational cohort study of uncertainties around postoperative critical care.15 (The full list of collaborators and their affiliations is included in the Supplementary material.) The aim of this survey was to describe and compare the critical care, enhanced care, and usual ward care availability for surgical patients in each of these countries, according to hospital types and health systems. We also aimed to investigate hospital factors associated with critical care bed capacity and with the likelihood of high-acuity bed availability.
Methods
We performed a survey in all hospital sites that expressed interest in participating in SNAP-2: EPICCS in the UK, Australia, and NZ.15 In the UK, sites were identified from a list of National Health Service (NHS) hospitals that undertake adult inpatient surgery, and from the list of hospitals that participated in the First Sprint National Anaesthesia Project (SNAP-1).16, 17, 18, 19 The UK sites were then invited to participate via approaches to the lead collaborators from SNAP-1 and the Royal College of Anaesthetists' (RCoA) network of Quality Audit and Research Coordinators (QuARCs). The QuARCs is a comprehensive network of researchers covering almost all UK NHS hospital trusts and have previously been instrumental in delivering the RCoA's National Audit Projects.20, 21 In Australia, all public hospitals accredited by the Australian and New Zealand College of Anaesthetists (ANZCA) to provide postgraduate anaesthesia training were invited to participate. The Australian Society of Anaesthetists (ASA) state representatives and ANZCA Clinical Trials Network (ANZCA CTN) contacted anaesthetic departments and anaesthetic department research leads via their respective national networks. In NZ, the Supportive Anaesthesia Trainee Audit and Research Network for NZ approached all public hospitals accredited by ANZCA based on the NZ Government Ministry of Health listings of all public hospitals providing adult inpatient surgical services. Ethical approval was not necessary, as no patient-level data were collected.
The survey was conducted between December 1, 2016 and March 31, 2017 in the UK, and between December 1, 2016 and January 31, 2018 in Australia and NZ. The lead collaborators at each site were asked to answer survey questions based on their own knowledge of their hospitals' structures and processes, and to approach senior hospital and nursing management teams for additional support to obtain information. Where hospital trusts and organisations operated across more than one geographical site, individual responses were requested for each location.
Questionnaire design
The survey was developed using a modified Delphi consensus method. A study steering group was convened with representatives from the RCoA, Faculty of Intensive Care Medicine (FICM), Intensive Care Society, Association of Anaesthetists of Great Britain and Ireland, Royal College of Surgeons (England), and lay representation (see Supplementary material). Draft questions were circulated amongst steering group members for anonymous feedback and evaluation (Round 1). The responses were collated by a facilitator at the National Institute of Academic Anaesthesia Health Services Research Centre. The draft questions were modified based on Round 1 feedback, and these questions were then used to construct a pilot questionnaire. The pilot questionnaire was recirculated to members of the steering group, and the survey was piloted in eight participating hospitals. A second cycle of anonymous feedback was then obtained (Round 2). The final survey was then constructed based on the responses from Round 2. (Final survey questions are reported in Supplementary File S1.) The survey questionnaire was designed in the UK. To facilitate international comparisons, no further changes were made to the questionnaire before it was distributed in Australia and NZ. All authors considered the terminology and definitions used in each country to be equivalent in their local contexts.
The survey was distributed electronically using online forms (FormAssembly; Veer West LLC, Bloomington, IN, USA) to all collaborators at sites in the UK, and electronically via e-mail to investigators at sites in Australia and NZ. To improve response rates in the UK, monthly reminders were sent to collaborators who had yet to respond, and reminder frequency was increased to weekly in the last month of the survey period. In Australia, individual ASA state representatives were given autonomy in following up on invitations to participate in the survey within their respective states, and two cycles of reminders were sent via the ANZCA CTN with a final reminder sent in January 2018. In NZ, correspondence was maintained with individual investigators at each site until data collection was completed.
The survey recorded hospital-level characteristics, including hospital size (total number of adult inpatient beds), number of adult ICU/HDU beds, types of tertiary services delivered, the presence or absence of an emergency department, nurse-to-patient staffing ratios, and the presence and characteristics of high-acuity care areas that were defined as ‘any other ward areas in the hospital which receive high-risk surgical patients for enhanced perioperative care’.
We defined surgical beds as those that would be used for any adult patient undergoing a non-obstetric inpatient procedure in an operating theatre or radiology suite.15
Statistical analyses
Descriptive statistics for normally distributed continuous data are reported as mean and standard deviation (sd), and for non-normally distributed data as median and inter-quartile range (IQR). For all analyses, P<0.05 was considered statistically significant. Critical care bed ratios were calculated per 100 hospital beds for each participating site, based on the number of critical care and hospital beds reported by survey respondents. These bed numbers were then aggregated by country and combined with published Organisation for Economic Co-operation and Development (OECD) indicator data on per capita hospital bed numbers to obtain critical care bed ratios per 100 000 population in each country.42 Univariate analysis was performed to compare the characteristics of hospitals, critical care units, and high-acuity care areas between each participating country, using appropriate statistical tests for continuous and categorical variables. Based on the hypothesis that critical care capacity is related to tertiary services provided, we investigated the association between critical care bed provision at each site and variables thought to influence critical care bed capacity, using negative binomial regression, as appropriate for count data. The response variable of critical care beds per 100 hospital beds was regressed against the following co-variates: number of hospital beds, tertiary services offered, country where the hospital was located, whether high-acuity care beds were present within the hospital, and whether the hospital had an emergency department. Relative ratios (RRs) were calculated to express the relative difference in critical care bed numbers associated with a particular variable, after adjusting for hospital size and other variables in the model. We further investigated the characteristics associated with the likelihood of hospitals having high-acuity care areas using logistic regression: the binary outcome variable of whether high-acuity care areas were present or absent was regressed against the following co-variates: number of hospital beds, whether tertiary services were offered, country, the critical-care-to-hospital-bed ratio, and whether the hospital had an emergency department.
Sensitivity analysis
Because of the lower national response rate from Australian sites compared with the UK and NZ, we compared the hospital characteristics of the respondent sites with published data of hospitals offering surgical services from the Australian Institute of Health and Welfare to determine if our survey sample was biased.22 Our collected data were matched by hospital name to this external openly accessible data set, which categorised hospitals by type (Supplementary File S1).
Statistical analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), with the following external packages enabled: tidyverse, tableone, and sjPlot.23, 24, 25 Negative binomial and logistic regression models were constructed using the glm command. The code for all analyses is available on request.
Results
We received responses from 309 hospitals across the UK, Australia, and NZ. In the UK, 257 hospitals responded out of the 263 invited to participate (response rate: 97.7%); these hospitals were nested within 141 English NHS Trusts, 13 Scottish NHS Boards, six Welsh Health Boards, and four Northern Irish Health and Social Care Trusts. Our sample therefore represented 94.8% of NHS secondary care organisations providing adult inpatient surgical services in the UK. In Australia, 107 hospitals were invited to participate, with 35 sites responding (response rate: 32.7%). In NZ, 18 hospitals were invited to participate, with 17 sites responding (response rate: 94.4%). Responding Australian hospitals were more likely to be medium to large hospitals, as classified by the Australian Institute of Health and Welfare, which offers a wider range of specialist services than the general population of Australian hospitals (Sensitivity Analysis; Supplementary File S1).
Hospital characteristics
The median reported hospital size in our sample was 429 beds (IQR=280–626; Table 1). Australian (median: 399 beds; IQR: 256–600 beds) and NZ (median: 315 beds; IQR: 193–540 beds) hospitals were not significantly different in size to those in the UK (median: 450 beds; IQR: 290–650 beds).
Table 1.
Summary of hospital characteristics. IQR, inter-quartile range
| Overall | UK | Australia | New Zealand | P-value | |
|---|---|---|---|---|---|
| N | 309 | 257 | 35 | 17 | |
| Total hospital beds (median [IQR]) | 429 (280, 626) | 450 (290, 650) | 399 (256, 600) | 315 (193, 540) | 0.095 |
| Emergency department present (%) | 256 (82.8) | 207 (80.5) | 33 (94.3) | 16 (94.1) | 0.058 |
| Total critical care beds (median [IQR]) | 12 (8, 22) | 12 (8, 21) | 14 (8, 26) | 9 (6, 16) | 0.435 |
| Total ventilated beds (median [IQR]) | 8 (6, 14) | 8 (6, 13) | 11 (5, 20) | 6 (4, 12) | 0.124 |
| Proportion of critical care beds per 100 hospital beds (median [IQR]) | 2.84 (2.11, 4.39) | 2.67 (2.07, 4.31) | 3.74 (3.02, 4.93) | 3.50 (2.55, 4.12) | 0.014 |
| Total general surgical ward beds (median [IQR]) | 120 (64, 189) | 121 (70, 189) | 90 (48, 156) | 87 (48, 195) | 0.309 |
| PACU present (%) | 7 (2.3) | 6 (2.3) | 1 (2.9) | 0 (0.0) | 0.797 |
| High-acuity care area present (%) | 92 (29.8) | 72 (28.0) | 14 (40.0) | 6 (35.3) | 0.304 |
| Tertiary services provided (%) | 178 (57.6) | 137 (53.3) | 27 (77.1) | 14 (82.4) | 0.003 |
The majority of responding hospitals were acute hospitals with emergency departments on-site (n=256; 82.9%). One hundred and seventy-eight hospitals (57.6%) provided tertiary services. However, higher proportions of hospitals in Australia (n=27; 77.1%) and NZ (n=14; 82.4%) were tertiary institutions than those in the UK (n=137; 53.3%). A sensitivity analysis performed indicated that our Australian data sample was weighted towards medium-to-large hospitals offering more specialist services (Supplementary Table S1).
Critical care beds
Most hospitals reported having on-site ICU/HDU facilities (n=283; 91.6%), with a median ratio of 2.84 (IQR: 2.11–4.39) critical care beds per 100 hospital beds. Four hundred and sixty separate critical care units were described within 283 hospitals across all three countries (Table 2). Of these units, 315 (68.5%) admitted patients from different specialties. However, 79 hospitals (17.2%) reported having at least one specialist critical care unit, and there were 145 such specialist units identified. Amongst these specialist units, 43 (28.3%) would admit patients from another specialty if necessary, with the remainder restricting admissions to patients from single specialties only (e.g. cardiothoracic or neurosurgery). The median number of critical care beds across all units was 10 (IQR: 6–15). The estimated number of critical care beds and ventilated beds per capita calculated using our sample was highest in Australia (Table 3).
Table 2.
Summary of critical care unit characteristics. HDU, high-dependency unit; IQR, inter-quartile range
| Overall | UK | Australia | New Zealand | P-value | |
|---|---|---|---|---|---|
| N | 460 | 397 | 40 | 23 | |
| Total critical care beds (median [IQR]) | 10 (6, 15) | 10 (7, 15) | 10 (7, 18) | 8 (6, 10) | 0.199 |
| Total ventilated beds (median [IQR]) | 6 (0, 10) | 7 (0, 10) | 7 (4, 15) | 5 (3, 8) | 0.098 |
| ICU/HDU/mixed (%) | 0.206 | ||||
| HDU | 136 (29.6) | 125 (31.6) | 6 (15.0) | 5 (21.7) | |
| ICU | 73 (15.9) | 61 (15.4) | 7 (17.5) | 5 (21.7) | |
| Mixed | 250 (54.5) | 210 (53.0) | 27 (67.5) | 13 (56.5) | |
| Specialty unit (%) | 0.304 | ||||
| Cardiothoracic | 41 (8.9) | 35 (8.8) | 4 (10.0) | 2 (9.1) | |
| General/mixed | 315 (68.6) | 263 (66.2) | 33 (82.5) | 19 (86.4) | |
| Medical | 27 (5.9) | 27 (6.8) | 0 (0.0) | 0 (0.0) | |
| Neurology/Neurosurgical | 19 (4.1) | 18 (4.5) | 1 (2.5) | 0 (0.0) | |
| Surgical | 30 (6.5) | 28 (7.1) | 1 (2.5) | 1 (4.5) | |
| Other | 27 (5.9) | 26 (6.5) | 1 (2.5) | 0 (0.0) | |
| Will admit off-specialty patients (%) | 43 (28.3) | 41 (30.6) | 1 (6.7) | 1 (33.3) | 0.146 |
Table 3.
Critical care beds per capita. The sum of the number of critical care beds was divided by the sum of all hospital beds within each country, and multiplied by 100, to obtain the average ratio of critical care beds to hospital beds in each country. This ratio was then multiplied by Organisation for Economic Co-operation and Development data on hospital beds per capita to obtain the per capita critical care bed numbers, rescaled to per 100 000 population
| Country | Critical care beds per 100 hospital beds | Ventilated beds per 100 hospital beds | High-acuity care beds per 100 hospital beds | Critical care beds per 100 000 population | Ventilated beds per 100 000 population | High-acuity care beds per 100 000 population |
|---|---|---|---|---|---|---|
| UK | 3.59 | 2.17 | 0.47 | 9.33 | 5.64 | 1.23 |
| Australia | 3.70 | 2.77 | 0.99 | 14.05 | 10.54 | 3.77 |
| New Zealand | 3.39 | 2.20 | 2.36 | 9.14 | 5.93 | 6.38 |
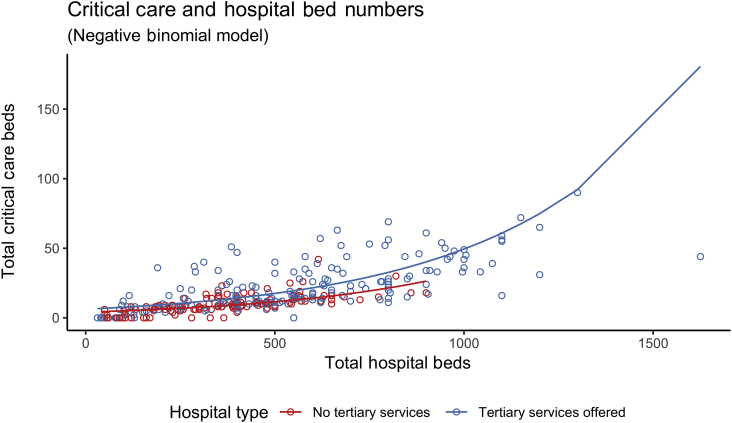
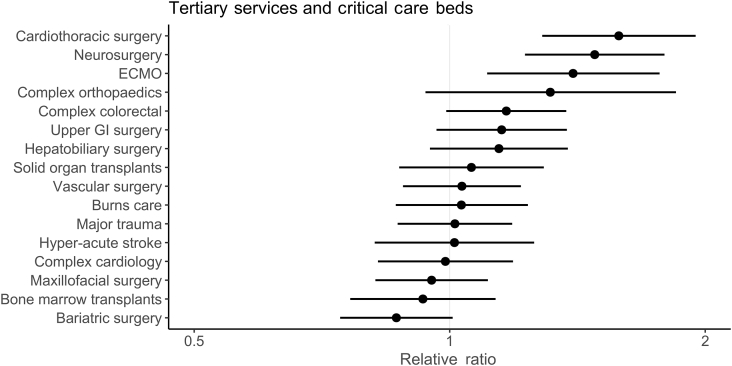
Hospitals offering tertiary services had 1.62 times (RR) as many critical care beds per 100 hospital beds than those that did not offer any tertiary services (95% confidence interval [CI]: 1.42–1.86; P<0.001; Fig. 1), after adjusting for other variables (Supplementary Table S2 for model coefficients). The provision of cardiothoracic (RR: 1.58; 95% CI: 1.29–1.95; P<0.001), neurosurgery (RR: 1.48; 95% CI: 1.23–1.79; P<0.001), and extracorporeal membrane oxygenation (RR: 1.40; 95% CI: 1.11–1.77; P=0.01) tertiary services were associated with increased proportions of critical care beds within hospitals (Fig. 2).
Fig 1.
Scatter plot of critical care beds vs. hospital size, with hospitals coloured by tertiary status. A line of best fit as estimated using a negative binomial regression model illustrates the higher number of critical care beds in hospitals offering tertiary services, compared to hospitals not offering tertiary services.
Fig 2.
Forest plot of associations between specialist services delivered and the relative availability of critical care beds per 100 hospital beds, after adjusting for hospital size, presence of enhance ward areas, presence of emergency department and country. ECMO = extra-corporeal membrane oxygenation; GI = gastrointestinal.
UK hospitals had a smaller proportion of critical care beds per 100 hospital beds (median: 2.67; IQR: 2.07–4.31) compared with hospitals in Australia (median: 3.74; IQR: 3.02–4.93) and NZ (median: 3.50; IQR: 2.55–4.12). However, after adjusting for tertiary services delivered and hospital size, the proportion of critical care beds to total hospital beds was lower in Australia (RR: 0.60; 95% CI: 0.49–0.75; P<0.001) and NZ (RR: 0.64; 95% CI: 0.49–0.85; P=0) than in the UK (Supplementary Table S3 for model coefficients). Neither the presence of an emergency department nor the presence of enhanced ward areas was associated with the proportion of critical care beds in any of the three countries.
High-acuity care areas
Ninety-six (31.1%) hospitals reported having high-acuity care areas where high-risk surgical patients could be admitted for postoperative management outside the operating theatre or critical care complexes: 72 hospitals in the UK (28.0% of hospitals), 14 in Australia (40.0%), and six in NZ (35.3%). A total of 147 such high-acuity care areas were identified (Table 4). These areas have a median of four beds (IQR: 3–8 beds), and a median nurse-to-patient ratio of 1:2 (IQR: 1:2–1:4). Patient care was led by surgeons in 73 (49.7%) of these high-acuity care areas. These areas were able to deliver a heterogeneous subset of interventions normally associated with critical care (Table 4), ranging from continuous observations and monitoring (n=128; 87.1%) to non-invasive ventilation or CPAP support (n=56; 38.1%).
Table 4.
Summary of high-acuity care area characteristics. IQR, inter-quartile range; NIV, non-invasive ventilation.
| Overall | UK | Australia | New Zealand | P-value | |
|---|---|---|---|---|---|
| N | 147 | 109 | 21 | 17 | |
| Total beds (median [IQR]) | 4 (3, 8) | 4 (3, 7) | 4 (4, 8) | 5 (4, 10) | 0.133 |
| Patient-to-nurse ratio (median [IQR]) | 2 (2, 4) | 2 (2, 4) | 2 (2, 4) | 2 (2, 3) | 0.983 |
| Responsible consultant (%) | 0.457 | ||||
| Intensivist | 7 (4.8) | 7 (6.4) | 0 (0.0) | 0 (0.0) | |
| Multi-specialty joint care | 34 (23.1) | 28 (25.7) | 3 (14.3) | 3 (17.6) | |
| Perioperative anaesthetist | 15 (10.2) | 13 (11.9) | 1 (4.8) | 1 (5.9) | |
| Surgeon | 73 (49.7) | 50 (45.9) | 13 (61.9) | 10 (58.8) | |
| Other specialty | 18 (12.2) | 11 (10.1) | 4 (19.0) | 3 (17.6) | |
| Able to provide continuous observations/monitoring (%) | 128 (87.1) | 94 (86.2) | 19 (90.5) | 15 (88.2) | 0.859 |
| Able to provide invasive blood pressure monitoring (%) | 83 (56.5) | 66 (60.6) | 10 (47.6) | 7 (41.2) | 0.220 |
| Able to manage vasoactive infusions (%) | 64 (43.5) | 46 (42.2) | 9 (42.9) | 9 (52.9) | 0.707 |
| Able to provide invasive ventilation (%) | 6 (4.1) | 6 (5.5) | 0 (0.0) | 0 (0.0) | 0.336 |
| Able to provide non-invasive ventilation/CPAP (NIV/CPAP) (%) | 56 (38.1) | 37 (33.9) | 12 (57.1) | 7 (41.2) | 0.129 |
| Able to manage epidural catheters (%) | 101 (68.7) | 74 (67.9) | 19 (90.5) | 8 (47.1) | 0.015 |
Larger hospitals (adjusted odds ratio [OR]: 1.97 for every sd increase in hospital bed numbers; 95% CI: 1.41–2.83; P<0.001) providing tertiary services (adjusted OR: 2.42; 95% CI: 1.24–4.84; P=0.01) were more likely to report having high-acuity care areas. Hospitals with emergency departments (adjusted OR: 0.27; 95% CI: 0.11–0.63; P<0.001) were less likely to report having these types of beds. Full coefficients for our logistic regression model are available in Supplementary Table S4.
After critical care and high-acuity bed numbers were considered together, the total potential per capita capacity for delivering at least some critical care to postoperative patients increased in all three countries (Table 3).
General surgical wards
Across all three countries, hospitals reported a median ratio of 28.3 surgical beds per 100 hospital beds (IQR: 21.3–36.0). The average surgical ward was reported as having a median of 26 beds (IQR: 22–30 beds). The median nurse-to-patient ratio during the daytime was 1:6 (IQR: 1:5–1:7; Table 5), and this ratio decreased to a median of 1:9 nurse to patients (IQR: 1:7–1:11) at night. General surgical ward nurses in the UK were responsible for more beds per nurse than in Australia or NZ, both in the day and at night (Table 5; P<0.001). The majority of UK (n=252; 98.1%) and NZ (n=16; 94.1%) hospitals reported staffing surgical wards with healthcare assistants to supplement the care delivered by nurses. In contrast, healthcare assistants were less commonly used in Australia with only 18 hospitals (51.4%) reporting their deployment on surgical wards.
Table 5.
Summary of general ward staffing levels. IQR, inter-quartile range
| Overall | UK | Australia | New Zealand | P-value | |
|---|---|---|---|---|---|
| N | 309 | 257 | 35 | 17 | |
| Number of beds (median [IQR]) | 26 (22, 30) | 25 (22, 30) | 30 (24, 32) | 28 (25, 30) | 0.008 |
| Number of nurses (day) (median [IQR]) | 4 (4, 6) | 4 (3, 5) | 8 (7, 9) | 6 (5, 8) | <0.001 |
| Beds-to-nurse ratio (day) (median [IQR]) | 5.71 (4.50, 7.00) | 6.00 (5.00, 7.50) | 3.75 (3.43, 4.00) | 4.45 (3.62, 5.00) | <0.001 |
| Number of nurses (night) (median [IQR]) | 3 (2, 4) | 3.00 (2.00, 3.00) | 4.00 (4.00, 4.50) | 3.00 (3.00, 3.62) | <0.001 |
| Beds-to-nurse ratio (night) (median [IQR]) | 9.00 (7.00, 11.00) | 9.33 (7.33, 11.83) | 6.86 (6.00, 8.00) | 8.67 (7.22, 9.33) | <0.001 |
| Healthcare assistants utilised (%) | 286 (92.6) | 252 (98.1) | 18 (51.4) | 16 (94.1) | <0.001 |
Discussion
We present a survey that provides a comprehensive overview of postoperative critical care facilities available for patients undergoing inpatient surgery in the UK, Australia, and NZ. Our study describes the critical care provision in hospitals within these countries, and quantifies the availability of high-acuity care areas where postoperative patients may receive critical care therapies outside of the traditional ICU/HDU setting. Hospitals in NZ were generally smaller compared with the UK and Australia. The proportion of hospital beds dedicated to critical care was similar across the three countries; however, the estimated per capita critical care capacity was highest in Australia. General surgery wards in Australia and NZ reported more favourable nurse-to-patient staffing ratios than in the UK. High-acuity care areas delivering some critical care interventions were present in all three countries, and these were of similar size and nurse staffing ratios. The total potential per capita capacity for delivering at least some critical care to postoperative patients increases after these enhanced care areas are taken into account.
Strengths and weaknesses
Our survey had nearly complete coverage of all UK and NZ public secondary care organisations that provide inpatient surgical care. The data collected are therefore likely to be an accurate representation of available postoperative facilities in both countries.
Whilst NHS England collects data on critical care bed numbers, these are aggregated at Trust level, and not individual hospital site level. The Scottish Intensive Care Society Audit Group publishes an annual audit report of critical care outcomes and facilities, against that we cross-checked with our results, and found high levels of agreement.26 To our knowledge, the national health authorities in Wales and Northern Ireland do not compile publicly accessible data of this nature for secondary analysis. The Australian and New Zealand Intensive Care Society (ANZICS) publishes an annual report with information on the total number of adult ICUs across Australia and NZ that includes numbers of paediatric intensive care beds.27 Our data are therefore comprehensive and robust, and contain information not routinely collected by national bodies in all three countries. A further key strength of this study is that we have been able to provide the first empirical description of perioperative high-acuity care areas.
There are also some weaknesses to this work. First, the response rate in Australia was lower than in the UK and NZ. A post hoc sensitivity analysis showed that the Australian hospitals sampled in our study were weighted towards medium-to-large major hospitals that provide postgraduate anaesthesia specialty training and are capable of delivering higher numbers of specialist services (Supplementary File S1). Second, a higher proportion of NZ hospitals that responded were tertiary institutions. Larger tertiary institutions in Australia and NZ may have had increased motivation to participate in our study, and survey dissemination via local networks may have favoured tertiary hospitals because of the nature of the networks used. (The anaesthesia trainee research networks relied upon to distribute the survey are more likely to be found within larger tertiary hospitals.) Third, private sector hospitals were not approached in our survey, and we therefore were not able to explore the pathways in those institutions, which we acknowledge may provide a substantial proportion of elective surgical care, especially in Australia, where the spend on private healthcare as a proportion of total healthcare expenditure is higher compared with UK and NZ (UK: 21.9%, Australia: 31.9%, and NZ: 21.1% of healthcare spend).28 These differences in our sample must be considered when evaluating the comparative data between the three countries. Finally, because of the difference in population distributions in Australia and NZ, these nations have a large number of geographically dispersed small rural hospitals, usually without critical care provision and linked to central hubs of secondary/tertiary care; these differences from the UK make direct comparisons of health systems difficult.
Defining critical care
Historically, the critical care bed capacity per capita in the UK, Australia, and NZ has been found to be low compared with many other developed health systems.13, 29 However, research in this area is made difficult by the lack of international consensus in critical care definitions. In the UK, Guidelines for the Provision of Intensive Care Services (GPICS) were published by the FICM and Intensive Care Society in 2015,30 following on from earlier publications that aimed to describe ICU/HDU standards in the UK.31, 32 In Australia and NZ, the College of Intensive Care Medicine defines minimum standards for ICU and HDU in separate documents.33, 34
A Level 0–3 classification system has been adopted in the UK, and it is referred to extensively in GPICS. Level 3 indicates care for complex patients requiring support for multi-organ failure and with a minimum of 1:1 nurse-to-patient ratio, whilst Level 2 indicates care for patients with single-organ support and a minimum 1:2 nurse-to-patient ratio. In contrast to the UK, Levels I–III ICU definitions in Australia and NZ refer not to patient dependency, but instead to multiple organisational factors relating to work practice/caseload, staffing requirements, operational requirements, design, and monitoring and equipment standards.33 In Australia and NZ, Level III ICUs are tertiary referral units for intensive care patients, whilst Levels I and II ICUs are rural units serving smaller populations where there are limited specialist services available, and where travel to specialist services may cause delay.33 Therefore, Levels I–III ICUs in Australia and NZ are all able to provide a period of mechanical ventilation, and HDUs do not come under this classification system.34
Other less clearly defined ‘high-acuity care’ areas
Beyond the aforementioned definitions, there are other patient care areas within the hospital that do not traditionally fall under the widely accepted umbrella of ICU/HDU critical care units. These have the ability to care for patients who require one or more interventions associated with critical care. For example, within the emergency department, resuscitation bays have the facilities to care temporarily for critically ill patients requiring intensive nursing/medical interventions. Another example would be the coronary care unit, which may have the ability to deliver 1:1 or 1:2 nursing, invasive blood pressure monitoring, continuous ECG telemetry, and inotropic/vasopressor support.
We sought to identify high-acuity care areas capable of delivering higher levels of postoperative care compared with usual ward-level care. We suggest that these high-acuity beds have evolved in the UK, Australia, and NZ to compensate for the low critical care capacity for high-risk patients. These areas may be thought of as ‘Level 1.5’ units, to borrow from the traditional UK classification system described earlier. Our survey suggests that many hospitals use such facilities to deliver postoperative critical care to patients.
Comparisons to existing literature
Using administrative panel data from multiple different sources, Adhikari and colleagues13 estimated the per capita ratio of critical care beds in a number of countries, and further estimated the number of ICU beds per 100 hospital beds. They reported 1.2 ICU beds per 100 hospital beds for the UK, and 1.5 ICU beds per 100 hospital beds for NZ public hospitals, but did not provide estimates for Australia. They also further estimated per capita ICU bed ratios of 3.5, 5.6, and 4.7 per 100 000 population for UK, Australia, and NZ, respectively. In a separate study of European critical care capacity, Rhodes and colleagues29 estimated 2.8 ICU beds per 100 hospital beds, and 6.6 ICU/intermediate care beds per 100 000 population for the UK in 2012. The ANZICS Centre for Outcome and Resource Evaluation reported 9.0 ICU beds per 100 000 population in Australia, and 5.3 ICU beds per 100 000 population in NZ.27 These numbers are similar to our estimates for ventilated critical care beds per 100 000 population for each country.
Whilst the critical care capacity estimates from our study differ from these previous estimates, our findings support previous suggestions that Australia and NZ critical care bed ratios are generally higher than in the UK. We propose that differences in our estimates may be caused by (i) variable definitions used for critical care, (ii) differences in sampling methodology, or (iii) changes in total hospital bed numbers and critical care bed numbers over time.
We asked respondents to provide the numbers of critical care beds in their hospitals, including both ICU and HDU beds. We used local collaborator-reported bed numbers for both the numerator and the denominator to arrive at our calculated ratios. In contrast, Adhikari and colleagues13 obtained estimates based on literature review, synthesising data from a number of different sources. Their primary sources were a 2005 paper published by Wunsch and colleagues,35 obtained from administrative data sets for the UK, and a 2006/2007 report by the ANZICS.36, 37 Rhodes and colleagues29 estimated critical care bed numbers using aggregated country-level data dating from 2010, combining data from a number of different administrative sources, including the European Commission database, the WHO, the Central Intelligence Agency World Factbook, and the OECD. Our results therefore add a reliable, updated, and empirical primary data source to the literature.
Using intermediate definitions for surgery, ∼8000 surgical procedures were performed per 100 000 population per year in the UK NHS between 2009 and 2014, with ∼3810 per 100 000 per year requiring overnight stay.38 In comparison, ∼4584 surgical admissions per 100 000 population per year occur in Australian public hospitals,39 and ∼4669 surgical procedures per 100 000 population per year are performed in NZ.1, 3 Combining the results from our study with data obtained from the literature, the availability of critical care beds in relation to volume of surgical activity performed in public hospitals can be approximated for each country (UK=2.5, Australia=3.1, and NZ=2.0 critical care beds per 1000 surgical procedures). However, these estimates may be limited by differences in the definitions used when accounting for surgical volume between the different sources.
Unanswered questions and future research
What is clear from our study is the prevalence of high-acuity beds in many of hospitals throughout the three countries studied. We propose that these high-acuity beds are being used to augment critical care capacity in hospitals where ICU/HDU beds may be insufficient to support clinical activity. However, we are unable to comment on patient case mix within these areas, or on the clinical effectiveness of treatment in these units. The high-acuity care areas likely represent a heterogeneous group of bed types. Further research is required to describe the detail of the structures and processes within these units, and the outcomes of patients admitted to them. We do not currently know if they provide good value care and whether they are a sufficient alternative to traditional ICU/HDU care for high-risk patients. Rapid expansion in their numbers cannot currently be recommended without further evaluation.
Other important factors that might influence the capacity to deliver postoperative care to high-risk patients also need further exploration. Particularly, the effects of hospital networking arrangements across large geographical regions were not explored in our study. Inter-hospital transfer is an established mechanism for diverting patients when critical care capacity may be inadequate in the transferring hospital, or when centralised tertiary services only available in the receiving hospital are required.40 There is evidence that patients transferred for non-clinical indications may have longer lengths of stay, but equivalent mortality outcomes, and therefore, critical care capacities, across regions may be important in resource planning beyond the immediate needs of a single hospital.41
Conclusions
There are differences between the UK, Australia, and NZ in postoperative provision of care, both in terms of critical care capacity and staffing levels in general surgical wards. There are no significant differences in critical care bed numbers as a proportion of total beds at each hospital between the three countries, after adjusting for hospital size and tertiary care provision. We identified and described high-acuity care areas that accommodate high-risk surgical patients for postoperative management. Per capita postoperative critical care availability was lowest in the UK after accounting for these high-acuity care beds. We suggest that high-acuity care areas may have developed to facilitate the provision of some aspects of critical care—in particular, more favourable nurse-to-patient ratios—outside the ICU and HDU, in order to meet service demand. However, the utility of these high-acuity beds requires further evaluation.
Authors' contributions
Study conception: SRM.
Data collection coordination: DJNW (UK); SP, PSM, SW (Australia); AMW, LMB, HAL, DC (New Zealand).
Data collection: lead collaborators.
Data linkage/cleaning: DJNW.
Data analysis: DJNW.
Input to analysis: SKH, SRM.
Drafting manuscript: DJNW.
Revising manuscript: all authors.
Declarations of interest
SRM is Director of the National Institute of Academic Anaesthesia Health Services Research Centre and the University College London Hospitals Surgical Outcomes Research Centre, and Associate National Clinical Director for elective care at National Health Service England. PSM is an editor for the British Journal of Anaesthesia. SP was the Chair of the Trainee Members Group of the Australian Society of Anaesthetists at the time of data collection. AMW, LMB, HAL, LF, DS, and DC. report no conflicts of interest.
Funding
National Institute of Academic Anaesthesia (Association of Anaesthetists of Great Britain and Ireland project grant), the Royal College of Anaesthetists, and the University College London Hospitals (UCLH) National Institute for Health Research (NIHR) Biomedical Research Centre in the UK (to Second Sprint National Anaesthesia Project: Epidemiology of Critical Care Provision After Surgery); NIHR Local Clinical Research Networks; Health Foundation (to SRM. and SKH); Royal College of Anaesthetists (to SRM); The London Clinic Hospital and the UCLH NIHR Surgical Outcomes Research Centre (to DJNW).
Acknowledgements
The authors thank all Second Sprint National Anaesthesia Project: Epidemiology of Critical Care Provision After Surgery (SNAP-2: EPICCS) site investigators and collaborators for contributing data to this study. The authors also thank the SNAP-2: EPICCS Study Steering Group for contributing to the study questionnaire construction, and David Highton for providing comments and suggestions to the manuscript.
Editorial decision: 31 December 2018
Handling editor: H.C. Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.12.026.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rose J., Weiser T.G., Hider P., Wilson L., Gruen R.L., Bickler S.W. Estimated need for surgery worldwide based on prevalence of diseases: a modelling strategy for the WHO Global Health Estimate. Lancet Glob Health. 2015;3:S13–S20. doi: 10.1016/S2214-109X(15)70087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Royal College of Anaesthetists . The Royal College of Anaesthetists; 2015. Perioperative medicine: the pathway to better surgical care.https://www.rcoa.ac.uk/sites/default/files/PERIOP-2014.pdf [accessed: 04 Jan 2018] [Google Scholar]
- 3.Weiser T.G., Haynes A.B., Molina G. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385:S11. doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 4.Khuri S.F., Henderson W.G., DePalma R.G. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. doi: 10.1097/01.sla.0000179621.33268.83. discussion 341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moonesinghe S.R., Harris S., Mythen M.G. Survival after postoperative morbidity: a longitudinal observational cohort study. Br J Anaesth. 2014;113:977–984. doi: 10.1093/bja/aeu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swart M., Carlisle J.B., Goddard J. Using predicted 30 day mortality to plan postoperative colorectal surgery care: a cohort study. Br J Anaesth. 2017;118:100–104. doi: 10.1093/bja/aew402. [DOI] [PubMed] [Google Scholar]
- 7.Eichenberger A.-S., Haller G., Cheseaux N., Lechappe V., Garnerin P., Walder B. A clinical pathway in a post-anaesthesia care unit to reduce length of stay, mortality and unplanned intensive care unit admission. Eur J Anaesth. 2011;28:859. doi: 10.1097/EJA.0b013e328347dff5. [DOI] [PubMed] [Google Scholar]
- 8.Findlay G.P., Goodwin A.P.L., Protopapa K., Smith N.C.E., Mason M. National Confidential Enquiry into Patient Outcome and Death (NCEPOD); 2011. Knowing the risk: a review of the peri-operative care of surgical patients.http://www.ncepod.org.uk/2011report2/downloads/POC_fullreport.pdf [accessed: 15 June 2016] [Google Scholar]
- 9.Anderson I., Eddleston J., Grocott M. The Royal College of Surgeons of England, Department of Health; 2011. The higher risk general surgical patient: towards improved care for a forgotten group.https://www.rcseng.ac.uk/-/media/files/rcs/library-and-publications/non-journal-publications/higher_risk_surgical_patient_2011_web.pdf [accessed: 21 Dec 2018] [Google Scholar]
- 10.Pearse R.M., Harrison D.A., James P. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearse R.M., Moreno R.P., Bauer P. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–1065. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The International Surgical Outcomes Study Group Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016;117:601–609. doi: 10.1093/bja/aew316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari N.K., Fowler R.A., Bhagwanjee S., Rubenfeld G.D. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batchelor A., Pittard A., Ripley A., Waeland D., Waldmann C. The Faculty of Intensive Care Medicine; 2017. Critical futures: a report on the first wave survey.https://www.ficm.ac.uk/sites/default/files/critical_futures_2017_1.pdf [accessed: 28 Feb 2018] [Google Scholar]
- 15.Moonesinghe S.R., Wong D.J.N., Farmer L., Shawyer R., Myles P.S., Harris S.K. SNAP-2 EPICCS: the second Sprint national anaesthesia project of critical care after surgery: protocol for an international observational cohort study. BMJ Open. 2017;7:e017690. doi: 10.1136/bmjopen-2017-017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker E.M.K., Bell M., Cook T.M., Grocott M.P.W., Moonesinghe S.R., for the SNAP-1 investigator group Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: a cross-sectional observational study. Br J Anaesth. 2016;117:758–766. doi: 10.1093/bja/aew381. [DOI] [PubMed] [Google Scholar]
- 17.NHS England . 2018. Cancelled elective operations.https://www.england.nhs.uk/statistics/statistical-work-areas/cancelled-elective-operations/ Available from: [Accessed: 26 Jan 2018] [Google Scholar]
- 18.NHS Scotland . Scottish Health on the Web; 2017. Organisations - Scotland’s health on the web.http://www.scot.nhs.uk Available from: [Accessed: 10 Apr 2018] [Google Scholar]
- 19.NHS Wales . 2006. NHS Wales hospitals.http://www.wales.nhs.uk/ourservices/directory/Hospitals Available from: [Accessed: 10 Apr 2018] [Google Scholar]
- 20.The National Institute of Academic Anaesthesia . 2018. Quality Audit and Research Coordinators (QuARCs)https://www.niaa-hsrc.org.uk/QUARCs?newsid=584 Available from: [Accessed: 13 Dec 2018] [Google Scholar]
- 21.The National Institute of Academic Anaesthesia The national audit projects (NAPs) 2018. https://www.nationalauditprojects.org.uk/ Available from: [Accessed: 13 Dec 2018]
- 22.Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; 2017. MyHospitals data.https://www.myhospitals.gov.au/about-the-data/data-overview [accessed: 12 Jul 2018] [Google Scholar]
- 23.Wickham H. 2017. Tidyverse: easily install and load the ’Tidyverse’.https://cran.r-project.org/package=tidyverse [accessed: 13 Sep 2018] [Google Scholar]
- 24.Yoshida K., Bohn J. 2018. Tableone: create ‘table 1’ to describe baseline characteristics.https://cran.r-project.org/web/packages/tableone/index.html [accessed: 28 Oct 2018] [Google Scholar]
- 25.Lüdecke D. Zenodo; 2018. Sjplot—data visualization for statistics in social science.https://zenodo.org/record/1308157 [accessed: 28 Oct 2018] [DOI] [Google Scholar]
- 26.Scottish Intensive Care Society Audit Group . 2017. Audit of critical care in Scotland 2017 reporting on 2016.https://www.sicsag.scot.nhs.uk/docs/2017/2017-08-08-SICSAG-Report.pdf?55 Edinburgh, Scotland, UK. [Google Scholar]
- 27.Australian and New Zealand Intensive Care Society . 2017. ANZICS Centre for outcome and resource evaluation adult patient database (APD) activity report 2016-2017.https://www.anzics.com.au/wp-content/uploads/2018/08/ANZICS-CORE-APD-Activity-Report-2016-17.pdf Carlton, Victoria, Australia. [Google Scholar]
- 28.Organisation for Economic Co-operation and Development (OECD) OECD Health Statistics; 2018. Health spending (indicator)https://data.oecd.org/healthres/health-spending.htm [accessed: 11 Jul 2018] [Google Scholar]
- 29.Rhodes A., Ferdinande P., Flaatten H., Guidet B., Metnitz P.G., Moreno R.P. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38:1647–1653. doi: 10.1007/s00134-012-2627-8. [DOI] [PubMed] [Google Scholar]
- 30.Masterson G., Baudouin S. Faculty of Intensive Care Medicine and Intensive Care Society; 2015. Guidelines for the provision of intensive care services.https://www.ficm.ac.uk/sites/default/files/GPICS%20-%20Ed.1%20%282015%29.pdf [accessed: 30 Sep 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Intensive Care Society . Intensive Care Society; 2009. Levels of critical care for adult patients.http://www.ics.ac.uk/EasysiteWeb/getresource.axd?AssetID=468&type=full&servicetype=Attachment [accessed: 19 Oct 2015] [Google Scholar]
- 32.Faculty of Intensive Care Medicine and Intensive Care Society . 2013. Core standards for intensive care units. [Google Scholar]
- 33.College of Intensive Care Medicine of Australia and New Zealand . 2011. Minimum standards for intensive care units.https://www.cicm.org.au/CICM_Media/CICMSite/CICM-Website/Resources/Professional%20Documents/IC-1-Minimum-Standards-for-Intensive-Care-Units.pdf [accessed: 18 Sep 2018] [Google Scholar]
- 34.College of Intensive Care Medicine of Australia and New Zealand . 2013. Guidelines on standards for high dependency units for training in intensive care medicine.http://cicm.org.au/CICM_Media/CICMSite/CICM-Website/Resources/Professional%20Documents/IC-13-Guidelines-on-Standards-for-High-Dependency-Units.pdf [accessed: 18 Sep 2018] [Google Scholar]
- 35.Wunsch H., Angus D.C., Harrison D.A. Variation in critical care services across north America and western Europe. Crit Care Med. 2008;36:2787–2793. doi: 10.1097/CCM.0b013e318186aec8. e1–9. [DOI] [PubMed] [Google Scholar]
- 36.Drennan K., Hart G.K., Hicks P. Australian and New Zealand Intensive Care Society; Carlton, Victoria, Australia: 2008. Intensive care resources & activity: Australia and New Zealand 2006/2007. [Google Scholar]
- 37.Martin J.M., Hart G.K., Hicks P. A unique snapshot of intensive care resources in Australia and New Zealand. Anaesth Intensive Care. 2010;38:149–158. doi: 10.1177/0310057X1003800124. [DOI] [PubMed] [Google Scholar]
- 38.Abbott T.E.F., Fowler A.J., Dobbs T.D., Harrison E.M., Gillies M.A., Pearse R.M. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth. 2017;119:249–257. doi: 10.1093/bja/aex137. [DOI] [PubMed] [Google Scholar]
- 39.Australian Institute of Health and Welfare . No. HSE 201; Canberra, Australia: 2018. Admitted patient care 2016-17: Australian hospital statistics. Report No. 84, Cat. [Google Scholar]
- 40.Whiteley S., Macartney I., Mark J., Barratt H.S., Binks R. 3rd ed. Intensive Care Society; London: 2011. Guidelines for the transport of the critically ill adult. [Google Scholar]
- 41.Barratt H., Harrison D.A., Rowan K.M., Raine R. Effect of non-clinical inter-hospital critical care unit to unit transfer of critically ill patients: a propensity-matched cohort analysis. Crit Care. 2012;16:R179. doi: 10.1186/cc11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Organisation for Economic Co-operation and Development (OECD) 2018. Hospital beds (indicator). OECD Health Statistics [Internet]http://data.oecd.org/healtheqt/hospital-beds.htm [accessed 2018 Jul 11]; Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.