In this issue of British Journal of Anaesthesia, Barnes and colleagues1 present consensus definitions of standardised end points in perioperative medicine for infection and sepsis. The authors, an international group of experts and trialists in perioperative medicine, undertook a systematic review of the literature to identify reported outcome measures related to infection or sepsis in clinical trials in the perioperative period. Of 1857 articles retrieved, 601 were scrutinised, and a total of 255 articles underwent outcome measure extraction. The extracted outcome measures (including outcome measures not reported but suggested by authors) were rated as critical, important, or of limited importance,2 and prioritised by the authors according to relevance using the Delphi method.3 Outcomes adjudicated as critical were subsequently rated and prioritised by the Standardised Endpoints for Perioperative (StEP) trials working group4 according to their validity, reliability, feasibility, and patient-centredness. After discussion and consensus, a total of 13 outcome measures including accompanying definitions were proposed as standardised outcomes in clinical trials in perioperative medicine within infection and sepsis.1 The authors are to be congratulated for a very important initiative with implications for patients, relatives, healthcare systems, and society.3
Patient-important outcomes
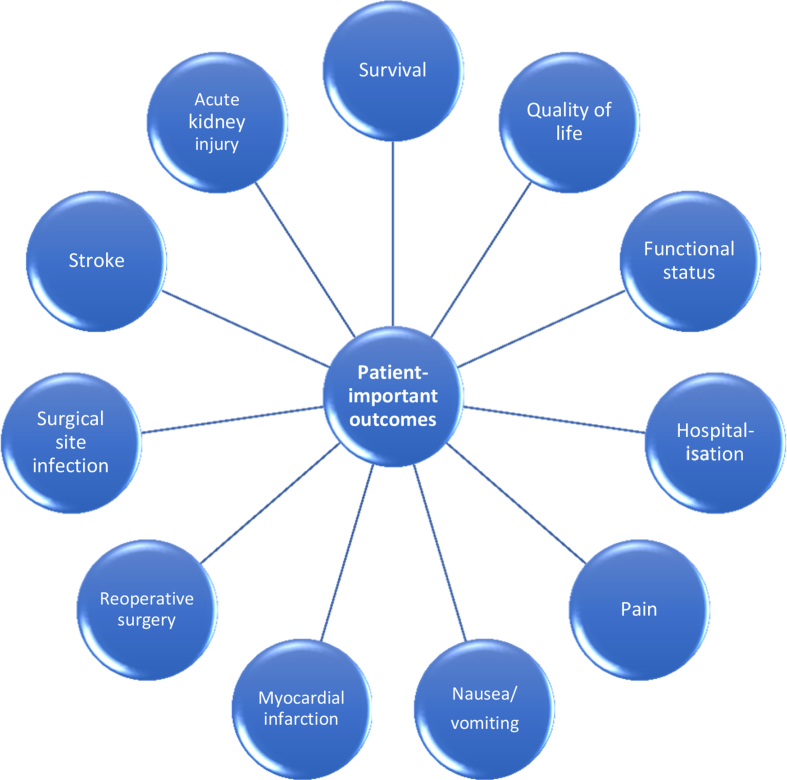
Evidence for the effectiveness of healthcare treatments should derive from high-quality randomised clinical trials or systematic reviews of trials that assess outcome measures relevant to patients5 (i.e. patient-important outcomes), including pain, nausea/vomiting, quality of life, postoperative complications, and survival6, 7, 8, 9 (Fig. 1). This is, however, rarely the case. Non-patient-centred outcomes (surrogate outcomes) are often used in clinical trials as substitutes for patient-important outcomes, including biomarkers, progression-free survival, vital signs, and radiological or histopathological examinations.10 In a recent systematic review, patient-important outcomes in 112 published randomised clinical trials in critically ill patients were reviewed.11 Only 24% of the primary outcomes and 22% of the secondary outcomes reported were adjudicated as patient-important. Mortality accounted for the vast majority of both primary and secondary patient-important outcomes, highlighting the lack of other patient-important outcomes than mortality (Fig. 1). Accordingly, patient-important outcomes should be prioritised over surrogate outcomes by trial groups, including within critical care and perioperative medicine.
Fig 1.
Examples of patient-important outcomes that apply to surgical patients.
The reasons for using surrogate outcomes and not patient-important outcomes are multiple. In general, surrogate outcomes are easier to collect and assess, which will result in shorter trial duration, smaller size, and lower costs.12 Importantly, meta-epidemiological data suggest that surrogate outcomes are associated with 40–50% larger treatment effects than trials reporting patient-important outcomes.13 This has the imminent risk that ineffective and, in worst cases, harmful treatments are used and recommended in daily clinical practice. This has been the case for several drugs,14 including hydroxyethyl starch.15
Core outcome measures and sets
In 2010 the Core Outcome Measures in Effectiveness Trials (COMET) initiative was established to develop a standardised collection of outcomes which should be measured and reported, as a minimum, in all trials within a specific clinical area.16 The overall aim of a core outcome set is to contribute to improvements in health and social care by helping patients and the public, practitioners, and policy makers to make informed decisions about the available healthcare treatments. This is done through standardisation and harmonisation of outcome reporting in trials by identifying outcomes perceived fundamental for decision making within a specific area.16 Clinical trial groups are now increasingly developing core outcome sets within their specific clinical areas, for example within appendicitis,17 acute respiratory distress syndrome,18 cardiac arrest,19 and now within perioperative medicine4 including hip fracture,20 renal end points,21 cancer outcomes,22 pulmonary complications,23 and patient comfort.24 Patient and public involvement is an integrated and prerequisite part in the development of core outcome sets, as this allows for nuanced assessments with a focus on patient preferences and views.16 The Standardised Endpoints for Perioperative Medicine working group4 are encouraged to consider patient and public involvement in future initiatives.
In conclusion, the initiative of the StEP trials working group4 is very welcome and much needed. Along with other similar initiatives including the COMET initiative,16 this should serve as an inspiration to other trial groups within specific clinical areas. Increased attention to patient-important outcomes and core outcome sets are much needed.
Declaration of interest
The author declares that they have no conflict of interest.
References
- 1.Barnes J., Hunter J., Harris S. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: infection and sepsis. Br J Anaesth. 2019;122:500–508. doi: 10.1016/j.bja.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt G.H., Oxman A.D., Kunz R. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Sinha I.P., Smyth R.L., Williamson P.R. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8:e1000393. doi: 10.1371/journal.pmed.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myles P.S., Grocott M.P., Boney O., Moonesinghe S.R., COMPAC-StEP Group Standardizing end points in perioperative trials: towards a core and extended outcome set. Br J Anaesth. 2016;116:586–589. doi: 10.1093/bja/aew066. [DOI] [PubMed] [Google Scholar]
- 5.Moher D., Schulz K.F., Altman D.G. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 6.Bourdaud N., Francois C., Jacqmarcq O. Addition of droperidol to prophylactic ondansetron and dexamethasone in children at high risk for postoperative vomiting. A randomized, controlled, double-blind study. Br J Anaesth. 2017;118:918–923. doi: 10.1093/bja/aex099. [DOI] [PubMed] [Google Scholar]
- 7.Ali M., Winter D.C., Hanly A.M., O'Hagan C., Keaveny J., Broe P. Prospective, randomized, controlled trial of thoracic epidural or patient-controlled opiate analgesia on perioperative quality of life. Br J Anaesth. 2010;104:292–297. doi: 10.1093/bja/aeq006. [DOI] [PubMed] [Google Scholar]
- 8.Dewinter G., Moens P., Fieuws S., Vanaudenaerde B., Van de Velde M., Rex S. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2017;118:576–585. doi: 10.1093/bja/aex038. [DOI] [PubMed] [Google Scholar]
- 9.Calvo-Vecino J.M., Ripolles-Melchor J., Mythen M.G. Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial) Br J Anaesth. 2018;120:734–744. doi: 10.1016/j.bja.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Svensson S., Menkes D.B., Lexchin J. Surrogate outcomes in clinical trials: a cautionary tale. JAMA Intern Med. 2013;173:611–612. doi: 10.1001/jamainternmed.2013.3037. [DOI] [PubMed] [Google Scholar]
- 11.Gaudry S., Messika J., Ricard J.D. Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review. Ann Intensive Care. 2017;7:28. doi: 10.1186/s13613-017-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming T.R., DeMets D.L. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 13.Ciani O., Buyse M., Garside R. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta-epidemiological study. BMJ. 2013;346:f457. doi: 10.1136/bmj.f457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moynihan R. Surrogates under scrutiny: fallible correlations, fatal consequences. BMJ. 2011;343:d5160. doi: 10.1136/bmj.d5160. [DOI] [PubMed] [Google Scholar]
- 15.Laake J.H., Tonnessen T.I., Chew M.S. The SSAI fully supports the suspension of hydroxyethyl-starch solutions commissioned by the European Medicines Agency. Acta Anaesthesiol Scand. 2018;62:874–875. doi: 10.1111/aas.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gargon E., Williamson P.R., Altman D.G., Blazeby J.M., Clarke M. The COMET initiative database: progress and activities update (2014) Trials. 2015;16:515. doi: 10.1186/s13063-015-1038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherratt F.C., Eaton S., Walker E. Development of a core outcome set to determine the overall treatment success of acute uncomplicated appendicitis in children: a study protocol. BMJ Paediatr Open. 2017;1:e000151. doi: 10.1136/bmjpo-2017-000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Needham D.M., Sepulveda K.A., Dinglas V.D. Core outcome measures for clinical research in acute respiratory failure survivors. An International Modified Delphi Consensus Study. Am J Respir Crit Care Med. 2017;196:1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haywood K., Whitehead L., Nadkarni V.M. COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the International Liaison Committee on Resuscitation. Resuscitation. 2018;127:147–163. doi: 10.1016/j.resuscitation.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell C.M., Black N., McCourt K.C. Development of a Core Outcome Set for studies evaluating the effects of anaesthesia on perioperative morbidity and mortality following hip fracture surgery. Br J Anaesth. 2019;122:120–130. doi: 10.1016/j.bja.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 21.McIlroy D.R., Bellomo R., Billings F.T., 4th Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: renal endpoints. Br J Anaesth. 2018;121:1013–1024. doi: 10.1016/j.bja.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Buggy D.J., Freeman J., Johnson M.Z., StEP-COMPAC Group Systematic review and consensus definitions for standardised endpoints in perioperative medicine: postoperative cancer outcomes. Br J Anaesth. 2018;121:38–44. doi: 10.1016/j.bja.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Abbott T.E.F., Fowler A.J., Pelosi P., StEP-COMPAC Group A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120:1066–1079. doi: 10.1016/j.bja.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Myles P.S., Boney O., Botti M. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br J Anaesth. 2018;120:705–711. doi: 10.1016/j.bja.2017.12.037. [DOI] [PubMed] [Google Scholar]