Abstract
The field of lung transplant has made significant advances over the last several decades. Despite these advances, morbidity and mortality remain high when compared with other solid organ transplants. As the field moves forward, the speed by which progress can be made will in part be determined by our ability to overcome several stumbling blocks, including donor shortage, proper selection of candidates, primary graft dysfunction, and chronic lung allograft dysfunction. The advances and developments surrounding these factors will have a significant impact on shaping the field within the coming years. In this review, we look at the current climate (ripe for expanding the donor pool), new technology (ex vivo lung perfusion and bioengineered lungs), cutting-edge innovation (novel biomarkers and new ways to treat infected donors), and evidence-based medicine to discuss current trends and predict future developments for what we hope is a bright future for the field of lung transplantation.
Abbreviations: BOS, bronchiolitis obliterans syndrome; CLAD, chronic lung allograft dysfunction; DAA, direct-acting antiviral; DCD, donation after circulatory death; EVLP, ex vivo lung perfusion; HCV, hepatitis C virus; LTX, lung transplant; PGD, primary graft dysfunction
Although the first human lung transplant (LTX) was performed by James Hardy in 1963,1 it was not until the mid-1980s—after a series of surgical and pharmacologic advances—that the operation became a clinical reality, increasing in its use ever since as a treatment for end-stage lung diseases. In the recent era, nearly 4,000 LTXs are performed around the world per year,2 with progress coming as a result of improvements to the processes of recipient and donor selection, surgical techniques, immunosuppression, and other posttransplant treatment regimens. There has similarly been a large increase in the numbers of patients listed.3, 4 Patient survival has improved and wait-list time has decreased as a result of both changes in allocation systems and changes in donor utilization.5, 6
Despite these favorable trends, the operation remains fraught with a mortality risk and much morbidity, largely because of the vagaries of immunosuppression. This morbidity and mortality are primarily related to primary graft dysfunction (PGD) and chronic lung allograft dysfunction (CLAD), which contribute to early and late complications of LTX, respectively.7 Additionally, there remains an overall donor shortage, and many candidates for LTX cannot survive to the point that a suitable donor becomes available.4, 8, 9
This paper offers background into the significant challenges that persist and describes the most significant work being done to ameliorate the shortcomings of caring for a patient who needs or who has had a LTX.
Status Quo and a Path Forward
As the field moves forward, the speed at which progress can be made will in part be determined by our ability to overcome several stumbling blocks. Four of the biggest stumbling blocks today include: (1) donor shortage, (2) proper selection of candidates for LTX, (3) PGD, and (4) CLAD.
With each of these, our understanding of the pathobiology and rationales has been greatly elucidated in recent years. Also with each of these, new approaches and technologies may greatly improve the care of LTX recipients in the era to come. The aim of the next section is to better define each of these problems and identify future directions aimed at addressing the main crux of each issue.
Addressing the Donor Shortage
Despite the increasing numbers of LTXs being performed around the world, there remains a very real problem with donor availability (and a resultant wait-list mortality problem). Advances such as implementation of a lung allocation score (implemented in the United States in 2005 and elsewhere in the ensuing years) significantly decreased deaths on the wait-list without compromising overall posttransplant outcomes, while improving median wait times; however, each year approximately 10% to 13% of listed candidates will die while on the waiting list as a graft shortage persists.4, 8, 9, 10 The problem is a combination of an overall shortage of available donors compounded by a low lung utilization rate from donors. To illustrate, lungs are used from eligible multiorgan donors only 15% to 20% of the time,11 and when looking at donation after circulatory death (DCD) lungs, < 2% of eligible lungs are transplanted in the United States.11 Most potential lung grafts are turned down at the time of evaluation because of concerns about the health of the lungs related to donor history, chest trauma, pneumonia, aspiration, or various ICU complications.4, 11, 12, 13
The problem of donor shortage appears to be worse in certain populations, specifically women (primarily thought to be because of shorter stature and a greater incidence of pretransplant allosensitization, making it more difficult to identify suitable donors),10, 14, 15, 16 patients with cystic fibrosis (primarily thought to be because of shorter stature and a difference in allocation systems in patients < 12 years of age),15, 17 and patients with idiopathic pulmonary hypertension (primarily because of severity of disease not being captured by pulmonary function testing).18, 19
Four ways in which we think the problem of donor shortage may be improved are: (1) use of more donors after circulatory death to increase the total number of donors, (2) use of extended criteria for donor selection, (3) use of the relatively new technology of ex vivo lung perfusion (EVLP) to increase utilization rate, and (4) utilization of bioengineered lungs.20, 21, 22, 23, 24
Donation After Circulatory Death
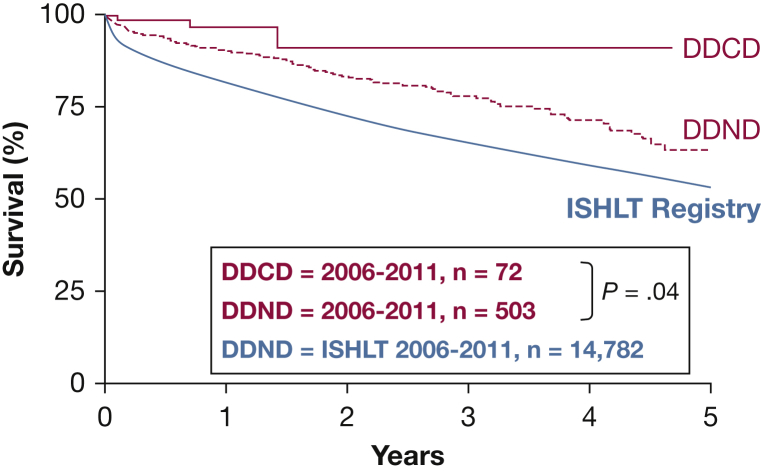
With this approach to lung donation, a potential donor in whom life-sustaining therapies are being withdrawn is declared dead after cardiac arrest, and the recovery team then procures organs, including lungs. Although Hardy’s first human LTX in 1963 used a DCD donor,1 historically most lung donors have had a declaration of brain death prior to lung recovery.13 This was in part because of brain death becoming codified into legislation. As such, organ donation after brain death became widely accepted. DCD was largely abandoned surrounding concerns of ischemia during the period between discontinuation of life support and organ procurement.13 The use of DCD in LTX became a clinical reality once again in a recent era13 and is becoming more common, with reports suggesting that up to 30% more donors can be identified in this way.25, 26 Although concerns exist about the logistics of the DCD recovery and about the quality of DCD lungs (because of potential lung injury around the time of withdrawal of life support from aspiration or from donor hypoxemia and hypotension—so-called warm ischemia), the growing experience with this method of donor recovery has demonstrated equal13, 27 and perhaps even superior outcomes because of avoidance of the cytokine storm and inflammatory state that often accompanies brain death (Fig 1).25, 27 The creation of a collaborative group and a DCD registry by the International Society for Heart and Lung Transplantation will facilitate further study and analysis of DCD outcomes and standardize the approach to its best use.
Figure 1.
Actuarial survival of DDCD donor lung transplant (LTX) recipients (P = .04) vs DDND LTx recipients vs ISHLT registry survival: 2006-2011. Four Australian LTx centers performed a combined 72 DDCD LTx and 503 DDND LTx between May 2006 and May 2011. For comparison, the registry survival of the ISHLT during the same period is also shown. DDCD = donation after determination of cardiac death; DDND = donation after determination of neurologic death; ISHLT = International Society for Heart and Lung Transplantation.
(Reprinted with permission from Levvey et al.25)
Most DCD donors consist of controlled DCD (Maastricht classification 3), but an untapped resource of donors is the so-called uncontrolled DCD (Maastricht classifications 2 and 5). In uncontrolled DCD, an unsuccessful resuscitation with declaration of death is followed by insertion of chest tubes with in situ cooling of the lungs and a preservation solution and intravenous heparin, followed by consent for donation and subsequent recovery of organs.12, 28 Teams in Spain have demonstrated the feasibility of this approach,12, 29, 30, 31 and efforts are underway to demonstrate feasibility in other locales.32 Several trials are underway which aim to optimize donor lung preservation in the preretrieval phase. Factors such as optimal ventilation, maintaining hemodynamic stability to protect end organ function, hormone therapy, and identifying optimal timing of lung retrieval will be of the utmost importance to fine tune modifiable acquired donor factors. The hope would be reduced PGD and improved long-term survival.28
Although logistically challenging, the combined use of uncontrolled DCD with EVLP in these lungs may increase the utility of EVLP and the usability of DCD lungs because it could allow for further assessment of the quality of the organs and facilitate timing and logistics of the transplant operation.12, 28 Use of uncontrolled DCD is likely to become more common in the coming years, but will not likely become ubiquitous.
EVLP
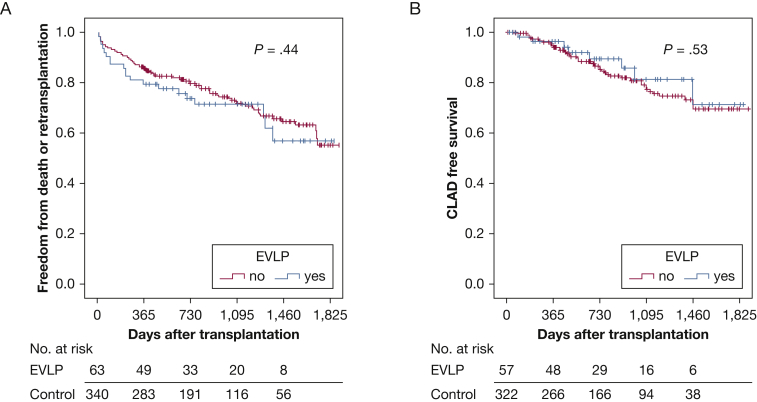
EVLP involves taking lungs of uncertain quality or in which further evaluation is desired and placing them on a device on which they are ventilated and perfused with an electrolyte and protein solution for a matter of some hours, allowing for an extended evaluation outside of the donor (and potentially also for therapeutic interventions).33, 34 The technology has been refined and enhanced in the last decade. The Toronto system was shown to allow lungs that were deemed unusable for direct transplantation to undergo EVLP for a period of up to 6 hours and to be transplanted successfully. Length of hospital stay and 30-day, 1-year, and 3-year mortality rates were similar to a control group who underwent standard transplantation without EVLP in the same era at the same transplant center (Fig 2).22, 34 A later report described a 5-year survival rate that was similar to conventional transplant and similar physiologic and functional outcomes and a similar rate of CLAD.22 A patented system based on this technology has been demonstrated to be safe and not inferior to standard transplantation.34 A different EVLP system that involves bringing a portable organ preservation system to the donor OR with normothermic perfusion during transport back to the recipient hospital has also been shown to have favorable safety and efficacy.33 Both EVLP technologies are being used in centers around the world and have increased the number of usable lungs by up to 30%.33, 34
Figure 2.
A, Kaplan-Meier curves depict the freedom from death or retransplantation of patients receiving EVLP-treated lungs compared with recipients of conventional donor lungs in the entire study cohort. The y axis depicts the proportion of patients free from death or retransplant, and the x axis shows days after transplant. The number of patients at risk is shown below the x axis at yearly time points. Log-rank analyses were used to determine statistical significance. B, Kaplan-Meier curves depict the freedom from CLAD in patients receiving effects of EVLP-treated lungs compared with recipients of conventional donor lungs in the entire study cohort. The y axis depicts the proportion of patients free from CLAD, and the x axis shows days after transplant. The number of patients at risk is shown below the x axis at yearly time points. CLAD = chronic lung allograft dysfunction; EVLP = ex vivo lung perfusion.
(Reprinted with permission from Tikkanen et al.22)
To date, EVLP has mostly been used as a tool to allow for extended evaluation of lungs that are of uncertain quality, rather than lungs that would otherwise be accepted for standard donation. However, there is also a possibility for EVLP to serve as a platform to optimize all lungs, even those that would normally be accepted for transplant without further testing. This long-asked question will hopefully soon be answered by the International Randomized Study of the TransMedics Organ Care System (OCS Lung) for Lung Preservation and Transplantation (INSPIRE) trial, which will be the first large, prospective, randomized controlled trial comparing normothermic preservation of standard donor lungs using the Organ Care System lung perfusion device (TransMedics, Inc) compared with cold storage in a noninferiority clinical study.35 It remains to be determined if EVLP will become standard of care, but the initial results are promising. In any event, it appears that use of EVLP will be used more often in the years to come, and may lead to increased numbers of donors (and therefore transplants).
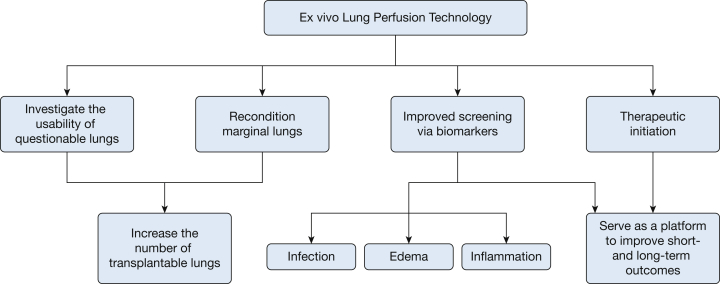
Additionally, the potential of EVLP as a tool to improve survival and decrease long-term complications by better screening through the use of molecular markers and as a therapeutic platform is very exciting. The next era in research and clinical care using EVLP promises to incorporate strategies to institute therapeutics during EVLP aimed at inflammation, edema, and infection in allografts, with a goal of making even more lungs useable and of making usable lungs even better.12 The Toronto group reported favorable outcomes in decreasing lung inflammation by instituting therapy with nebulized IL-10 therapy during EVLP with an adenoviral vector.12, 36 Treatment with antibacterial therapy could ameliorate donor-derived bacterial infections, and a recent study using ultraviolet light therapy of the perfusate in the EVLP circuit showed a reduction in levels of hepatitis C virus (HCV), suggesting that such treatment could make infected lungs usable in uninfected recipients, which would open up a large donor population for use.12 The possibilities with EVLP continue to grow (Fig 3).
Figure 3.
Schematic of the possible future roles of ex vivo lung perfusion technology.
Extended Criteria for Donor Selection
In recent years, because of the unacceptable gap between supply (lungs accepted for transplantation) and demand (patients listed for LTX), successive guidelines regarding lung donor selection have proposed more liberal use of donors with extended criteria.21, 26, 37 Some examples of these extended criteria include older donors (age > 55 years), increased smoking history, more medical comorbidities, abnormal chest radiograph at time of donation, Po2/Fio2 ratio < 300, and known drug abuse.21, 23, 37 As we look at the future of LTX, we foresee many centers accepting more of these marginal donors for transplantation that would have otherwise been excluded in the past. Several reports have suggested that use of such donors is relatively safe and effective.23, 26 Given that wait-list mortality by definition means that life is shortened, use of such donors would increase survival even if it leads to some compromise in overall transplant survival. For example, a recent registry analysis of UK LTX data indicated that while a donor with a positive history of smoking has a negative influence on postoperative outcomes, the survival of the recipient is improved with a positive smoking history donor, compared with waiting for a suitable, nonsmoking donor organ to become available.37, 38 With that in mind, judicious use of select donors who do not meet ideal criteria can be justified and is becoming more common in many centers, even without the use of EVLP.20, 23
Additionally, in the United States, the opioid epidemic has led to a sharp increase in the number of organ donors39; however, there exists the problem of increased HCV infections within this population of donors.40 In an era of effective direct-acting antiviral (DAA) therapy against HCV, we see great potential in further expanding the donor pool. A case report by Khan et al,41 first described successful treatment of donor-acquired HCV with DAA treatment. This was followed shortly thereafter by a case series of 12 HCV-naïve patients who underwent heart transplant using hearts from HCV-positive donors, who were treated with DAA therapy with excellent results.42 Moving forward, we see potential to expand the donor pool by combining DAA therapies with platforms such as EVLP (to reduce viral loads through the use of ultraviolet C light) for donors with HCV.41, 42, 43
Bioengineered Lungs
Bioengineered artificial lung technology (using recipient cells grown on decellularized lung scaffold) to provide lung replacement for patients with end-stage lung disease is being developed, but clinical reality remains premature as of now.24, 44 Although elusive, the possibility is real and if realized would mean LTX without the need for immunosuppression, wait-lists, or concerns related to immunosuppression, including infection, cytopenias, renal failure, and even perhaps chronic rejection.45 Therefore, what are the issues now preventing progress and what will the field of bioengineered lungs look like in 10 years?
Currently, support by synthetic membranes mimicking lungs is limited by several factors, which will need to be addressed in the future for this concept to remain a viable reality.
-
1.
Size: the size of current synthetic membrane oxygenators are incompatible with placement within the body. Because the native human lung provides an area of 70 m2 of gas exchange, future models will need to work to maximize the surface area to volume ratio to remain viable candidates.24, 44
-
2.
Resilience: current synthetic membranes are limited to a period of hours to days, lasting weeks at best. During this time, the patient’s mobility is greatly restricted because of the constraints of the current devices. Mobility and quality of life are greatly decreased. Paired with a high mortality, the benefits rarely outweigh the risks of using these devices at this time.24, 44
-
3.
Thrombogenicity: endothelial cells of the lung are naturally thromboresistant, unlike many of the synthetic membranes currently under study. With current synthetic membranes, a high level of anticoagulation is needed to deter deposition of clot, and there remains a problem in terms of the longevity of these synthetic grafts.24
These issues have led researchers to look at using native lung as scaffolding for tissue engineering. The process involves whole lung decellularization to erase immunogenicity of the native lung and then repopulate the decellularized scaffolding with new lung epithelial cells using recipient-derived tissue engineering.46 Again, the major stumbling block for in vivo implantation remains the resilience of these models to function long term, and thrombogenicity. Future directions in the coming years will likely aim to develop techniques targeted at increasing the overall robustness. If progress if made, it may be conceivable that donor lungs could become scaffolding of bioengineered lung grafts for recipients.44, 45, 46
Addressing Proper Selection of Candidates for LTX
The appropriate selection of LTX recipients has a large impact on outcomes and survival.47 Additionally, it is imperative that physicians in the community outside of large transplant centers understand the importance of early referral for LTX evaluation in patients who have a lung disease that is amenable to LTX.47 Early referral (too soon for LTX) can be of particular value in identifying relative contraindications to LTX that may be modifiable (ie, obesity, substance abuse, chronic infections), and the patient can work to improve those factors prior to listing. Indeed, one of the major aims of LTX is finding the correct timing of candidate listing, a so-called Goldilocks window, to ensure patients are not listed too early—but even more importantly that they are not listed too late and risk wait-list mortality. Also, at the front end, efforts aimed at delaying the need for LTX are now a reality with newer drugs in use for diseases such as idiopathic pulmonary fibrosis, idiopathic pulmonary hypertension, and cystic fibrosis.48, 49, 50, 51 These advances in pretransplant care have allowed for later listing in some individuals and work to lengthen the clock (time until need for LTX) and contribute to an overall decrease in time to death for these end-stage lung diseases.52 Additionally, transplanting patients with chronic infections such as HIV and HCV are becoming a clinical reality.53, 54 Novel drug therapy that can be used before and/or after LTX to eradicate or control infection is leading to the development of a more inclusive pool of transplant candidates.41, 42, 53, 54
Addressing PGD
PGD is defined as acute graft dysfunction in the first 72 h after LTX. PGD has been better defined and is graded according to radiographic appearance and Po2/Fio2 ratio.55, 56, 57 Severe PGD (grade 3) is the most common cause of early mortality and has also been associated with later dysfunction of the graft, namely in the form of bronchiolitis obliterans syndrome (BOS)58; therefore, those surviving this initial insult remain at risk for long-term morbidity and mortality. Although there are likely many different etiologies of the inflammatory state associated with PGD, the most significant mechanism is thought to be noncardiogenic pulmonary edema and diffuse alveolar damage resulting from an ischemia-reperfusion injury. The refined definition has allowed for a more organized study through a collaborative study group called the Lung Transplant Outcomes Group, with resultant improved identification of risk factors and pathobiology.55
One of the issues that still remains is heterogeneity of the severity and duration inherent within the graded PGD criteria.57 This has led to the hypothesis that grade 3 PGD is made up of subgroups, each with distinct mechanisms. In one recent study, latent class analysis was used to identify two main phenotypes in grade 3 PGD, each with distinct risk factors for development.57 Better characterization of the most severe grade of PGD may lead to a better understanding of the mechanisms underlying each PGD phenotype and improved potential for early identification and development of techniques or therapies for its prevention.
Recent studies which aimed to define key pathways involved in the development of PGD may indicate that response to either pathogens (likely donor-derived) or response to cell and tissue damage signals are key in the development of PGD.59 Indeed, many acute phase reactants and cytokines that are involved in inflammation from the inflammatory cascade and innate immunity, such as long pentraxin-3, angiopoietin-2, and IL-1B, have been found to be upregulated in patients with severe PGD (grade 3) and are thought to contribute to the pathogenesis of PGD.59, 60, 61 It is hypothesized that these molecules can be upregulated or augmented in response to higher amounts of ischemia or perfusion, and donor-specific characteristics such as infection or smoking.59, 62 One of the most promising biomarkers of LTX rejection includes donor-derived cell-free DNA. As this technology develops, the possibility of recognizing subclinical rejection months earlier by a single blood draw and allowing for initiation of early treatment may become a reality.63, 64
Further exploration of these pathways and molecules in the future may lead us to therapeutic targets or the development of on-site screening tools to better inform our decisions on whether to accept potential donor lungs before LTX occurs. Additionally, the platform in which these potential therapeutics or diagnostic testing could be used may very well be the EVLP circuit.12, 65
Addressing CLAD
The syndrome of CLAD remains the Achilles heel in the long-term morbidity and mortality of LTX, limiting the 5-year survival to approximately 55%.66 Indeed, as many as one-half of all patients can be shown to have CLAD at the 5-year mark after LTX, and it remains the primary cause of death after the third year.67, 68 CLAD is an all-encompassing term for chronic graft dysfunction after LTX, inclusive of the more recognized phenotype of BOS.66 CLAD is defined as “a persistent (at least 3 weeks), often unexplained decline in pulmonary function (FEV1 with or without FVC) ≥ 10% from baseline (baseline defined as the average of the 2 best post-transplant values for FEV1 and FVC obtained at least 3 weeks apart).”66 Therefore, a decrease in spirometry should prompt an investigation into other underlying treatable etiologies. When CLAD is the likely cause of FEV1 decline by exclusion of other conditions, further investigation is needed to gather appropriate data to further identify specific CLAD phenotype.69 Although the most common phenotype of CLAD is BOS, the phenotype of restrictive allograft syndrome has been characterized in a recent era70 and has been shown to have an accelerated course with worse outcomes after retransplant.71 Table 1 compares the distinct entities of BOS and restrictive allograft syndrome.66, 72, 73
Table 1.
| Entity | Classic BOS | RAS |
|---|---|---|
| Pulmonary function testing | Obstructive | Restrictive |
| HRCT thoracic imaging |
|
|
| Histopathology |
|
|
| Clinical course | Typically progressive, but may stabilize; individuals may have coexistent chronic bacterial infection | Tends to be relentlessly progressive; may start as or coincide with BOS |
| Proposed mechanism | Cytotoxic/proinflammatory lymphocyte (increased CD8T, NKT-like, and NK cells in small airways) | Antibody-mediated rejection (increased levels of immunoglobulins and complement proteins) |
| Risk factors for development |
|
|
| Potential therapies |
|
|
| Median survival after diagnosis, y | 2.5 | 1.5 |
BOS = bronchiolitis obliterans syndrome; CD8T = cytotoxic t-cell; CMW = cytomegalovirus; DAD = diffuse alveolar damage; GERD = gastroesophageal reflux disease; HRCT = high-resolution CT; NK, natural killer cell; NKT, natural killer t-cell; PGD = primary graft dysfunction; RAS = restrictive allograft syndrome.
(Adapted with permission from Verleden et al.66)
In recent years, quantitative BAL research has helped to illicit some of the biomarkers and cell lines upregulated within the subtypes of CLAD; however, the underlying mechanisms and therapies remain elusive.72, 73 Most strategies currently aim at the prevention of gastroesophageal reflux disease and use of azithromycin, whereas other treatments have had small or no impact.69, 74 There is much room to grow in preventing and developing innovative treatments for CLAD. As previously discussed, severe PGD is associated with later graft dysfunction such as CLAD.58 In parallel to early recognition and treatment of LTX rejection, there is hope for expanded use of donor-derived cell-free DNA as a biomarker for the identification of acute and chronic rejection. With early identification, adaptation of immunosuppressive drugs can be used, thereby curbing graft dysfunction and lending to a reduction in the incidence of CLAD.63, 64
With more precise definitions and classifications within the realm of CLAD, our hope is that future research will be able to better elicit the underlying mechanisms and therefore identify more targeted therapies with a goal of improving long-term LTX survival.
Summary
There have been recent advances on multiple fronts within the field of LTX. New technologies (eg, EVLP, bioengineered lungs) alongside increased utilization of DCD donors and use of extended criteria donor selection hold great promise for the coming future to address the problem of donor shortage. Additionally, the innovation of drug therapies to treat chronic infections such as HCV and HIV will likely expand the reality of LTX to more individuals than in a previous era. Although the future of LTX is bright, it will be predicated on discerning mechanisms and pathways to detect, prevent, and treat PGD and CLAD—in a noble effort to drive down LTX morbidity and mortality. The discovery and increased use of particular biomarkers will aid in this quest. It is important to keep in mind that although these novel developments are exciting and driving the field of LTX forward, future directions and adoption should be based in evidence-based care whenever possible. The future is upon us.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. F. D. has received research support from Lung Biotechnology PBC, Roche LTD, Boehringer Ingelheim, and FibroGen; has received honoraria for a Speakers Bureau for Genentech and Boehringer Ingelheim; and has participated in Advisory Boards for Genentech. None declared (K. A. Y.).
References
- 1.Hardy J.D., Webb W.R., Dalton M.L., Walker G.R. Lung homotransplantation in man: report of the initial case. JAMA. 1963;186(12):1065–1074. doi: 10.1001/jama.1963.63710120001010. [DOI] [PubMed] [Google Scholar]
- 2.Hachem R.R. Advancing lung transplantation. Clin Transpl. 2015;31:239–247. [PubMed] [Google Scholar]
- 3.Nathan S.D. The future of lung transplantation. Chest. 2015;147(2):309–316. doi: 10.1378/chest.14-1748. [DOI] [PubMed] [Google Scholar]
- 4.Valapour M., Skeans M.A., Heubner B.M. OPTN/SRTR 2013 annual data report: lung. Am J Transplant. 2015;15(suppl 2):1–28. doi: 10.1111/ajt.13200. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb J. Lung allocation. J Thorac Dis. 2017;9(8):2670–2674. doi: 10.21037/jtd.2017.07.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mooney J.J., Hedlin H., Mohabir P.K. Lung quality and utilization in controlled donation after circulatory determination of death within the United States. Am J Transplant. 2016;16(4):1207–1215. doi: 10.1111/ajt.13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orens J.B., Garrity E.R. General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6(1):13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 8.De Meester J., Smits J.M., Persijn G.G., Haverich A. Lung transplant waiting list: differential outcome of type of end-stage lung disease, one year after registration. J Heart Lung Transplant. 1999;18(6):563–571. doi: 10.1016/s1053-2498(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 9.Yusen R.D., Shearon T.H., Qian Y. Lung transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 pt 2):1047–1068. doi: 10.1111/j.1600-6143.2010.03055.x. [DOI] [PubMed] [Google Scholar]
- 10.Weill D. Access to lung transplantation. The long and short of it. Am J Respir Crit Care Med. 2016;193(6):605–606. doi: 10.1164/rccm.201511-2257ED. [DOI] [PubMed] [Google Scholar]
- 11.Charles E.J., Huerter M.E., Wagner C.E. Donation after circulatory death lungs transplantable up to six hours after ex vivo lung perfusion. Ann Thorac Surg. 2016;102(6):1845–1853. doi: 10.1016/j.athoracsur.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeb J., Keshavjee S., Cypel M. Expanding the lung donor pool. Curr Opin Organ Transplant. 2015;20(5):498–505. doi: 10.1097/MOT.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 13.Krutsinger D., Reed R.M., Blevins A. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 2015;34(5):675–684. doi: 10.1016/j.healun.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Sell J.L., Bacchetta M., Goldfarb S.B. Short stature and access to lung transplantation in the United States. A cohort study. Am J Respir Crit Care Med. 2016;193(6):681–688. doi: 10.1164/rccm.201507-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeshan B.C., Rossano J.W., Beck N. Lung transplant waitlist mortality: Height as a predictor of poor outcomes. Pediatr Transplant. 2015;19(3):294–300. doi: 10.1111/petr.12390. [DOI] [PubMed] [Google Scholar]
- 16.Wille K.M., Harrington K.F., deAndrade J.A., Vishin S., Oster R.A., Kaslow R.A. Disparities in lung transplantation before and after introduction of the lung allocation score. J Heart Lung Transplant. 2013;32(7):684–692. doi: 10.1016/j.healun.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blatter J., Sweet S. Lung transplantation in cystic fibrosis: trends and controversies. Pediatr Allergy Immunol Pulmonol. 2015;28(4):237–243. doi: 10.1089/ped.2015.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan K.M. Idiopathic pulmonary arterial hypertension and equity of donor lung allocation in the era of the lung allocation score. Am J Respir Crit Care Med. 2009;180(5):385–387. doi: 10.1164/rccm.200906-0976ED. [DOI] [PubMed] [Google Scholar]
- 19.George M.P., Champion H.C., Pilewski J.M. Lung transplantation for pulmonary hypertension. Pulm Circ. 2011;1(2):182–191. doi: 10.4103/2045-8932.83455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotecha S., Hobson J., Fuller J. Continued successful evolution of extended criteria donor lungs for transplantation. Ann Thorac Surg. 2017;104(5):1702–1709. doi: 10.1016/j.athoracsur.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Aigner C., Winkler G., Jaksch P. Extended donor criteria for lung transplantation—a clinical reality. Eur J Cardiothorac Surg. 2005;27(5):757–761. doi: 10.1016/j.ejcts.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Tikkanen J.M., Cypel M., Machuca T.N. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J Heart Lung Transplant. 2015;34(4):547–556. doi: 10.1016/j.healun.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 23.Somers J., Ruttens D., Verleden S.E. A decade of extended-criteria lung donors in a single center: Was it justified? Transpl Int. 2015;28(2):170–179. doi: 10.1111/tri.12470. [DOI] [PubMed] [Google Scholar]
- 24.Dorrello N.V., Guenthart B.A., O’Neill J.D. Functional vascularized lung grafts for lung bioengineering. Sci Adv. 2017;3(8):e1700521. doi: 10.1126/sciadv.1700521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levvey B.J., Harkess M., Hopkins P. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am J Transplant. 2012;12(9):2406–2413. doi: 10.1111/j.1600-6143.2012.04193.x. [DOI] [PubMed] [Google Scholar]
- 26.Meers C., Van Raemdonck D., Verleden G.M. The number of lung transplants can be safely doubled using extended criteria donors; a single-center review. Transpl Int. 2010;23(6):628–635. doi: 10.1111/j.1432-2277.2009.01033.x. [DOI] [PubMed] [Google Scholar]
- 27.Cypel M., Levvey B., Van Raemdonck D. Favorable outcomes of donation after cardiac death in lung transplantation: a multicenter study. J Heart Lung Transplant. 2013;32(4):S15. [Google Scholar]
- 28.Munshi L., Keshavjee S., Cypel M. Donor management and lung preservation for lung transplantation. Lancet Respir Med. 2013;1(4):318–328. doi: 10.1016/S2213-2600(12)70064-4. [DOI] [PubMed] [Google Scholar]
- 29.de Antonio D.G., Marcos R., Laporta R. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant. 2007;26(5):529–534. doi: 10.1016/j.healun.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-de-Antonio D., Campo-Cañaveral J.L., Crowley S. Clinical lung transplantation from uncontrolled non–heart-beating donors revisited. J Heart Lung Transplant. 2012;31(4):349–353. doi: 10.1016/j.healun.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Meneses J.C., Gámez P., Mariscal A. Development of a non–heart-beating donor program and results after the first year. Transplant Proc. 2012;44(7):2047–2049. doi: 10.1016/j.transproceed.2012.07.092. [DOI] [PubMed] [Google Scholar]
- 32.Ortega-Deballon I., Hornby L., Shemie S.D. Protocols for uncontrolled donation after circulatory death: a systematic review of international guidelines, practices and transplant outcomes. Crit Care. 2015;19(1):268. doi: 10.1186/s13054-015-0985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machuca T.N., Cypel M. Ex vivo lung perfusion. J Thorac Dis. 2014;6(8):1054–1062. doi: 10.3978/j.issn.2072-1439.2014.07.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cypel M., Yeung J.C., Liu M. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 35.Van Raemdonck D., Neyrinck A., Cypel M., Keshavjee S. Ex-vivo lung perfusion. Transpl Int. 2015;28(6):643–656. doi: 10.1111/tri.12317. [DOI] [PubMed] [Google Scholar]
- 36.Cypel M., Liu M., Rubacha M. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1(4):4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 37.Sommer W., Kühn C., Tudorache I. Extended criteria donor lungs and clinical outcome: results of an alternative allocation algorithm. J Heart Lung Transplant. 2013;32(11):1065–1072. doi: 10.1016/j.healun.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Bonser R.S., Taylor R., Collett D., Thomas H.L., Dark J.H., Neuberger J. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet. 2012;380(9843):747–755. doi: 10.1016/S0140-6736(12)60160-3. [DOI] [PubMed] [Google Scholar]
- 39.Stehlik J., Smits J., Jarcho J.A., Lehman R., Cherikh W., Mehra M.R. The opioid use crisis and organ transplantation: a comparison of the United States and Europe. J Heart Lung Transplant. 2018;37(4):S188. [Google Scholar]
- 40.Zibbell J.E., Asher A.K., Patel R.C. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. doi: 10.2105/AJPH.2017.304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan B., Singer L.G., Lilly L.B. Successful lung transplantation from hepatitis C positive donor to seronegative recipient. Am J Transplant. 2017;17(4):1129–1131. doi: 10.1111/ajt.14137. [DOI] [PubMed] [Google Scholar]
- 42.Schlendorf K.H., Zalawadiya S., Shah A.S. Early outcomes using hepatitis C–positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant. 2018;37(6):763–769. doi: 10.1016/j.healun.2018.01.1293. [DOI] [PubMed] [Google Scholar]
- 43.Galasso M., Patel A., Summers C. Using ultraviolet C irradiation as a sterilization method for organ perfusion systems. J Heart Lung Transplant. 2018;37(4):S156. [Google Scholar]
- 44.Wagner W.R., Griffith B.P. Reconstructing the lung. Science. 2010;329(5991):520–522. doi: 10.1126/science.1194087. [DOI] [PubMed] [Google Scholar]
- 45.Ott H.C., Clippinger B., Conrad C. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 46.Wobma H., Vunjak-Novakovic G. Tissue engineering and regenerative medicine 2015: a year in review. Tissue Eng Part B Rev. 2016;22(2):101–113. doi: 10.1089/ten.teb.2015.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weill D., Benden C., Corris P.A. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 48.King T.E., Bradford W.Z., Castro-Bernardini S. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 49.Richeldi L., du Bois R.M., Raghu G. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 50.Taichman D.B., Ornelas J., Chung L. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146(2):449–475. doi: 10.1378/chest.14-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rafeeq M.M., Murad H.A. Cystic fibrosis: current therapeutic targets and future approaches. J Transl Med. 2017;15(1):84. doi: 10.1186/s12967-017-1193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hook J.L., Lederer D.J. Selecting lung transplant candidates: Where do current guidelines fall short? Expert Rev Respir Med. 2012;6(1):51–61. doi: 10.1586/ers.11.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doucette K.E., Halloran K., Kapasi A., Lien D., Weinkauf J.G. Outcomes of lung transplantation in recipients with hepatitis C virus infection. Am J Transplant. 2016;16(8):2445–2452. doi: 10.1111/ajt.13796. [DOI] [PubMed] [Google Scholar]
- 54.Kern R.M., Seethamraju H., Blanc P.D. The feasibility of lung transplantation in HIV-seropositive patients. Ann Am Thorac Soc. 2014;11(6):882–889. doi: 10.1513/AnnalsATS.201402-083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diamond J.M., Lee J.C., Kawut S.M. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187(5):527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christie J.D., Carby M., Bag R., Corris P., Hertz M., Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 57.Shah R.J., Diamond J.M., Cantu E. Latent class analysis identifies distinct phenotypes of primary graft dysfunction after lung transplantation. Chest. 2013;144(2):616–622. doi: 10.1378/chest.12-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki Y., Cantu E., Christie J.D. Primary graft dysfunction. Semin Respir Crit Care Med. 2013;34(3):305–319. doi: 10.1055/s-0033-1348474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cantu E., Lederer D.J., Meyer K. Gene set enrichment analysis identifies key innate immune pathways in primary graft dysfunction after lung transplantation. Am J Transplant. 2013;13(7):1898–1904. doi: 10.1111/ajt.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diamond J.M., Lederer D.J., Kawut S.M. Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant. 2011;11(11):2517–2522. doi: 10.1111/j.1600-6143.2011.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diamond J.M., Porteous M.K., Cantu E. Elevated plasma angiopoietin-2 levels and primary graft dysfunction after lung transplantation. PLoS One. 2012;7(12):e51932. doi: 10.1371/journal.pone.0051932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diamond J.M., Porteous M.K., Roberts L.J. The relationship between plasma lipid peroxidation products and primary graft dysfunction after lung transplantation is modified by donor smoking and reperfusion hyperoxia. J Heart Lung Transplant. 2016;35(4):500–507. doi: 10.1016/j.healun.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck J., Oellerich M., Schulz U. Donor-derived cell-free DNA is a novel universal biomarker for allograft rejection in solid organ transplantation. Transplant Proc. 2015;47(8):2400–2403. doi: 10.1016/j.transproceed.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 64.Zou J., Duffy B., Slade M. Rapid detection of donor cell free DNA in lung transplant recipients with rejections using donor-recipient HLA mismatch. Hum Immunol. 2017;78(4):342–349. doi: 10.1016/j.humimm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsin M.K., Zamel R., Cypel M. Metabolic profile of ex vivo lung perfusate yields biomarkers for lung transplant outcomes. Ann Surg. 2018;267(1):196–197. doi: 10.1097/SLA.0000000000002016. [DOI] [PubMed] [Google Scholar]
- 66.Verleden G.M., Raghu G., Meyer K.C., Glanville A.R., Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33(2):127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 67.Thabut G., Mal H. Outcomes after lung transplantation. J Thorac Dis. 2017;9(8):2684–2691. doi: 10.21037/jtd.2017.07.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verleden S.E., Vos R., Vanaudenaerde B.M., Verleden G.M. Chronic lung allograft dysfunction phenotypes and treatment. J Thorac Dis. 2017;9(8):2650–2659. doi: 10.21037/jtd.2017.07.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verleden G.M., Vos R., Vanaudenaerde B. Current views on chronic rejection after lung transplantation. Transpl Int. 2015;28(10):1131–1139. doi: 10.1111/tri.12579. [DOI] [PubMed] [Google Scholar]
- 70.Sato M., Hwang D.M., Waddell T.K., Singer L.G., Keshavjee S. Progression pattern of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2013;32(1):23–30. doi: 10.1016/j.healun.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Verleden S.E., Todd J.L., Sato M. Impact of CLAD phenotype on survival after lung retransplantation: a multicenter study. Am J Transplant. 2015;15(8):2223–2230. doi: 10.1111/ajt.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vandermeulen E., Verleden S.E., Bellon H. Humoral immunity in phenotypes of chronic lung allograft dysfunction: a broncho-alveolar lavage fluid analysis. Transpl Immunol. 2016;38(2016):27–32. doi: 10.1016/j.trim.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Hodge G., Hodge S., Yeo A. BOS is associated with increased cytotoxic proinflammatory CD8 T, NKT-Like, and NK cells in the small airways. Transplantation. 2017;101(10):2469–2476. doi: 10.1097/TP.0000000000001592. [DOI] [PubMed] [Google Scholar]
- 74.Ruttens D., Verleden S.E., Vandermeulen E. Prophylactic azithromycin therapy after lung transplantation: post hoc analysis of a randomized controlled trial. Am J Transplant. 2016;16(1):254–261. doi: 10.1111/ajt.13417. [DOI] [PubMed] [Google Scholar]