Abstract
Background
Lung cancer is a leading cause of morbidity and mortality worldwide, and the incidence continues to rise. Although many prognostic factors have been identified, the clinical characteristics and outcomes in Korean lung cancer patients are not well defined.
Methods
Of the 23,254 new lung cancer cases registered at the Korea Central Cancer Registry in 2013, total 489 patients from 19 hospitals were abstracted by the Korean Central Cancer Registry. The clinical data retrospectively analyzed, patients were followed up until December 2015.
Results
The median age was 69 years (interquartile range, 60–74 years); 65.4% were male and 62.1% were ever-smokers. Cough was the most common initial symptom (33.5%); 13.1% of patients were asymptomatic. While squamous cell carcinoma was the most common subtype in male patients (37.2%), adenocarcinoma was the most frequent histological type in all patients (48.7%) and females (76.3%). The majority of patients received treatment (76.5%), which included surgery, radiation therapy, and chemotherapy. Older age (hazard ratio [HR], 1.037), lower body mass index (HR, 0.904), ever-smoker (HR, 2.003), small cell lung cancer (HR, 1.627), and distant metastasis (HR, 3.990) were independent predictors of mortality. Patients without symptoms (HR, 0.387) and without treatment (HR, 0.364) were associated with a favorable outcome in multivariate Cox analysis.
Conclusion
Lung cancer in Korea occurs predominantly in elderly patients, with adenocarcinoma being the most frequent subtype. The prognosis was poorer in ever-smokers and older, malnourished, and untreated patients with advanced lung cancer.
Keywords: Lung Neoplasms, Epidemiology, Korea, Mortality, Survival
Introduction
Lung cancer is one of the most common cancers and is a leading cause of morbidity and mortality worldwide1. Although antismoking policies have resulted in a reduction in smoking rate, the incidence of lung cancer continues to increase2,3. In addition, the proportion of never-smokers developing lung cancer is higher than before4. Although recent studies show that the epidemiology of lung cancer in Korea has changed in line with international observations3,5, few studies provide clinical information on lung cancer based on nationwide registry.
The first national lung cancer survey in Korea was conducted in 1997 by the Korean Academy of Tuberculosis and Respiratory Diseases, at which point squamous cell carcinoma was the most frequent histological subtype6. However, the second national lung cancer survey conducted by the Korean Association for the Study of Lung Cancer in 2005 showed that adenocarcinoma was the most common histological subtype and that the absence of symptoms at diagnosis was a favorable prognostic factor for patients with non-small cell lung cancer (NSCLC)7. Because the clinical characteristics and outcomes of patients with lung cancer were expected to differ over time, we analyzed data from a population-based nationwide cancer registry. In this study, we aimed to investigate the clinical characteristics, histological types, and prognostic factors associated with lung cancer in Korea.
Materials and Methods
1. Study populations and methods
In 2013, 23,254 new cases of lung cancer were registered nationwide at Korean Central Cancer Registry (KCCR). To investigate the detailed clinical characteristics, treatment information, and outcomes of Korean lung cancer patients, the Korean Association for Lung Cancer (KALC) and KCCR selected data using systematic sampling method for initial analysis. First, 12 national or regional cancer centers were selected according to the number of lung cancer patients. Subsequently, two additional hospitals were selected by the KCCR and five by the KALC. Each of the national or regional cancer centers and the two hospitals selected by the KCCR recruited 10–20 patients; the five hospitals selected by the KALC registered approximately 50 patients.
Data regarding age, sex, body mass index (BMI), smoking history, histopathologic type, symptoms, performance status (PS), Eastern Corporative Oncology Group score, clinical stage (according to the seventh edition of the TNM International Staging System), treatment modality, and survival status were collected according to a standardized protocol. Patients were followed up until October 2016. Survival data for all patients were obtained from medical records, telephone interviews, and/or the record of National Health Insurance of Korea. The study protocol was reviewed and approved by the Institutional Review Board at the National Cancer Center. Informed consent was waived due to the retrospective nature of the study.
2. Statistical analysis
Comparison of baseline characteristics between survivor and non-survivor was performed using a Mann–Whitney U test for continuous variables and a chi-square or Fisher exact test for categorical data. All p-values were two-tailed, with statistical significance set at p<0.05. Risk factors for mortality were analyzed using the Cox proportional hazards model, and Kaplan-Meier survival analysis was used to evaluate differences in survival rates. Statistical analyses were performed using SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
1. Patient characteristics
A total of 489 lung cancer patients were enrolled at the 19 sites, the baseline characteristics of which are summarized in Table 1. The median patient age was 69 years (interquartile range, 60–74 years), 65.4% of patients were male, and 62.1% of patients were ever-smokers. Of the male patients, 88.6% were former or current smokers, while only 11.5% of the female patients had a history of smoking. Cough (33.5%) was the most common symptom at the time of diagnosis, and 13.1% of patients were asymptomatic. Among patients with NSCLC, proportions of asymptomatic patients in each clinical stage were 29.2% (in stage I), 20.5% (in stage II), 7.0% (in stage III), and 6.3% (in stage IV), respectively. PS score data were available from 351 patients, and 14.2% of patients showed poor PS (PS ≥2). The proportion of NSCLC patients at each clinical stage was as follows: stage I, 25.6%; stage II, 9.5%; stage III, 22.9%; and stage IV, 42.0%. According to the sex, the clinical stages of NSCLC patients were I (19.0%), II (9.0%), III (28.0%), and IV (44.0%) in male patients and I (28.8%), II (9.6%), III (10.3%), and IV (51.3%) in female patients, respectively. In small cell lung cancer (SCLC) patients, 36.8% were at a limited stage of disease and 52.6% were at an extensive stage. Among patients with adenocarcinoma, epidermal growth factor receptor (EGFR) mutations were found in 13.4% of patients and 31.7% of patients performed EGFR mutation test, respectively. A total 76.5% of patients were treated by surgery, chemotherapy, or radiation therapy.
Table 1. Baseline characteristics of lung cancer patients.
| Characteristic | Total (n=489) | Survivor (n=212) | Non-survivor (n=277) | p-value |
|---|---|---|---|---|
| Age | 69 (60‒74) | 63 (56‒72) | 71 (64‒76) | <0.001 |
| Male sex | 320 (65.4) | 120 (56.6) | 200 (72.2) | <0.001 |
| BMI, kg/m2 | 22.6 (20.3‒24.7) | 23.4 (21.2‒25.3) | 22.1 (19.7‒24.0) | <0.001 |
| Ever smoker | 298 (62.1) | 108 (52.2) | 190 (69.6) | <0.001 |
| Symptoms | ||||
| Asymptomatic | 64 (13.1) | 48 (22.6) | 16 (5.8) | <0.001 |
| Cough | 164 (33.5) | 53 (25.0) | 111 (40.1) | <0.001 |
| Sputum | 114 (23.3) | 39 (18.4) | 75 (27.1) | 0.024 |
| Dyspnea | 99 (20.2) | 30 (14.2) | 69 (24.9) | 0.003 |
| Hoarseness | 4 (0.8) | 1 (0.5) | 3 (1.1) | 0.637 |
| Hemoptysis | 32 (6.5) | 15 (7.1) | 17 (6.1) | 0.678 |
| Weight loss | 33 (6.7) | 10 (4.7) | 23 (8.3) | 0.117 |
| Performance status | <0.001 | |||
| 0–1 | 301 (85.8) | 140 (94.0) | 161 (79.7) | |
| 2–4 | 50 (14.2) | 9 (6.0) | 41 (20.3) | |
| Clinical staging (NSCLC) | <0.001 | |||
| I | 94 (25.6) | 87 (52.1) | 7 (3.5) | |
| II | 35 (9.5) | 23 (13.8) | 12 (6.0) | |
| III | 84 (22.9) | 29 (17.4) | 55 (27.5) | |
| IV | 154 (42.0) | 28 (16.8) | 126 (63.0) | |
| Clinical staging (SCLC) | 0.001 | |||
| Limited | 21 (36.8) | 9 (90.0) | 12 (25.5) | |
| Extensive | 30 (52.6) | 1 (10.0) | 29 (61.7) | |
| Unknown | 6 (10.5) | 0 (0) | 6 (12.8) | |
| EGFR mutation status (in adenocarcinoma) | 0.697 | |||
| Positive | 32 (13.4) | 18 (12.5) | 14 (14.9) | |
| Negative | 69 (29.0) | 40 (27.8) | 29 (30.9) | |
| Not checked | 137 (57.6) | 86 (59.7) | 51 (54.3) | |
| Any treatment after diagnosis | 374 (76.5) | 189 (89.2) | 185 (66.8) | <0.001 |
Values are expressed are expressed as number (%) or median (interquartile range).
BMI: body mass index; NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer; EGFR: epidermal growth factor receptor.
During follow-up, there were 277 patient deaths (56.6%). The non-survival group was characterized by older age, a higher proportion of males, lower median BMI, and a higher proportion of ever-smokers when compared with the surviving group. In addition, the non-survival group had fewer asymptomatic patients, lower PS, and a higher incidence of metastatic disease than patients who survived the follow-up period. The surviving group had a higher proportion of patients who received treatment, particularly surgery.
2. Histological classification
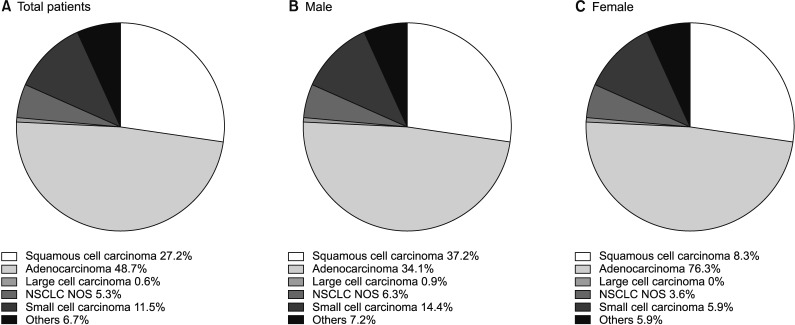
In the overall population, the most frequent histological subtype was adenocarcinoma (48.7%), followed by squamous cell carcinoma (27.2%) and SCLC (11.5%). While squamous cell carcinoma (37.2%) was the leading subtype in male patients, adenocarcinoma showed the highest incidence in female patients, accounting for 76.3% of lung cancers (Figure 1).
Figure 1. Proportion of lung cancer histological type based on Korean Nation-wide Lung Cancer Registry. (A) In total patients. (B) In male gender. (C) In female gender. NSCLC NOS: non-small cell lung cancer not otherwise specified.
3. Initial treatment
In patients with NSCLC, 47.2% of patients underwent surgery, followed by chemotherapy (27.9%) and radiation therapy (23.7%), after initial diagnosis. By contrast, chemotherapy (69.8%) was the most common treatment modality in patients with SCLC and only four patients received surgery after the initial SCLC diagnosis (Table 2).
Table 2. Initial treatment of patients with lung cancer.
| NSCLC (n=316) | SCLC (n=43) | p-value | |
|---|---|---|---|
| Treatment modality | <0.001 | ||
| Operation | 149 (47.2) | 4 (9.3) | |
| Radiation therapy | 75 (23.7) | 9 (20.9) | |
| Chemotherapy | 88 (27.9) | 30 (69.8) | |
| CCRT | 4 (1.3) | 0 (0) |
Values are expressed are expressed as number (%)
NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer; CCRT: concurrent chemoradiation therapy.
4. Risk factors for mortality
The median follow-up period was 504 days (interquartile range, 182–1,038 days). On univariate Cox analysis, age, male sex, lower BMI, smoking history, absence of symptoms, weight loss, poor PS, SCLC, metastatic disease, and no treatment were all significant predictors of mortality in patients with lung cancer. In multivariate Cox analysis, age, lower BMI, smoking history, absence of symptoms, SCLC, metastatic disease, and no treatment were seen to be meaningful predictors of mortality in total subjects (Table 3).
Table 3. Risk factor for mortality in patients with lung cancer assessed by Cox proportional hazards model.
| Parameter | HR | 95% CI | p-value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.048 | 1.035–1.061 | <0.001 |
| Male sex | 1.751 | 1.345–2.280 | <0.001 |
| Under weight patient (BMI<18.5) | 2.009 | 1.384–2.915 | <0.001 |
| BMI, kg/m2 | 0.906 | 0.869–0.945 | <0.001 |
| Ever smoker | 1.714 | 1.323–2.220 | <0.001 |
| Asymptomatic patient | 0.265 | 0.160–0.439 | <0.001 |
| Weight loss | 1.577 | 1.029–2.417 | 0.037 |
| Performance status 2–4 | 3.135 | 2.206–4.456 | <0.001 |
| Small cell lung cancer | 2.488 | 1.807–3.425 | <0.001 |
| Distant metastasis | 4.142 | 3.179–5.397 | <0.001 |
| Any treatment | 0.242 | 0.187–0.314 | <0.001 |
| Multivariate analysis | |||
| Age | 1.037 | 1.020–1.055 | <0.001 |
| BMI, kg/m2 | 0.904 | 0.864–0.945 | <0.001 |
| Ever smoker | 2.003 | 1.394–2.876 | <0.001 |
| Asymptomatic patient | 0.387 | 0.195–0.765 | 0.006 |
| Small cell lung cancer | 1.627 | 1.077–2.458 | 0.021 |
| Distant metastasis | 3.990 | 2.812–5.661 | <0.001 |
| Any treatment | 0.364 | 0.253–0.522 | <0.001 |
HR: hazard ratio; CI: confidence interval; BMI: body mass index.
While treatment, age, and weight loss were significant prognostic factor in stage I–II NSCLC patients, meaningful prognostic factor in stage III–IV NSCLC patients were treatment status, age, sex, BMI, and PS. In addition, although proportion of asymptomatic patients in stage III–IV NSCLC was relatively low, absence of symptoms was significant prognostic factor in these patients (Supplementary Table S1).
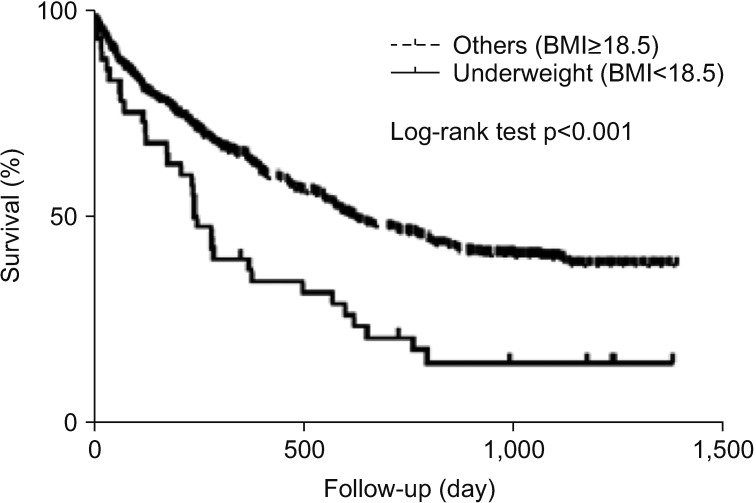
When underweight patients (BMI <18.5) were evaluated in total patients, Kaplan-Meier survival analysis showed that the survival period of this sub-population was significantly shorter than that of the remaining patients (p<0.001) (Figure 2).
Figure 2. Kaplan-Meier survival curves of the lung cancer patients with under-weight (BMI <18.5 kg/m2) and others (BMI ≥18.5 kg/m2).
Discussion
This study evaluated the characteristics and recent trends among lung cancer patients in Korea, showing that lung cancer occurs predominantly in elderly patients (median age, 69 years). In addition, histological and clinical characteristics differed from those in the two previous nationwide surveys. Ever-smokers, the elderly, those with a low BMI, and patients with advanced lung cancer had a poor clinical outcome, while asymptomatic patients and those who received anti-tumor treatment had a favorable prognosis.
According to the KCCR, 23,254 new lung cancer cases were diagnosed in Korea in 2013 (men, 69.8%; women, 30.2%)8. The crude incidence rate was 45.8 per 100,000, and the age-standardized lung cancer incidence rate (age-adjusted to the world standard population) was 27.4 per 100,000. In addition, lung cancer (crude mortality rate, 49.5 per 100,000) was the leading cause of death in both sexes. However, the 5-year relative survival rate for lung cancer has increased from 11.3% (1993–1995) to 23.5% (2009–2013).
The incidence of adenocarcinoma had been increasing in both men and women, and it is the most common type of lung cancer in Korea. This finding is in agreement with previous studies worldwide, and there are some possible hypotheses for this observation9,10. First, the development of low-tar and filtered cigarettes may result in smokers inhaling more deeply, which promotes peripheral tumors such as adenocarcinoma11. Also, changes in cigarette composition, such as an increase in the dose of potent tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), may have contributed to the rising incidence in adenocarcinoma12,13. However, in the present study, a substantial proportion of adenocarcinoma cases (58.5%) occurred in patients who had never smoked, and occupational risk, air pollution, and genetic susceptibility could, therefore, be risk factors in these patients14,15,16.
The median age of our study population was 69 years, and the age at diagnosis was higher than that observed in the two previous nationwide studies in Korea6,7. Although younger patients have a more favorable prognosis than older patients in general7,17, the incidence of lung cancer in the elderly is expected to continue to increase18. In addition, older age may in itself be an important treatment consideration due to physiologic changes associated with aging, a variety of comorbidities, and toxicities associated with treatment19. To clarify these considerations, further studies focusing on elderly patients will be required.
Smoking is a well-established cause of lung cancer20. According to the Korea National Health and Nutrition Examination Survey, the prevalence of current cigarette smoking in 2013 was 42.1% in male adults and 6.2% in females21. Although the smoking rate has been decreasing in men, no significant change was seen in women2,21. In agreement with previous studies, smoking was seen to be an independent prognostic factor in the present study, even after adjustment22,23,24. It would appear that vigorous antismoking policies, such as smoking cessation medication and motivational interviewing, are still required to reduce the rate of smoking among both sexes25,26.
Our data, when compared with those of previous studies, showed that the proportion of female lung cancer patients has been steadily increasing in Korea, and recent studies suggest that some genetic and hormonal factors may play a role27,28,29. In addition, the proportion of patients with stage I–II NSCLC and good performance (PS 0–1), and asymptomatic patients, was higher. These results may reflect improved lung cancer screening processes, including low-dose computed tomography (CT) in high risk patients30. An increase in the proportion of patients with stage IV NSCLC could be explained by the use of positron emission tomography with CT and brain magnetic resonance imaging, also possibly as a result of changing TNM stage31,32.
Over three-quarters of patients received treatment including surgery, radiation therapy, and chemotherapy. As almost half of the NSCLC patients received surgery, this may have resulted in the improved prognosis seen in NSCLC patients compared with SCLC patients, in the present study. Also, a number of molecularly targeted therapies and immune checkpoint inhibitors have become available for use in clinical practicerecently33,34, further studies are required to evaluate the efficacy of these new agents in Korean patients.
Interestingly, low BMI was associated with poor outcome, particularly in underweight patients. Although previous studies showed similar results35,36, the precise mechanism underlying this observation has not yet been clarified. These findings are, however, comparable to those of other chronic lung diseases, such as chronic obstructive lung disease and idiopathic pulmonary fibrosis37,38, and a possible explanation may be related to the effect of several cytokines or malnutrition39,40. The association between mortality and low BMI in lung cancer patients requires further evaluation in future studies.
There are several limitations to this study. First, our analysis was based on a sample of the full database and the study number was relatively small. Although patients were included from 19 centers, it may not be possible to generalize our results to the full population. Secondly, due to the retrospective design, some clinical information could not be extracted. Also, social factors and comorbid conditions were unavailable for most patients and this could have resulted in unknown bias. However, this population-based study will help to understand changes in the epidemiology of lung cancer in Korea over time and serve as reference for future studies.
In conclusion, the characteristics of lung cancer in Korea have changed over time, showing an increase in the proportion of adenocarcinoma, female patients, asymptomatic status, and early stage lung cancer. Our data also show that prognosis is poorer in ever-smokers, the elderly, and malnourished and untreated patients with advanced lung cancer. Future advancement in the management of lung cancer requires early detection and individualized therapy.
Acknowledgments
This study was supported by the Health Promotion Fund, Ministry of Health & Welfare, Republic of Korea (1660680).
The data used for this study were provided by the Korean Association for Lung Cancer & Ministry of Health and Welfare, Korean Central Cancer Registry.
Footnotes
- Conceptualization: Choi CM.
- Methodology: Choi CM.
- Formal analysis: Kim HC, Choi CM.
- Data curation: Jung CY, Cho DG, Jeon JH, Lee JE, Ahn JS, Kim SJ, Kim Y, Kim YC, Kim JE, Lee B, Won YJ, Choi CM.
- Writing - original draft preparation: Kim HC.
- Writing - review and editing: Won YJ, Choi CM.
- Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Risk factor for mortality in patients with NSCLC according to clinical stage assessed by Cox proportional hazards model
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Park EJ, Koh HK, Kwon JW, Suh MK, Kim H, Cho SI. Secular trends in adult male smoking from 1992 to 2006 in South Korea: age-specific changes with evolving tobacco-control policies. Public Health. 2009;123:657–664. doi: 10.1016/j.puhe.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Park JY, Jang SH. Epidemiology of lung cancer in Korea: recent trends. Tuberc Respir Dis. 2016;79:58–69. doi: 10.4046/trd.2016.79.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho J, Choi SM, Lee J, Lee CH, Lee SM, Kim DW, et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin J Cancer. 2017;36:20. doi: 10.1186/s40880-017-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin A, Oh CM, Kim BW, Woo H, Won YJ, Lee JS. Lung cancer epidemiology in Korea. Cancer Res Treat. 2017;49:616–626. doi: 10.4143/crt.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C, Kang KH, Koh Y, Chang J, Chung HS, Park SK, et al. Characteristics of lung cancer in Korea, 1997. Lung Cancer. 2000;30:15–22. doi: 10.1016/s0169-5002(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 7.In KH, Kwon YS, Oh IJ, Kim KS, Jung MH, Lee KH, et al. Lung cancer patients who are asymptomatic at diagnosis show favorable prognosis: a Korean Lung Cancer Registry Study. Lung Cancer. 2009;64:232–237. doi: 10.1016/j.lungcan.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48:436–450. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. doi: 10.1016/j.lungcan.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer. 2003;41:245–258. doi: 10.1016/s0169-5002(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 11.Gray N. The consequences of the unregulated cigarette. Tob Control. 2006;15:405–408. doi: 10.1136/tc.2006.017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control. 2008;17:198–204. doi: 10.1136/tc.2007.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 14.Gallus S, Negri E, Boffetta P, McLaughlin JK, Bosetti C, La Vecchia C. European studies on long-term exposure to ambient particulate matter and lung cancer. Eur J Cancer Prev. 2008;17:191–194. doi: 10.1097/CEJ.0b013e3282f0bfe5. [DOI] [PubMed] [Google Scholar]
- 15.Ahn YS, Kang SK. Asbestos-related occupational cancers compensated under the Industrial Accident Compensation Insurance in Korea. Ind Health. 2009;47:113–122. doi: 10.2486/indhealth.47.113. [DOI] [PubMed] [Google Scholar]
- 16.Seow WJ, Matsuo K, Hsiung CA, Shiraishi K, Song M, Kim HN, et al. Association between GWAS-identified lung adenocarcinoma susceptibility loci and EGFR mutations in never-smoking Asian women, and comparison with findings from Western populations. Hum Mol Genet. 2017;26:454–465. doi: 10.1093/hmg/ddw414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Choi CM, Kim SG. The younger patients have more better prognosis in limited disease small cell lung cancer. Tuberc Respir Dis. 2016;79:274–281. doi: 10.4046/trd.2016.79.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 19.Dawe DE, Ellis PM. The treatment of metastatic non-small cell lung cancer in the elderly: an evidence-based approach. Front Oncol. 2014;4:178. doi: 10.3389/fonc.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 21.Choi S, Kim Y, Park S, Lee J, Oh K. Trends in cigarette smoking among adolescents and adults in South Korea. Epidemiol Health. 2014;36:e2014023. doi: 10.4178/epih/e2014023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Lee J, Park YS, Lee CH, Lee SM, Yim JJ, et al. Impact of smoking on mortality of patients with non-small cell lung cancer. Thorac Cancer. 2014;5:43–49. doi: 10.1111/1759-7714.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferketich AK, Niland JC, Mamet R, Zornosa C, D'Amico TA, Ettinger DS, et al. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer. 2013;119:847–853. doi: 10.1002/cncr.27824. [DOI] [PubMed] [Google Scholar]
- 24.Sardari Nia P, Weyler J, Colpaert C, Vermeulen P, Van Marck E, Van Schil P. Prognostic value of smoking status in operated non-small cell lung cancer. Lung Cancer. 2005;47:351–359. doi: 10.1016/j.lungcan.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Taylor GMJ, Taylor AE, Thomas KH, Jones T, Martin RM, Munafo MR, et al. The effectiveness of varenicline versus nicotine replacement therapy on long-term smoking cessation in primary care: a prospective cohort study of electronic medical records. Int J Epidemiol. 2017;46:1948–1957. doi: 10.1093/ije/dyx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim G, Park I, Park S, Song S, Kim H, Kim S. Effectiveness of smoking cessation using motivational interviewing in patients consulting a pulmonologist. Tuberc Respir Dis. 2014;76:276–283. doi: 10.4046/trd.2014.76.6.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauk N, Kubik A, Zatloukal P, Krepela E. Lung cancer in women. Lung Cancer. 2005;48:1–9. doi: 10.1016/j.lungcan.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Shriver SP, Bourdeau HA, Gubish CT, Tirpak DL, Davis AL, Luketich JD, et al. Sex-specific expression of gastrin-releasing peptide receptor: relationship to smoking history and risk of lung cancer. J Natl Cancer Inst. 2000;92:24–33. doi: 10.1093/jnci/92.1.24. [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty S, Ganti AK, Marr A, Batra SK. Lung cancer in women: role of estrogens. Expert Rev Respir Med. 2010;4:509–518. doi: 10.1586/ers.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest. 2004;126:114–121. doi: 10.1378/chest.126.1.114. [DOI] [PubMed] [Google Scholar]
- 31.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–1209. [PubMed] [Google Scholar]
- 32.Postmus PE, Brambilla E, Chansky K, Crowley J, Goldstraw P, Patz EF, Jr, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol. 2007;2:686–693. doi: 10.1097/JTO.0b013e31811f4703. [DOI] [PubMed] [Google Scholar]
- 33.Roh MS. Molecular pathology of lung cancer: current status and future directions. Tuberc Respir Dis. 2014;77:49–54. doi: 10.4046/trd.2014.77.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon SH. Immunotherapy for non-small cell lung cancer. Tuberc Respir Dis. 2014;77:111–115. doi: 10.4046/trd.2014.77.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SW, Kim MY, Lee YP, Ryu YJ, Lee SJ, Lee JH, et al. Clinical features and prognostic factors in elderly koreans with advanced non-small-cell lung cancer in a tertiary referral hospital. Tuberc Respir Dis. 2013;75:52–58. doi: 10.4046/trd.2013.75.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsunaga T, Suzuki K, Imashimizu K, Banno T, Takamochi K, Oh S. Body mass index as a prognostic factor in resected lung cancer: obesity or underweight, which is the risk factor? Thorac Cardiovasc Surg. 2015;63:551–557. doi: 10.1055/s-0035-1554964. [DOI] [PubMed] [Google Scholar]
- 37.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 38.Alakhras M, Decker PA, Nadrous HF, Collazo-Clavell M, Ryu JH. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest. 2007;131:1448–1453. doi: 10.1378/chest.06-2784. [DOI] [PubMed] [Google Scholar]
- 39.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 40.Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. 2002;56(Suppl 3):S46–S49. doi: 10.1038/sj.ejcn.1601485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk factor for mortality in patients with NSCLC according to clinical stage assessed by Cox proportional hazards model