Case Presentation
A 54-year-old man presented with a 6-month history of progressive dyspnea occurring at rest and with minimal exertion. His medical history was notable for hypertension, non-insulin-dependent diabetes, coronary artery disease, and factor V Leiden heterozygosity. His social history was notable for a 43-pack-year smoking history but was otherwise unremarkable.
Physical Examination Findings
An initial chest radiograph showed bilateral reticular opacities (Fig 1). Vital signs were notable for mild resting tachypnea, with a respiratory rate of 20 breaths/min, a pulse rate of 93 beats/min, and BP of 130/73 mm Hg. Lung auscultation was notable for fine bibasilar crackles. Examination of the heart, abdomen, extremities, and skin were normal.
Figure 1.
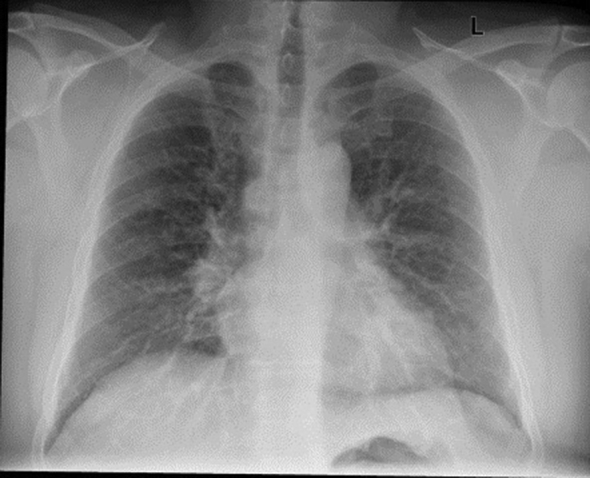
Chest radiograph showing bilateral diffuse reticular opacities.
Diagnostic Studies
Initial laboratory studies included a complete blood count, which was notable for mild normocytic anemia, with a hemoglobin level of 13.5 g/dL and a hematocrit level of 39.9%. Electrolyte level and hepatic function studies were normal. He underwent a cardiac evaluation, including an ECG and an echocardiogram, which were normal.
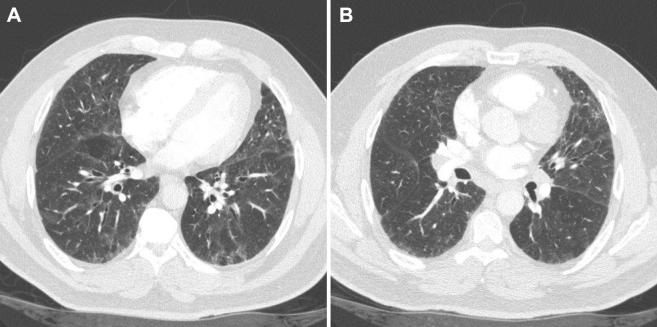
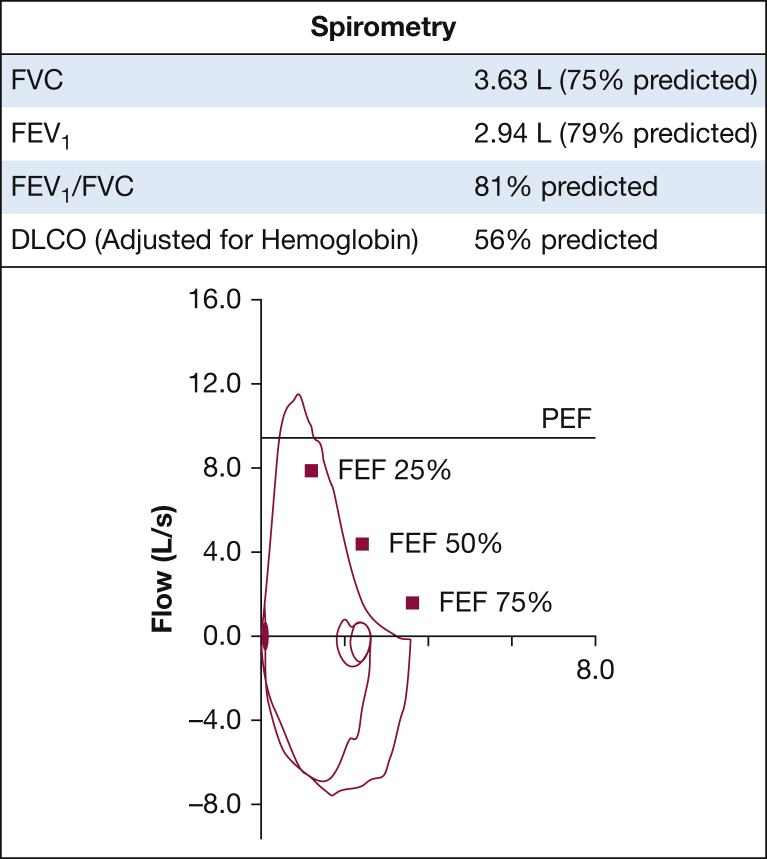
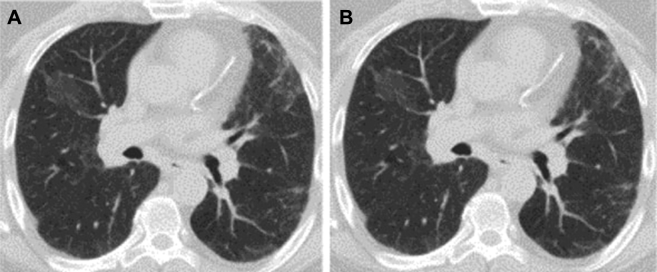
A CT scan of the chest showed diffuse areas of ground glass opacities, mosaic attenuation, and interlobular septal thickening (Fig 2). There was also mild hilar and mediastinal lymphadenopathy noted. Spirometry showed a restrictive ventilatory defect with a diffusing capacity for carbon monoxide of 56%, consistent with diffusion impairment (Fig 3). A methacholine challenge test was negative, and there was no evidence of desaturation on exercise oximetry.
Figure 2.
A, B, Representative images of chest CT scan demonstrating bilateral ground glass opacities with areas of mosaic attenuation.
Figure 3.
Pulmonary function tests.
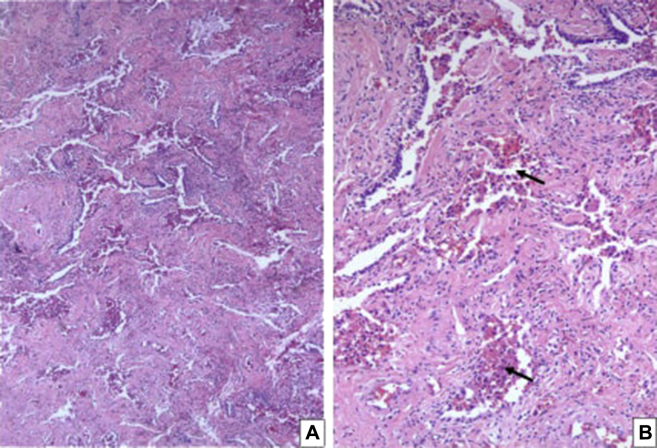
Bronchoscopy showed normal airway mucosa, and BAL in the left upper lobe had a normal cell count and cytology with no evidence of infection on bacterial and fungal culture. The patient underwent a video-assisted thoracoscopic wedge resection of the left upper lobe, which showed fibrotic interstitial lung disease in association with respiratory bronchiolitis-like areas (Fig 4) accompanied by peribronchiolar and alveolar septal fibrosis (which was more pronounced in a subpleural distribution pattern).
Figure 4.
A, B, Surgical wedge lung biopsy from patient showing respiratory bronchiolitis-like areas with pigmented (smoker’s) macrophages within the respiratory bronchioles and adjacent alveolar spaces (arrows). There is significant peribronchiolar and alveolar septal fibrosis, arguing against a diagnosis of respiratory bronchiolitis interstitial lung disease. A, Hematoxylin-eosin stain (low [40×] magnification). B, Hematoxylin-eosin stain (intermediate [100×] magnification).
What is the diagnosis?
Diagnosis: Smoking-related interstitial fibrosis.
Discussion
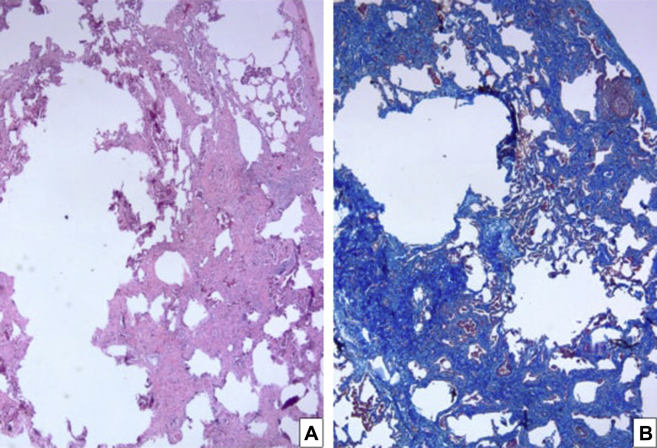
Smoking is associated with a heterogeneous group of diffuse interstitial lung disorders. These diseases have distinct clinicopathologic features manifesting as progressive respiratory symptoms in the setting of lung function impairment from smoking exposure. The well-established smoking-associated lung diseases include pulmonary Langerhans cell histiocytosis, respiratory bronchiolitis-associated interstitial lung disease, desquamative interstitial pneumonia, and idiopathic pulmonary fibrosis. Because of the presence of macrophages within bronchioles and alveolar spaces, initially a respiratory bronchiolitis diagnosis was considered on histopathologic examination of the wedge biopsy obtained from this patient (Fig 4). However, significant interstitial fibrosis is not a feature of respiratory bronchiolitis (there is usually no or minimal peribronchiolar fibrosis in respiratory bronchiolitis interstitial pneumonia). In addition, there was alveolar wall distortion and destruction leading to irregular emphysematous change (Fig 5) in this case. There were also areas of extensive peribronchiolar metaplasia (lambertosis) (Fig 4). No Langerhans cells or eosinophils were noted in the interstitium and alveolar spaces.
Figure 5.
A, Surgical wedge lung biopsy from patient showing severe alveolar septal fibrosis with associated irregular emphysematous change (left side) in a subpleural distribution. A portion of the pleural surface is included in the right upper portion of the pictures (Hematoxylin-eosin stain, low [20×] magnification). B, Trichrome stain (low [20×] magnification).
A distinct histologic pattern of fibrosis called smoking-related interstitial fibrosis (SRIF) is being increasingly recognized in lung biopsies of patients with an extensive smoking history. The distinctive histology of SRIF includes a uniform pattern of fibrosis with an absence of temporal heterogeneity associated with alveolar septal wall thickening by deposition of densely eosinophilic hyalinized collagen and metaplastic smooth muscle in the subpleural parenchyma (Figure 5, Figure 6). There is also the presence of emphysematous changes (Fig 5) with pigmented macrophages within alveolar spaces and respiratory bronchioles and a paucity of inflammatory cells in the interstitium (Fig 5).
Figure 6.
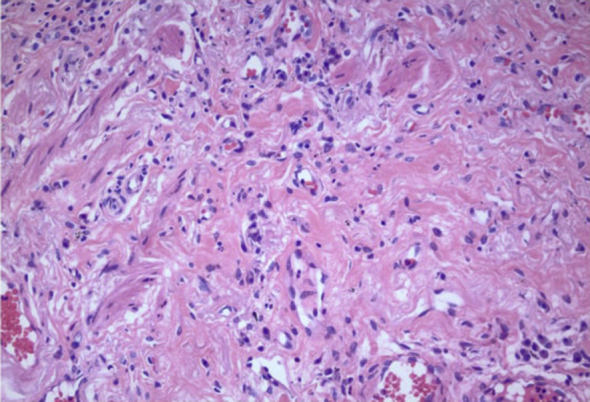
High (200×) magnification of surgical lung biopsy from the patient showing prominent hyalinized collagen with no or minimal inflammation and interspersed (metaplastic) smooth muscle bundles in the thickened alveolar walls (hematoxylin-eosin stain).
SRIF has been reported exclusively among smokers with a mean age of 65 years (range, 52-77 years) and an average smoking history of 48 pack-years (range, 16-80 pack-years). It occurs equally among sexes, with dyspnea and cough being the most common presenting complaints. Imaging of the chest may show ground glass opacities in the dependent portions of the lungs.
Smoking cessation is thought to be the most effective treatment intervention, and no specific pharmacologic treatment has been described. Serial pulmonary function tests may allow for assessment of disease progression after smoking cessation. Although the prognosis is relatively favorable with a mean survival of 8 years and 5-year survival rate of 85% compared with pulmonary Langerhans cell histiocytosis (74%), respiratory bronchiolitis-associated interstitial lung disease (75%), and idiopathic pulmonary fibrosis (40%), it still remains poor overall. Pulmonary hypertension has been reported to be associated with SRIF; however, there have been no other reports of such an association, and this patient did not have evidence of pulmonary hypertension on echocardiography.
Clinical Course
The patient was informed about the diagnosis, was informed about its association with smoking, and was referred to tobacco cessation counseling. He was prescribed nicotine replacement therapy and was able to quit smoking after 4 months, during which he noted a significant improvement of his dyspnea. Repeat chest imaging a year later showed improvement in the ground glass infiltrates (Fig 7).
Figure 7.
A, B, Representative images of chest CT scan demonstrating bilateral areas of mosaic attenuation that partially improved after smoking cessation at follow-up 1 y later.
Clinical Pearls
-
1.
SRIF is a distinct histologic pattern of lung injury in smokers characterized by a patchy (but striking) alveolar septal uniform fibrosis composed of densely eosinophilic hyalinized collagen and admixed metaplastic smooth muscle bundles. Although the process involves mainly subpleural lung parenchyma, it may also be present around alveolar ducts and bronchioles (centrilobular distribution). Smoker’s macrophages are typically noted in the airways and airspaces. Emphysematous change may accompany the fibrosis.
-
2.
Diagnosis is made by surgical lung biopsy.
-
3.
Prognosis is favorable compared with some other interstitial lung diseases, with an estimated 5-year survival rate of 85%.
-
4.
Smoking cessation is the only known treatment intervention; however, the specific effects of cessation on prognosis for SRIF are yet to be determined.
Acknowledgments
Author contributions: L. H., S. T., A. A., and S. A. take full responsibility for the content of the manuscript. L. H. and S. T. made substantial contributions to the content and the writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Other contributions: CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met.
Footnotes
Dr Ahmad is currently at the Division of Pulmonary and Critical Care, Mayo Clinic (Rochester, MN).
Suggested Readings
- McAdams H.P., Rosado-de-Christenson M.L., Wehunt W.D., Fishback N.F. The alphabet soup revisited: the chronic interstitial pneumonias in the 1990s. Radiographics. 1996;16(5):1009–1033. doi: 10.1148/radiographics.16.5.8888388. [DOI] [PubMed] [Google Scholar]
- Caminati A., Harari S. Smoking-related interstitial pneumonias and pulmonary Langerhans cell histiocytosis. Proc Am Thorac Soc. 2006;3(4):299–306. doi: 10.1513/pats.200512-135TK. [DOI] [PubMed] [Google Scholar]
- Yousem S.A. Respiratory bronchiolitis-associated interstitial lung disease with fibrosis is a lesion distinct from fibrotic nonspecific interstitial pneumonia: a proposal. Mod Pathol. 2006;19(11):1474–1479. doi: 10.1038/modpathol.3800671. [DOI] [PubMed] [Google Scholar]
- Katzenstein A.L., Mukhopadhyay S., Zanardi C., Dexter E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol. 2010;41(3):316–325. doi: 10.1016/j.humpath.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Katzenstein A.L. Smoking-related interstitial fibrosis (SRIF), pathogenesis and treatment of usual interstitial pneumonia (UIP), and transbronchial biopsy in UIP. Mod Pathol. 2012;25(suppl 1):S68–S78. doi: 10.1038/modpathol.2011.154. [DOI] [PubMed] [Google Scholar]
- Katzenstein A.L. Smoking-related interstitial fibrosis (SRIF): pathologic findings and distinction from other chronic fibrosing lung diseases. J Clin Pathol. 2013;66(10):882–887. doi: 10.1136/jclinpath-2012-201338. [DOI] [PubMed] [Google Scholar]
- Katzenstein A.L. Smoking-related interstitial fibrosis (SRIF): pathologic findings and distinction from other chronic fibrosing lung diseases. Postgrad Med J. 2014;90(1068):597–602. doi: 10.1136/postgradmedj-2012-201338rep. [DOI] [PubMed] [Google Scholar]
- Primiani A., Dias-Santagata D., Iafrate A.J., Kradin R.L. Pulmonary adenocarcinoma mutation profile in smokers with smoking-related interstitial fibrosis. Int J Chron Obstruct Pulmon Dis. 2014;9(1):525–531. doi: 10.2147/COPD.S61932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K.J., Jin G.Y., Jung H.N. Differentiating smoking-related interstitial fibrosis (SRIF) from usual interstitial pneumonia (UIP) with emphysema using CT features based on pathologically proven cases. PLoS One. 2016;11(9):e0162231. doi: 10.1371/journal.pone.0162231. [DOI] [PMC free article] [PubMed] [Google Scholar]