Abstract
Background
Tissue factor (TF) canonically functions as the initiator of the coagulation cascade. TF levels are increased in inflamed airways and seem to be important for tumor growth and metastasis. We hypothesized that airway epithelia release TF as part of a wound repair program.
Objectives
The goal of this study was to evaluate whether airway epithelia release TF in response to pro-inflammatory stimuli and to investigate roles of TF in cell growth and repair.
Methods
Airway epithelial cells were exposed to 10 μg/mL of lipopolysaccharide or 1 ng/mL of transforming growth factor β (TGF-β). TF and TGF-β messenger RNA and protein were measured in cell lysate and culture media, respectively. Signaling pathways were evaluated by using selective agonists and inhibitors. Airway epithelia were mechanically injured in the presence of TF and tissue factor pathway inhibitor to investigate their roles in wound repair.
Results
TF protein levels increased in cell media after exposure to lipopolysaccharide (P < .01) but only in growing cells, and this action was blocked when exposed to an extracellular signal-regulated kinase inhibitor or a “small” worm phenotype and mothers against Decapentaplegic inhibitor. TF protein also increased in the presence of TGF-β (P < .05). Exposure to TF pathway inhibitor decreased the rate of cell growth by 60% (P < .05), and exposure to TF increased the rate of airway healing after injury by 19% (P < .05).
Conclusions
Growing airway epithelia release TF when exposed to lipopolysaccharide or TGF-β. TF reduces wound-healing time in airway epithelia and therefore may be important to airway recovery after injury.
Key Words: inflammation, remodeling, tissue factor, transforming growth factor β, wound healing
Abbreviations: ALI, air-liquid interface; ELISA, enzyme-linked immunosorbent assay; FVII, Factor VII; FVIIa, activated Factor VII; LPS, lipopolysaccharide; MEK/ERK, mitogen-activated protein kinase/extracellular signal-regulated kinases; NHBE, normal human bronchial epithelium; SMAD, “small” worm phenotype and mothers against Decapentaplegic; TF, tissue factor; TFPI, tissue factor pathway inhibitor; TGF-β, transforming growth factor β
Tissue factor (TF) is a 47-kDa transmembrane glycoprotein that structurally resembles class II cytokine receptors and canonically functions as an initiator of the coagulation cascade.1 Vascular endothelium, fibroblasts, smooth muscle cells, and epithelial cells constitutively express TF.2 When exposed to Factor VII (FVII), which is present in circulating blood, TF activates FVII (FVIIa) through binding to FVIIa and forming a complex that triggers the Factor X pathway of coagulation.3, 4, 5 This action leads to self-amplified activation of FVII, thrombin production, and eventually clot formation and tissue growth. Lipopolysaccharide (LPS) can also induce expression of TF in monocytes and vascular endothelium via mitogen-activated protein kinase/extracellular signal-regulated kinases (MEK/ERK), activator protein 1, and nuclear factor-κB, which also leads to thrombin/fibrin clot formation in blood.4 An endogenous inhibitor of TF called tissue factor pathway inhibitor (TFPI) balances TF activity in vivo by interfering with TF/FVIIa complexes as well as Factor X.6, 7
TF has also been identified in serum and other biofluids. Serum TF levels increase during disseminated intravascular coagulation,8 hemorrhage,5 and disorders that increase capillary permeability such as sepsis and systemic inflammatory disorders.9, 10 TF has been measured in BAL fluid from subjects with inflammatory airway diseases, including asthma, pneumonia, and ARDS.9, 11, 12, 13, 14 These findings were initially attributed to vascular leakage into the airway lumen; however, Park et al15 reported that cultured normal human bronchial epithelium (NHBE) produced and released TF in cell culture in response to cyclic stress.
We hypothesized that an inflammatory stimulus (ie, LPS) would induce airway epithelial release of TF and that this release may influence cell growth and repair.
Materials and Methods
Details on experimental and statistical methods used can be found in e-Appendix 1.
Evaluating TF Release From NHBE in Response to Inflammation
Growing, submerged NHBE cells were exposed to 10 μg/mL Escherichia coli LPS added to 500 μL cell media for either 3 or 24 h. TF messenger RNA was measured according to polymerase chain reaction in those exposed to LPS for 3 h. TF protein was measured by enzyme-linked immunosorbent assay (ELISA) in the media of those exposed to LPS for 24 h. Similar experiments were conducted by using fully differentiated airway epithelia grown at an air-liquid interface (ALI), described in e-Appendix 1.
MEK/ERK Signaling Pathway Evaluation
Cells were exposed to a MEK/ERK-selective inhibitor (PD98059, 20 μM) in the presence of LPS 10 μg/mL or vehicle. TF protein in cell media was measured according to ELISA after 24 h of exposure. To assess the effect of the MEK/ERK inhibitor, phosphorylation of MEK/ERK was evaluated by using Western blot analysis.
Evaluating the Effects of Inflammation on TGF-β in NHBE
TGF-β is associated with tissue remodeling in airway disease.16, 17, 18 To evaluate the effects of LPS on TGF-β and investigate a potential relationship between TF and wound healing, cells were exposed to LPS 10 μg/mL for 24 h as described earlier. TGF-β protein was evaluated in cell media and lysate according to ELISA.
Evaluating TF Release From NHBE in Response to TGF-β Exposure
We investigated the effect of TGF-β on TF release. NHBE was exposed to 1 ng/mL of TGF-β for 24 h, and TF protein in cell media was then quantified according to ELISA.
TGF-β Signaling Pathway Evaluation
TGF-β activates the TGF-β receptor complex initiating “small” worm phenotype and mothers against Decapentaplegic (SMAD) and SMAD-independent signaling pathways.18 Cells were exposed to a SMAD-selective inhibitor (SB431542, 20 μM) in the presence of LPS 10 μg/mL or vehicle as described earlier. TF protein in cell media was measured according to ELISA after 24 h of exposure.
Effects of TFPI on Airway Epithelial Cell Growth
NHBE cells were grown in submerged culture in the presence of 0, 250, 500, and 1,000 pg/mL of TFPI. Confluence was evaluated by using light microscopy before each media change. Confluence percentage was calculated by using ImageJ software (US National Institutes of Health) to count cells’ light microscopy images (https://imagej.nih.gov/ij). Lactate dehydrogenase levels in each group were measured to assess the cytotoxic effects of TFPI.
Effects of TF and TFPI on Airway Epithelial Wound Repair
NHBE cells were differentiated at ALI to ciliated columnar epithelium. A scratch was produced across the midline of the transwell by using a sterile pipette tip. Cultures were exposed to 0, 250, 500, or 1,000 pg/mL of TF or TFPI. Scratch size was evaluated by using light microscopy every 4 h until the TF exposure group scratch was healed; ciliary beat was verified every 4 h to confirm cell viability. Confluence percentage was verified by using ImageJ software and compared between groups.
Results
TF Protein Release From NHBE Increases Following LPS Exposure But Only in Undifferentiated Epithelial Cells and Is Mediated by MEK/ERK Signaling
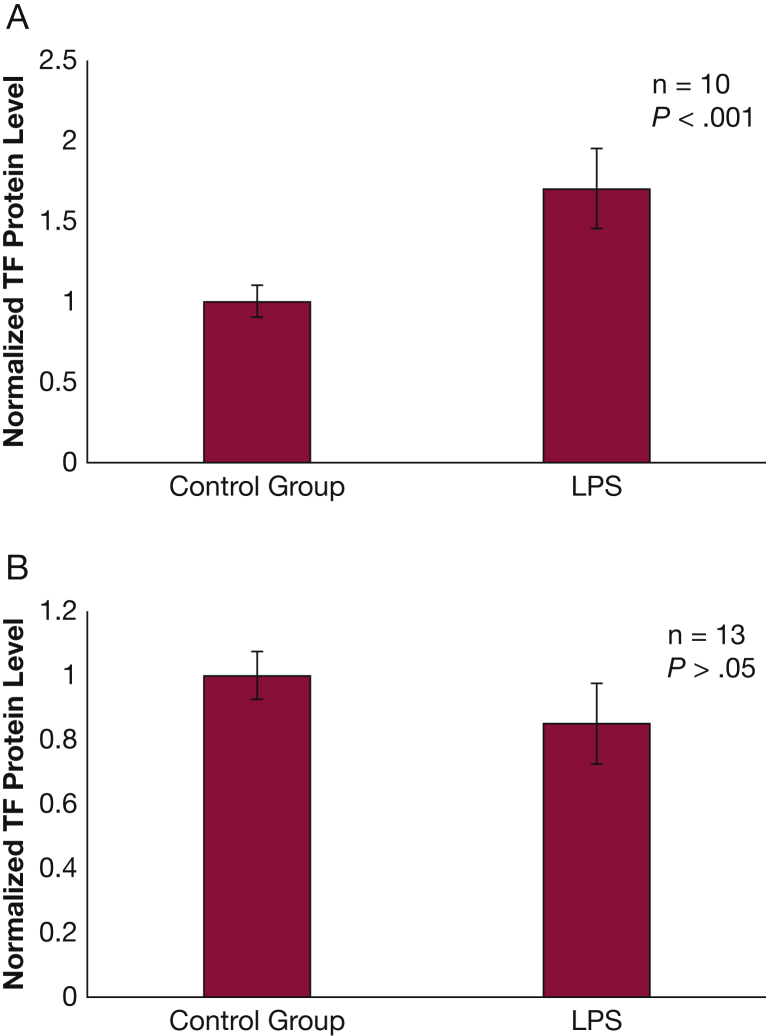
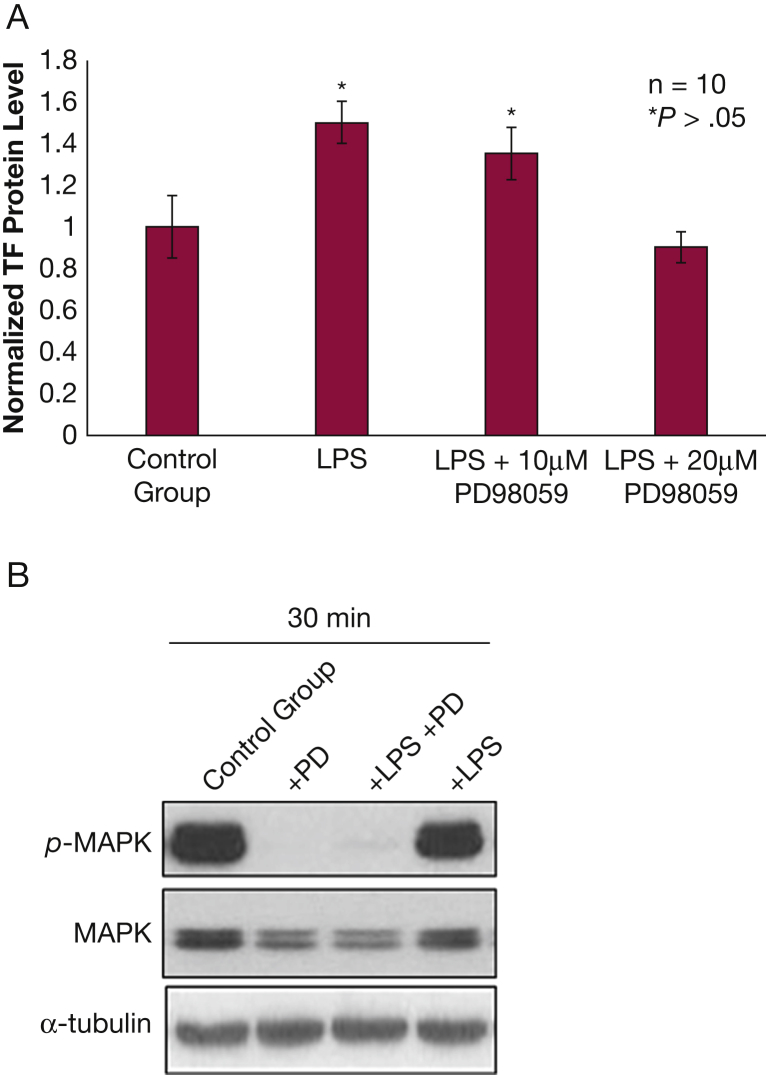
Submerged NHBE cells exposed to LPS exhibited a nearly twofold increase (P < .001) in released TF protein compared with the control groups (Fig 1A). TF messenger RNA levels were not affected by exposure to LPS (Fig 1B). However, no increase in TF was observed in fully differentiated NHBE grown at ALI when exposed to LPS (e-Fig 1). PD98059 exposure resulted in a dose-dependent decrease in LPS-induced TF protein release from NHBE (Fig 2A). PD98059 blocks MEK/ERK phosphorylation (Fig 2B).
Figure 1.
Normal human bronchial epithelium releases tissue factor (TF) protein when exposed to LPS. A, Normal human bronchial epithelium exposed to 10 μg/mL of LPS for 24 h released > 50% more TF protein than those exposed to vehicle (P < .001). Three different cell lines were used to verify this observation, and data were normalized to reflect change vs control instead of reporting protein concentrations due to significant variation in relative concentrations between the cell lines. B, TF messenger RNA did not increase after exposure to 10 μg/mL of LPS for 3 h. Results normalized to reflect change vs control instead of reporting protein concentrations due to significant variation in relative concentrations between the cell lines. Results are reported as means with error bars indicating SEs. LPS = lipopolysaccharide.
Figure 2.
–TF release from normal human bronchial epithelium (NHBE) following LPS exposure is mediated by mitogen-activated protein kinase. A, NHBE exposed to 10 μg/mL of LPS for 24 h release more TF than control (P < .05); NHBE exposed to 10 μg/mL of LPS for 24 h in the presence of 10 μM PD98059, a mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor, do not release more TF than control. B, PD98059 inhibits MAPK phosphorylation at baseline and after LPS exposure. See Figure 1 legend for expansion of other abbreviations.
LPS Exposure Increases TGF-β in NHBE Cells
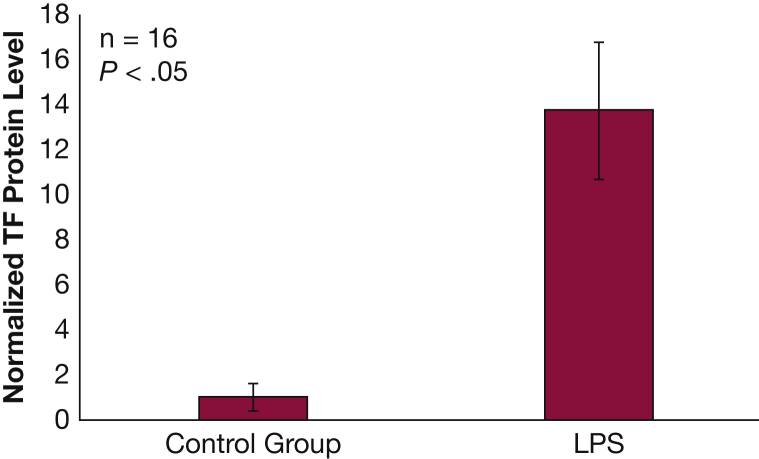
NHBE cells exposed to LPS exhibited a 10-fold increase in activated TGF-β protein in cell lysate compared with control (P < .05) (Fig 3). There was no change in TGF-β protein in cell media.
Figure 3.
NHBE transforming growth factor β protein levels increase following exposure to LPS. Levels of transforming growth factor β protein in cell lysate increased in NHBE after 24 h exposure to 10 μg/mL of LPS (P < .05). Results are reported as means with error bars indicating SEs. See Figure 1, Figure 2 legends for expansion of abbreviations.
TF Protein Is Released From NHBE Following Exposure to TGF-β and Is Mediated by SMAD Signaling
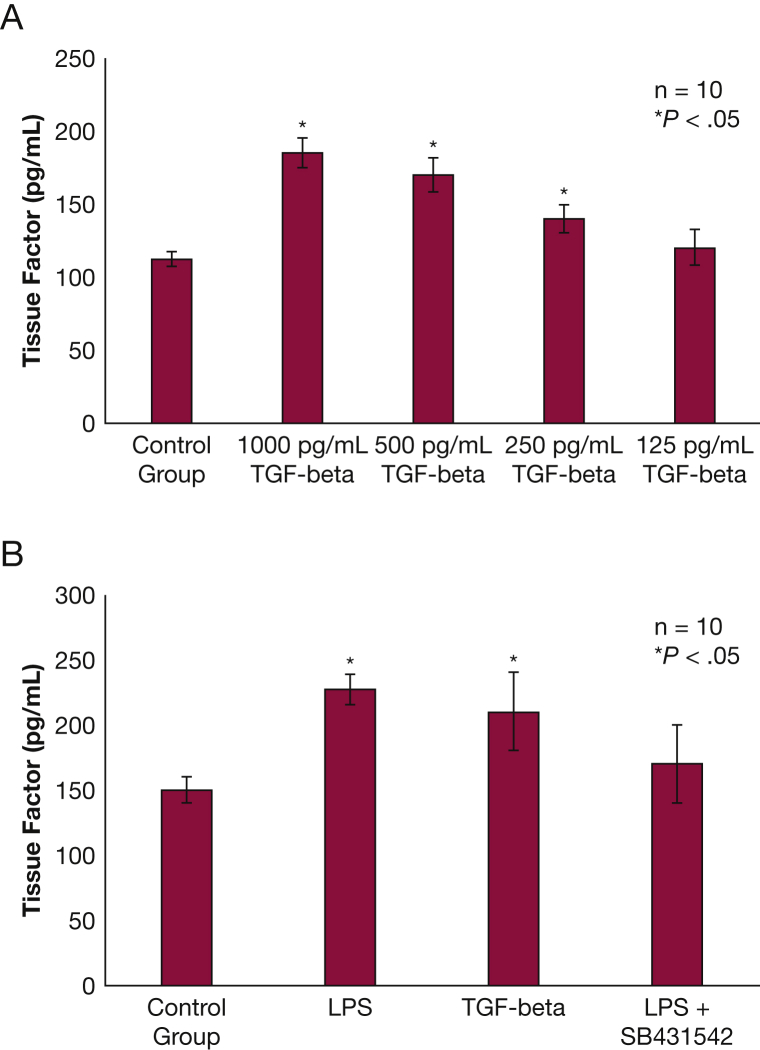
NHBE cells exposed to TGF-β exhibited a dose-dependent increase in released TF protein compared with the control groups (P < .05) (Fig 4A). TF messenger RNA levels were not affected by TGF-β exposure. NHBE treated with SB431542 did not release TF when exposed to LPS (Fig 4B).
Figure 4.
TF release from NHBE following LPS exposure is mediated by TGF-β and “small” worm phenotype and mothers against Decapentaplegic inhibitor signaling. A, NHBE exposed to TGF-β for 24 h release TF in a dose-dependent manner (P < .05). B, When NHBE were exposed to LPS 10 μg/mL of or TGF-β 1 ng/mL for 24 h, they released more TF compared with control (P < .05). When cells were exposed to LPS 10 μg/mL + SB431542 (a “small” worm phenotype and mothers against Decapentaplegic inhibitor ) 20 μM, this inhibited TF release (P < .05). All results are reported as means with error bars indicating SEs. TGF-β = transforming growth factor β. See Figure 2, Figure 4 legends for expansion of other abbreviations.
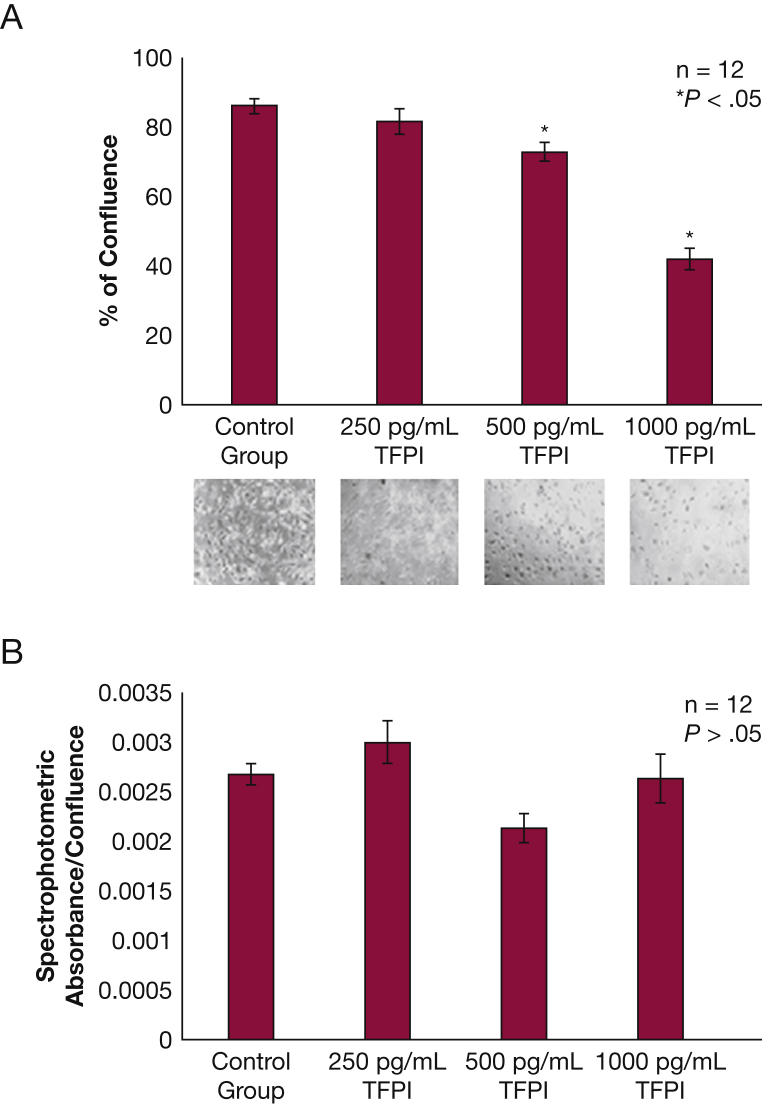
NHBE Exposed to TFPI Do Not Reach Confluence in Culture
NHBE exposed to TFPI grew significantly slower than the control group, reaching < 50% confluence by the time the control group was fully confluent (P < .05) (Fig 5A). This outcome was dose dependent. Levels of lactate dehydrogenase did not differ between any of the groups (P > .05) (Fig 5B), indicating that the lower number of cells was not caused by cytotoxicity.
Figure 5.
NHBE exposed to TFPI do not reach confluence in culture. A, NHBE exposed to 1 ng/mL or 500 pg/mL of TFPI grew significantly slower under submerged conditions than those exposed to 250 pg/mL of TFPI and the control group (P < .05). Images of each exposure group are below their respective column in the bar graph. Results are reported as means with error bars indicating SEs. B, Lactate dehydrogenase levels were not significantly different between the control or treatment groups (P > .05). TFPI = tissue factor pathway inhibitor. See Figure 2 legend for expansion of other abbreviations.
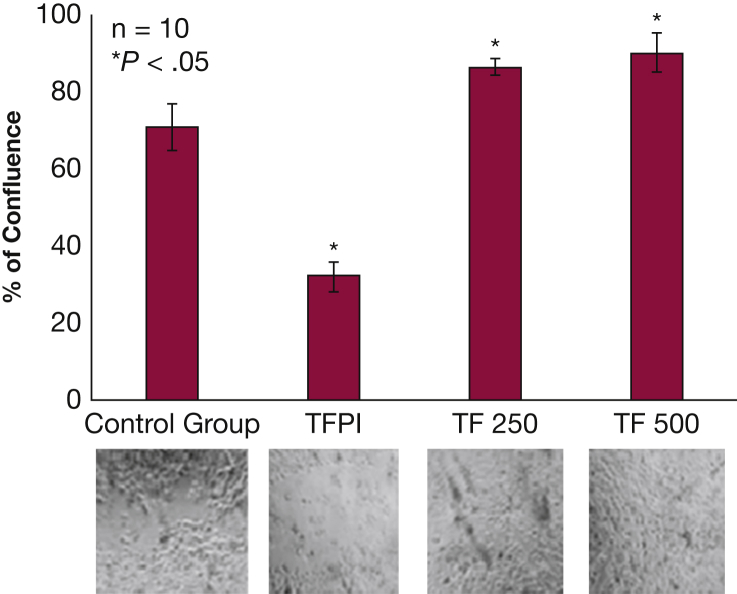
TF Decreases Healing Time in Differentiated NHBE
NHBE cells, uniformly scratched across the midline, regrew differently when exposed to TF or TFPI compared with the control group (P < .05) (Fig 6). After 55 h, those cells exposed to 500 pg/mL of TF had grown to a mean of 90% confluence within the scratch. In contrast, those cells exposed to 1 ng/mL of TFPI had grown to a mean of 32% confluence within the scratch after 55 h. The control group had grown to a mean 71% of confluence at the same time point. Ciliary beat was observed by using light microscopy immediately following scratch and every 4 h until the TF-treated group became confluent, suggesting that the scratch procedure did not alter the ciliary function of the cells in culture. Ciliary beat was also verified in the cells that filled the scratch in the TF-treated group.
Figure 6.
TF decreases healing time in differentiated NHBE. NHBE differentiated as pseudostratified ciliated columnar epithelium in air-liquid interface conditions were scratched along the midline. Those exposed to 1 ng/mL of TFPI regrew more slowly than the control group (P < .05); those exposed to 250 or 500 pg/mL of TF regrew more rapidly than the control group (P < .05). Images of each exposure group are below their respective column in the bar graph. Results are reported as means with error bars indicating SEs. See Figure 1, Figure 2, Figure 5 legends for expansion of other abbreviations.
Discussion
The canonical function of TF as initiator of the coagulation and subsequent clot formation is integral to wound healing.1, 4, 19 TF also increases cell proliferation and the rate of tumor metastasis.19 The increased levels of TF found in the inflamed airway, including during asthma, plastic bronchitis, bronchopulmonary dysplasia, and ARDS,10, 11, 12, 13, 14, 20, 21 suggests that TF might have local airway roles as well. The airway source of TF, its mechanism for production and release, and the function of TF within airways are largely unknown.
In vitro dysregulation of TF via selective inhibitors or knock-down models leads to systemic hemorrhage, and fatal pulmonary hemorrhage occurs in TF knockout mice shortly following birth.22 TF levels are increased at sites of active wound healing along the leading edge of healing cutaneous tissue.19 Inhibition of TF by administration of TFPI, its endogenous inhibitor, is associated with delayed wound closure and increased clotting time.19, 22 This outcome is consistent with our findings that TF decreased healing time of differentiated NHBE, and TFPI prevented NHBE from healing or reaching confluence (Figure 5, Figure 6).
LPS is a well-described initiator of inflammatory responses.23 LPS binds to Toll-like receptor 4 and activates intracellular signaling pathways, including MEK/ERK.23, 24 We have previously reported that 10 μg/mL of LPS will activate MEK/ERK in NHBE without causing cell death.24 LPS exposure resulted in similar TF release from NHBE as that reported after cyclic stress,15 suggesting that NHBE Toll-like receptor 4 activation by LPS leads to TF release mediated by MEK/ERK signaling (Fig 2). Although we validated that inhibition of MEK/ERK signaling by PD98059 reduced LPS-induced TF release in a dose-dependent manner, MAPK phosphorylation was similar in the control group and LPS group. This finding indicates that MAPK itself is not responsible for increased TF release from NHBE.
TGF-β regulates many of the processes surrounding wound healing and is increased in many cell types following exposure to LPS.16, 17, 18 Local increases of TGF-β in the airways are associated with airway remodeling in severe asthma, which is believed to involve a dysregulated and excessive wound healing process.17, 18 The LPS-induced TGF-β increase along with the TF release are consistent with a local repair function of TF within the airways. We observed no increase in TGF-β release into culture media, which could be consistent with SMAD-dependent TF release. This outcome may be due to the rapid binding of TGF-β to TGF-β receptors upon its release or deactivation of TGF-β following release. Of note, the ELISA used for our experiments only detects activated TGF-β.
Other groups have reported that subjects with severe asthma had higher levels of TF in their sputum than subjects with moderate asthma or control subjects, even while undergoing treatment.11 A significant increase in released TF protein from NHBE following exposure to cyclic stress pressure levels similar to those reported in the asthma airway has also been reported.15 When placed in the context of our finding that TF increases cell proliferation and airway repair, this outcome suggests that the robust cell proliferation response may contribute to airway remodeling and that TF might play an important role in remodeling.
The submerged cell culture conditions we used prevent cells from fully differentiating to pseudostratified ciliated columnar epithelia. We initially exposed differentiated cells grown in an ALI to LPS and observed no increase in TF (e-Fig 1). Submerged, undifferentiated cells are actively growing and dividing, similar to wound healing, which is probably why there was no detectable release of TF from differentiated NHBE when exposed to LPS.
The present study has several limitations. Most notably, we did not investigate the effects of extracellular TF on unaltered airway epithelium. The effects of TF and TFPI on NHBE cell cycle regulation and potential overgrowth, as we have previously reported following exposure to TGF-β, were also not evaluated.24 However, our results are not significantly affected by these limitations.
Conclusions
Our data from different cell lines using stepwise inhibition and stimulation showed that NHBE released TF and up-regulated TGF-β in response to LPS exposure. This release is regulated by MEK/ERK and SMAD signaling pathways and may function as a local repair mechanism. This potential mechanism is supported by the novel finding that TF decreased NHBE healing time and TFPI prevented NHBE cell growth and wound healing. On the basis of these findings, inhibitors of TF and the pathways leading to its release during inflammation could be therapeutic targets for severe asthma and airway remodeling.
Acknowledgments
Author contributions: M. D. D. took the lead in writing the manuscript, study design, and execution. I. S., S. K., and K. K. contributed to manuscript drafting, revision, and study design. Y. O. and Q. C. contributed to study design and execution. B. K. R. contributed guidance for study concept, design, manuscript drafting, revisions, and mentorship; and is the guarantor of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. D. D. has received investigator-initiated research awards from Mallinckrodt Pharmaceuticals and the Children’s Hospital of Richmond Foundation. B. K. R. has received research grants and awards from the National Institutes of Health, the US Food and Drug Administration, McNeil Consumer Pharmaceuticals, Gilead Pharmaceuticals, Fisher & Paykel, Boehringer Ingelheim, and Children’s Hospital of Richmond Foundation over the past 2 years. None declared (I. S., S. K., K. K., Y. O., Q. C.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: Melissa Yopp, MSHA, assisted in experimental design and technique training. Judith Voynow, MD, Roland Pittman, PhD, Vijay Lyall, PhD, John F. Hunt, MD, Alison Montpetit, PhD, and Vladimir Bogdanov, PhD, provided suggestions and comments.
Additional information: The e-Appendix and e-Figure can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This research was funded in part by a research and training award from the American Respiratory Care Foundation (M. D. D.).
Supplementary Data
References
- 1.Eilertsen K.E., Osterud B. Tissue factor: (patho)physiology and cellular biology. Blood Coagul Fibrinolysis. 2004;15(7):521–538. doi: 10.1097/00001721-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Witkowski M., Landmesser U., Ranch U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc Med. 2016;26(4):297–303. doi: 10.1016/j.tcm.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Aberg M., Eriksson O., Siegbahn A. Tissue factor noncoagulant signaling: mechanisms and implications for cell migration and apoptosis. Semin Thromb Hemost. 2015;41(7):691–699. doi: 10.1055/s-0035-1564046. [DOI] [PubMed] [Google Scholar]
- 4.Osterud B., Bjorklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32(1):11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- 5.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36(2):104–107. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj M.S., Birktoft J.J., Steer S.A. Structure and biology of tissue factor pathway inhibitor. Thromb Haemost. 2001;86(4):959–972. [PubMed] [Google Scholar]
- 7.Mast A.E. Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein. Arterioscler Thromb Vasc Biol. 2016;36(1):9–14. doi: 10.1161/ATVBAHA.115.305996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asakura H., Kamikubo Y., Goto A. Role of tissue factor in disseminated intravascular coagulation. Thromb Res. 1995;80(3):217–224. doi: 10.1016/0049-3848(95)00170-v. [DOI] [PubMed] [Google Scholar]
- 9.Tang H., Ivanciu L., Popescu N. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. Am J Pathol. 2007;171(3):1066–1077. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todoroki H., Nakamura S., Higure A. Neutrophils express tissue factor in a monkey model of sepsis. Surgery. 2000;127(2):209–216. doi: 10.1067/msy.2000.103027. [DOI] [PubMed] [Google Scholar]
- 11.Brims F.J., Chauhan A.J., Higgins B., Shute J.K. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax. 2009;64(12):1037–1043. doi: 10.1136/thx.2009.114439. [DOI] [PubMed] [Google Scholar]
- 12.Schouten M., Van der Pol M.A., Levi M., van der Poll T., van der Zee J.S. Early activation of coagulation after allergen challenge in patients with allergic asthma. J Thromb Haemost. 2009;7(9):1592–1594. doi: 10.1111/j.1538-7836.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- 13.Cunha L.G., Jr., Assis M.C., Machado G.B. Potential mechanisms underlying the acute lung dysfunction and bacterial extrapulmonary dissemination during Burkholderia cenocepacia respiratory infection. Respir Res. 2010;11(1):4. doi: 10.1186/1465-9921-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laterre P.F. Beyond antibiotics in severe community-acquired pneumonia: the role and rationale for tissue factor pathway inhibition. Crit Care. 2008;12(suppl 6):S4. doi: 10.1186/cc7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J.A., Sharif A.S., Tschumperlin D.J. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J Allergy Clin Immunol. 2012;130(6):1375–1383. doi: 10.1016/j.jaci.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pakyari M., Farrokhi A., Maharlooei M.K., Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care. 2013;2(5):215–224. doi: 10.1089/wound.2012.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halwani R., Al-Muhsen S., Al-Jahdali H., Hamid Q. Role of transforming growth factor-β in airway remodeling and asthma. Am J Respir Cell Mol Biol. 2011;44(2):127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 18.Aschner Y., Downey G.P. Transforming growth factor-b: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol. 2016;54(5):647–655. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman M., Monroe D.M. The multiple roles of tissue factor in wound healing. Front Biosci (Schol Ed) 2012;1(4):713–721. doi: 10.2741/s295. [DOI] [PubMed] [Google Scholar]
- 20.Rancourt R.C., Veress L.A., Guo X., Jones T.N., Hendry-Hofer T.B., White C.W. Airway tissue factor dependent coagulation activity in response to sulfur mustard analog 2-chloroethyl ethyl sulfide. Am J Physiol Lung Cell Mol Physiol. 2012;302(1):L82–L92. doi: 10.1152/ajplung.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palojarvi A., Andersson S., Siitonen S., Janer C., Petaja J. High tissue factor in lungs and plasma associates with respiratory morbidity in preterm infants. Acta Paediatr. 2012;101(4):403–409. doi: 10.1111/j.1651-2227.2011.02537.x. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen B., Holscher T., Sato Y. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105(7):2777–2782. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 23.Knapp S. LPS and bacterial lung inflammation models. Drug Disc Today Dis Models. 2009;6(4):113–118. [Google Scholar]
- 24.Tanabe T., Rubin B.K. Airway goblet cells secrete pro-inflammatory cytokines, chemokines, and growth factors. Chest. 2016;149(3):714–720. doi: 10.1378/chest.15-0947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.