Abstract
The present study was designed to evaluate the antihypertensive activity and cardioprotective effects of magnesium taurate against cadmium chloride (CdCl2)-intoxicated albino rats. Sprague Dawley male albino rats (120–150 g) were divided into five groups having six animals in each group. Hypertension and cardiotoxicity were induced in animals by administration of CdCl2 (0.5 mg/kg/day, i.p.) for four weeks. Magnesium taurate (2 and 4 mg/kg/day) was administered orally after induction of hypertension (after two weeks) in their respective groups concurrently with CdCl2 for next two weeks. Amlodipine (3 mg/kg/day, p.o.) was used as a standard and administered after induction of hypertension. Blood pressure was monitored biweekly by using non-invasive blood pressure system and biochemical parameters and histopathology of the heart were evaluated after four weeks of the experimental protocol. During the four weeks of the experimental protocol, the toxic control group showed significant elevation of systolic and diastolic blood pressure concomitant with augmentation of cardiotoxicity as indicated by reduction in myocardial antioxidants including glutathione peroxidase, catalase, superoxide dismutase, reduced glutathione and increased malondialdehyde level in heart as compared to the normal group. The oral administrations of magnesium taurate significantly restored the blood pressure, myocardial antioxidants and malondialdehyde level as compared to toxic control group. In addition, histopathological examination showed that magnesium taurate treatments substantially reduced the myocardial damages against CdCl2 treatment. The results suggest that magnesium taurate has prominent antihypertensive and cardioprotective activity via its potent antioxidant activity and can be used as a nutrition supplement to improve the cardiovascular health.
Keywords: Magnesium taurate, Hypertension, Cardiotoxicity, Cadmium chloride, Amlodipine
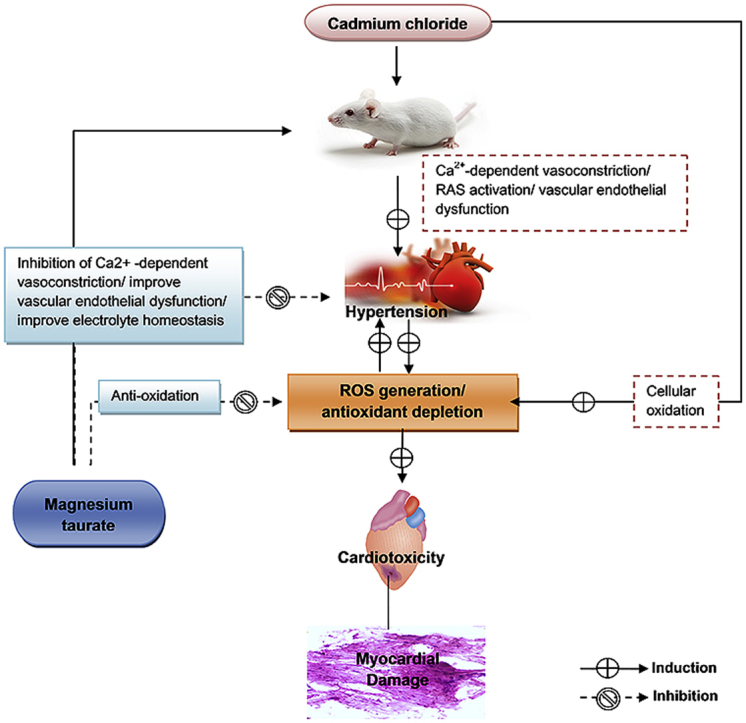
Graphical abstract
1. Introduction
Hypertension is a common cause of cardiovascular diseases and major cause of morbidity and mortality because of its association with arteriosclerosis, coronary heart disease, stroke, myocardial infarction, cerebrovascular diseases, and renal disease.1, 2, 3 The cardiovascular system can affect a variety of mechanism including, environment pollutants. Cadmium (Cd2+) is an environmental toxicant, exposure to human via industrial contamination, food sources and tobacco smoke.4 Cd2+ exposure can affect several organs such as liver, kidneys, eyes, reproductive system and cardiovascular system.5, 6, 7, 8 Increased oxidative stress during Cd2+ toxicity can affect the vascular wall and myocardial tissues, thereby leading to hypertension and cardiotoxicity.5 Literature survey revealed that cardiovascular system is a critical target of Cd2+ toxicity, leading to an increased risk of cardiovascular disease.9, 10 Moreover, epidemiological studies demonstrate a positive association between hypertension and blood Cd2+ level.11, 12 Plethora of literatures illustrate that Cd2+ exposure can affect the availability of vasodilator nitric oxide, oxidative defense system, renin angiotensin system, and calcium associated contractile activity in the cardiovascular system. These various mechanisms are involved in the development of hypertension and cardiotoxicity.5, 13, 14, 15, 16 Enhanced oxidative stress in the heart, vascular wall, and kidney is the characteristic feature of cardiovascular diseases.17 Numerous studies indicate that antioxidants and nutrition supplements may improve the cardiovascular health. Antioxidants scavenge free radicals and regulate gene expression and signaling regulatory pathways, consequently prevent cell death.18 Among the pharmacological treatments, various studies concerning lifestyle change with the regular physical activity and nutritional supplements may improve the cardiovascular health.
Observational studies suggest that magnesium ion (Mg2+) and taurine have potential nutritional value for regulation of hypertension and cardiovascular physiology.19, 20, 21, 22, 23, 24 Mg2+ is the second most abundant intracellular ion and regulates various biological activity in the cardiovascular system.19 Mg2+ modulates the production and release of nitric oxide, improve the endothelial dependent vasodilation, inhibits the Ca2+ influx, and activates the Ca2+ATPase and Na+K+ATPase resulting in the alteration of arterial smooth muscle tone, and blood pressure. Moreover, reduced extracellular Mg2+ trigger the Ca2+ influx by Ca2+ channels and activates inositol-triphosphate (IP-3) mediated mobilization of intracellular Ca2+.19, 20, 25, 26 Taurine (2-aminoethanesulphonic acid) is the most abundant, semi-essential, sulfur-containing amino acid, which can be derived in the body from diet and synthesized from the cysteine amino acid.27 Taurine supplementation has shown several potential beneficial effects on cardiovascular system. It regulates the endothelial function, nitric oxide production, renin-angiotensin system, oxidative stress system, and sympathoadrenal system.21, 23, 24, 27 Moreover, depletion of Mg2+ and taurine can accelerate the development of hypertension.28, 29 Therefore, magnesium taurate (MgT), a complex of Mg2+ and taurine (Fig. 1) may ameliorate the hypertension and cardioprotective effects. In this perceptive, we investigated the possible antihypertensive and cardioprotective effects of MgT against the CdCl2-induced hypertension in rat as an experimental model.
Fig. 1.
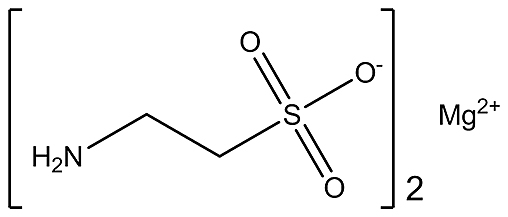
The molecular structure of magnesium taurate.
2. Material and methods
2.1. Drugs and dosing
Magnesium taurate was purchased from Cardiovascular research limited, California. CdCl2 was purchased from Himedia Chemicals, Mumbai, India. Other chemicals and reagents used were of analytical grade. A stock solution of MgT (2 mg/ml) and amlodipine (3 mg/ml) was prepared by suspending in 0.3% w/v solution of carboxymethyl cellulose solution as the vehicle and administered by the oral route according to their dose. Stock solution of MgT (2 ml/kg) was administerd orally at two doses level 1 ml/kg/day for 2 mg/kg/day and 2 ml/kg/day for 4 mg/kg/day in their respective group. Stock solution of amlodipine (3 mg/ml) was administerd orally at 1 ml/kg for 3 mg/kg/day dose. A stock solution (0.5 ml/kg) of CdCl2 was prepared by dissolving in distilled water and administered via intraperitoneal route (0.5 ml/kg/day). The effective doses of the drugs CdCl2, MgT, and amlodipine were selected based on the previous reports.30, 31, 32 All the doses were administered daily during 10:00 am to 11:00 am.
2.2. Animals
Sprague Dawley male albino rats (120–150 g, 12–15 weeks old) were used in the present study. They were housed under standard environmental condition (22 ± 2 °C, with 55 ± 5% humidity and 12 h light/dark cycle), in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Govt. of India. Animals were allowed to have free access to standard pellet diet with water ad-libitum under hygienic conditions. The whole experimental protocol was approved by the Institutional Animals Ethics Committee (Reg. NO.- 994/GO/ERe/S/06/CPCSEA) of Institute of Pharmaceutical Sciences, Guru Ghasidas Vishwavidyalaya, Bilaspur (C.G.) India and the experiments on animals were conducted as per the ethical principles and guidelines provided by CPCSEA, Govt. of India.
2.3. Experimental design
The albino rats, which were normal regarding the blood pressure examination at the baseline after an acclimatization period of seven days, were selected for the present study. The selected animals were divided into five groups having six animals in each group. Group I (normal) animals received 0.3% carboxymethyl cellulose (10 ml/kg/day, p.o.) for four weeks. Hypertension was induced in groups II to V by administration of CdCl2 (0.5 mg/kg/day, i.p.) for four weeks.33 By the end of the second week, induction of hypertension was observed in all the animals of groups II to V. After induction of hypertension, group II (toxic control) continued to receive CdCl2 only, while group III (standard) received amlodipine (3 mg/kg/day, p.o.) and group IV to V received MgT at two dose levels (2 and 4 mg/kg/day, p.o.) respectively, concurrently with CdCl2 from the beginning of the third week to fourth week.
2.4. Measurement of blood pressure and heart rate
The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were monitored biweekly by using non-invasive blood pressure system (CODA-08 Channel, Kent scientific, USA).
2.5. Preparation of heart homogenate
Animals were sacrificed after completion of experimental protocol (four weeks). The hearts were isolated and washed with cold saline. Each heart was homogenized in 10% w/v of 0.1 M potassium phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 rpm for 1 h. The supernatant was separated and stored at 2–8 °C for further biochemical analysis.34
2.6. Cardiac biochemical assay
Enzymatic antioxidants such as glutathione peroxidase (GPx) was assayed by using method of Tappel (1978),35 catalase (CAT) activity was determined by using method of Sinha, (1972),36 and superoxide dismutase (SOD) was measured by using method of Kakkar et al. (1984).37 Non-enzymatic antioxidant, reduced glutathione (GSH) was estimated by using Ellman's reagent.38 Malondialdehyde (MDA), an end product of lipid peroxidation was estimated in the heart homogenate by colorimetric methods of Ohkawa, (1979).39
2.7. Histopathological examination of heart
One heart from the each group was fixed in 10% buffered neutral formalin solution. After fixation, tissue was embedded in paraffin wax, cut about 5 μm, and mounted on slides, stained with hematoxylin and eosin (H&E). The slides were viewed under a trinocular microscope (AXIOCAM-ERC-5S, Germany) at various magnifications (10x and 40x) to find out the changes in the microscopic features of tissues under study. The myocardial damage was scored depending upon myocardial damages.40 Score 00: normal (no changes), score 01: mild (focal myocyte damage with mild degree of inflammation), score 02: moderate (degeneration in cardiac muscle fibres), score 03: marked (complete loss of cross striation, loss of cardiomyocyte boundary, degeneration in cardiac muscle fibres, necrosis, vacuolization, edematous fluid, fatty degeneration and infiltration of inflammatory cells).
2.8. Statistical analysis
All tested data were expressed as mean ± SEM. Assessments of results were performed using analysis of variance (ANOVA). The level of statistical significance was set at P < 0.05. Statistical analysis was performed using GraphPad Prism version 5.0 (Graph pad Software Inc., CA. USA).
3. Results
3.1. Effect on blood pressure
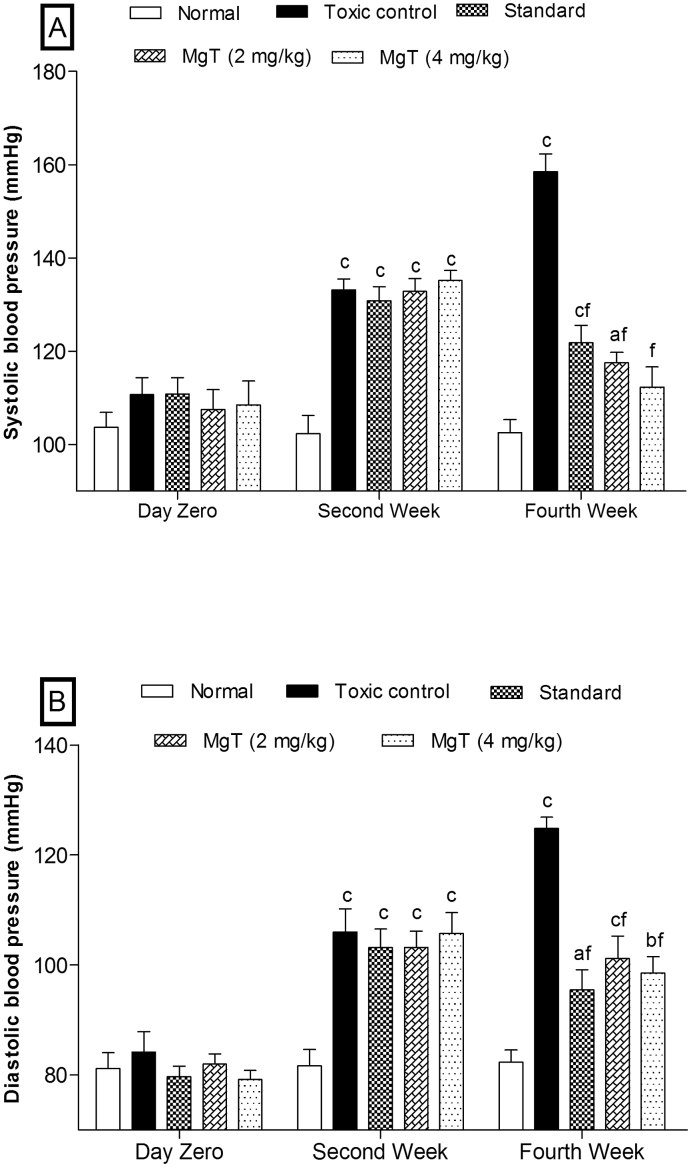
The CdCl2-treated rats (Groups II to V) showed significant (P < 0.001) elevation in blood pressure in terms of SBP and DBP within two weeks as compared to the normal group (Fig. 2). The Group II (toxic control) showed significant (P < 0.001) induction of hypertension in a time dependent manner (day zero to fourth week) as compared to the normal group. Whereas, the next two weeks (third to four weeks) oral administration of amlodipine (3 mg/kg) in group III (standard) and MgT in groups IV and V at 2 and 4 mg/kg led to significant (P < 0.001) reduction in the blood pressures as compared to toxic control group. The results indicate that MgT at 4 mg/kg has profound antihypertensive activity than amlodipine (3 mg/kg) and MgT (2 mg/kg).
Fig. 2.
Effect of magnesium taurate on (A) systolic blood pressure and (B) diastolic blood pressure. After monitoring of baseline at day zero animals of Group II to V (toxic control, standard, and MgTs) were treated with CdCl2 for two weeks for inducing hypertension thereafter amlodipine and magnesium taurate were administered in their respective group for next two weeks. Values are expressed as mean ± SEM; n = 6 in each; aP < 0.05;bP < 0.01;cP < 0.001 when compared to normal and dP < 0.05;eP < 0.01;fP < 0.001 when compare to toxic control (two-way ANOVA followed by Bonferroni post-hoc test).
3.2. Effect on cardiac antioxidants marker
Table 1 shows that chronic exposure of CdCl2 to the animals of group II to V substantially reduced the myocardial antioxidants like CAT, GPx, GSH, and SOD as compared to the normal group. In CdCl2 alone treated rats (toxic control group), the level of antioxidants (CAT, GPx, GSH, and SOD) was significantly (P < 0.001) reduced as compared to the normal group. While CdCl2 co-administered with MgT in MgT-treated groups (group IV and V), the level of antioxidants was significantly (P < 0.001), increased as compared to the normal group. The amlodipine-treated rats (standard) also showed significant (P < 0.001) increase in the level of myocardial antioxidants as compared to toxic control group. The results indicate that MgT has similar ability to restore the antioxidants level as that of amlodipine.
Table 1.
Effect of magnesium taurate on CAT, GPx, GSH, SOD, and MDA level in heart.
| CAT (μmoles of H2O2 consumed/min/mg protein) |
GPx (μmoles GSH oxidised/min/mg protein) |
GSH (μmoles/g tissue) |
SOD (U/min/mg protein) |
MDA (μmoles/g tissue) |
|
|---|---|---|---|---|---|
| Normal | 33.40 ± 2.08 | 2.40 ± 0.07 | 6.95 ± 0.10 | 9.50 ± 0.54 | 5.30 ± 0.25 |
| Toxic control | 11.20 ± 1.06c | 0.94 ± 0.07c | 4.26 ± 0.05c | 3.82 ± 0.21c | 8.48 ± 0.29c |
| Standard | 26.40 ± 1.20bf | 2.10 ± 0.06bf | 6.67 ± 0.11af | 7.34 ± 0.19cf | 6.76 ± 0.26bf |
| MgT (2 mg/kg) | 24.00 ± 1.22cf | 1.59 ± 0.07cf | 5.32 ± 0.11cf | 6.34 ± 0.28cf | 6.74 ± 0.26bf |
| MgT (4 mg/kg) | 26.80 ± 1.28bf | 1.93 ± 0.05cf | 6.37 ± 0.04cf | 8.12 ± 0.28bf | 6.26 ± 0.30af |
Values are expressed as mean ± SEM; n = 6 in each; aP < 0.05;bP < 0.01;cP < 0.001 when compared to normal and dP < 0.05;eP < 0.01;fP < 0.001 when compare to toxic control (one-way ANOVA followed by Newman Keuls post hoc test). CAT: catalase; GPx: glutathione peroxidase; GSH: reduced glutathione; SOD: superoxide dismutase; MDA: malondialdehyde.
3.3. Effect on lipid peroxidation
The toxic control group showed significant (P < 0.001) elevation in MDA level as compared to the normal group while MgT and amlodipine-treated groups showed significant (P < 0.001) depletion in MDA level as compared to toxic control group. The results (Table 1) indicate that MgT treatments diminish the lipid peroxidation level induced by CdCl2 administrations.
3.4. Histopathological studies
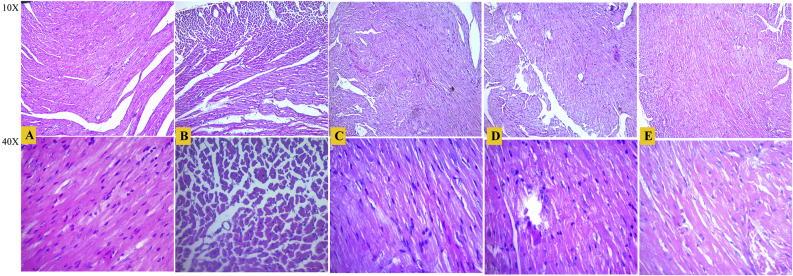
Fig. 3 shows the results of histopathological examination of the myocardial tissues. The myocardial tissue of normal group showed no pathological changes (score 00) and had the clear integrity of myocardial cell membrane, cardiomyocyte boundary, and cardiac muscle fibers. The CdCl2 treatments to the toxic control group led to marked myocardial damages (score 03), it caused loss of cross striation, loss of cardiomyocyte boundary, degeneration in cardiac muscle fibers, necrosis, vacuolization, and fatty degeneration. The amlodipine and MgT (2 mg/kg) treated groups showed moderate degree (score 02) of myocardial damages while MgT at 4 mg/kg dose level showed mild degree (score 01) of myocardial damages. The results indicate that MgT at 4 mg/kg dose level potentially reduces the progression of myocardial damages and has prominent cardioprotective effects than amlodipine and MgT (2 mg/kg).
Fig. 3.
Histopathological section (hematoxylin and eosin stained microscopic sections with 10x and 40x magnification) of heart tissue of the different experimental groups. (A) normal, (B) toxic control, (C) standard, (D) MgT (2 mg/kg), and (E) MgT (4 mg/kg).
4. Discussion
The Cd2+, a potent toxicant metal, is widely dispersed in the environment and exposed to the human via industrial contamination, food sources and tobacco smoke.4 Cd2+ exposure can cause a variety of harmful effects in human and animals. It produces toxic effects in several organs, including cardiovascular system.16, 41 The Cd2+ exposure affects the cardiovascular system via several mechanisms. It produces vascular endothelial dysfunction, reduces nitric oxide (NO) level,15, 16 activates renin-angiotensin system,13 disrupts Ca2+ channel,5 and induces oxidative stress14, 33 that cause the induction of hypertension and further exacerbates the cardio toxicity. Therefore, in this study we used CdCl2-induced hypertension animal model for inducing hypertension and cardiotoxicity.
In the present investigation, chronic administration of CdCl2 to the animals of toxic control group induced significant elevation in systolic and diastolic blood pressure for a consecutive four weeks. Whereas, MgT treatment concurrently with CdCl2 from the third week to fourth week significantly reduced the elevated blood pressure suggesting that MgT potentially abolishes the hypertensive effects of CdCl2. The anti-hypertensive action of MgT might be due to multiple protective effects of Mg2+ and taurine on the cardiovascular system. Mg2+ acts as a natural calcium antagonist and regulates the cell membrane function, smooth muscle contraction, vascular endothelial function, and electrolyte homeostasis.19, 26, 42 Taurine regulates the blood pressure via modulation of vascular endothelial activity, NO level, myocardial cGMP/cAMP ratio, renin-angiotensin system, and antioxidant defense mechanism.21, 23, 24
Antioxidant defense system plays a pivotal role in organ protection. Antioxidant enzymes, such as CAT, GPx, and SOD, mitigate the toxic effects of reactive oxygen species (ROS).43 The SOD catalyzes conversion of superoxide radical and hydrogen peroxide, it also inhibits oxidative inactivation of nitric oxide, consequently preventing peroxynitrite formation and endothelial dysfunction.44 CAT decomposes the hydrogen peroxide to water and oxygen.45 GPx regulates the hydrogen peroxide content within the cell.46 Thus, antioxidant enzymes protect organ damage from oxidative stress. The disruption of antioxidant defense system elevates the ROS/antioxidant ratio in oxidative stress condition that is responsible for the oxidation of cellular proteins and lipids, resulting in oxidative organ damage.47 The CdCl2 administration significantly reduced the enzymatic antioxidants (CAT, GPx, and SOD) level in the toxic control myocardial tissues. However, MgT treatment significantly restored these antioxidants, suggesting that MgT prevents oxidative damage of myocardial tissues against CdCl2 exposure. The restoration of antioxidant defense system by MgT may be attributed to potent antioxidant ability of Mg2+ and taurine, which was corroborated with various researchers.24, 26, 48, 49
In addition, MgT treatment significantly enhanced the GSH level (non-enzymatic antioxidants) and reduced MDA level (an end product of lipid peroxidation) in myocardial tissues of MgT-treated group as compared to toxic control group. The histopathological studies also indicate the restoration of damage caused by CdCl2 after treatment with MgT. The increased GSH and decreased MDA level can be attributed to the protective effects of MgT against CdCl2-induced oxidative cellular damage. GSH is a major intracellular non-protein –SH compound that plays an important role in protecting cells against electrophiles and free radicals. GSH maintains cell membrane protein –SH groups in the reduced form, and scavenges free radical by neutralizing hydroxyl ion.47 MDA is generated by peroxidation of polyunsaturated fatty acids that can be accelerated by ROS. MDA may further react with proteins, phospholipids, and nucleic acid leading to structural modifications.47, 50 The myocardial protection shown by MgT might have been mediated via restoration of basal myocardial antioxidants enzyme activities.
Thus, we can conclude that MgT alleviates the cardiovascular protection by restoring hypertension and myocardial antioxidant defense system. The results suggest that MgT can be used as a nutritional supplement to manage cardiovascular complications.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
Authors are thankful to Department of Pharmacology, Institute of Pharmaceutical Sciences, Guru Ghasidas Vishwavidyalaya, Bilaspur (C.G.) India for providing necessary facilities to carry out this work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Tanahashi N. Hypertension associated with cerebrovascular disease. Nihon Rinsho. 2015;73(11):1864–1870. [PubMed] [Google Scholar]
- 2.Franklin S.S., Wong N.D. Hypertension and cardiovascular disease: contributions of the framingham heart study. Glob Heart. 2013;8(1):49–57. doi: 10.1016/j.gheart.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Tedla F.M., Brar A., Browne R., Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens. 2011;2011:132405. doi: 10.4061/2011/132405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faroon O., Ashizawa A., Wright S. Agency for Toxic Substances and Disease Registry (ATSDR) Atlanta, GA, U.S.A: Public Health Service, Department of Health and Humans Services, Centers for Disease Control; 2012. Toxicological profile for cadmium.http://www.ncbi.nlm.nih.gov/books/NBK158838/ [PubMed] [Google Scholar]
- 5.Bernhoft R.A. Cadmium toxicity and treatment. ScientificWorldJournal. 2013;2013:394652. doi: 10.1155/2013/394652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuypers A., Plusquin M., Remans T. Cadmium stress: an oxidative challenge. Biometals. 2010;23(5):927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 7.Fowler B.A. Monitoring of human populations for early markers of cadmium toxicity: a review. Toxicol Appl Pharmacol. 2009;238(3):294–300. doi: 10.1016/j.taap.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Langford-Smith A., Tilakaratna V., Lythgoe P.R., Clark S.J., Bishop P.N., Day A.J. Age and smoking related changes in metal ion levels in human lens: implications for cataract formation. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houston M.C. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern Ther Health Med. 2007;13(2):S128–S133. [PubMed] [Google Scholar]
- 10.Messner B., Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals. 2010;23(5):811–822. doi: 10.1007/s10534-010-9314-4. [DOI] [PubMed] [Google Scholar]
- 11.Eum K.D., Lee M.S., Paek D. Cadmium in blood and hypertension. Sci Total Environ. 2008;407(1):147–153. doi: 10.1016/j.scitotenv.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Lee B.K., Kim Y. Association of blood cadmium with hypertension in the Korean general population: analysis of the 2008-2010 Korean national health and nutrition examination survey data. Am J Ind Med. 2012;55(11):1060–1067. doi: 10.1002/ajim.22078. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed M.A. Effect of angiotensin II type 1 receptor blocker on renal function, arterial blood pressure and parathyroid hormone related protein over expression in cadmium induced nephrotoxicity in adult male rats. Int J Physiol Pathophysiol Pharmacol. 2013;5(2):109–119. [PMC free article] [PubMed] [Google Scholar]
- 14.Kukongviriyapan U., Pannangpetch P., Kukongviriyapan V., Donpunha W., Sompamit K., Surawattanawan P. Curcumin protects against cadmium-induced vascular dysfunction, hypertension and tissue cadmium accumulation in mice. Nutrients. 2014;6(3):1194–1208. doi: 10.3390/nu6031194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nwokocha C.R., Baker A., Douglas D., McCalla G., Nwokocha M., Brown P.D. Apocynin ameliorates cadmium-induced hypertension through elevation of endothelium nitric oxide synthase. Cardiovasc Toxicol. 2013;13(4):357–363. doi: 10.1007/s12012-013-9216-0. [DOI] [PubMed] [Google Scholar]
- 16.Sangartit W., Kukongviriyapan U., Donpunha W. Tetrahydrocurcumin protects against cadmium-Induced hypertension, raised arterial stiffness and vascular remodeling in mice. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffrin E.L. Antioxidants in hypertension and cardiovascular disease. Mol Interv. 2010;10(6):354–362. doi: 10.1124/mi.10.6.4. [DOI] [PubMed] [Google Scholar]
- 18.Young I.S., Woodside J.V. Antioxidants in health and disease. J Clin Pathol. 2001;54(3):176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha A.R., Umbelino B., Correia M.L., Neves M.F. Magnesium and vascular changes in hypertension. Int J Hypertens. 2012;2012:754250. doi: 10.1155/2012/754250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houston M. The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens. 2011;13(11):843–847. doi: 10.1111/j.1751-7176.2011.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J., Xu X., Yang J., Wu G., Sun C., Lv Q. Antihypertensive effect of taurine in rat. Adv Exp Med Biol. 2009;643:75–84. doi: 10.1007/978-0-387-75681-3_8. [DOI] [PubMed] [Google Scholar]
- 22.Touyz R.M. Role of magnesium in the pathogenesis of hypertension. Mol Asp Med. 2003;24(1–3):107–136. doi: 10.1016/s0098-2997(02)00094-8. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y.-J., Arneja A.S., Tappia P.S., Dhalla N.S. The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol. 2008;13(2):57–65. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q., Yang J., Wu G. Effects of taurine on myocardial cGMP/cAMP ratio, antioxidant ability, and ultrastructure in cardiac hypertrophy rats induced by isoproterenol. Adv Exp Med Biol. 2013;776:217–229. doi: 10.1007/978-1-4614-6093-0_21. [DOI] [PubMed] [Google Scholar]
- 25.Barbagallo M., Dominguez L.J., Galioto A., Pineo A., Belvedere M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res. 2010;23(3):131–137. doi: 10.1684/mrh.2010.0214. [DOI] [PubMed] [Google Scholar]
- 26.Sontia B., Touyz R.M. Role of magnesium in hypertension. Arch Biochem Biophys. 2007;458(1):33–39. doi: 10.1016/j.abb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q., Wang B., Li Y. Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: randomized, double-Blind, placebo-controlled study. Hypertension. 2016;67(3):541–549. doi: 10.1161/HYPERTENSIONAHA.115.06624. [DOI] [PubMed] [Google Scholar]
- 28.Bo S., Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol. 2008;19(1):50–56. doi: 10.1097/MOL.0b013e3282f33ccc. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffari M.S., Patel C., Abdelsayed R., Schaffer S.W. Accelerated NaCl-induced hypertension in taurine-deficient rat: role of renal function. Kidney Int. 2006;70(2):329–337. doi: 10.1038/sj.ki.5001503. [DOI] [PubMed] [Google Scholar]
- 30.Balaraman R., Gulati O.D., Bhatt J.D., Rathod S.P., Hemavathi K.G. Cadmium-induced hypertension in rats. Pharmacology. 1989;38(4):226–234. doi: 10.1159/000138541. [DOI] [PubMed] [Google Scholar]
- 31.Choudhary R., Bodakhe S.H. Magnesium taurate prevents cataractogenesis via restoration of lenticular oxidative damage and ATPase function in cadmium chloride-induced hypertensive experimental animals. Biomed Pharmacother. 2016;84:836–844. doi: 10.1016/j.biopha.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Choudhary R., Bodakhe S.H. Olmesartan, an angiotensin II receptor blocker inhibits the progression of cataract formation in cadmium chloride induced hypertensive albino rats. Life Sci. 2016;167:105–112. doi: 10.1016/j.lfs.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Khan S.A., Choudhary R., Singh A., Bodakhe S.H. Hypertension potentiates cataractogenesis in rat eye through modulation of oxidative stress and electrolyte homeostasis. J Curr Ophthalmol. 2016;28(3):123–130. doi: 10.1016/j.joco.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantrell C.R., Borum P.R. Identification of a cardiac carnitine binding protein. J Biol Chem. 1982;257(18):10599–10604. [PubMed] [Google Scholar]
- 35.Tappel A.L. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 36.Sinha A.K. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 37.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132. [PubMed] [Google Scholar]
- 38.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 39.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Asdaq S.M., Inamdar M.N. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. 2010;17(13):1016–1026. doi: 10.1016/j.phymed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Mollaoglu H., Gokcimen A., Ozguner F. Caffeic acid phenethyl ester prevents cadmium-induced cardiac impairment in rat. Toxicology. 2006;227(1–2):15–20. doi: 10.1016/j.tox.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Korkmaz S., Ekici F., Tufan H.A., Aydın B. Magnesium: effect on ocular health as a calcium channel antagonist. J Clin Exp Invest. 2013;4(2):244–251. [Google Scholar]
- 43.Halliwell B. Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol. 1989;70(6):737–757. [PMC free article] [PubMed] [Google Scholar]
- 44.Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abucayon E., Ke N., Cornut R. Investigating catalase activity through hydrogen peroxide decomposition by bacteria biofilms in real time using scanning electrochemical microscopy. Anal Chem. 2014;86(1):498–505. doi: 10.1021/ac402475m. [DOI] [PubMed] [Google Scholar]
- 46.Li C., Shi L., Chen D., Ren A., Gao T., Zhao M. Functional analysis of the role of glutathione peroxidase (GPx) in the ROS signaling pathway, hyphal branching and the regulation of ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet Biol. 2015;82:168–180. doi: 10.1016/j.fgb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Pandey K.B., Rizvi S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3(1):2–12. doi: 10.4161/oxim.3.1.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiny K.S., Kumar S.H., Farvin K.H., Anandan R., Devadasan K. Protective effect of taurine on myocardial antioxidant status in isoprenaline-induced myocardial infarction in rats. J Pharm Pharmacol. 2005;57(10):1313–1317. doi: 10.1211/jpp.57.10.0010. [DOI] [PubMed] [Google Scholar]
- 49.Zhou J., Li Y., Yan G. Protective role of taurine against morphine-induced neurotoxicity in C6 cells via inhibition of oxidative stress. Neurotox Res. 2011;20(4):334–342. doi: 10.1007/s12640-011-9247-x. [DOI] [PubMed] [Google Scholar]
- 50.Ho E., Karimi Galougahi K., Liu C.-C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1(1):483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]