Abstract
ARDS is an acute inflammatory pulmonary process triggered by severe pulmonary and systemic insults to the alveolar-capillary membrane. This causes increased vascular permeability and the development of interstitial and alveolar protein-rich edema, leading to acute hypoxemic respiratory failure. Supportive treatment includes the use of lung-protective ventilatory strategies that decrease the work of breathing, can improve oxygenation, and minimize ventilator-induced lung injury. Despite substantial advances in supportive measures, there are no specific pharmacologic treatments for ARDS, and the overall hospital mortality rate remains about 40% in most series. The pathophysiology of ARDS involves interactions among multiple mechanisms, including immune cell infiltration, cytokine storm, alveolar-capillary barrier disruption, cell apoptosis, and the development of fibrosis. Here we review some new developments in the molecular basis of lung injury, with a focus on possible novel pharmacologic interventions aimed at improving the outcomes of patients with ARDS. Our focus is on platelet-endothelial cell adhesion molecule-1, which contributes to the maintenance and restoration of vascular integrity following barrier disruption. We also highlight the wingless-related integration site signaling pathway, which appears to be a central mechanism for lung healing as well as for fibrotic development.
Key Words: endothelial injury, mechanical ventilation, platelet-endothelial cell adhesion molecule, pulmonary fibrosis, ventilator-induced lung injury, wingless-related integration site
Abbreviations: AEC, alveolar epithelial cell; ECM, extracellular matrix; MMP, matrix metallopeptidase; MMP7, matrix metallopeptidase-7 (or matrilysin); PECAM1, platelet-endothelial cell adhesion molecule-1; VEGF, vascular endothelial growth factor; VILI, ventilator-induced lung injury; Wnt, wingless-related integration site; Wnt-5A, wingless-related integration family member 5A
ARDS is a very severe form of acute hypoxemic respiratory failure associated with an intense inflammatory pulmonary edema. Clinically, it is characterized by bilateral pulmonary infiltrates on chest imaging and severe hypoxemia (as assessed by Pao2/Fio2 ratio) that is refractory to oxygen therapy.1 The most common disease processes causing ARDS are sepsis and pneumonia. Despite advances in the general treatment and ventilatory support of patients with ARDS, there are no specific pharmacologic therapies to treat ARDS and, according to recent observational studies, the overall hospital mortality rate remains about 40%.2 Most patients with ARDS die as a result of multisystem organ failure and not due to lung failure (hypoxemia) per se.3
The pathophysiology of ARDS is multifactorial and includes inflammation, barrier disruption, interstitial and airspace edema, cell injury, and cell death.4 Diffuse alveolar damage is the pathologic hallmark of ARDS. As ARDS progresses through the exudative and proliferative phases, lung injury can increase, potentially leading to a fibrotic phase and worsening lung function. Lung-protective mechanical ventilation along with other adjunctive approaches (eg, prone position, judicious use of neuromuscular blocking agents) has been shown to decrease mortality.4 Over the past two decades there has been intense—but to date, unsuccessful—research to develop pharmacologic therapies to treat ARDS.
In this short review, we discuss some new mechanistic insights at the molecular level that may have clinical implications for the development of novel therapies to modulate ventilator-associated damage and improve outcomes of patients with ARDS. We have chosen to focus on platelet-endothelial cell adhesion molecule-1 (PECAM1) since it is essential for maintaining endothelial cell junctions, and on wingless-related integration site (Wnt) signaling since it appears to be key to tissue remodeling and wound closure. We postulate that pharmacologic manipulation of these two molecules has great potential in maintaining the integrity, or repairing the alveolar-capillary membrane. Specifically, we will discuss the role of PECAM1 in the modulation of alveolar-capillary permeability and the production of inflammatory mediators; and the effects of Wnt signaling in the modification of cell death, fibrosis, and lung repair and regeneration.
Targeting the Integrity of Lung Endothelium in ARDS
In patients with ARDS there is injury to the alveolar-capillary membrane,5, 6, 7 leading to increased permeability and pulmonary edema.8, 9 Movement of plasma across the lung vascular bed results in the accumulation of protein-rich edema fluid in the pulmonary interstitium and the airspaces, even at normal pulmonary vascular pressure and without physical rupture of these structures.
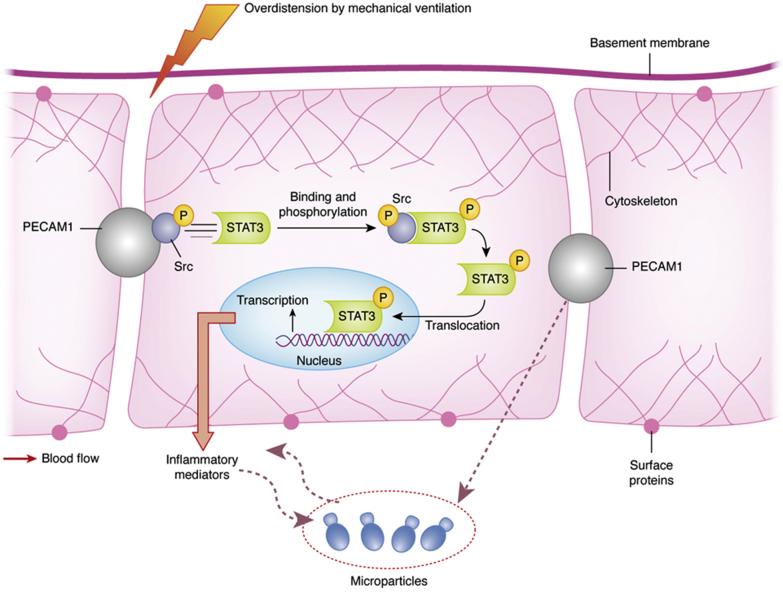
The search for biomarkers of alveolar-capillary barrier damage has been a challenging journey.7, 10 The alveolar-capillary barrier contains proteins that form tight and adherent junctions. CD31, also termed platelet-endothelial cell adhesion molecule-1 (PECAM1), is a 130-kDa transmembrane protein that is a member of the immunoglobulin superfamily of adhesion molecules constitutively expressed and localized at endothelial cell-cell junctions.11 Synthesis of PECAM1 is tightly regulated within the vasculature. There is increasing evidence that PECAM1 contributes to the maintenance of vascular integrity in resting cells, as well as playing a role in restoration of vascular integrity following barrier disruption.12 Blood flowing through the pulmonary vasculature during ventilatory-induced repetitive cyclic opening and closing of alveoli inflicts shear stress on pulmonary capillary endothelial cells (Fig 1). The force generated is transmitted through cytoskeletal elements to sites of cell-cell adherence, where PECAM1 responds by activating downstream signaling pathways, including Src family kinase members, STAT-3 (signal transducer and activator of transcription-3), and integrins on the basal membrane of the cells.13, 14 Extreme mechanical stress causes progressive changes in the shape and leads to loss of functional PECAM1.15
Figure 1.
Proposed platelet-endothelial cell adhesion molecule-1 (PECAM1) and Src signaling pathway activation in ARDS and in ventilator-induced lung injury. The combined effects of inflammatory mediators and repetitive opening and closing of alveoli during mechanical ventilation inflict shear stress on the endothelial cells of the pulmonary capillary blood vessels. The resultant mechanical force is transmitted through cytoskeletal elements to sites of cell-to-cell adhesion, where a transmembrane protein, known as PECAM1, responds by activating downstream signaling pathways, including those involving Src and signal transducer and activator of transcription-3 (STAT-3). The STAT-3 transcription factor may play a role in initiating the inflammatory response seen in ventilator-induced lung injury.
PECAM1 can be cleaved from endothelial cells by a number of mechanisms including shear stress,16 resulting in a secreted, shed form of protein (sPECAM1) that is soluble and can be released into the circulation, exerting proinflammatory effects.17, 18, 19 Inflammatory cells that have been activated can undergo shedding of the PECAM1 extracellular domain, which leads to loss of cell-cell adherence17 and contributes to the increase in circulating sPECAM1.18 Thus, circulating sPECAM1 levels may be measured as a biomarker to identify and monitor the development of ARDS and ventilator-induced lung injury (VILI), and persistently high sPECAM1 levels may indicate ongoing injury. Using a clinically relevant experimental model of ARDS and VILI in animals with or without sepsis, Villar et al20 showed that expression of PECAM1 in the lungs is modulated by mechanical ventilation. Their data strongly suggested that loss of PECAM1 contributes to increased barrier permeability with subsequent edema formation, leukocyte infiltration, and severe hypoxemia. Deficiency in the murine endothelial PECAM1 gene impedes repair of vascular integrity21 and prolongs bleeding times.22 Heterogeneous expression of PECAM1 has been reported in the pulmonary vessels of patients who died of ARDS23 and in the lungs of children who received prolonged mechanical ventilation for bronchopulmonary dysplasia.24
There is experimental evidence implicating mechanical ventilation in pulmonary and distal organ endothelial activation and inflammation.25 Recovery from acute lung injury requires restoration of an intact endothelial barrier to ensure appropriate removal of pulmonary edema fluid. Since PECAM1 is essential for reestablishing endothelial integrity, it is an interesting target for the treatment of ARDS, sepsis, and VILI. In a mouse model of septic shock, Maas et al26 transplanted bone marrow cells expressing PECAM1 into mice that were deficient in PECAM1. Transplanted animals were resistant to endotoxic shock, and the integrity of the vascular barrier was better maintained after endotoxin challenge. Thus, there might be great potential for innovative therapies using genetically modified mesenchymal stromal cells expressing PECAM1 to reassemble the endothelial cell junctions in the treatment of ARDS and VILI.27 We propose that upregulation of PECAM1 and/or reducing sPECAM1 through extracorporeal removal28 or pharmacologic inhibition29, 30 might be a novel therapeutic strategy in ARDS and VILI.
Attenuation of Lung Fibrosis to Enhance Lung Repair and Regeneration
As diffuse alveolar damage progresses in patients with ARDS, severe abnormal repair of alveolar epithelium and pulmonary capillary endothelium can develop into a fibrotic phase, resulting in poor lung function and death.5 The fibroproliferative process observed in patients with ARDS is characterized by migration of mesenchymal cells, and deposit of connective tissue in the alveolar airspace and vascular wall. Survival from ARDS depends on the alveolar epithelium being repaired, a process initiated by proliferation and migration of endogenous progenitor alveolar epithelial type II cells (AECII), with their subsequent differentiation into alveolar type I cells (AECI).31 Meanwhile, lung fibroblast proliferation and migration occur and are important for lung repair after injury.32, 33 However, repetitive or/and sustained injury to the alveolar epithelium can lead to loss of ventilated alveolar units and a vigorous fibroblastic response, as a result of uncontrolled deposition of extracellular matrix (ECM) and collagen, leading to destruction of lung parenchymal architecture.33, 34
Pulmonary fibrosis is a pathologic response in patients with ARDS, independent of the underlying inciting event that caused the initial lung injury. The tissue repair process includes coordinated cellular infiltration along with deposition of ECM and reepithelialization. In addition, the use of injurious ventilatory strategies plays a potential and additive role to further accelerate ECM remodeling,33, 35 contributing to lung fibrosis and loss of lung function during ARDS.36, 37, 38
Among other molecules and pathways, β-catenin-mediated Wnt signaling appears to be an important mechanism in the context of pulmonary repair and fibrosis.39, 40, 41 β-Catenin protein is a central component of the endothelial adherens junctional complex, plays an important role in regulating microvascular permeability by its direct linkage to vascular endothelial cadherin, and plays an essential role in the wingless/Wnt signaling pathway.42 Although the precise cellular and molecular mechanisms by which β-catenin regulates endothelial permeability in health and disease are not well understood, overexpression of β-catenin protects against endothelial dysfunction and increased microvascular permeability.
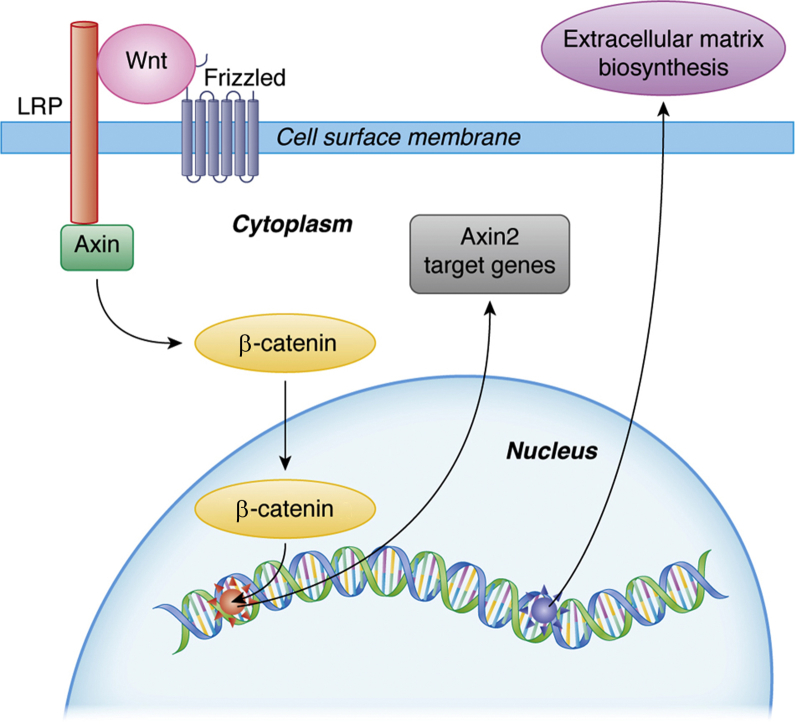
Wnt proteins attach to cell surface receptors of the Frizzled family,41 as well as to other transmembrane proteins (eg, lipoprotein receptor-related protein), which leads to cytosolic accumulation of β-catenin. This then leads to translocation to the nucleus, where it participates in gene transcription and subsequent regulation of various genes, including Axin2 and Lgr5 (Fig 2). Axin2, also known as axis inhibition protein-2 or conductin, is thought to play an important role in stabilizing β-catenin in the Wnt signaling pathway.43 The leucine-rich G protein-coupled receptor-5 (LGR5) belongs to a glycoprotein hormone receptor superfamily, and it modulates signaling through the Wnt pathway after binding to its cognate ligand R-spondin.44 Wnt signaling pathways, which are mediated by either paracrine or autocrine mechanisms, are regulated at multiple points.45 Wnt signaling stimulates tissue remodeling through matrix metallopeptidases (MMPs) as well as other gene products such as cyclin D1 and vascular endothelial growth factor (VEGF).40, 46, 47
Figure 2.
Proposed molecular Wnt signaling pathway in lung fibrosis during ARDS. Wnt signaling begins when a Wnt protein binds to a Frizzled family receptor. To facilitate Wnt signaling, coreceptors such as lipoprotein receptor-related protein (LRP) and others may be required. On activation of the receptor, Axin is removed from the receptor complex and activates β-catenin. β-Catenin moves into the nucleus, binds to a transcription factor on DNA, and activates transcription of target genes. Wnt = wingless-related integration site.
The Wnt signaling pathways comprise a family of highly evolutionarily conserved secreted glycoproteins, which trigger many signaling pathways that control a number of processes including cell proliferation, differentiation, and migration.40 Three Wnt signaling pathways have been characterized: the canonical Wnt pathway, the noncanonical planar cell polarity pathway, and the noncanonical Wnt/calcium pathway. The distinction of canonical and noncanonical Wnt signaling pathways is subject to debate, and a combined Wnt pathway has been suggested.48 Expression of the nuclear transcriptional factor β-catenin can be decreased in AECII when there is destruction of alveolar walls and/or abnormal repair processes associated with deposition of ECM, such as in patients with COPD.49 Reduced canonical Wnt/β-catenin signaling is linked to decreased lung repair as a result of a shift from canonical to noncanonical Wnt signaling.50
Wnt-5A is a macrophage-derived molecule that is highly specific for macrophage activation, and is thought to play a role in pulmonary disorders.51 Villar et al37, 52 investigated whether Wnt/β-catenin signaling is activated early in experimental animals exposed to VILI and acute lung injury, as well as in patients with ARDS. They assessed lung protein levels of Wnt-5A and target genes (eg, MMP7, cyclin D1, VEGF) implicated in profibrotic alterations of injured tissues.52 They used a clinically relevant experimental model of sepsis-induced ARDS, and found marked collagen deposition and increased Wnt-5A and MMP7 protein levels. Similar findings were observed in lung biopsies of patients who died early of sepsis-related ARDS. These results reinforced the concept that Wnt-5A and β-catenin pathway contribute to early repair of the lung.40, 53 This is in accord with increasing clinical evidence of fibrotic changes in patients in the early stages of ARDS.54 Investigators have recently demonstrated that inhibition of Wnt-5A in a murine model of COPD attenuated lung tissue damage, improved lung function, and reestablished expression of alveolar epithelial cell surface markers.55 Wnt-5A signaling is also involved in a process termed the epithelial-mesenchymal transition,51, 56 in which cells switch to a fibroblast-like phenotype from an epithelial phenotype.
Wnt signaling is a double-edged sword. Early activation of Wnt signaling during septic-induced ARDS or VILI may represent a signal from damaged epithelium to regenerate.37, 52, 56, 57 Thus, the Wnt signaling pathway represents a possible therapeutic target for patients with ARDS, since the development of pulmonary fibrosis is associated with poor clinical outcomes in these patients.36 MMPs, which are regulated by Wnt signaling, are required for proteolytic degradation of the ECM. Although it is not clear why resolution of ARDS is associated with fibrosis in some patients but not in others, the selective regulation of the Wnt/β-catenin signaling pathway can have antiinflammatory and antifibrotic effects.58
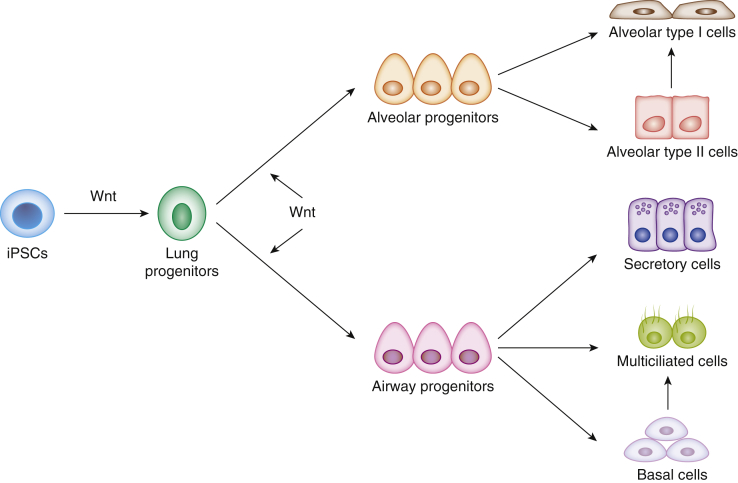
In mammalian organisms, multiple tissues, including the lung, are constantly undergoing regeneration, with older cells constantly dying, and new cells being regenerated. Normally, cellular proliferation in the lung is low compared with other organs. However, lung injury causes activation of stem/progenitor cell populations59 (Fig 3). The Wnt signaling pathway participates in stem cell activity and is involved in stem cell control, as a proliferative and self-renewal signal60, 61 giving shape to tissues, as cells are proliferating. Resident pluripotent stem cells differentiate into mesoderm and endoderm progenitor cells via Wnt signaling.62 Reprogramming to induce the generation of pluripotent stem cells represents a very interesting approach for repair. McCauley et al63 demonstrated that Wnt signaling is a potent regulator of proximal and distal epithelial patterning in both mouse and human induced pluripotent stem cells. These progenitor cells can differentiate into endothelial and vascular smooth muscle cells. Inhibition of the Wnt pathway markedly decreases tissue regeneration. Thus, Wnt signals can be exploited to enable propagation of stem cells as self-renewing lung epithelial and endothelial cells.
Figure 3.
Schematic depicting proposed pathway for the generation of proximal or distal epithelial lineages in the lung on activation of human induced pluripotent stem cell (iPSC)-derived airway epithelium via Wnt signaling.
Conclusions
In patients with ARDS there is increased alveolar-capillary permeability with protein-rich edema formation as a result of endothelial damage. PECAM1 is a transmembrane protein that connects adjacent endothelial cells and plays a role in the regulation of inflammation, leukocyte migration, and vascular responses during sepsis. We propose that upregulation of membrane PECAM1 may be a novel therapeutic strategy in ARDS and VILI.
Injury to alveolar epithelial cells activates pulmonary fibroblasts, inducing their transformation into matrix-producing myofibroblasts. The Wnt signaling pathways coordinate lung repair after injury, and hence the therapeutic manipulation of Wnt signaling in endogenous stem cells may be exploited for tissue renewal and regeneration during early ARDS.
Acknowledgments
Financial/nonfinancial disclosure: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: Funded in part by grants from the Instituto de Salud Carlos III, Spain [Grant PI016/00049] and the Canadian Institutes of Health Research, Canada [Grant FDN143285].
References
- 1.Villar J. What is the acute respiratory distress syndrome? Respir Care. 2011;56(10):1539–1545. doi: 10.4187/respcare.01395. [DOI] [PubMed] [Google Scholar]
- 2.Villar J., Blanco J., Kacmarek R.M. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care. 2016;22(1):1–6. doi: 10.1097/MCC.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 3.Villar J., Martínez D., Mosteiro F. Is overall mortality the right composite endpoint in clinical trials of acute respiratory distress syndrome? Crit Care Med. 2018;46(6):892–899. doi: 10.1097/CCM.0000000000003022. [DOI] [PubMed] [Google Scholar]
- 4.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 5.Tomashefski J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21(3):435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 6.Schuster D.P. What is acute lung injury? What is ARDS? Chest. 1995;107(6):1721–1726. doi: 10.1378/chest.107.6.1721. [DOI] [PubMed] [Google Scholar]
- 7.Villar J., Slutsky A.S. Golden anniversary of the acute respiratory distress syndrome: still much work to do! Curr Opin Crit Care. 2017;23(1):4–9. doi: 10.1097/MCC.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya J., Matthay M.A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 9.Villar J., Blanco J., Zhang H., Slutsky A.S. Ventilator-induced lung injury and sepsis: two sides of the coin? Minerva Anestesiol. 2011;77(6):647–653. [PubMed] [Google Scholar]
- 10.García-Laorden M.I., Lorente J.A., Flores C., Slutsky A.S., Villar J. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017;5(14):283. doi: 10.21037/atm.2017.06.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodfin A., Voisin M.B., Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27(12):2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 12.Privratsky J.R., Paddock C.M., Florey O. Relative contribution of PECAM1 adhesion and signaling to the maintenance of vascular integrity. J Cell Sci. 2011;124(Pt 9):1477–1485. doi: 10.1242/jcs.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzime E., Irani-Tehrani M., Kiosses W.B. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 14.Akst J. Full speed ahead. Scientist. 2009;23(December) https://www.the-scientist.com/?articles.view/articleNo/27816/title/Full-Speed-Ahead/ [Google Scholar]
- 15.Tarbell J.M. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87(2):320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naganuma Y., Satoh K., Yi Q., Asazuma N., Yatomi Y., Ozaki Y. Cleavage of platelet endothelial cell adhesion molecule-1 (PECAM-1) in platelets exposed to high shear stress. J Thromb Haemost. 2004;2(11):1998–2008. doi: 10.1111/j.1538-7836.2004.00954.x. [DOI] [PubMed] [Google Scholar]
- 17.Ilan N., Mohsenin A., Cheung L., Madri J.A. PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. FASEB J. 2001;15(2):362–372. doi: 10.1096/fj.00-0372com. [DOI] [PubMed] [Google Scholar]
- 18.Fornasa G., Groyer E., Clement M. TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. J Immunol. 2010;184(10):5485–5492. doi: 10.4049/jimmunol.0902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrera-Benitez N., Valladares F., García-Hernández Altered profile of circulating endothelial-derived microparticles in ventilator-induced lung injury. Crit Care Med. 2015;43(12):e551–e559. doi: 10.1097/CCM.0000000000001280. [DOI] [PubMed] [Google Scholar]
- 20.Villar J., Muros M., Cabrera-Benitez N. Soluble platelet-endothelial cell adhesion molecule-1, a biomarker of ventilator-induced lung injury. Crit Care. 2014;18(2):R41. doi: 10.1186/cc13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graesser D., Solowiej A., Bruckner M. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109(3):383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahooti S., Graeser D., Patil S. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157(1):75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller A.M., Cronen C., Müller K.M. Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J Pathol. 2002;198(2):270–275. doi: 10.1002/path.1186. [DOI] [PubMed] [Google Scholar]
- 24.Bhatt A.J., Pryhuber G.S., Huyck H. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1 and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164(10 Part 1):1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 25.Hegeman M.A., Hennus M.P., Heijnen C.J. Ventilator-induced endothelial activation and inflammation in the lung and distal organs. Crit Care. 2009;13(6):R182. doi: 10.1186/cc8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maas M., Stapleton M., Bergom C., Mattson D.L., Newman D.K., Newman P.J. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288(1):H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- 27.Wilson J.G., Liu K.D., Zhuo H. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelhafeez A.H., Jeziorczak P.M., Schaid T.R. Clinical CVVH model removes endothelium-derived microparticles from circulation. J Extracell Vesicles. 2014;3(1) doi: 10.3402/jev.v3.23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roseblade A., Luk F., Rawling T. Cell-derived microparticles: new targets in the therapeutic management of disease. J Pharm Pharm Sci. 2013;16(2):238–253. doi: 10.18433/j3989x. [DOI] [PubMed] [Google Scholar]
- 30.Bellingan G., Brealey D., Mancebo J. Comparison of the efficacy and safety of FP-1201-lyo (intravenously administered recombinant human interferon beta-1a) and placebo in the treatment of patients with moderate or severe acute respiratory distress syndrome: study protocol for a randomized controlled trial. Trials. 2017;18(1):536. doi: 10.1186/s13063-017-2234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimabukuro D.W., Sawa T., Gropper M.A. Injury and repair in lung and airways. Crit Care Med. 2003;31(8 suppl):S524–S531. doi: 10.1097/01.CCM.0000081437.06466.B3. [DOI] [PubMed] [Google Scholar]
- 32.Chesnut A.N., Matthay M.A., Tibayan F.A., Clark J.G. Early detection of type III procollagen peptide in acute lung injury: pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997;156(3 Pt 1):840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 33.Marshall R.P., Bellingan G., Webb S. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162(5):1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 34.Selman M., Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res. 2002;3(1):31. doi: 10.1186/rr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deheinzelin D., Jatene F.B., Saldiva P.H., Brentani R.R. Upregulation of collagen messenger RNA expression occurs immediately after lung damage. Chest. 1997;112(5):1184–1188. doi: 10.1378/chest.112.5.1184. [DOI] [PubMed] [Google Scholar]
- 36.Rocco P.R.M., Dos Santos C., Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva Anestesiol. 2009;75(12):730–740. [PubMed] [Google Scholar]
- 37.Villar J., Cabrera N.E., Valladares F. WNT/β-catenin signaling is modulated by mechanical ventilation in an experimental model of acute lung injury. Intensive Care Med. 2011;37(7):1201–1209. doi: 10.1007/s00134-011-2234-0. [DOI] [PubMed] [Google Scholar]
- 38.Cabrera-Benitez N.E., Laffey J.G., Parotto M. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121(1):189–198. doi: 10.1097/ALN.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Königshoff M., Kramer M., Balsara N. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crosby L.M., Waters C.M. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logan C., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 42.Sawant D.A., Tharakan B., Hunter F.A., Smythe W.R., Childs E.W. Role of β-catenin in regulating microvascular endothelial cell hyperpermeability. J Trauma. 2011;70(2):481–488. doi: 10.1097/TA.0b013e31820b3ed7. [DOI] [PubMed] [Google Scholar]
- 43.Song X., Wang S., Li L. New insights into the regulation of Axin function in canonical Wnt signaling pathway. Protein Cell. 2014;5(3):186–193. doi: 10.1007/s13238-014-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuijers J., Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31(12):2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malinauskas T., Jones E.Y. Extracellular modulators of Wnt signaling. Curr Opin Struct Biol. 2014;29:77–84. doi: 10.1016/j.sbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Morrisey E.E. Wnt signaling and pulmonary fibrosis. Am J Pathol. 2003;162(5):1393–1397. doi: 10.1016/S0002-9440(10)64271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson W.J., Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thrasivoulou C., Millar M., Ahmed A. Activation of intracellular calcium by multiple Wnt ligands and translocation of β-catenin into the nucleus: a convergent model of Wnt/Ca2+ and Wnt/β-catenin pathway. J Biol Chem. 2013;288(50):35651–35659. doi: 10.1074/jbc.M112.437913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Z., Lao T., Qiu W. A chronic obstructive pulmonary disease susceptibility gene, FAM13A, regulates protein stability of β-catenin. Am J Respir Crit Care Med. 2016;194(2):185–197. doi: 10.1164/rccm.201505-0999OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemeth M.J., Topol L., Anderson S.M., Yang Y., Bodine D.M. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci USA. 2007;104(39):15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pongracz J.E., Stockley R.A. Wnt signaling in lung development and diseases. Respir Res. 2006;7:15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villar J., Cabrera-Benitez N., Ramos-Nuez A. Early activation of pro-fibrotic WNT5A in sepsis-induced acute lung injury. Crit Care. 2014;18(5):568. doi: 10.1186/s13054-014-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Königshoff M., Balsara N., Pfaff E.M. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3(5):e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichikado K., Muranaka H., Gushima Y. Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: a prospective observational cohort study. BMJ Open. 2012;2(2):e000545. doi: 10.1136/bmjopen-2011-000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baarsma H.A., Skronka-Wasek W., Mutze K. Noncanonical WNT5a signaling impairs endogenous lung repair in COPD. J Exp Med. 2017;214(1):143–163. doi: 10.1084/jem.20160675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabrera-Benitez N.E., Parotto M., Post M. Mechanical stress induces lung fibrosis by epithelial-mesenchymal transition. Crit Care Med. 2012;40(2):510–517. doi: 10.1097/CCM.0b013e31822f09d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Königshoff M., Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol. 2010;42(1):21–31. doi: 10.1165/rcmb.2008-0485TR. [DOI] [PubMed] [Google Scholar]
- 58.Henderson W.R., Jr., Chi E.Y., Ye X. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107(32):14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y., Chen X., Yang X., Ei-Hashash A. Stem cells in lung repair and regeneration: current applications and future promise. J Cell Physiol. 2018;233(10):6414–6424. doi: 10.1002/jcp.26414. [DOI] [PubMed] [Google Scholar]
- 60.Lee J.H., Rawlins E.L. Developmental mechanisms and adult stem cells for therapeutic lung regeneration. Dev Biol. 2018;433(2):166–176. doi: 10.1016/j.ydbio.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 61.Clevers H., Loh K.M., Nusse R. Stem cell signaling: an integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 62.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18(5):523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 63.McCauley K.B., Hawkins F., Serra M. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell. 2017;20(6):844–857. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]