Abstract
Background
The factors determining peak susceptibility of the developing brain to anaesthetics are unclear. It is unknown why postnatal day 7 (P7) male rats are more vulnerable to anaesthesia-induced memory deficits than littermate females. Given the precocious development of certain regions in the female brain during the neonatal critical period, we hypothesised that females are susceptible to anaesthetic brain injury at an earlier time point than previously tested.
Methods
Female rats were exposed to isoflurane (Iso) 1 minimum alveolar concentration or sham anaesthesia at P4 or P7. Starting at P35, rats underwent a series of behavioural tasks to test their spatial and recognition memory. Cell death immediately after anaesthesia was quantified by Fluoro-Jade C staining in select brain regions, and developmental expression of the chloride transporters KCC2 and NKCC1 was analysed by immunoblotting in male and female rats at P4 and P7.
Results
Female rats exposed to Iso at P4 displayed impaired spatial, object-place, -context, and social recognition memory, and increased cell death in the hippocampus and laterodorsal thalamus. Female rats exposed at P7 exhibited only decreased performance in object-context compared with control. The ratio of NKCC1/KCC2 expression in cerebral cortex was higher in P4 females than in P7 females, and similar to that in P7 males.
Conclusions
Female rats exposed to Iso at P4 are sensitive to anaesthetic injury historically observed in P7 males. This is consistent with a comparably immature developmental state in P4 females and P7 males. The window of anaesthetic vulnerability correlates with sex-specific cortical expression of chloride transporters NKCC1 and KCC2. These findings suggest that both sex and developmental age play important roles in determining the outcome after early anaesthesia exposure.
Keywords: anaesthetic neurotoxicity, general anaesthesia, memory, neurodevelopment, sex factors
Editor's key points.
-
•
The mechanisms for the greater vulnerability to anaesthesia-induced memory deficits in neonatal male rats than littermate females were examined.
-
•
Female rats exposed to isoflurane at postnatal day 4 were more sensitive to anaesthetic neurotoxicity and memory deficits compared with data reported for postnatal day 7 females, but comparable to data for postnatal day 7 males.
-
•
This age- and sex-dependent sensitivity was associated with greater expression of NKCC1 relative to KCC2 chloride transporters, which is associated with gamma-aminobutyric acid (GABA)ergic excitotoxicity.
-
•
Both sex and developmental age play important roles in determining the outcome after early anaesthesia exposure, and must be considered in designing future studies.
Exposure to general anaesthetics during early postnatal development is associated with lasting cognitive deficits in rodent and non-human primate models, with evidence for a similar outcome in humans.1, 2 Despite the potentially harmful effect of anaesthetics, their use is unavoidable in young children who require surgery. As such, it is important to define the critical period during development in which the brain may be most susceptible to anaesthesia in order to determine who is at risk and to guide scheduling of anaesthesia for paediatric patients when possible. Early brain development is influenced by sex-specific factors; exposure at postnatal Day 7 (P7) to the volatile anaesthetic isoflurane (Iso) leads to decreased recognition memory in male but not female rats.3 This underscores differential timing or mechanism of anaesthesia susceptibility between the sexes.
The brain developmental timeline differs slightly by region between males and females. Expression of some proteins that are critical for neurogenesis, synaptogenesis, and neuronal excitation and inhibition change on a day-to-day basis during early rodent brain development. Some of these developmental processes occur earlier in certain regions of the female brain at P7 compared with littermate males, which could be a protective factor from the detrimental effect of anaesthesia observed in males.4, 5, 6 Thus, the effects of anaesthesia exposure in females at an earlier age, when the brain may be more developmentally similar to males at a later stage and more vulnerable to insult, is an important area of investigation.
P7 is often used as a standard age at which the immature rodent brain is particularly sensitive to anaesthetics. The majority of studies establishing this were performed using male or mixed-sex groups (without differentiation between sexes) and with large differences between ages at exposure.7 This has led to a gap in our understanding of how anaesthesia exposure interacts with neurodevelopmental changes that occur in a sex-dependent manner. Much of the development of the rodent brain occurs in the first 2 postnatal weeks, which roughly translates to the late third trimester and first few years of human life.8, 9 The developmental expression of chloride transporters KCC2 and NKCC1 has instrumental roles in normal brain development, and is vital to the regulation of gamma-aminobutyric acid (GABA) function, which is regulated differently between males and females during early development.4, 5, 10, 11 Given the precocious development of certain regions in the female brain during the critical neonatal period, we hypothesised that females are susceptible to anaesthetic-induced brain injury and behavioural deficits at an earlier time than previously tested.
Methods
Animals
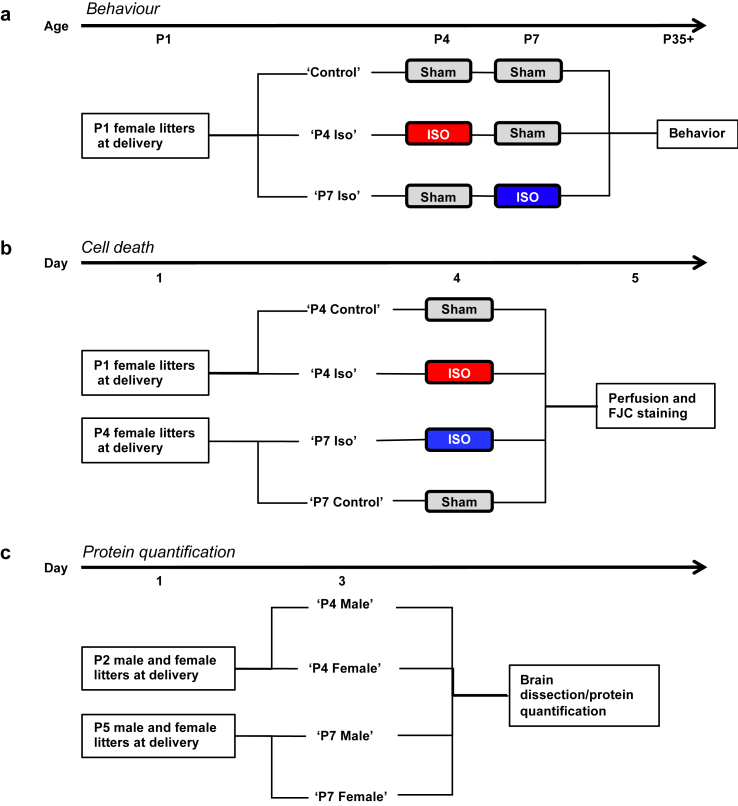
All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee at the University of California, San Francisco, CA, USA. Sprague Dawley dams purchased from Charles River Laboratories (South San Francisco CA) delivered with P1 or P4 female-only litters were used for all behaviour and cell death experiments. Male and female litters were used for RNA and protein expression experiments. Litters were housed with a reverse 12 h light–dark cycle. Standard chow and water were provided ad libitum. Maternal effects were minimised by cross fostering through obtaining same-sex litters that contain pups from multiple dams, and by randomly selecting pups from each dam to be included in experimental groups. After weaning at P21 for the behaviour cohort, animals were housed in groups of three rats per cage and subjected to a series of behavioural tasks. Experimental timelines for each cohort are depicted in Figure 1.
Fig 1.
Experimental schemas. (a) Female littermates were exposed to isoflurane (Iso) or sham conditions at postnatal Day 4 (P4) and P7 (control, n=12; P4 Iso, n=12; P7 Iso, n=14). All groups underwent behavioural testing starting at P35. (b) Females were exposed to Iso at P4 (n=5) or P7 (n=5) or sham (n=4 for both P4 and P7 control groups) and perfused 18–24 h later. Brain tissue was stained with Fluoro-Jade C (FJC) to detect neurodegeneration. (c) Fresh brain tissue was dissected from females and males at P4 and P7 (n=5 per group) for protein quantification.
Anaesthesia
Animals were removed from the dams and anaesthetised with Iso for 4 h as described.3 Briefly, Iso was delivered into an anaesthetic chamber through a humidified gas line with 1:1 oxygen and air (Supplementary Fig. S1). Pup temperatures and chamber gas concentrations were continuously monitored and recorded. Control pups were separated from the dams and placed in a temperature-controlled sham anaesthesia chamber for 30 min. This was less than the total anaesthetic duration to minimise maternal separation stress in the awake animals. In the behaviour cohort, there was 14% mortality (two/14) in the P4 Iso group and no deaths (zero/14) in the P7 Iso group. In the histology cohort, there was a 17% mortality rate (one/six) in both groups.
Barnes maze
The Barnes maze tests spatial learning and memory.12 The maze consisted of an elevated black circular platform with 20 evenly spaced holes, one of which led to an escape box hidden under the platform. The maze was surrounded by a black curtain and visual cues. Training and probe test trials were tracked with EthoVision XT 11.5 (Noldus; Leesburg VA) using a GigE camera (Basler Inc; Exton PA) suspended above the platform. Animals were placed into the escape box for a 2-min habituation period, followed immediately by the first training session. Animals underwent one training session per day for 4 days. Each training session ended when the rat entered the escape box or 4 min passed, whichever came first. The platform and escape box were cleaned with ethanol 70% between each testing session. Barnes maze was performed at P35-42.
Recognition tasks
Recognition tasks (novel object, object-place, object-context, and social recognition) were performed at P55-74 as described.3
Fluoro-Jade C staining
Acute neuronal death was assessed with Fluoro-Jade C 0.001% (Millipore, Billerica, MA, USA) staining, a fluorescent marker specific for degenerating neurons. Postnatal animals were anaesthetised and transcardially perfused with phosphate-buffered saline and paraformaldehyde 4% 12–18 h after anaesthesia exposure at P4 or P7. Brains were sectioned to 60 μm thickness and every other section was mounted and stained as described.1
Stereology
Stereological analysis was performed using the optical fractionator (Stereo Investigator 10, MBF Bioscience; Williston VT). A fluorescence microscope (Nikon Eclipse 80i, Nikon; Melville NY) equipped with a fluorescent lamp and a CX9000 camera (MBF Biosciences; Williston VT) was used. Using a 4× objective, outline border tracing was done for the hippocampus, laterodorsal thalamus, and mediodorsal thalamus. All mounted sections that contained the hippocampus were imaged for eight to nine sections per rat. The counting frame size was set to 300×300 and the systematic random sampling grid was set to 75% of the region of interest for the control and 50% for the Iso group. Cells were counted using a 40× objective. Because of volumetric differences in brain regions between ages, results are reported as estimated cell population μm−3 rather than an absolute value.
Protein quantification
Five male and five female rats at P4 and P7 were killed and the cerebral cortex, hippocampus, and thalamus removed. Tissue was immediately homogenised in a radioimmunoprecipitation assay buffer (Boston Bioproducts; Ashland MA) with a protease inhibitor cocktail (Fisher Scientific; South San Francisco CA ). Protein concentration was measured with an ND-1000 spectrophotometer (Nanodrop Technologies; Wilmington DE) and proteins were separated using Tris-glycine polyacrylamide 7.5% gels (BioRad; Hercules CA) at 120 V for 1 h. Protein was transferred to polyvinylidene fluoride membranes by semi-dry transfer. Membranes were blocked with 5% non-fat dry milk then incubated with primary antibody overnight at 4°C: rabbit anti-NKCC1 1:1000 (#14581 Cell Signaling Technologies; Danvers MA), rabbit anti-GAPDH 1:2000 (#1440. Cell Signaling Technologies; Danvers MA), rabbit anti-KCC2 1:1000 (#07-432. Millipore; Hayward CA). After washing, blots were incubated in secondary antibody (goat anti-rabbit HRP 1:1000; Life Technologies; Carlsbad CA #A16104) at room temperature for 1 h. Membranes were washed and developed with Picco Enhanced Chemiluminescent Substrate for KCC2 and GAPDH for 1 min, and Femto ECL SuperSignal substrate (ThermoFisher; South San Francisco CA) for NKCC1 for 30 s. Blots were imaged with a ChemiDoc MP imager (BioRad; Hercules CA) and Image Lab 6.0 software (BioRad; Hercules CA) was used to determine band densities. Densities for all samples were converted to relative densities for each protein of interest, normalised to GAPDH loading controls which were measured in the manner above, and displayed as expression relative to GAPDH.
Results
Spatial reference memory is impaired in adult female rats exposed to Iso at P4 but not at P7
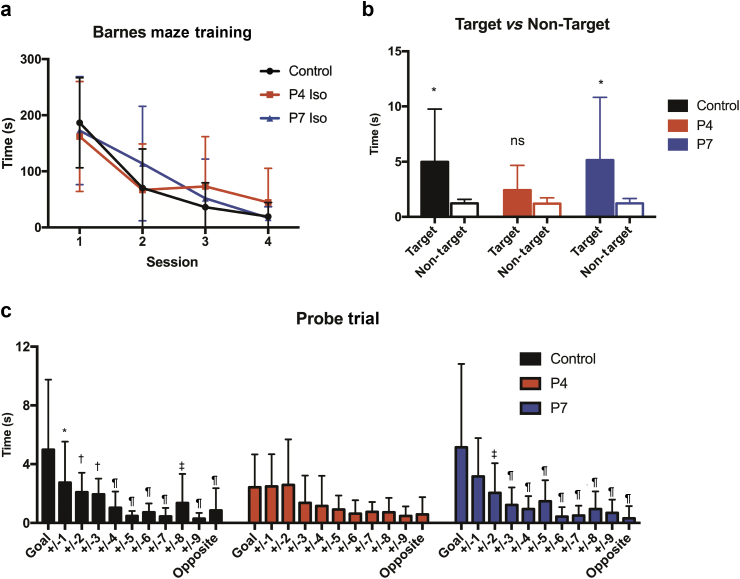
All groups (P4 and P7 exposed females and controls) did similarly well in learning the location of the target hole during the four consecutive daily training sessions of the Barnes maze, regardless of anaesthetic exposure or age at which they were exposed (Fig. 2a). All groups exhibited decreasing latency times across training sessions, with no effect of treatment [two-way repeated measures ANOVA, interaction P=0.16, session P<0.0001, treatment P=0.87, subjects (matching) P<0.0001].
Fig 2.
Female rats exposed to isoflurane (Iso) at P4 but not P7 have impaired spatial memory in adulthood. (a) Average latency to reach the escape box during training sessions of Barnes maze. There was a significant effect of session (P<0.0001) but not treatment (P=0.8742) (two-way repeated measures analysis of variance). (b) Cumulative duration of time spent investigating the target (goal hole) vs the average of all non-target holes during Barnes maze probe trial, 1 week after training completion. Control P=0.0235, P4 Iso P=0.0923, P7 Iso P=0.0248; paired t-test, two-tailed. (c) Cumulative duration of time spent investigating the goal compared with neighbouring holes (average of two holes equidistant from goal). Not significant if no asterisk, *P<0.05, †P<0.01, ‡P<0.001, ¶P<0.0001). Mean and standard deviation shown for all graphs.
Rats underwent a probe trial 1 week after the completion of training to test spatial reference memory. During the 90-s probe trial, the P4 Iso group did not spend more time investigating the target hole when compared with the average of all non-target holes in the maze (P=0.0923, paired t-test) (Fig. 2b). Comparison of time spent investigating the target hole to the neighbouring holes revealed that the P4 Iso group did not distinguish the target from any other holes of varying distances in the maze (two-way ANOVA, multiple comparisons, adjusted P>0.1 for target vs neighbouring holes, Fig. 2c). In contrast, the P7 Iso-treated and control animals spent significantly more time at the target hole compared with the average of all non-target holes (P7 Iso P=0.025, control P=0.024, target vs non-target, paired t-test).
There were no differences between groups in performance on the elevated plus maze (Supplementary Fig. S2), indicating similar levels of baseline anxiety.
Associative object recognition, but not object discrimination, is impaired in female rats exposed to Iso at P4 but not at P7
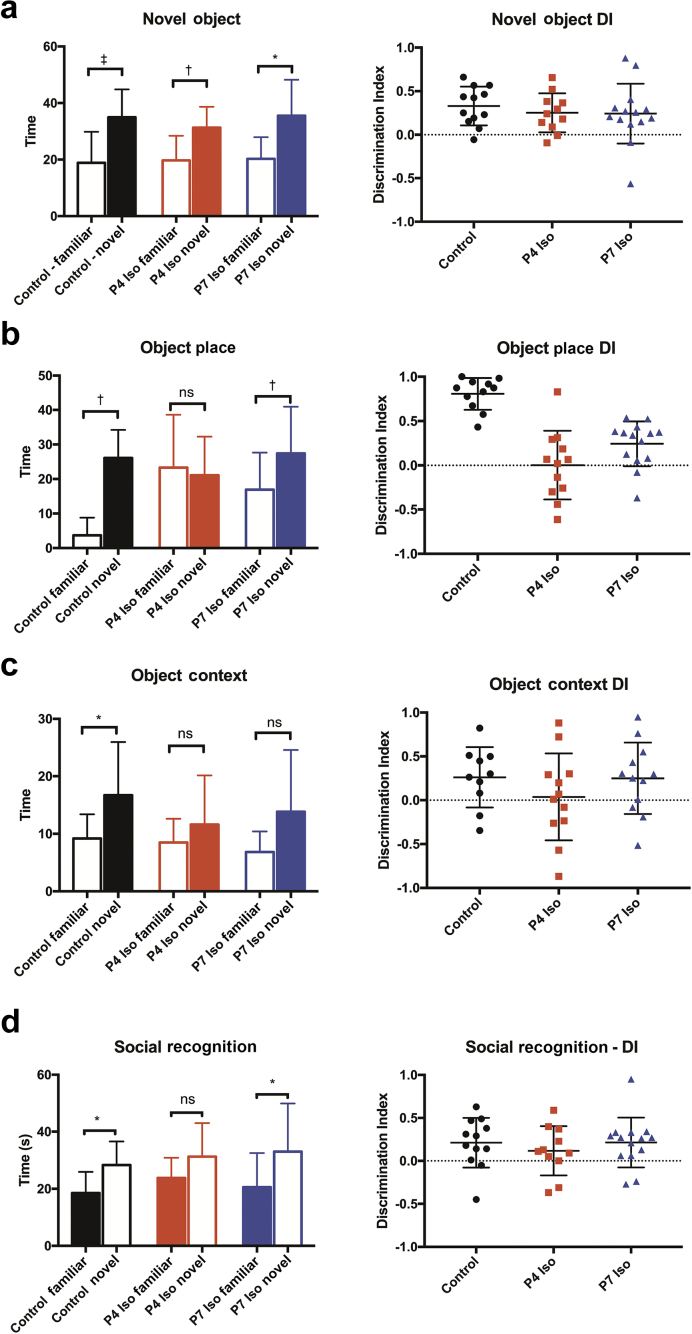
In the novel object recognition task, all groups spent more time investigating the novel object over the familiar object (control P=0.0005, P4 Iso P=0.0054, P7 Iso P=0.035, ratio paired t-test of novel vs familiar object investigation time). The discrimination index [DI, (time spent investigating novel object–time spent investigating familiar object)/(total investigation time)] was significantly above zero for all three groups (control P=0.0003, P4 Iso P=0.0039, P7 Iso P=0.0204, one sample t-test with a theoretical mean=0), indicating that all three groups successfully recognised the familiar object (Fig. 3a).
Fig 3.
Isoflurane (Iso)-induced associative recognition memory impairment is dependent on precise age of postnatal exposure. (a) All three groups spent more time investigating a novel object over a familiar object in the novel object recognition task, left (control P=0.0005, P4 Iso P=0.0054, P7 Iso P=0.0348; ratio paired t-test). Performance on recognition tasks was further assessed by the discrimination index (DI), right (control P=0.003, P4 Iso P=0.0204; one sample t-test against hypothetical mean=0). (b) The P4 Iso group was impaired in the object place recognition task, which requires subjects to identify an object in a previously seen location (familiar vs novel: control P=0.0014, P4 Iso P=0.8763, P7 Iso P=0.0033; ratio paired t-test; DI: control P<0.0001, P4 Iso P=0.9783, P7 Iso P=0.0031; one sample t-test against hypothetical mean=0). (c) The P4 Iso and P7 Iso groups were impaired in the object context recognition task, which tests subjects’ ability to recognise an object and its associated context in which it was previously seen (familiar vs novel: control P=0.0475, P4 Iso P=0.8006, P7 Iso P=0.0753; ratio paired t-test; DI: control P<0.04, P4 Iso P=0.7961, P7 Iso P=0.0576; one sample t-test against hypothetical mean=0). (d) The P4 Iso group was impaired in social recognition memory and did not spend more time on average investigating a novel juvenile rat over a previously seen juvenile (familiar vs novel: control P=0.0113, P4 Iso P=0.1252, P7 Iso P=0.0478; ratio paired t-test; DI: control P=0.0278, P4 Iso P=0.2040, P7 Iso P=0.0164; one sample t-test against hypothetical mean=0). All graphs are shown with mean and standard deviation. *P<0.05,† P<0.01, ‡ P<0.001, ns = not significant.
The object place recognition task tested whether rats were able to recognise an object and its associated spatial location. The control and P7 Iso groups spent more time investigating the object in the new location, however the P4 Iso group was not able to discriminate between the novel and familiar location (control P=0.0014, p4 Iso P=0.8763, P7 Iso P=0.0033, ratio paired t-test). The DIs reflected the same pattern (control P<0.0001, P4 Iso P=0.98, P7 Iso P=0.0031, one sample t-test) (Fig. 3b).
Both the P4 Iso and P7 Iso groups were impaired in the object-context recognition task, failing to spend more time investigating the object associated with a different context than in the original exposure trial (control P=0.048, P4 Iso P=0.80, P7 Iso P=0.075 ratio paired t-test) and did not have a DI significantly above zero (control P=0.04, P4 Iso P=0.80, P7 Iso P=0.58) (Fig. 3c).
Social recognition is impaired in P4 isoflurane-exposed female rats
All groups spent a similar amount of time investigating a caged juvenile female rat over an identical empty cage, indicating normal social investigatory behaviour. However, the P4 Iso group did not spend significantly more time investigating a novel juvenile rat compared with the previously seen one (P=0.12), indicating impaired social recognition memory. Conversely, both P7 Iso and control groups spent more time with the novel juvenile over the familiar one (control P=0.011, P7 Iso P=0.048) (Fig. 3d).
Females have greater cell death after isoflurane exposure at P4 than at P7
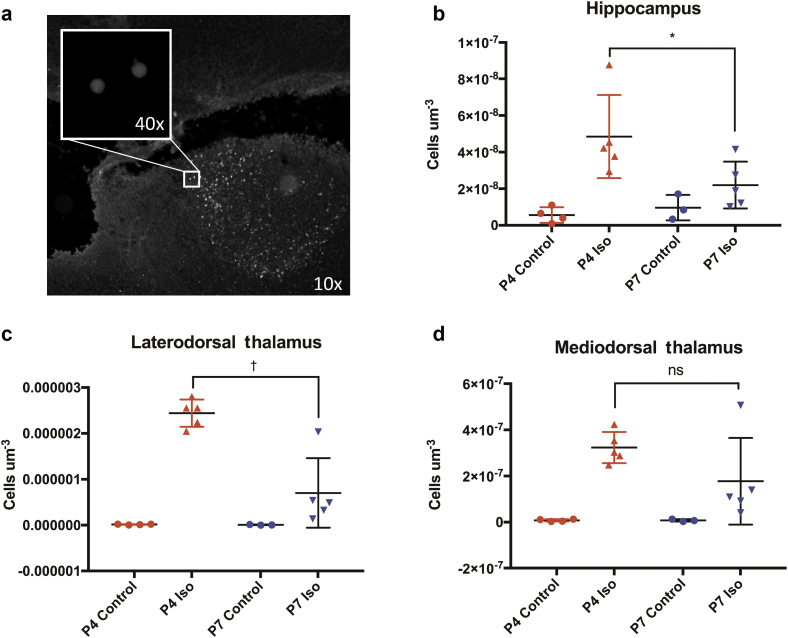
We assessed neurodegeneration in the hippocampus, medial dorsal thalamus (MDT), and lateral dorsal thalamus (LDT) as these regions contribute to recognition, spatial memory, or both13, 14 and display significant cell death after early anaesthesia exposure.3, 15 All brain regions regardless of age showed significant cell death after Iso treatment (hippocampus P=0.0025, MDT P=0.0007, LDT P<0.0001; two-way ANOVA). The hippocampus and LDT showed greater cell death in the P4 Iso group than the P7 Iso group (hippocampus P=0.029, LDT P<0.0001; post hoc Sidak's multiple comparisons test) (Fig 4a–d).
Fig 4.
Isoflurane induces higher rates of acute neurodegeneration in P4 females than P7 females in the hippocampus and laterodorsal thalamus. (a) Representative image of Fluoro-Jade C (FJC) staining in the laterodorsal thalamus of a P4 isoflurane-exposed rat, 10× (inset, 40×). (b) Number of neurodegenerating cells, fluorescently labelled with FJC+, per um3 in the hippocampus. Two-way ANOVA shows effect of treatment (P=0.0025) but not age (P=0.1516); no interaction (P=0.0588). Post hoc Sidak’s multiple comparison test shows significantly higher cell death in P4 Iso compared with P7 Iso (P=0.0288). (c) FJC+ cells in the laterodorsal thalamus. Two-way ANOVA shows effect of age (P=0.0018) and treatment (P<0.0001), interaction P=0.002. (d) FJC+ cells in the mediodorsal thalamus. Two-way ANOVA shows effect of treatment (P=0.0007) but not age (P=0.2055); no interaction (P=0.2052). Mean and standard deviation shown. * P<0.05, † P<0.001, ns = not significant.
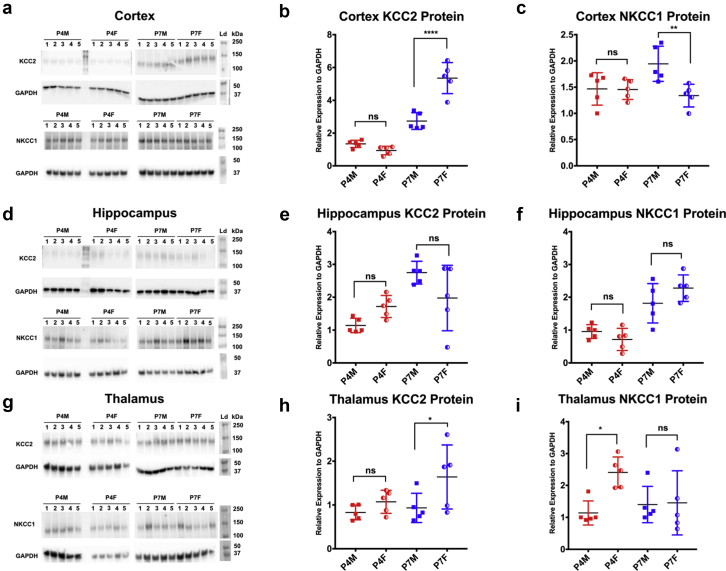
Sex-specific developmental expression of NKCC1 and KCC2
In cerebral cortex at P4, both males and females expressed similar amounts of NKCC1 and KCC2 (post hoc Sidak). However, by P7 the female cortex expressed more KCC2 (P<0.0001) and less NKCC1 (P=0.005) than in males (sex P=0.021 and interaction P=0.025; two-way ANOVA) (Fig 5a–c). A similar result was seen in the thalamus where KCC2 expression was higher in P7 females than in males (P=0.039) (Fig. 5h). These data suggest that, although similar on P4, the cortex and thalamus of males have a less mature chloride gradient than those of females at P7, as established by the transporter stoichiometry.
Fig 5.
Sex-specific developmental expression of NKCC1 and KCC2 protein. Immunoblots of KCC2 and NKCC1 with glyceraldehyde-3-phosphate dehydrogenase loading control for cortex (a), hippocampus (d) and thalamus (g) at ages P4 and P7 for both males and females (n=5 per group). (b) Quantification of western blot data for KCC2 and NKCC1 with individual data points and standard deviations displayed. Two-way analysis of variance of KCC2 levels showed differences in sex, age, and interaction. Sex: F(1,16)= 19.41 P=0.0004, age: F(1,16)=133.6 13 P<0.0001, interaction: F(1,16)=36.13 P<0.0001). Post hoc multiple comparison Sidak’s test showed a significant difference between KCC2 protein in males and females at P7 (P<0.0001). (c) Two-way analysis of variance of NKCC1 levels showed a difference by sex [F(1,16)=6.556 P=0.021] and interaction [F(1,16)=6.104 P=0.0251] but not age [F(1,16)=2.259 P=0.1523]. Sidak’s test showed a decrease in protein levels in females compared with males (P=0.0052). These results are consistent with a more mature expression pattern in females relative to males at P7. This pattern was not consistent across the hippocampus (d–f) which showed no difference in expression. KCC2 levels in the thalamus (g–i) were increased in females compared with males at P7 (Sidak’s P=0.039) similar to the cortex, but were dissimilar in that NKCC1 levels were no different at P7.
Protein expression in the hippocampus did not exhibit the same pattern as observed in the cortex. There was an increase in hippocampal expression of KCC2 from P4 to P7 across development with no difference between males and females. There was an increase in expression of NKCC1 from P4 to P7, with a significantly higher concentration of NKCC1 in females (P<0.0001) (Fig 5d–f). The thalamus also showed higher NKCC1 concentrations at P4 in females compared with males at the same age (Fig 5g–i). These different patterns of expression likely represent different maturation states of the perinatal brain across regions. mRNA transcript concentrations of these transporters were measured using quantitative real-time polymerase chain reaction (qRT—PCR) in male and female rats, but there were no significant differences to account for the difference in protein concentrations at the same time points (Supplementary Fig. S3).
Discussion
Our findings indicate that postnatal female rats are more vulnerable to lasting cognitive impairment after anaesthesia exposure at P4 compared with P7. Both spatial reference memory and associative recognition memory are impaired in P4 Iso-exposed female rats, although spatial working memory and novel object recognition remained intact. We have reported that males exposed to anaesthetic at P7 have a worse cognitive outcome than females. The results presented here provide evidence that females are at risk for anaesthetic injury during an earlier window of vulnerability. One potential explanation for this shift is the sex- and age-specific difference in expression of the chloride transporters NKCC1 and KCC2. Our results show differences in protein expression of these molecules between males and females which predict a more mature chloride gradient in females at P7 than P4.
It is unclear how anaesthesia-induced neuroapoptosis is associated with behavioural outcomes, despite being a major focus of most early anaesthesia neurotoxicity studies. Male and female rats have similar levels of neurodegeneration after anaesthesia exposure at P7 despite differences in behavioural outcomes,2 and prevention of cell death does not always reverse cognitive deficits.16 Our finding that cell death is higher in the hippocampus and LDT of females exposed to Iso at P4 than at P7 provides a possible explanation to the cognitive deficit seen later in life, however, it is unlikely the primary mechanism leading to the behavioural outcome given previous studies. We suggest that, rather than cell death being responsible for the lasting outcome, it is the function or circuit formation of these young neurones during this specific critical period of development that is interrupted and later manifests as behavioural impairment. This hypothesis is further supported by findings recently showing that maintaining synapses,17 rather than preventing cell death,16 protects from cognitive impairment. Our results, showing a difference in cognitive outcome in females at P4 vs P7 and a difference in the expression of chloride transporters, lend additional evidence to this argument. At P7, some of the connections may now be formed in females, but not yet fully developed in males, affording the intact circuits an adult-like protection during the anaesthetic exposure.
The mechanisms of action of most general anaesthetics are poorly understood, however anaesthetics such as Iso modulate inhibition by allosterically enhancing the response of GABAA receptors to GABA.18 During prenatal to early postnatal brain development, GABA exerts an excitatory effect on GABAergic neurones as a result of a reversed chloride gradient in immature neurones that is established by a higher NKCC1/KCC2 expression ratio.19, 20 This NKCC1/KCC2 ratio changes rapidly in the postnatal period and is critical to normal developmental processes. Our work supports previous evidence that differential expression of these molecules is sex-dependent, which we hypothesise underlies the different behavioural outcomes in response to Iso. Specifically, the higher NKCC1/KCC2 protein expression ratio in the P7 cortex might predispose males to anaesthesia-induced cognitive deficit. In females, the change in NKCC1/KCC2 protein ratio between P4 and P7 could underlie the sensitivity to Iso toxicity at P4 but not P7. The differences in mRNA and protein expression suggest that NKCC1 and KCC2 are post-translationally modified.21, 22 KCC2 also plays a role in synapse stabilisation through cytoskeletal interactions that is independent of its chloride transporter function,23, 24 providing another possible mechanism by which a testosterone-mediated delay of KCC2 expression4 leads to slower maturation of neural circuitry in males. Expression of KCC2 and NKCC1 is critical to early brain development and exhibits marked sex-specific postnatal developmental expression. This differential expression may set critical developmental boundaries that dictate when anaesthetics are most harmful and can further inform us about the timing and mechanism of lasting anaesthetic injury.
Limitations
There are limitations to this study that should be considered when interpreting the results. We used concentrations of Iso over 4 h determined to be 1 MAC for female rats at P7.1 We chose to expose both P4 and P7 animals to identical Iso conditions to isolate age as the primary variable. In human infants, the MAC for Iso increases until approximately 4 months of age, at which point the MAC peaks and then decreases for the remainder of the infant's life.25 It is unknown how MAC for these narrowly defined ages in this study directly translate between species. Based on human MAC, it is possible that MAC at P4 is lower than at P7 in rats, which could translate to greater exposure in the P4 group despite identical inspired concentrations in the P7 group.
Not all animals survived the anaesthetic exposure used, so performance of those animals could not be evaluated. Further, it is not possible to control physiologic variables during anaesthesia in neonatal rodents to the same degree as in humans. The exposure protocol produces some physiologic derangement and blood gas abnormalities making it unlike a normal anaesthetic in human children. We chose to use this paradigm in order to directly compare our results with previous studies and to allow interpretation of the subtle difference in age at exposure and sex of the animals.
Drawing conclusions about human exposure to anaesthesia from our data is difficult because of the above limitations inherent in using a model species. However, the data do provide insight into how a single early event can produce life-long behavioural changes during vulnerable critical periods of cognitive development and circuit wiring, and how those periods may differ depending on age and sex. Although it remains unknown if and how these sex differences and specific ages translate to humans,26 our findings support the need to balance sex in clinical trials, which often have an over-representation of male subjects,27, 28, 29 and further investigate vulnerable patient subgroups.
In conclusion, our findings show that females, like males, are susceptible to cognitive deficits after anaesthesia exposure, but the window of vulnerability is shifted earlier in rats. Careful attention to age and sex at the time of exposure and the specific outcomes observed will be important in future studies. Determining the role of sex hormones in the difference between males and females is key to further understanding how and why these sex- and age-based differences in the critical period exist.
Authors' contributions
Conceptualised and designed the experiments: JWS, JSR.
Performed and analysed data from behavioural experiments: JSR.
Performed the NKCC1/KCC2 experiments and analysed resulting data: GC.
Performed and analysed cell death experiments: JSR, DM, YE.
Contributed to the writing and editing of the manuscript, and responsible for contents: all authors.
Acknowledgements
We thank Jason Leong, Claudia Tang, and Nicole Yabut for technical assistance.
Handling editor: H.C. Hemmings Jr
Editorial decision: 05 December 2018
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.12.008.
Declaration of interests
The authors declare that they have no conflicts of interest.
Funding
National Institutes of Health Grant R01 GM112831 (JWS), the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant TL1 TR001871 (JSR), and Ruth L. Kirschstein National Research Service Award T32 GM08440 (GAC).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Clausen N.G., Kähler S., Hansen T.G. Systematic review of the neurocognitive outcomes used in studies of paediatric anaesthesia neurotoxicity. Br J Anaesth. 2018;120:1255–1273. doi: 10.1016/j.bja.2017.11.107. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary J.D., Warner D.O. What do recent human studies tell us about the association between anaesthesia in young children and neurodevelopmental outcomes? Br J Anaesth. 2017;119:458–464. doi: 10.1093/bja/aex141. [DOI] [PubMed] [Google Scholar]
- 3.Lee B.H., Chan J.T., Kraeva E., Peterson K., Sall J.W. Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology. 2014;83:9–17. doi: 10.1016/j.neuropharm.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuñez J.L., McCarthy M.M. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrot-Sinal T.S., Sinal C.J., Reader J.C., Speert D.B., Mccarthy M.M. Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol. 2007;19:302–308. doi: 10.1111/j.1365-2826.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 6.Weinhard L., Neniskyte U., Vadisiute A. Sexual dimorphism of microglia and synapses during mouse postnatal development. Dev Neurobiol. 2018;78:618–626. doi: 10.1002/dneu.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jevtovic-Todorovic V., Hartman R.E., Izumi Y. Early exposure to common anaesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clancy B., Darlington R.B., Finlay B.L. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 9.Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murguía-Castillo J., Beas-Zárate C., Rivera-Cervantes M.C., Feria-Velasco A.I., Ureña-Guerrero M.E. NKCC1 and KCC2 protein expression is sexually dimorphic in the hippocampus and entorhinal cortex of neonatal rats. Neurosci Lett. 2013;552:52–57. doi: 10.1016/j.neulet.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 11.Galanopoulou A.S. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld C.S., Ferguson S.A. Barnes maze testing strategies with small and large rodent models. J Vis Exp. 2014 doi: 10.3791/51194. e511944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Groen T., Kadish I., Wyss J.M. The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behav Brain Res. 2002;136:329–337. doi: 10.1016/s0166-4328(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell A.S., Chakraborty S. What does the mediodorsal thalamus do? Front Syst Neurosci. 2013;7:37. doi: 10.3389/fnsys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratmann G., May L.D., Sall J.W. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 16.Schilling J.M., Kassan A., Mandyam C. Inhibition of p75 neurotrophin receptor does not rescue cognitive impairment in adulthood after isoflurane exposure in neonatal mice. Br J Anaesth. 2017;119:465–471. doi: 10.1093/bja/aew299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearn M.L., Schilling J.M., Jian M. Inhibition of RhoA reduces propofol-mediated growth cone collapse, axonal transport impairment, loss of synaptic connectivity, and behavioural deficits. Br J Anaesth. 2018;120:745–760. doi: 10.1016/j.bja.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia P.S., Kolesky S.E., Jenkins A. General anaesthetic actions on GABA(A) receptors. Curr Neuropharmacol. 2010;8:2–9. doi: 10.2174/157015910790909502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina I., Friedel P., Rivera C. Current view on the functional regulation of the neuronal K+-Cl− cotransporter KCC2. Front Cell Neurosci. 2014;8:27. doi: 10.3389/fncel.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M., Fukuda A. Development and regulation of chloride homeostasis in the central nervous system. Front Cell Neurosci. 2015;9:371. doi: 10.3389/fncel.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M., Wang J., Jiang J. APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. eLife. 2017;6 doi: 10.7554/eLife.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbato C., Ruberti F., Pieri M. MicroRNA-92 modulates K(+) Cl(−) co-transporter KCC2 expression in cerebellar granule neurons. J Neurochem. 2010;113:591–600. doi: 10.1111/j.1471-4159.2009.06560.x. [DOI] [PubMed] [Google Scholar]
- 23.Li H., Khirug S., Cai C. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56:1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 24.Fiumelli H., Briner A., Puskarjov M. An ion transport-independent role for the cation-chloride cotransporter kcc2 in dendritic spinogenesis in vivo. Cereb Cortex. 2014;23:378–388. doi: 10.1093/cercor/bhs027. [DOI] [PubMed] [Google Scholar]
- 25.Eger E.I. Age, minimum alveolar anaesthetic concentration, and minimum alveolar anaesthetic concentration-awake. Anesth Analg. 2001;93:947–953. doi: 10.1097/00000539-200110000-00029. [DOI] [PubMed] [Google Scholar]
- 26.Sun L.S., Li G., Miller T.L. Association between a single general anaesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glatz P., Sandin R.H., Pedersen N.L., Bonamy A.K., Eriksson L.I., Granath F. Association of anaesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2016;313 doi: 10.1001/jamapediatrics.2016.3470. e163470. [DOI] [PubMed] [Google Scholar]
- 28.Davidson A.J., Disma N., de Graaff J.C. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano S.G., Vutskits L., Jevtovic-Todorovic V. Thinking, fast and slow: highlights from the 2016 BJA seminar on anaesthetic neurotoxicity and neuroplasticity. Br J Anaesth. 2017;119:443–447. doi: 10.1093/bja/aex238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.