Abstract
Background
A pulmonary hypertensive crisis (PHC) can be a life-threatening condition. We established a PHC model by exposing rats with monocrotaline (MCT)-induced pulmonary hypertension to acute hypoxia, and investigated the effects of vasopressin, phenylephrine, and norepinephrine on the PHC.
Methods
Four weeks after MCT 60 mg kg−1 administration i.v., right ventricular systolic pressure (RVSP), systolic BP (SBP), mean BP (MBP), cardiac index (CI), and pulmonary vascular resistance index (PVRI) were measured. PHC defined as an RVSP exceeding or equal to SBP was induced by changing the fraction of inspiratory oxygen to 0.1. Rats were subsequently treated by vasopressin, phenylephrine, or norepinephrine, followed by assessment of systemic haemodynamics, isometric tension of femoral and pulmonary arteries, cardiac function, blood gas composition, and survival.
Results
PHC was associated with increased RV dilatation and paradoxical septal motion. Vasopressin increased MBP [mean (standard error)] from 52.6 (3.8) to 125.0 (8.9) mm Hg and CI from 25.4 (2.3) to 40.6 (1.8) ml min−1 100 g−1 while decreasing PVRI. Vasopressin also improved RV dilatation, oxygenation, and survival in PHC. In contrast, phenylephrine increased MBP from 54.8 (2.3) to 96.8 (3.2) mm Hg without improving cardiac pump function. Norepinephrine did not alter MBP. Vasopressin contracted femoral but not pulmonary arteries, whereas phenylephrine contracted both arterial beds. Hence, improvements with vasopressin in PHC might be associated with decreased PVRI and selective systemic vasoconstriction.
Conclusions
In this rat model of a PHC, vasopressin, but not phenylephrine or norepinephrine, resulted in better haemodynamic and vascular recovery.
Keywords: hypertension, pulmonary hypertension, hypoxia, norepinephrine, phenylephrine, vasopressin
Editor's key points.
-
•
There is no treatment guideline for the diagnosis and treatment of a pulmonary hypertensive crisis (PHC) in pulmonary hypertension.
-
•
In a rat model for pulmonary hypertension, the authors compared vasopressin, norepinephrine, and phenylephrine in the treatment of PHC induced by acute hypoxia.
-
•
Vasopressin was the most beneficial in improving systemic haemodynamics, cardiac function, and survival during a PHC in this rat model.
In patients with pulmonary hypertension (PH) from congenital heart diseases, a pulmonary hypertensive crisis (PHC) is a life-threatening situation characterised by a sudden increase in pulmonary vascular resistance, right heart failure, and systemic hypotension during the perioperative period of cardiovascular surgeries.1 This crisis also can occur in idiopathic pulmonary arterial hypertension, sarcoidosis, pulmonary fibrosis during cardiac2 and non-cardiac surgeries,3, 4 and lung transplantation.5, 6 Management of the crisis is challenging, because there is no treatment guideline or expert consensus specifying concrete agents as standard medication and diagnostic criteria for PHC.1, 7, 8 In addition, no animal model of PHC has been reported to investigate PHC management to date, despite the availability of various models of PH.9, 10 Thus, there are few comparative data on the agents for PHC treatment either clinically or experimentally.
At present, vasopressors/inotropes including norepinephrine and phenylephrine have been used as rescue therapy11 to raise the systemic BP above the pulmonary arterial pressure to maintain coronary perfusion,1, 5, 11 even though the level of evidence for this therapy was shown to be low in a systematic review12 and in guidelines.7 Recently, vasopressin has been used in the management of PHC in cardiac11, 13, 14 and in non-cardiac surgery.2 Vasopressin has a constrictive effect on the systemic vasculature15, 16, 17, 18, 19 and a vasodilatory effect on the pulmonary artery via endothelial nitric oxide synthase (eNOS).15, 16, 17 Nonetheless, endothelial function of the pulmonary artery has been impaired, and the effects of vasopressin on pulmonary artery pressure have not been consistent in PH patients12, 20 and in animal models.9, 19 The vasodilatory effect of vasopressin on the pulmonary arteries may also be uncertain in PH,17, 21 and data justifying the use of vasopressin for PHC remain limited.12, 22
We aimed to develop an experimental model of PHC by creating monocrotaline (MCT)-induced PH in rats10, 23 which were subsequently exposed to acute hypoxia. Then, we examined whether vasopressin, norepinephrine, and phenylephrine could improve haemodynamics, cardiac motion, oxygenation, and survival period during PHC using the developed PHC model. We also measured isometric tension of pulmonary and femoral arteries to investigate the underlying modifying mechanism of these agents.
Methods
Inflammation and endothelial cell injury in whole pulmonary arteries are involved in the genesis of PHC,1, 24 and acute hypoxia is an important trigger inducing PHC.24 We reproduced PHC by MCT-induced PH in rats which were subsequently exposed to acute hypoxia.10 All experimental protocols were approved by the Institutional Animal Care and Use Committee of Yokohama City University School of Medicine (F-A-17-057) and performed according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Animal handling was in accordance with guidelines for proper conduct of animal experiments of the Science Council of Japan. All chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Monocrotaline-induced pulmonary hypertension in rats
Eight-week-old male Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) were divided into the PH group (n=182), which received MCT 60 mg kg−1 s.c., and the control group (n=90), which received saline.23 Rats were housed up to three per cage and had free access to water and food with constantly maintained room temperature and humidity under a 12–12 h light–dark cycle (light phase 7:00–19:00). For some experiments, PH rats were further divided into the vasopressin, norepinephrine, phenylephrine, and saline treatment groups. Animals were euthanised with an i.v. or i.p. injection of pentobarbital sodium (120 mg kg−1) at the end of the experiment or to remove pulmonary and femoral artery rings.
Haemodynamic measurements
Four weeks after each injection, we measured systolic blood pressure (SBP), mean BP (MBP), right ventricular systolic pressure (RVSP) as an indicator of pulmonary artery pressure23, 25 and cardiac output (CO), as described previously.23 SBP, MBP, and RVSP were examined simultaneously through catheters in the femoral artery and right ventricle, respectively (PowerLab system, ADInstruments, Colorado Springs, CO, USA) under positive pressure ventilation with sevoflurane 4 vol% at a fraction of inspiratory oxygen (FiO2) of 1.0 (SN-480-7 ventilator, Shinano, Tokyo, Japan). CO was measured twice at each measurement by the thermodilution technique. Cardiac index (CI) was calculated as the ratio of CO to body weight.23, 26 Pulmonary vascular resistance index (PVRI) was defined as RVSP divided by CI.23, 26 The left femoral vein was cannulated with a catheter to administer drugs. Local anaesthesia was achieved with lidocaine 0.5% during every surgical procedure. Adequate pain relief was ensured by confirming a lack of pedal withdrawal response and righting reflex to pain stimuli before and during the surgical procedures. The absence of increases in HR and MBP during the surgical procedures was also confirmed.
Echocardiographic studies
Two-dimensional echocardiography was performed using a Sonos 5500 (Hewlett-Packard, Andover, MA, USA). The right ventricular inner area (RVA) and left ventricular inner area (LVA) in diastole were measured in the short-axis view at the level of the papillary muscles. Right ventricular dilatation was evaluated by the ratio of RVA to LVA (RVA/LVA) in a blinded manner. Cardiac septal motion was examined in the same view.
Induction of a pulmonary hypertensive crisis
After obtaining the basal values of SBP, MBP, RVSP, CI, and echocardiography in the PH rats, we induced the PHC state by exposing them to acute hypoxia. We changed FiO2 from 1.0 to 0.1. PHC was defined as RVSP exceeding SBP or being equal to SBP (within 5 mm Hg). The time to obtain the PHC state from starting FiO2 0.1 was recorded. In some animals, FiO2 was returned to 1.0 some 20 min after the induction.
Administration of vasopressin, phenylephrine, and norepinephrine during a pulmonary hypertensive crisis
During PHC, vasopressin, norepinephrine, and phenylephrine were administered continuously via the femoral vein catheter. Preliminarily, we obtained dose-dependent responses of SBP to each agent. The doses of vasopressin, phenylephrine, and norepinephrine to increase SBP up to the physiological limit of 180–200 mm Hg were 1.5 μg kg−1 h−1, 0.3 mg kg−1 h−1, and 12 μg kg−1 h−1, respectively, in the control rats. If the doses did not increase the collapsed MBP in PHC, an additional higher dose was administered. Administration was started from a lower dose in all experiments. When constant values of MBP and RVSP were obtained for 1 min during administration, the haemodynamic values and CO were recorded. Then, the administered dose was increased. Each agent was administered at the rate of 2 ml h−1 using a precise syringe pump (STC-525; Terumo, Tokyo, Japan). The duration of administration of the agents was also recorded. We also examined the haemodynamic responses to the agents in control rats exposed to acute hypoxia at FiO2 0.1 and in PH rats without PHC.
Assessment of oxygenation
PaO2 was measured in the PH rats. Arterial blood (0.4 ml) was drawn through the femoral artery catheter and was analysed at three time points (1.2 ml withdrawn in total): before, during PHC, and after administration of the agents at maximum dose (ABL800Flex blood gas analyser; Radiometer, Brønshøj, Denmark).
Survival
We examined the survival rate at 120 min after the induction of PHC in the PH rats with administration of the agents.
Isometric tension in pulmonary and femoral arteries
Four weeks after MCT or saline injection, we prepared ring samples from pulmonary and femoral arteries in rats for measurements of isometric tension as described previously.27, 28 The ring samples were pre-contracted with norepinephrine 100 nM. The concentration-response curves to vasopressin and acetylcholine for relaxation of the pulmonary artery rings from control and PH rats were constructed. Some samples from control rats were pre-treated with the nitric oxide synthase (NOS) inhibitor NG-nitro-L-arginine monomethyl ester (L-NAME) 30 μM 20 min before contraction with norepinephrine. The relaxation is expressed as a percentage of the norepinephrine-induced contraction.
Pulmonary and femoral artery rings from control and PH rats were contracted with KCl 65 mM and washed. To construct the concentration response curves for contraction, the ring samples were exposed to increasing concentrations of vasopressin and phenylephrine. The results are expressed as a percentage of the KCl-induced contraction. Preliminarily, we confirmed histological changes such as increased medial wall thickness and muscularisation of the pulmonary arteries in the MCT-induced PH rats (data not shown).
Data analysis
All values are shown as mean (standard error). CI and HR were analysed using one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test. MBP and isometric tension data were analysed with two-way ANOVA followed by the Tukey-Kramer post hoc test. The log-rank test was used to assess the significance of the Kaplan-Meier analysis of survival in the PHC model. Statistical significance was set at P<0.05. All statistical analyses were performed with Prism 6 version 6.04 (GraphPad Prism 6, La Jolla, CA, USA).
Results
Monocrotaline-induced pulmonary hypertension
RVSP in the PH group was significantly higher than that in the control group [37.2 (0.6) vs 73.1 (2.1) mm Hg, P<0.0001], whereas SBP was slightly but significantly lower [148.8 (1.2) vs 142.9 (1.9) mm Hg, P<0.05]. CI in the PH group was significantly lower than that in the control group [44.9 (1.1) vs 36.0 (1.0) ml min−1100 g−1, P<0.0001] (n=24 each).
Haemodynamic and echocardiographic evaluation
After changing FiO2 from 1.0 to 0.1, SBP was significantly decreased compared with the basal level, whereas RVSP was increased slightly and became equal to or exceeded the SBP (n=12; Fig. 1a and b). The ventilation at FiO2 0.1 also significantly decreased CI and increased PVRI (Fig. 1c and d). After returning FiO2 from 0.1 to 1.0, SBP, CI, and PVRI also returned to basal levels (Fig. 1b–d). On echocardiography, the RVA/LVA was significantly increased in the PH rats compared with control rats (Fig. 1e, f, and h). After PHC induction, the ratio was further increased (Fig. 1g and h) (n=12 in control and n=12 in PHC rats). Moreover, interventricular septal flattening and paradoxical motion were also observed in PHC (Video 2). The times to obtain the PHC state from starting FiO2 0.1 until the return to basal levels at FiO2 1.0 were 6.4 (2.2) and 6.0 (1.6) min, respectively. The induction of PHC and return to basal levels appeared to cause a similar change in each animal. Therefore, we considered that MCT-induced PH with acute hypoxia at FiO2 0.1 could reproduce acute right heart failure mimicking the PHC state.
Fig 1.
Haemodynamic and echocardiographic changes with pulmonary hypertension (PH) in the induction of pulmonary hypertensive crisis (PHC). (a) Simultaneous measurements of systolic BP (SBP) and right ventricular systolic pressure (RVSP) during PHC induction at FiO2 0.1. Changes in (b) SBP, RVSP, (c) cardiac index (CI), and (d) pulmonary vascular resistance index (PVRI) at FiO2 1.0, FiO2 0.1, and after returning FiO2 to 0.1 induction (n=12) in PH rats. Two-dimensional echocardiography in the short-axis view at the level of the papillary muscles in (e) control rats and rats with (f) PH at FiO2 1.0 and (g) PH at FiO2 0.1. The right ventricular inner area (RVA) and left ventricular inner area (LVA) in diastole are shown. (h) The ratio of RVA to LVA (n=12 in control and PH rats). Values are given as mean (standard error). *P<0.05 compared with FiO2 1.0. †P<0.05 compared with FiO2 0.1. LV, left ventricle; RV, right ventricle.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.bja.2019.01.014
The following are the Supplementary data related to this article:
Effects of vasopressin, phenylephrine, and norepinephrine on haemodynamic parameters
We measured the haemodynamic changes in response to i.v. administration of vasopressin, norepinephrine, and phenylephrine during PHC. We determined maximum doses of vasopressin, phenylephrine, and norepinephrine to be 1.5 μg kg−1 h−1, 2 mg kg−1 h−1, and 24 μg kg−1 h−1, respectively, to raise the collapsed MBP in PHC to the basal level except norepinephrine (n=6 with each agent). The times to obtain the PHC state in the vasopressin, phenylephrine, and norepinephrine groups were 7.3 (2.9), 6.2 (2.2), and 7.2 (2.6) min, respectively. Administration of vasopressin 1.5 μg kg−1 h−1 significantly increased decompensated MBP without increasing RVSP (Fig. 2a). Vasopressin 1.5 μg kg−1 h−1 also significantly increased CI compared with that before administration (Fig. 2d) and significantly decreased PVRI (Fig. 2g).
Fig 2.
Haemodynamic changes in mean BP (MBP), right ventricular pressure (RVSP), HR, cardiac index (CI), and pulmonary vascular resistance index (PVRI) in response to vasopressin (AVP), phenylephrine (PE), and norepinephrine (NE) administrations during pulmonary hypertensive crisis (PHC) at FiO2 0.1 in PH rats. Changes in (a) MBP, RVSP, and HR, (d) CI, and (g) PVRI in response to AVP (0.5–1.5 μg kg−1 h−1) were examined, as were changes in (b, c) MBP, RVSP, and HR in response to PE (0.3–2.0 mg kg−1 h−1) and NE (12–24 μg kg−1 h−1), (e, f) CI, and (h, i) PVRI. Values are given as mean (standard error). *P<0.05 compared with that at FiO2 1.0. †P <0.05 compared with that at FiO2 0.1 without treatment (n=6 each).
In contrast, administration of phenylephrine 2 mg kg−1 h−1 during PHC significantly increased not only MBP, but also RVSP more than those before administration while not significantly improving CI (Fig. 2b, e, and h). Administrations of vasopressin 0.5 μg kg−1 h−1 and phenylephrine 0.3 mg kg−1 h−1 had little effect on the haemodynamics of PHC (Fig. 2a, b, d, e, g, and h).
The administration of norepinephrine 12 kg−1 h−1 did not increase MBP and CI, but did decrease HR (Fig. 2c and f). Because norepinephrine 24 μg kg−1 h−1 appeared to worsen MBP and arrhythmia, we did not examine higher doses of norepinephrine any further. The approximate duration of administration of the agents was as follows: vasopressin 0.5 and 1.5 μg kg−1 h−1: 18 and 14 min; phenylephrine 0.3 and 2 mg kg−1 h−1: 11 and 8 min; and norepinephrine 12 and 24 μg kg−1 h−1: 11 and 10 min. The entire duration of hypoxia in these experiments was 30–40 min.
Effects of vasopressin, phenylephrine, and norepinephrine on cardiac motion
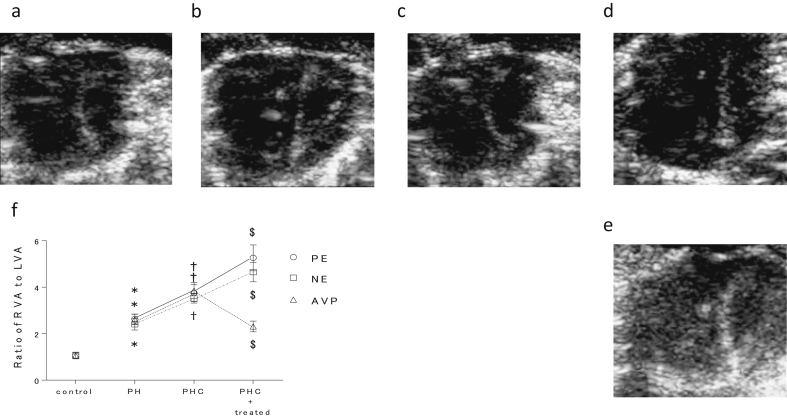
We examined the effects of vasopressin, phenylephrine, and norepinephrine by echocardiography in control rats (n=18) and in PH rats with PHC (n=6 each with vasopressin, phenylephrine, and norepinephrine). RVA/LVA in PHC was significantly increased compared with the basal value (Fig. 3a, b, and f) and was significantly reversed with vasopressin 1.5 μg kg−1 h−1 (Fig. 3c and f) (Video 3). Vasopressin also improved interventricular septal flattening and paradoxical motion. In contrast, phenylephrine 2 mg kg−1 h−1 further increased RVA/LVA over that before phenylephrine treatment and did not improve the septal motion (Fig. 3d and f) (Video 4). Norepinephrine 12 μg kg−1 h−1 also increased RVA/LVA (Fig. 3e and f) and caused arrhythmia (Video 5). Because norepinephrine 24 μg kg−1 h−1 appeared to worsen haemodynamics with arrhythmia compared with norepinephrine 12 μg kg−1 h−1 in PHC as mentioned above (Fig. 2c), we used norepinephrine 12 μg kg−1 h−1 for this experiment.
Fig 3.
Echocardiographic changes in right ventricular dilatation and septal flattening in response to vasopressin (AVP), phenylephrine (PE), and norepinephrine (NE) administration during pulmonary hypertensive crisis (PHC) at FiO2 0.1 in pulmonary hypertensive (PH) rats. The short-axis view at the level of the papillary muscles in (a) PH (PH), (b) during pulmonary hypertensive crisis (PHC), and (c) during PHC with AVP 1.5 μg kg−1 h−1 and with (d) PE 2.0 mg kg−1 h−1, and (e) NE 12 μg kg−1 h−1. (f) The ratio of right ventricular inner area (RVA) to left ventricular inner area (LVA) in control, PH at FiO2 1.0, PH at FiO2 0.1, and PH at FiO2 0.1 with administration of AVP 1.5 μg kg−1 h−1, PE 2.0 mg kg−1 h−1, and NE 12 μg kg−1 h−1. Values are given as mean (standard error). *P<0.05 compared with control, †P<0.05 compared with PH at FiO2 1.0, ‡P <0.05 compared with PH at FiO2 0.1 (n=6 each). LV, left ventricle; RV, right ventricle.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.bja.2019.01.014
The following are the Supplementary data related to this article:
Effects of vasopressin and phenylephrine on PaO2
When FiO2 was changed from 1.0 to 0.1 in PH rats to induce PHC, PaO2 obviously decreased in the vasopressin and phenylephrine groups [44.1 (2.3) vs 4.6 (0.3) kPa and 43.2 (5.6) vs 4.3 (0.2) kPa, respectively] (n=5 each). During the PHC state, vasopressin 1.5 μg kg−1 h−1 significantly improved PaO2 [6.5 (0.5) kPa, P<0.05], whereas phenylephrine 2 mg kg−1 h−1 significantly decreased PaO2 [3.1 (0.3) kPa, P<0.05] compared with that before each treatment. We could not measure PaO2 in the norepinephrine group because of the fragile haemodynamics as mentioned above.
Effects of vasopressin, phenylephrine, and norepinephrine on survival
With vasopressin 1.5 μg kg−1 h−1 administration (n=6), five rats survived through the entire observational period of 120 min in PHC (Fig. 4). With saline (n=6), only 40% of the rats survived at 120 min. With the phenylephrine 2 mg kg−1 h−1 (n=6) and norepinephrine 12 μg kg−1 h−1 (n=6) treatments, all rats died before 120 min. A Kaplan-Meyer analysis of survival rate in PHC showed that vasopressin significantly improved the survival rate compared with saline, phenylephrine, and norepinephrine. Phenylephrine and norepinephrine worsened the survival rate compared even with saline.
Fig 4.
Kaplan-Meier survival curves at 120 min during pulmonary hypertensive crisis (PHC) at FiO2 0.1 with administration of vasopressin (AVP) 1.5 μg kg−1 h−1 (a: n=6), saline (b: n=6), phenylephrine (PE) 2.0 mg kg−1 h−1 (c: n=6), and norepinephrine (NE) 12 μg kg−1 h−1 (d: n=6). The administration rate of each agent was fixed at 2 ml h−1. *P<0.05 compared with saline (log-rank test).
Effects of vasopressin, norepinephrine, and phenylephrine on haemodynamic parameters in control rats exposed to acute hypoxia
We examined haemodynamic changes in response to the i.v. administration of vasopressin, phenylephrine, and norepinephrine in control rats exposed to acute hypoxia (n=6 for each drug). After changing the FiO2 from 1.0 to 0.1, MBP decreased significantly compared with the basal levels, whereas RVSP did not change significantly (Supplementary Fig. S1). The times to obtain a constant state of SBP and RVSP with hypoxia in the vasopressin, phenylephrine, and norepinephrine groups were 7.0 (2.6), 7.8 (2.6), and 7.3 (1.8) min, respectively. The ventilation slightly, but not significantly, decreased CI and increased PVRI. Administration of vasopressin 1.5 μg kg−1 h−1 and phenylephrine 2 mg kg−1 h−1 significantly increased MBP and CI compared with those before administration. Vasopressin 0.5 μg kg−1 h−1 and phenylephrine 0.3 mg kg−1 h−1 had little effect on the haemodynamics. Although norepinephrine 24 μg kg−1 h−1 had a small effect on MBP, it significantly improved CI compared with that before administration.
Effects of vasopressin, norepinephrine, and phenylephrine on haemodynamic parameters in pulmonary hypertensive rats
We examined haemodynamic changes in response to the i.v. administration of vasopressin, norepinephrine, and phenylephrine in PH rats without PHC at FiO2 1.0. Vasopressin 1.5 μg kg−1 h−1 significantly increased MBP while decreasing RVSP compared with the basal levels (Supplementary Fig. S2). CI and HR at vasopressin 1.5 μg kg−1 h−1 were significantly lower than the basal levels. MBP with phenylephrine 0.1 and 0.3 mg kg−1 h−1 was significantly increased, and RVSP with phenylephrine 0.3 mg kg−1 h−1 was significantly higher than that at the basal level. CI, PVRI, and HR did not change with phenylephrine administration. Administration of norepinephrine 12 μg kg−1 h−1 and 24 μg kg−1 h−1 did not increase MBP and had little effect on CI and PVRI.
Relaxation effect of vasopressin and acetylcholine on isometric tension of pulmonary artery rings
Vasopressin produced concentration-dependent relaxations of pulmonary artery ring samples from control rats whose rings had been contracted with norepinephrine 100 nM (n=4) (Fig. 5a). The relaxation was greatly inhibited by pre-treatment with L-NAME 30 μM (n=4). However, vasopressin did not cause relaxation of pulmonary artery rings from PH rats (n=4). Acetylcholine also produced concentration-dependent relaxations of pulmonary artery ring samples from control rats (n=10). The relaxation was impaired in pulmonary artery rings from PH rats (n=10) (Fig. 5b).
Fig 5.
Mechanical responses of the pulmonary artery (PA) and femoral artery (FA). Relaxation responses of the PA to vasopressin (AVP) during contraction with norepinephrine 100 nM in control and pulmonary hypertensive (PH) rats, and the effect of NG-nitro-L-arginine monomethyl ester (L-NAME) 30 μM on the relaxation (a). Relaxation induced by AVP (1–300 nM) was expressed as a percentage of the 100 nM norepinephrine-induced contraction. *P<0.05 compared with control (n=4). Contractile responses of PA and FA to (b) AVP and to (c) phenylephrine (PE) in PH rats. Contraction is expressed as a percentage of that induced by KCl 65 mM. Values are given as mean (standard error). *P<0.05 compared with PA (n=8 in AVP, n=6 in PE).
Constrictive effect of vasopressin and phenylephrine on isometric tension of pulmonary and femoral artery rings
Vasopressin produced dose-dependent contraction of the femoral artery but not pulmonary artery ring samples in PH rats (Fig. 5c) (n=8 in vasopressin, n=6 in phenylephrine). In contrast, phenylephrine caused dose-dependent contraction of both pulmonary and femoral artery ring samples (Fig. 5d) (n=8 in vasopressin, n=6 in phenylephrine).
Discussion
Report a novel rat model of PHC created by exposing rats with MCT-induced PH to acute hypoxia. Induction of the PHC showed a critically decreased SBP equal to or exceeded by RVSP, increased PVRI, and decreased CI. This model also showed exacerbated right ventricle dilatation, interventricular septal flattening, and paradoxical septal motion, indicating aggravation of right heart failure. Induction of PHC reproducibly showed similar haemodynamic changes with a similar time course in all 30 PH rats. Therefore, this model appeared to reproduce the haemodynamics and cardiac motion of PHC at least in a certain situation. Returning FiO2 to 1.0 also recovered all haemodynamic parameters, and thereby we consider that this model could be reversible and endurable enough to examine PHC management as a PHC model.
We also showed that vasopressin returned the haemodynamic parameters and cardiac motion to basal values in the PH rats and improved oxygenation and the survival period in PHC, whereas norepinephrine and phenylephrine worsened them in this PHC model. Vasopressin 1.5 μg kg−1 h−1 significantly increased MBP and CI while decreasing PVRI in PHC. Similarly, vasopressin improved the RVA/LVA ratio, septal motion, and PaO2 and greatly prolonged the survival period during PHC compared with phenylephrine and norepinephrine. These results are consistent with previous reports showing efficacy of vasopressin for PHC management.2, 11, 13, 14, 29 In contrast, phenylephrine 2 mg kg−1 h−1 further increased PVRI and worsened CI, RVA/LVA, and septal motion compared with their values before the treatment. Phenylephrine is known to improve right coronary perfusion in RV failure while worsening RV function attributable to increased PVRI.12 Because phenylephrine 2 mg kg−1 h−1 increased not only MBP, but also RVSP in PHC, the pressure gradient between the MBP and RVSP to drive right coronary artery perfusion probably could not be improved. Increased RVSP could also attenuate left ventricular dilation and worsen septal motion even further. Norepinephrine at 12 and 24 μg kg−1 h−1 caused lethal arrhythmias and was not capable of maintaining MBP in PHC. Norepinephrine can dose-dependently cause arrhythmias, and the sympathetic nervous system is upregulated in heart failure.30 Although norepinephrine 12 μg kg−1 h−1 was not a high dose for PH management,12 right heart failure from PH may affect the sensitivity to norepinephrine. Combined treatments with pulmonary vasodilators might be a more appropriate way to use norepinephrine practically. In the control group, acute hypoxia with FiO2 0.1 induced similar effects on haemodynamics to those in the PH rats, although to a lesser degree. It is probable that control rats may be more tolerant to hypoxia than PH rats. Acute hypoxia slightly, but not significantly, increased PVRI in the control rats. Sevoflurane has been reported to have a vasodilatory effect31 and to reduce hypoxic pulmonary vasoconstriction31, 32 in a dose-dependent manner via a variety of mechanisms.33, 34, 35 Therefore, in this study, although the precise mechanism for the absence of an increase in PVRI is obscure, it is possible that sevoflurane 4% could affect this lack of increase. As with the PHC rats, vasopressin also significantly recovered the decreased MBP and increased CI in the control rats with hypoxia. Hypoxia-induced decrease in MBP and the vasopressin- and phenylephrine-induced increases in MBP did not affect HR in the PH and control rats. These results suggested impaired baroreflex control of HR. Because sevoflurane is known to reduce baroreflex in a dose-dependent manner,35, 36, 37 sevoflurane 4% might have affected the reflex in the present study.
Vasopressin improved PaO2 in PHC. These results are consistent with previous reports showing improved oxygenation in PH in infants with congenital heart disease38, 39 and in adults undergoing cardiac surgery.13 Vasopressin also improved oxygenation more than epinephrine did in a post-resuscitation model, because of a lower ventilation-perfusion mismatch.40 In the present study, an increased CI with vasopressin and subsequent increase in pulmonary blood flow at least could contribute to the increases in oxygen delivery and in PaO2. This increased PaO2 also could improve cardiac motion and the duration of survival in PHC. In contrast, the decreased PaO2 with phenylephrine in PHC may be a result of a decrease in CI. Taken together, these results suggest that vasopressin may be useful in the management of PHC at least in this situation.
In isometric tension measurements, vasopressin showed a selective vasoconstrictive effect on the femoral artery, but not the pulmonary artery rings from PH rats. These results are consistent with previous reports in humans15 and chronic hypoxia-induced PH rats.19 The selective constrictive effect appeared to be consistent with an increase in MBP, but not RVSP with vasopressin in PHC, which increased the pressure gradient between them.
In contrast, phenylephrine constricted both the pulmonary and femoral artery rings. Therefore, it may be reasonable that phenylephrine increased both MBP and RVSP with little increase in the pressure gradient. In this study, although vasopressin decreased PVRI in PHC, the relaxation effect of vasopressin on the pulmonary artery rings from MCT-induced PH rats was significantly reduced compared with that from the control rats. The relaxation effect in control rats was also inhibited by pre-treatment with L-NAME. Vasodilation of the pulmonary artery with vasopressin has been reported to be through the eNOS/NO pathway.16, 17, 41 However, various studies of PH using MCT-induced rats42 reported impaired activity of eNOS, decreased eNOS expression,43, 44 eNOS activity,42, 45 NO product,45, 46 cyclic guanosine monophosphate content,47 and endothelium-dependent relaxation46, 48 in pulmonary artery. These data may be consistent with our results of impaired relaxation of pulmonary artery with vasopressin in PH. In contrast, Resta and colleagues49 reported a modest increase of eNOS expression and augmented vasodilation with vasopressin in isolated lung from MCT-induced PH rats. As Nakazawa and colleagues46 noted, the discrepancy between these results in MCT-induced PH rats might be because of the difference in the preparations used such as pulmonary artery rings and isolated lungs. Although the mechanism of the decrease in PVRI with vasopressin remains to be elucidated, a selective increase in MBP, but not RVSP, and a decrease in PVRI with vasopressin might contribute to improvements of CI, PaO2, and survival rate.
In addition, vasopressin 1.5 μg kg−1 h−1 increased MBP in both PHC and PH similarly, whereas phenylephrine 2 mg kg−1 h−1 was required to increase MBP in PHC, although phenylephrine 0.3 mg kg−1 h−1 could increase MBP significantly in PH. The vasoconstrictive effect of vasopressin was preserved in hypoxia, acidosis,50, 51 catecholamine-refractory septic shock,52 and cardiopulmonary resuscitation compared with catecholamine.6, 11, 44 These findings also support the use of vasopressin in the management of PHC.
Interestingly, in the PH rats without PHC, although vasopressin 1.5 μg kg−1 h−1 significantly increased MBP, it decreased CI to below its basal levels. These results are consistent with previous reports that vasopressin decreases CI and RVSP while increasing SBP in rats under normal18 and chronic hypoxic conditions,19 and in canines with acute PH.9 Our study and these previous studies were performed under states of maintained circulation, and the SBP was higher than the pulmonary arterial pressure (PAP). Collectively, a beneficial effect of vasopressin on CI appeared to be exerted more in a collapsed than maintained circulation. These assumptions may be supported by the findings that vasopressin improved refractory severe hypotension in non-cardiac53 and cardiac surgeries14 and in septic shock.52
Although an MCT-induced PH model has been used to assess the effects of drugs in clinical settings,54 this model is now known to have some limitations when translated to human PH. Because it lacks the complete pathological changes of severe and chronic PH in humans such as plexiform lesions, this model may represent acute/subacute damage of curable PH.10 Inflammation and vasoactivity have also been thought to be important mechanistic components of this PH model.55
However, PHC can arise from various types of PH at various levels of severity.1, 2, 3, 4, 5, 6 PHC is heterogeneous and complex, and although the factors that promote PHC are not completely understood, inflammation, endothelial dysfunction, and pulmonary artery contractility may be important.1, 11, 24, 56 Indeed in the perioperative period of congenital heart diseases with cardiopulmonary bypass, these factors have been associated with PHC.1, 56 In such cases, PH stage can be acute or subacute and curable because surgery must be performed before the onset of high PVR and irreversible pathological changes.57 Therefore, it seems possible that MCT-induced PH could be applied for PHC experiments on these terms.
Similarly, hypoxia could also be an important trigger of PHC, but various other stimuli can also be triggers.8 Thus, it might be possible that our PHC model in this study could represent acute right heart failure mimicking PHC at least in a certain situation. However, the development of various PHC states, using other known PH than MCT-induced models, may provide better understanding.
Although we examined single treatment with vasopressor, other inotropes and pulmonary vasodilators such as dobutamine and prostacyclin analogue1, 7 have been applied for rescue of PHC.11 Investigation of combined treatments using a PHC model may provide more appropriate management of PHC.
In conclusion, we reproduced an experimental model of PHC for the first time, to our knowledge, which showed that vasopressin, but not norepinephrine and phenylephrine, might be useful for PHC management. These results could justify future studies of PHC management focussing on vasopressin.
Authors' contributions
Collected and analysed data: YS, SO.
Wrote the initial draft of the manuscript: YS.
Designed the study: YM.
Interpreted data and revised the manuscript: YM, TG.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 17K11058.
Acknowledgements
We greatly thank Yusuke Nakazawa for technical assistance.
Editorial decision: 08 January 2019
Handling editor: C. Boer
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.01.014.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 2
Multimedia component 3
Multimedia component 4
References
- 1.Brunner N., de Jesus Perez V.A., Richter A. Perioperative pharmacological management of pulmonary hypertensive crisis during congenital heart surgery. Pulm Circ. 2014;4:10–24. doi: 10.1086/674885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilkington S.A., Taboada D., Martinez G. Pulmonary hypertension and its management in patients undergoing non-cardiac surgery. Anaesthesia. 2015;70:56–70. doi: 10.1111/anae.12831. [DOI] [PubMed] [Google Scholar]
- 3.Sato K., Saji T., Kaneko T., Takahashi K., Sugi K. Unexpected pulmonary hypertensive crisis after surgery for ocular malignant melanoma. Life Sci. 2014;118:420–423. doi: 10.1016/j.lfs.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Carmosino M.J., Friesen R.H., Doran A., Ivy D.D. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104:521–527. doi: 10.1213/01.ane.0000255732.16057.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabanal J.M., Real M.I., Williams M. Perioperative management of pulmonary hypertension during lung transplantation (a lesson for other anaesthesia settings) Rev Esp Anestesiol Reanim. 2014;61:434–445. doi: 10.1016/j.redar.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Schisler T., Marquez J.M., Hilmi I., Subramaniam K. Pulmonary hypertensive crisis on induction of anesthesia. Semin Cardiothorac Vasc Anesth. 2016;21:105–113. doi: 10.1177/1089253216652222. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N., Humbert M., Vachiery J.-L. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 8.Abman S.H., Hansmann G., Archer S.L. Pediatric pulmonary hypertension guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 9.Leather H.A., Segers P., Berends N., Vandermeersch E., Wouters P.F. Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension. Crit Care Med. 2002;30:2548–2552. doi: 10.1097/00003246-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Stenmark K.R., Meyrick B., Galie N., Mooi W.J., McMurtry I.F. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 11.Kaestner M., Schranz D., Warnecke G., Apitz C., Hansmann G., Miera O. Pulmonary hypertension in the intensive care unit. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102:ii57–ii66. doi: 10.1136/heartjnl-2015-307774. [DOI] [PubMed] [Google Scholar]
- 12.Price L.C., Wort S.J., Finney S.J., Marino P.S., Brett S.J. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14:R169. doi: 10.1186/cc9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tayama E., Ueda T., Shojima T. Arginine vasopressin is an ideal drug after cardiac surgery for the management of low systemic vascular resistant hypotension concomitant with pulmonary hypertension. Interact Cardiovasc Thorac Surg. 2007;6:715–719. doi: 10.1510/icvts.2007.159624. [DOI] [PubMed] [Google Scholar]
- 14.Scheurer M.A., Bradley S.M., Atz A.M. Vasopressin to attenuate pulmonary hypertension and improve systemic blood pressure after correction of obstructed total anomalous pulmonary venous return. J Thorac Cardiovasc Surg. 2005;129:464–466. doi: 10.1016/j.jtcvs.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Currigan D.A., Hughes R.J., Wright C.E., Angus J.A., Soeding P.F. Vasoconstrictor responses to vasopressor agents in human pulmonary and radial arteries: an in vitro study. Anesthesiology. 2014;121:930–936. doi: 10.1097/ALN.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 16.Evora P., Pearson P.J., Schaff H.V. Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. V1-receptor-mediated production of nitric oxide. Chest. 1993;103:1241–1245. doi: 10.1378/chest.103.4.1241. [DOI] [PubMed] [Google Scholar]
- 17.Russ R.D., Walker B.R. Role of nitric oxide in vasopressinergic pulmonary vasodilatation. Am J Physiol. 1992;262:H743–H747. doi: 10.1152/ajpheart.1992.262.3.H743. [DOI] [PubMed] [Google Scholar]
- 18.Walker B.R., Haynes J., Jr., Wang H.L., Voelkel N.F. Vasopressin-induced pulmonary vasodilation in rats. Am J Physiol. 1989;257:H415–H422. doi: 10.1152/ajpheart.1989.257.2.H415. [DOI] [PubMed] [Google Scholar]
- 19.Jin H.K., Yang R.H., Chen Y.F., Thornton R.M., Jackson R.M., Oparil S. Hemodynamic effects of arginine vasopressin in rats adapted to chronic hypoxia. J Appl Physiol. 1989;66:151–160. doi: 10.1152/jappl.1989.66.1.151. [DOI] [PubMed] [Google Scholar]
- 20.Mols P., Hallemans R., Van Kuyk M. Hemodynamic effects of vasopressin, alone and in combination with nitroprusside, in patients with liver cirrhosis and portal hypertension. Ann Surg. 1984;199:176–181. doi: 10.1097/00000658-198402000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin H.K., Chen Y.F., Yang R.H., McKenna T.M., Jackson R.M., Oparil S. Vasopressin lowers pulmonary artery pressure in hypoxic rats by releasing atrial natriuretic peptide. Am J Med Sci. 1989;298:227–236. doi: 10.1097/00000441-198910000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Enomoto M., Pan J., Shifrin Y., Belik J. Age dependency of vasopressin pulmonary vasodilatory effect in rats. Pediatr Res. 2014;75:315–321. doi: 10.1038/pr.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koga M., Mizuno Y., Watanabe I., Kawakami H., Goto T. Role of VPAC2 receptor in monocrotaline-induced pulmonary hypertension in rats. J Appl Physiol. 2014;117:383–391. doi: 10.1152/japplphysiol.00861.2013. [DOI] [PubMed] [Google Scholar]
- 24.Adatia I., Beghetti M. Early postoperative care of patients with pulmonary hypertension associated with congenital cardiac disease. Cardiol Young. 2009;19:315–319. doi: 10.1017/S1047951109990175. [DOI] [PubMed] [Google Scholar]
- 25.Christou H., Morita T., Hsieh C.M. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–1229. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- 26.Dahal B.K., Kosanovic D., Pamarthi P.K. Therapeutic efficacy of azaindole-1 in experimental pulmonary hypertension. Eur Respir J. 2010;36:808–818. doi: 10.1183/09031936.00140309. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno Y., Isotani E., Huang J., Ding H., Stull J.T., Kamm K.E. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol. 2008;295:C358–C364. doi: 10.1152/ajpcell.90645.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuno Y., Isotani E., Ohno K., Nagai A., Imamura M., Azuma H. Involvement of accumulated NOS inhibitors and endothelin-1, enhanced arginase, and impaired DDAH activities in pulmonary dysfunction following subarachnoid hemorrhage in the rabbit. Vascul Pharmacol. 2008;48:21–31. doi: 10.1016/j.vph.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig E.B., Starc T.J., Chen J.M. Intravenous arginine-vasopressin in children with vasodilatory shock after cardiac surgery. Circulation. 1999;100 doi: 10.1161/01.cir.100.suppl_2.ii-182. II-182–Ii-6. [DOI] [PubMed] [Google Scholar]
- 30.Lohse M.J., Engelhardt S., Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 31.De Hert S., Moerman A. Sevoflurane. F1000Res. 2015;4:626. doi: 10.12688/f1000research.6288.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibe Y., Gui X., Uno H., Shiokawa Y., Umeda T., Suekane K. Effect of sevoflurane on hypoxic pulmonary vasoconstriction in the perfused rabbit lung. Anesthesiology. 1993;79:1348–1353. doi: 10.1097/00000542-199312000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Jarman J., Maharaj C.H., Higgins B.D. Role of potassium and calcium channels in sevoflurane-mediated vasodilation in the foeto-placental circulation. BMC Anesthesiol. 2009;9:4. doi: 10.1186/1471-2253-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matta B.F., Heath K.J., Tipping K., Summors A.C. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology. 1999;91:677–680. doi: 10.1097/00000542-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Ebert T.J., Harkin C.P., Muzi M. Cardiovascular responses to sevoflurane: a review. Anesth Analg. 1995;81:S11–S22. doi: 10.1097/00000539-199512001-00003. [DOI] [PubMed] [Google Scholar]
- 36.Umehara S., Tanaka M., Nishikawa T. Effects of sevoflurane anesthesia on carotid-cardiac baroreflex responses in humans. Anesth Analg. 2006;102:38–44. doi: 10.1213/01.ane.0000183651.10514.9a. [DOI] [PubMed] [Google Scholar]
- 37.Eger E.I., 2nd New inhaled anesthetics. Anesthesiology. 1994;80:906–922. doi: 10.1097/00000542-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Malikiwi A., Sasi A., Tan K., Sehgal A. Vasopressin as an adjunct therapy for pulmonary hypertension: a case report. Eur J Pediatr. 2014;173:1651–1654. doi: 10.1007/s00431-013-2225-y. [DOI] [PubMed] [Google Scholar]
- 39.Steinhorn R.H. Advances in neonatal pulmonary hypertension. Neonatology. 2016;109:334–344. doi: 10.1159/000444895. [DOI] [PubMed] [Google Scholar]
- 40.Loeckinger A., Kleinsasser A., Wenzel V. Pulmonary gas exchange after cardiopulmonary resuscitation with either vasopressin or epinephrine. Crit Care Med. 2002;30:2059–2062. doi: 10.1097/00003246-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Sai Y., Okamura T., Amakata Y., Toda N. Comparison of responses of canine pulmonary artery and vein to angiotensin II, bradykinin and vasopressin. Eur J Pharmacol. 1995;282:235–241. doi: 10.1016/0014-2999(95)00343-j. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki A., Doi S., Mizutani S., Azuma H. Roles of accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, and attenuated nitric oxide synthase activity in endothelial cells for pulmonary hypertension in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1480–L1487. doi: 10.1152/ajplung.00360.2006. [DOI] [PubMed] [Google Scholar]
- 43.Pei Y., Ma P., Wang X. Rosuvastatin attenuates monocrotaline-induced pulmonary hypertension via regulation of Akt/eNOS signaling and asymmetric dimethylarginine metabolism. Eur J Pharmacol. 2011;666:165–172. doi: 10.1016/j.ejphar.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 44.Tyler R.C., Muramatsu M., Abman S.H. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol. 1999;276:L297–L303. doi: 10.1152/ajplung.1999.276.2.L297. [DOI] [PubMed] [Google Scholar]
- 45.Ou Z.J., Wei W., Huang D.D. L-arginine restores endothelial nitric oxide synthase-coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J P Endocrinol Metab. 2010;298:E1131–E1139. doi: 10.1152/ajpendo.00107.2010. [DOI] [PubMed] [Google Scholar]
- 46.Nakazawa H., Hori M., Ozaki H., Karaki H. Mechanisms underlying the impairment of endothelium-dependent relaxation in the pulmonary artery of monocrotaline-induced pulmonary hypertensive rats. Br J Pharmacol. 1999;128:1098–1104. doi: 10.1038/sj.bjp.0702878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee M.Y., Tsai K.B., Hsu J.H., Shin S.J., Wu J.R., Yeh J.L. Liraglutide prevents and reverses monocrotaline-induced pulmonary arterial hypertension by suppressing ET-1 and enhancing eNOS/sGC/PKG pathways. Sci Rep. 2016;6:31788. doi: 10.1038/srep31788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirmagul B., Ilgin S., Atli O., Usanmaz S.E., Demirel-Yilmaz E. Assessment of the endothelial functions in monocrotaline-induced pulmonary hypertension. Clin Exp Hypertens. 2013;35:220–227. doi: 10.3109/10641963.2012.721838. [DOI] [PubMed] [Google Scholar]
- 49.Resta T.C., Gonzales R.J., Dail W.G., Sanders T.C., Walker B.R. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am J Physiol. 1997;272:H806–H813. doi: 10.1152/ajpheart.1997.272.2.H806. [DOI] [PubMed] [Google Scholar]
- 50.Dunser M.W., Mayr A.J., Ulmer H. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107:2313–2319. doi: 10.1161/01.CIR.0000066692.71008.BB. [DOI] [PubMed] [Google Scholar]
- 51.Fox A.W., May R.E., Mitch W.E. Comparison of peptide and nonpeptide receptor-mediated responses in rat tail artery. J Cardiovasc Pharmacol. 1992;20:282–289. doi: 10.1097/00005344-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Gordon A.C., Wang N., Walley K.R., Ashby D., Russell J.A. The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest. 2012;142:593–605. doi: 10.1378/chest.11-2604. [DOI] [PubMed] [Google Scholar]
- 53.Khan K.K., Khan F.H. Pulmonary hypertensive crisis and its efficient management. A Case report and literature review. J Pak Med Assoc. 2017;67:936–938. [PubMed] [Google Scholar]
- 54.Nogueira-Ferreira R., Vitorino R., Ferreira R., Henriques-Coelho T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: a network approach. Pulm Pharmacol Ther. 2015;35:8–16. doi: 10.1016/j.pupt.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Arroyo J.G., Farkas L., Alhussaini A.A. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 56.Lindberg L., Olsson A.K., Jogi P., Jonmarker C. How common is severe pulmonary hypertension after pediatric cardiac surgery? J Thorac Cardiovasc Surg. 2002;123:1155–1163. doi: 10.1067/mtc.2002.121497. [DOI] [PubMed] [Google Scholar]
- 57.Gatzoulis M.A., Alonso-Gonzalez R., Beghetti M. Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev. 2009;18:154–161. doi: 10.1183/09059180.00003309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 2
Multimedia component 3
Multimedia component 4