Key Points
Oxylipins and plasticizers from end-of-storage RBC supernatants accumulate ∼20-fold in the bloodstream of transfusion recipients.
Posttransfusion levels of oxylipins and arginine metabolites negatively correlate with hemodynamic properties.
Abstract
Cold storage of blood for 5 to 6 weeks has been shown to impair endothelial function after transfusion and has been associated with measures of end-organ dysfunction. Although the products of hemolysis, such as cell-free plasma hemoglobin, arginase, heme, and iron, in part mediate these effects, a complete analysis of transfused metabolites that may affect organ function has not been evaluated to date. Blood stored for either 5 or 42 days was collected from 18 healthy autologous volunteers, prior to and after autologous transfusion into the forearm circulation, followed by metabolomics analyses. Significant metabolic changes were observed in the plasma levels of hemolytic markers, oxidized purines, plasticizers, and oxidized lipids in recipients of blood stored for 42 days, compared with 5 days. Notably, transfusion of day 42 red blood cells (RBCs) increased circulating levels of plasticizers (diethylhexyl phthalate and derivatives) by up to 18-fold. Similarly, transfusion of day 42 blood significantly increased circulating levels of proinflammatory oxylipins, including prostaglandins, hydroxyeicosatrienoic acids (HETEs), and dihydroxyoctadecenoic acids. Oxylipins were the most significantly increasing metabolites (for 9-HETE: up to ∼41-fold, P = 3.7e-06) in day 42 supernatants. Measurements of arginine metabolism confirmed an increase in arginase activity at the expense of nitric oxide synthesis capacity in the bloodstream of recipients of day 42 blood, which correlated with measurements of hemodynamics. Metabolic changes in stored RBC supernatants impact the plasma metabolome of healthy transfusion recipients, with observed increases in plasticizers, as well as vasoactive, pro-oxidative, proinflammatory, and immunomodulatory metabolites after 42 days of storage.
Visual Abstract
Introduction
Transfusion of red blood cell (RBC) concentrates is the most common in-hospital medical procedure worldwide, with over 90 million people transfused every year. Blood transfusion is a life-saving intervention for chronically or massively transfused recipients, who rely on altruistically donated blood to restore systemic oxygenation secondary to bleeding or anemia. In the light of these numbers, it is self-evident why blood storage in the blood bank is a logistic necessity that makes ∼11 million units of blood available for routine transfusion every year in the United States alone.1 However, as RBCs are stored in the blood bank, they progressively accumulate a series of morphological2 and biochemical3 modifications collectively referred to as the storage lesion. The introduction of omics technologies, in particular, metabolomics, to the field of transfusion medicine4 has advanced our understanding of the kinetics and extent of the metabolic storage lesion, which is a direct indicator of RBC physiology and function.
High-energy phosphate compounds such as adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (DPG) are relevant mediators of RBC homeostasis and gas transport function because they control energy metabolism, ion and redox homeostasis, structural membrane integrity, and hemoglobin-oxygen–binding kinetics.3 For example, proton pumps5 and glutathione synthesis,6,7 phosphatidylserine recycling to the inner lipid membrane bilayer,8 or regulatory phosphorylation of structural proteins,9-11 and, most and foremost, hemoglobin-oxygen binding and off-loading12 are processes finely tuned by ATP and DPG availability. Leveraging classic biochemical tools and understanding of RBC biology, over the past 100 years, transfusion scientists have designed novel storage additives that minimize storage-induced depletion of RBC ATP and DPG, with excellent results in the case of alkaline additives,13 rejuvenation solutions14,15 or oxygen-controlled storage strategies (eg, anaerobic storage).16,17 Recent metabolomics studies expanded our understanding of the impact of RBC processing and storage strategies, including storage additives, on the metabolic storage lesion.18-27 The advent of studies that leveraged the power of stable isotope-labeled tracers revealed the impact of RBC storage on metabolic rewiring and the kinetics of this process,6,28-32 ultimately identifying 3 metabolic phases that can be exploited to engineer novel storage additives through an iterative process that combines systems biology–based predictions and metabolomics testing of novel solutions.33
Despite these advances, reassuring evidence from randomized clinical trials on the age of blood34-37 prompted the inevitable question, “Cui prodest?” Although it is beyond the scope of this manuscript to comment on the controversies related to the interpretations of these trials,38 the potential for improvement of current storage strategies clearly emerges if we consider the body of literature that is still burgeoning on the negative impact of the storage lesion on posttransfusion recoveries,39 intravascular/extravascular hemolysis (which in turn negatively impacts vasodilatory capacity and circulating non–transferrin-bound iron) and transfusion outcomes in animal models (eg, of sepsis),40 as well as retrospective studies41 and controlled small-scale clinical trials.42,43 A number of these studies suggest that a combination of (1) the extreme of storage age of the unit (5-6 weeks), (2) the donor’s genetic susceptibility to storage and stress hemolysis, (3) the number of such units transfused, and (4) the susceptibility of the host (infection, diabetes, etc) are all potential contributing factors to the adverse effects of transfusion. For example, our research groups have identified common variables such as sex (especially in males), African American ethnicity, and sickle cell trait status as potential genetic modulators of erythrocyte viability during prolonged cold storage.25,44-48
The disconnect between large-scale randomized clinical trials and laboratory studies can be at least in part explained by the failure of the latter to appreciate the impact on blood quality of variables almost as critical as the age of blood. Some of these caveats are being addressed by the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) omics study. Within the framework of this study, RBCs from ∼14 000 healthy donor volunteers were tested for their propensity to hemolyze (either spontaneously or following oxidative and osmotic insult). Such a large-scale laboratory study revealed for the first time that donor genetics (eg, ancestry, ethnicity, sex, etc) does indeed impact not only hemolytic propensity,44 but also metabolic variance over storage.49 These results suggest that novel storage additives may be tailored not only to counteract the storage lesion, but also to minimize the RBC metabolic toll of storage on a donor-to-donor basis. Such an approach would enable the introduction of personalized additives, continuing the tradition of transfusion medicine as a pioneer discipline in the field of personalized medicine, owing to its century-long attention to donor and recipient biologies (eg, blood-group matching).
To define improved storage strategies based on metabolomics data, critical information should be obtained by directly testing the impact of blood storage and stored blood transfusion on recipient vascular and endothelial function. Recently, we performed a small-scale study to determine the impact of blood transfusion on sickle cell recipients’ plasma and RBC metabolism.50 However, that study was limited by the number of subjects enrolled (n = 8) and other biological confounders (ie, recipients underwent exchange transfusion of >10 U of blood from different donors and ∼15 storage days of age). In this view, here we performed a metabolomics study of blood from 18 healthy volunteers, who received autologous transfusion of blood stored for either 5 or 42 days. Such a controlled study was coupled to investigations of hemodynamics as a function of blood flow following acetylcholine (Ach)-stimulated vasodilation for 30 minutes. We previously published that the transfusion of autologous blood stored for 42 days, compared with 5 days, reduced endothelium-dependent vasodilation elicited by acetylcholine infusion. Interestingly, while impairing endothelial-dependent blood flow, the transfusion of aged autologous blood increased basal nitric oxide (NO)-independent blood flow. Similar studies have been performed by other investigators in human subjects and rodent models showing aged stored blood impairs NO-dependent and endothelial-dependent blood flow responses, an observation described as endothelial dysfunction.42 Expanding on those studies, here we report for the first time the results from a controlled clinical trial on the impact of the age of blood on recipients’ plasma metabolism and blood flow.
Materials and methods
Overview of the study
The investigation enrolled a total of 18 healthy individuals at the University of Pittsburgh who were evaluated with strain-gauge plethysmography before and after infusion of autologous blood stored for either 5 days or 42 days to evaluate forearm blood flow and vasodilatory responses to acetylcholine. Inclusion and exclusion criteria are reported in supplemental Methods and in prior studies.42 The institutional review board at the University of Pittsburgh (#PRO09100113) approved the study. Written informed consent was obtained from subjects. A National Heart, Lung, and Blood Institute independent data safety and monitoring board monitored the study.
Blood donation
Standard protocols were adopted, as described in prior publications.42
Blood infusion
The IV access procedure was extensively described in prior publications.42 On the first testing day (range, 5-7 days), the subjects received 0.5 U of blood, ∼138 ± 9 mL (mean plus or minus standard deviation), transfused through a brachial artery line into the nondominant arm. On the second testing day, performed 5 weeks later (range, 40-42 days), the remainder of the unit was transfused. Blood was intraarterially transfused for a total duration ranging from 9 to 12 minutes. The intra-arterial infusion protocol (described in detail in Risbano et al42) allowed for exposure of the forearm circulation to the equivalent of a 2- to 4-U transfusion volume over 9 to 12 minutes and allowed for collection of venous blood from the same arm antecubital vein. The protocol thus modeled a larger systemic transfusion exposure, while maintaining volunteer safety. The volume of blood in each 0.5 U influenced the duration of each transfusion. On average, the first transfusion of 140 ± 16 mL and second transfusion of 138 ± 22 mL lasted ∼11.1 ± 1.2 and 10.9 ± 1.4 minutes, respectively. The sequentially increasing rates of transfusion were 5 mL per minute for 2 minutes (300 mL/h), 10 mL per minute for 2 minutes (600 mL/h), and finally 15 mL per minute until the unit was fully transfused (900 mL/h). Before the study, we confirmed that transfusion of red cells through a 20-gauge brachial artery angiocatheter at infusion rates of 300, 600, and 900 mL per hour through the same infusion pump, filter, and tubing system did not produce any artifactual hemolysis.42
Forearm blood-flow measurements
Forearm blood-flow measurements were performed with venous occlusion strain-gauge plethysmography (Hokanson, Bellevue, WA). Eighteen participants underwent the same protocol on days 5 and 42 with the exception of the age of blood infused. These subjects received sequential infusion of Ach, autologous RBC transfusion, and the repeat Ach infusion. This protocol allowed for direct measurement of the effects of transfusion on baseline blood flow, and on acetylcholine-dependent blood flow. Subjects refrained from drinking alcohol and caffeine for 8 hours and fasted overnight. Nonsteroidal anti-inflammatory drugs were not permitted 1 week before to the study. All studies were performed in the morning to reduce circadian variation, in a quiet, temperature-controlled room.
Ach stimulation
Miochol-E powder (Novartis Pharmaceuticals, Inc, Hanover, NJ) was used as the source of sterile Ach in this study. The University of Pittsburgh Medical Center Investigational Drug Service prepared the Ach solution for use. Ach acts as an endothelium-dependent vasodilator, stimulating endothelial cell muscarinic receptors to increase intracellular calcium and activate the endothelial NO synthase enzyme to synthesize NO from arginine, which relaxes vascular smooth muscle.51 Ach was continuously infused into the brachial artery with 3 sequentially increasing doses, each for 3 minutes at 7.5, 15, and 30 μg per minute before and after the intraarterial infusion of autologous blood. These Ach doses corresponded to infusion rates of 15, 30, and 60 mL per hour. Ach was infused with a normal saline carrier at decreasing rates of 105, 90, and 60 mL per hour, respectively, to maintain a constant fluid rate of 120 mL per hour to control for volume effects on blood-flow measurements.
Metabolomics and lipidomics
Sample extraction.
Metabolomics analyses were performed on 20 μL of either supernatants or plasma at a 1/25 dilution in a methanol-to-acetonitrile-to-water ratio of 5:3:2 vol/vol and pure methanol extraction (Optima; Thermo Fisher Scientific, San Jose, CA) prior to vortexing and centrifugation, as described.52,53 Supernatants were analyzed via ultra-high-pressure liquid chromatography coupled to mass spectrometry (UHPLC-MS).
UHPLC-MS analysis.
The analytical platform uses a Vanquish UHPLC system (Thermo Fisher Scientific) coupled online to a Q Exactive mass spectrometer (Thermo Fisher Scientific), as extensively described in the extended supplemental Methods as well as in prior work.49,50,52,54 Metabolite assignments, isotopologue distributions, and correction for expected natural abundances of deuterium, 13C, and 15N isotopes were performed using MAVEN (Princeton, NJ),55 against an in-house library of deuterated lipid standards (SPLASH LIPIDOMIX Mass Spec Standard; Avanti Lipids). Discovery mode analysis was performed with standard workflows using Compound Discoverer (Thermo Fisher Scientific).
Statistical analysis
Significance was determined through the 2-tailed paired Student t test (Microsoft Excel, Redmond, CA; GraphPad Prism 5.0, Prism, San Diego, CA) for paired measurements in the bag at days 5 and 42 and in plasma from recipients of blood stored for 5 or 42 days (prior to and after transfusion), elaborated in supplemental Table 1.
Results
Metabolomics analyses of in vitro supernatants of RBCs stored for 5 and 42 days
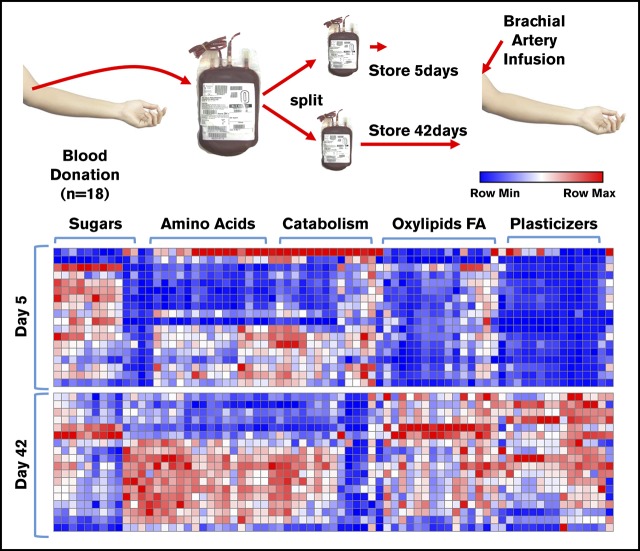
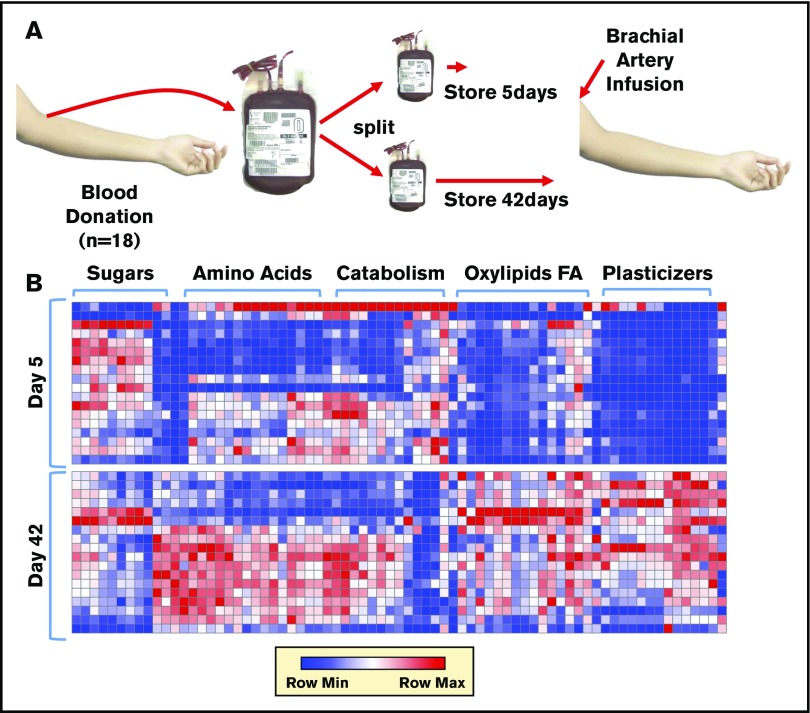
A packed RBC unit contains plasma mixed with adenine, dextrose, sorbitol, sodium chloride, and mannitol (ADSOL) additive solution. Metabolomics analyses were performed on these matched supernatants of RBC concentrates from 18 donors stored for either 5 or 42 days (Figure 1A). Results are reported in a tabulated form in supplemental Table 1 (metabolomics) and supplemental Table 2 (lipidomics), or as heat maps with hierarchical clustering in Figure 1B (a vectorial [scalable] version is provided in supplemental Figure 1). Storage promoted the consumption of sugars (eg, glucose) and other substrates from the storage additive solution 1, ADSOL (eg, adenine), while promoting the accumulation of amino acids, carboxylates, free fatty acids, oxylipins, and plasticizers (Figure 1B; supplemental Figure 1). Results are comparable to previous observations from RBCs stored in other additives, such as saline, adenine, glucose, mannitol,33,56 AS-3,6,21 and AS-5.20
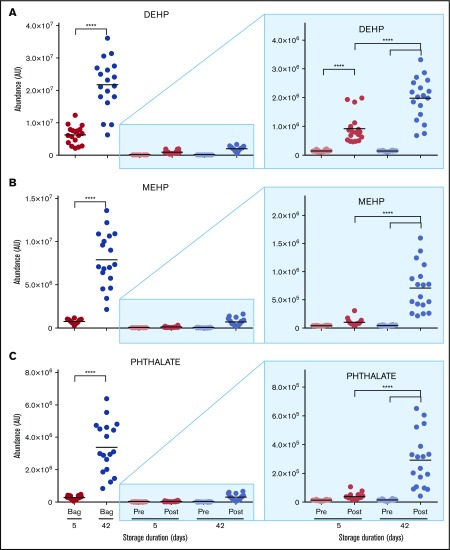
Figure 1.
Metabolomics analysis of blood components post-storage and autologous transfusion. (A) Overview of the experimental design. Briefly, 18 healthy volunteers donated a unit of blood. Packed RBCs were thus split and stored for 5 and 42 days, respectively, prior to autologous reinfusion via brachial artery. (B) A heat map summarizing the main metabolic changes in the supernatants of units stored for 5 (top) and 42 days (bottom). Color codes indicate row minimum (min; blue) to maximum (max; red) upon z-score normalization across values measured in all samples. FA, fatty acids.
Transfusion impacts in vivo plasma metabolism of autologous recipients as a function of storage age
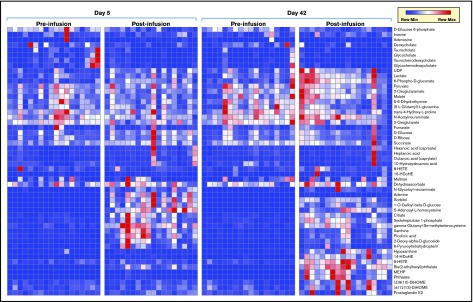
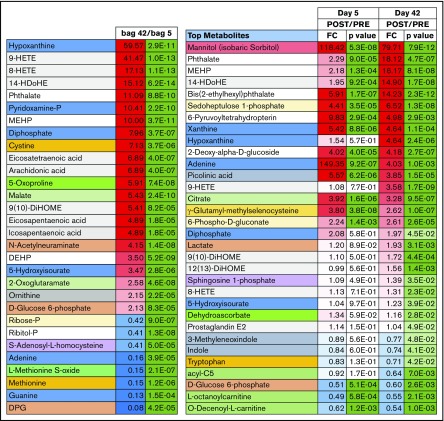
After transfusion, the plasma leaving the forearm collected from the antecubital vein was collected and metabolites in the plasma collected. Transfusion of RBC units stored for either 5 or 42 days significantly impacted plasma metabolism of healthy autologous recipients (Figure 2; vectorial version of the heat map in supplemental Figure 2). Raw data, fold-changes, and P values of pre- vs posttransfusion measurements are reported in supplemental Table 1, whereas the most significant changes in the plasma metabolome as a function of transfusion of day 5 and day 42 blood are highlighted in Figure 3. Transfusion (independent from storage duration) had a dilution effect on circulating metabolites like bile acids, including conjugated bile acids taurocholate, taurochenodeoxycholate, and glycocholate and glycochenodeoxycholate, which clustered together in the heat map in Figure 2. Minor (storage-independent) increases in plasma levels of short-chain fatty acids hexanoate, heptanoate, and octanoate were observed upon transfusion in a donor-dependent fashion (Figure 2). Transfusion increased circulating levels of metabolites involved in purine metabolism (eg, S-adenosyl-homocysteine, xanthine) and anticoagulant/additive solution components (eg, adenine, citrate, more markedly so upon transfusion of fresher blood; Figure 2). In the latter category, increases in mannitol (a component of storage additives) were noted upon transfusion, though orthogonal chromatographic approaches would be required to disambiguate the contribution of eventual isobaric isomers such as sorbitol (Figure 2). On the other hand, plasma levels of purine oxidation products (eg, hypoxanthine), oxylipins (including several prostaglandins, hydroxyeicosatrienoic acids [HETEs], and hydroxyoctadecenoic acids [HOMEs]), were exclusively increased by transfusion of day 42 blood (Figure 2).
Figure 2.
Metabolic phenotypes of plasma pre- and postinfusion of day 5 or day 42 RBC concentrates. HDoHE, hydroxydocosahexaenoic acid; MEHP, mono-ethylhexyl phthalate; UDP, uridine diphosphate.
Figure 3.
Highlights of the most significant changes in the plasma metabolome following transfusion of day 5 or day 42 stored RBCs. DEHP, bis(2-ethylhexyl)phthalate.
Transfusion impacts circulating levels of glucose and glucose-oxidation products
Blood storage from day 5 to day 42 corresponds to ∼50% consumption of glucose in the hyperglycemic additive solution (111 mM57; ie, ∼22-fold higher than physiological levels) and the generation of lactate to ∼30 mM levels (>30-fold higher than physiological). Despite considerations related to dilution (herein we transfused a single split unit to an autologous healthy recipient), clear increases in both plasma glucose (especially in fresher units) and lactate (especially in day 42 units) were observed, as expected (supplemental Figure 3). Similarly, posttransfusion plasma levels of hexose phosphates and ribose phosphate were decreased by transfusion of both fresh and day 42 blood, whereas phosphogluconate and sedoheptulose phosphate (markers of the pentose phosphate pathway) were increased two- to sixfold posttransfusion independent of storage duration (supplemental Figure 3). Posttransfusion increases in the plasma levels of these metabolites could be explained by increased hemolysis. On the other hand, detection of low levels of these intracellular metabolites in plasma prior to transfusion may be explained by minimal hemolysis from preanalytical procedures, despite efforts to minimize this phenomenon.
Storage age impacts posttransfusion levels of oxidized purines
Storage additives like ADSOL are rich in purine substrates to fuel ATP synthesis, such as adenine (∼2 mM57; >2000-fold higher than physiological levels in plasma). During storage, adenine is rapidly consumed within the first 2 weeks, though adenine supplementation after storage day 10 does not provide significant metabolic advantage with respect to ATP levels31 in the absence of early/end-of-storage metabolic rejuvenation.26,58 Transfusion of day 5 RBCs (but not day 42) results in significant increases in plasma adenine (supplemental Figure 4). Similarly, guanine consumption during storage results in significant decreases in circulating guanine only upon transfusion of day 42 RBCs (supplemental Figure 4). Circulating adenine and guanine can be metabolized systemically, though it can also fuel hydrogen peroxide–generating reactions catalyzed by xanthine oxidase, a reaction that also produces xanthine, hypoxanthine, urate, and hydroxyisourate. Of note, in higher mammals, the enzymatic conversion of hydroxyisourate to allantoin/allantoate is hampered by the lack of a functional uricase enzyme, which makes hydroxyisourate a final metabolic product of this pathway. Of note, all metabolites in the purine oxidation pathway increase significantly upon transfusion, especially of day 42 blood (supplemental Figure 4).
Blood transfusion increases circulating levels of tricarboxylates and some dicarboxylates
Transfusion of either day 5 and 42 blood significantly increased circulating levels of citrate (up to threefold from baseline when recipients were transfused either day 5 or 42 blood), whereas no significant changes were observed in the posttransfusion levels of succinate and malate and α-ketoglutarate (supplemental Figure 5). No significant changes were observed in the levels of glutamine and glutamate, which participate in transamination reactions involving ketoglutarate (supplemental Figure 5). Of note, α-ketoglutarate levels in day 5 and 42 supernatants are significantly lower than baseline circulating levels of this metabolite, suggesting a likely dilution effect upon transfusion.
Consumption of circulating methionine is faster upon transfusion of day 42 blood
Building on pioneering evidence from Clarke59-62 and Ingrosso,63-66 we recently reported that methionine consumption in vivo and in vitro is a marker of activation of the protein damage-repair mechanism that ensues upon oxidative damage to key RBC structural and functional proteins.64 Building on this evidence, here we report that circulating methionine levels in transfused recipients are lower upon transfusion of day 42 (borderline significant), but not day 5 blood (supplemental Figure 6). Although this result may be also in part explained by dilution effect (supernatant levels of methionine decrease significantly in day 42 units), an alternative explanation of this phenomenon may be attributable to the impact of transfused RBCs as scavengers of plasma methionine. Methionine consumption in RBCs would fuel protein damage-repair pathways and result in significant increases in plasma RBCs and plasma S-adenosylhomocysteine, as observed upon transfusion (supplemental Figure 6). However, increases in plasma S-adenosylhomocysteine may also be consistent with increased hemolysis.
Lipidomics analyses reveal significant posttransfusion increases in plasma levels of plasticizers and oxylipins as a function of storage age
The most significant impact of storage age on posttransfusion plasma metabolism relates to the lipid compartment. Although no significant changes were observed in posttransfusion circulating levels of cholesterol, cholesteryl-esters, or other sterol hormones (supplemental Table 2), transfusion of day 42 RBCs increased circulating levels of plasticizers (diethylhexyl phthalate and derivative) by up to 18-fold (Figure 4; supplemental Table 1).
Figure 4.
Overview of plasticizers in the supernatants of RBCs stored for 5 and 42 days and in the plasma of autologous recipients pre- and posttransfusion of the same units. (A) DEHP. (B) MEHP. (C) Phthalate. ****P < .0001 (2-tailed paired Student t test).
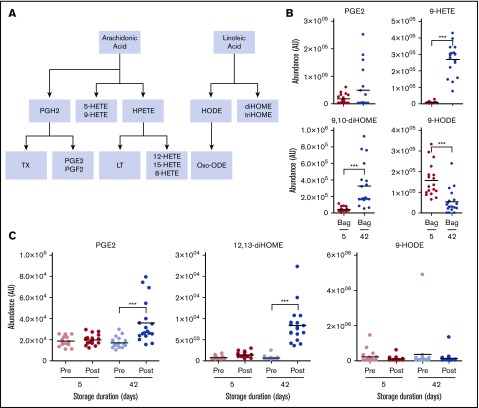
Similarly, transfusion of day 42 blood significantly increased circulating levels of proinflammatory oxylipins, including eicosanoids and linoleic acid oxidized derivatives prostaglandins, HETEs, and diHOMEs (Figure 5). Of note, oxylipins were almost the most significantly increasing metabolites (for 9-HETE: up to ∼41-fold, P = 3.7e-06; supplemental Table 1) when comparing day 42 vs day 5 supernatants.
Figure 5.
Overview of eicosanoids and oxylipins in the supernatants of RBCs stored for 5 and 42 days and in the plasma of autologous recipients pre- and posttransfusion of the same units. (A) Outline of eicosanoid and oxylipin metabolism. (B) Levels of eicosanoids in RBC supernatants at 5 and 42 days. (C) Levels of eicosanoids in autologous recipient plasma. ***P < .001; ****P < .0001 (2-tailed paired Student t test). HPETE, hydroperoxyeicosatetraenoic acid; LT, leukotriene; Oxo-ODE, oxo-octadecadienoic acid; PGE2, prostaglandin E2; PGF2, prostaglandin F2; PGH2, prostaglandin H2; TX, thromboxane.
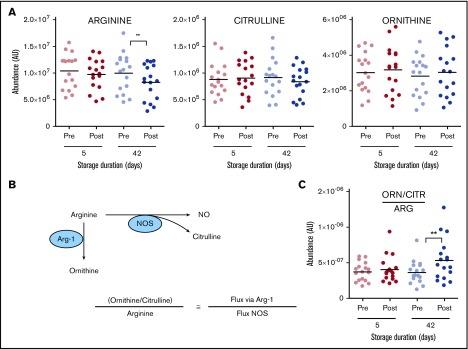
Transfusion of day 42 blood impairs NO metabolism
Previous studies have reported that transfusion of day 42 resulted in altered NO metabolism through mechanisms of cell-free plasma hemoglobin-dependent scavenging and RBC lysis-dependent increases in circulating arginase-1.42 Metabolic testing of the same sample from the study by Risbano et al42 confirmed these observations by reporting significant decreases in plasma arginine following transfusion of day 42 (less markedly so for day 5) blood (Figure 6). Determination of circulating levels of ornithine (a product of arginase activity) and citrulline (a product of NO synthase activity) allowed for the conclusion that transfusion of day 42 blood significantly increases fluxes through the arginase-1 pathway (Figure 6), potentially impacting NO-mediated vasodilation. The arginine/ornithine plus citrulline ratio has been shown to correlate with both RBC and vascular arginase 167 and 268 activity, respectively.
Figure 6.
Overview of arginine (ARG) and NO metabolism in the supernatants of RBCs stored for 5 and 42 days and in the plasma of autologous recipients pre- and posttransfusion of the same units. (A) Arginine and its downstream metabolites. (B) Outline of arginine metabolism by NO synthase (NOS) and arginase. (C) Determination of arginine fate. **P < .01 (2-tailed paired Student t test). CTR, citrulline; ORN, ornithine.
Metabolic correlates to Ach-stimulated vasodilation
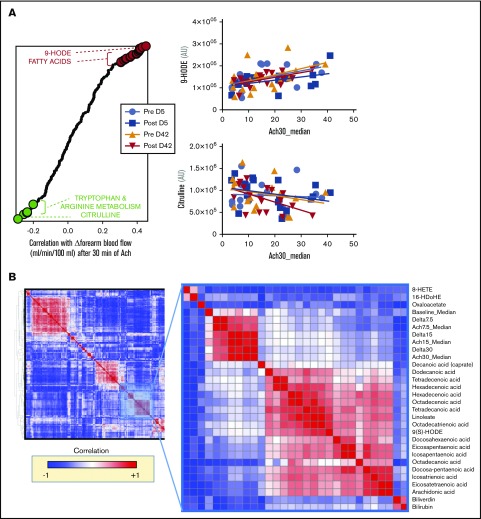
Blood flow was determined at baseline and after 30 minutes of Ach-stimulated vasodilation (30 μg/min), as described in Risbano et al.42 In this prior study, impairments in Ach stimulation–dependent changes in blood flow correlated with markers of hemolysis, such as plasma levels of RBC-derived arginase 1 and cell-free hemoglobin. Here, we correlated the same measurements of Ach-dependent blood-flow stimulation to metabolomics measurements of day 5 and day 42 RBC supernatants, as well as to metabolic measurements of posttransfusion metabolic changes in autologous recipients receiving either day 5 or day 42 blood. Results are shown in the graph in Figure 7 and are extensively reported in a tabulated format in supplemental Table 1. Of note, free fatty acids and some specific lipid peroxidation products (9-hydroxyoctadecadienoic acid [9-HODE]) resulted in being the top positive correlates between Δ-blood flow (ie, positive correlation with Ach-stimulated vasodilation). On the other hand, other oxylipins (including 9-HETE), tryptophan metabolites (eg, kynurenine) and metabolites involved in arginine metabolism (eg, citrulline, especially following transfusion of day 42 blood) showed significant negative correlation with Δ-blood flow (ie, negative correlation with Ach-stimulated vasodilation) (Figure 7). Unsupervised clustering of Δ measurements (calculated as posttransfusion changes upon baseline) confirmed coclustering of blood-flow measurements and with metabolic correlates such as oxylipins, free fatty acids, and 2 markers of hemolysis biliverdin and bilirubin (Figure 7).
Figure 7.
Metabolic correlates to Ach-stimulated vasodilation. (A) Top metabolites from stored RBC supernatants that positively (red) or negatively (green) correlate with changes in forearm blood flow after 30 minutes from stimulation with Ach. (B) Hierarchical clustering analysis of correlations indicates that physiological correlates clustered close to a node including oxylipins, free fatty acids, and markers of hemolysis like biliverdin and bilirubin.
Discussion
RBC storage in the blood bank is characterized by a series of metabolic changes, though it is unclear whether and to what extent these alterations correlate or etiologically contribute to clinically relevant parameters. In the present study, we performed metabolomics analyses on RBC supernatants and plasma from 18 autologous healthy blood donors who received one-half of a unit of their blood, both after 5 and 42 days of storage, infused into the forearm circulation via the brachial artery. Such a controlled study design, in which each autologous donor received his or her own blood upon 5- or 42-day-long storage, allows analysis of signals that could have been otherwise lost owing to biological variability, recognizing that this phenomenon was still observed to a minimal extent in this study with some donor/recipients’ blood storing differently from others. This study modeled a relatively high concentration of blood in the forearm circulation, and the design allowed for control of biological variables such as the impact of donor/recipient biologics on transfusion outcomes.
In this study, subjects were also tested for Ach-stimulated vasodilatory capacity before and after transfusion. Ach stimulation of the Ach receptor stimulates endothelial cell-mediated NO synthesis and release, which in turn promotes vasodilation. Prior studies have shown that transfusion of day 42 blood significantly increases plasma arginase 1 and cell-free hemoglobin levels, factors reducing synthesis (by mechanism of substrate consumption) or bioavailability (via direct scavenging) of vasodilatory NO.42 Here, we confirm and expand on this evidence, by providing direct measurements of (1) hemolysis-derived metabolites (eg, pentose phosphate pathway intermediates, which are not actively released in the plasma); (2) purine oxidation products (including hypoxanthine, a marker of posttransfusion recovery54 and its oxidation byproduct hydroxyisourate); and (3) pre- and posttransfusion levels of circulating arginine, ornithine, and citrulline. Through equations derived from the law of mass action, we infer decreased fluxes from arginine to citrulline for the synthesis of NO following transfusion of day 42 (but not day 5) stored blood. Although further studies will be necessary (for example with the use of stable isotope-labeled tracers such as 15N-arginine), we provide direct evidence of the impact of storage-induced metabolic regulation of NO metabolism and its correlation to Ach-stimulated NO vasodilatory function in vivo.
Interestingly, in this very cohort, Risbano et al42 had reported the counterintuitive finding that, despite impaired endothelial function (Ach-dependent blood flow changes) following transfusion of day 42 blood, basal blood flow actually increased in the 42-day blood transfusion group in comparison with the same subjects receiving blood stored for only 5 days. This basal effect was never explained. This observation is suggestive that some NO-independent (Ach-independent) factor independently contributes to increases in blood flow upon transfusion of day 42 blood. Here, we observed significant negative correlations between Ach-stimulated vasodilation and several oxylipins. This is relevant for several reasons: (1) oxylipins are the class of metabolites with the highest storage-dependent increases in stored RBCs and supernatants in this and other studies6,24; (2) this class of metabolites has been associated with RBC posttransfusion recoveries in animal models39; and (3) proinflammatory oxylipins promote inflammation and vasodilation.69 Consistent with the latter point, >90% of healthy volunteers enrolled in the present study reported an increased sensation of “warmth” and “pins and needles” in the forearm during the infusion of 42-day blood (rich in oxylipins), but not with infusion of fresh blood.42 Of note, dietary supplementation of polyunsaturated fatty acids may limit the pool of arachidonic and linoleic acid available for the synthesis of some of these oxylipins.69 How increases in oxylipins may affect vascular and end-organ function has not been considered to date and deserves additional study. Finally, it is worth noting that a potential player in the Ach-stimulation assay could be represented by RBC acetylcholinesterase (AChE). Mature RBCs and stored RBC-derived vesicles have been reported to have a functional AChE, an enzyme whose activity decreases in RBC during aging in vivo or after 1 day of storage, or in RBC-derived particles during aging in vitro.70
Similarly, we noted that transfusion of day 42 blood promoted minor (<twofold) albeit significant (P < .05) increases in the circulating levels of tryptophan catabolites (eg, picolinic acid), tricarboxylates (eg, citrate), or sphingolipids (eg, sphingosine 1-phosphate), all metabolites with an increasingly appreciated role in immunometabolism and inflammation.71-74
Finally, consistent with previous observations in sickle cell recipients undergoing exchange transfusion,46 we observed significant increases in circulating levels of plasticizers following transfusion of day 42 blood. End-of-storage levels of plasticizers (400 μM to 1 mM) in packed RBC supernatants75 and calculated ∼10-fold dilutions (one 450-mL unit in 5 L of recipients’ blood) may reach levels consistent with toxicity and other untoward long-term outcomes (eg, endocrine dysfunction and deriving infertility) reported in animal models.76 Increased odds ratios for such complications may have been missed by randomized clinical trials on the age of blood that exclusively tested survival or other short-term outcomes (eg, duration of hospitalization).
In conclusion, in the present study, we describe the impact of storage duration on the metabolome and lipidome of stored RBC supernatants at days 5 and 42. We thus explore the impact of fresh or stored blood transfusion on the plasma metabolome of autologous healthy volunteers. As a result, we identify (1) metabolites uniquely increasing in recipients of fresh units, mostly limited to components of storage additives and anticoagulants (eg, citrate, adenine, glucose); (2) plasma metabolites increasing or decreasing upon transfusion, independently of storage age (concentration or dilution effects); and (3) metabolites increasing in recipients of day 42 units, in particular purine-oxidation products, plasticizers, and proinflammatory oxylipins. The latter group may help explain the counterintuitive increase in posttransfusion blood flow observed in recipients of day 42 blood, despite the impairment of NO synthesis capacity upon transfusion of older blood, herein confirmed by direct measurements of substrate and products of arginine metabolism.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by funds from the Boettcher Webb-Waring Investigator Award (A.D.), the Shared Instrument grant by the National Institutes of Health Office of the Director (S10OD021641). M.T.G. received research support from National Institutes of Health, National Heart, Lung, and Blood Institute grant 2R01HL098032, the Institute for Transfusion Medicine (Vitalant), and the Hemophilia Center of Western Pennsylvania.
Authorship
Contribution: A.D., J.A.R., Y.Z., and M.T.G. designed the metabolomics studies; T.K., D.J.T., C.D., S.B., J.B., S.J., M.G.R., and M.T.G. designed the clinical study and collected the samples; A.D., J.A.R., S.G., and K.A. performed metabolomics analyses; A.D. prepared the figures and drafted the first version of the manuscript; A.D., J.A.R., T.K., and M.T.G. polished the first version manuscript; and all of the authors contributed to the finalization of the manuscript.
Conflict-of-interest disclosure: A.D. is a consultant for Hemanext Inc and founder of Omix Technologies Inc. M.T.G. is a coinventor of pending patent applications and planned patents directed to the use of recombinant neuroglobin and heme-based molecules as antidotes for carbon monoxide poisoning, which have recently been licensed by Globin Solutions, Inc. M.T.G. is also a shareholder, advisor, and director in Globin Solutions, Inc. Additionally, and unrelated to carbon monoxide poisoning, M.T.G. is a coinventor on patents directed to the use of nitrite salts in cardiovascular diseases, which have been licensed by United Therapeutics and Hope Pharmaceuticals, and is a coinvestigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguat as a treatment for patients with sickle cell disease. The remaining authors declare no competing financial interests.
Correspondence: Angelo D’Alessandro, Department of Biochemistry and Molecular Genetics, University of Colorado–Anschutz Medical Campus, 12801 East 17th Ave, Aurora, CO 80045; e-mail: angelo.dalessandro@ucdenver.edu; and Mark T. Gladwin, Division of Pulmonary Allergy and Critical Care Medicine, School of Medicine, University of Pittsburgh, 1218 Scaife Hall, 3550 Terrace St, Pittsburgh, PA 15261; e-mail: gladwinmt@upmc.edu.
References
- 1.Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56(9):2173-2183. [DOI] [PubMed] [Google Scholar]
- 2.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55(1):205-219. [DOI] [PubMed] [Google Scholar]
- 4.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56(4):980-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatto C, Milanick M. Red blood cell Na pump: insights from species differences. Blood Cells Mol Dis. 2009;42(3):192-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325-336. [DOI] [PubMed] [Google Scholar]
- 7.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion. 2011;51(7):1450-1459. [DOI] [PubMed] [Google Scholar]
- 8.Dinkla S, Peppelman M, Van Der Raadt J, et al. Phosphatidylserine exposure on stored red blood cells as a parameter for donor-dependent variation in product quality. Blood Transfus. 2014;12(2):204-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinalducci S, Longo V, Ceci LR, Zolla L. Targeted quantitative phosphoproteomic analysis of erythrocyte membranes during blood bank storage. J Mass Spectrom. 2015;50(2):326-335. [DOI] [PubMed] [Google Scholar]
- 10.Azouzi S, Romana M, Arashiki N, et al. Band 3 phosphorylation induces irreversible alterations of stored red blood cells. Am J Hematol. 2018;93(5):E110-E112. [DOI] [PubMed] [Google Scholar]
- 11.Boivin P. Role of the phosphorylation of red blood cell membrane proteins. Biochem J. 1988;256(3):689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apheresis Sci. 2010;43(1):69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess JR, Hill HR, Oliver CK, Lippert LE, Greenwalt TJ. Alkaline CPD and the preservation of RBC 2,3-DPG. Transfusion. 2002;42(6):747-752. [DOI] [PubMed] [Google Scholar]
- 14.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion. 2011;51(7):1574-1579. [DOI] [PubMed] [Google Scholar]
- 15.Inglut C, Kausch K, Gray A, Landrigan M. Rejuvenation of stored red blood cells increases oxygen release capacity [abstract]. Blood. 2016;128(22). Abstract 4808. [Google Scholar]
- 16.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8(4):220-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosyst. 2013;9(6):1196-1209. [DOI] [PubMed] [Google Scholar]
- 18.D’Alessandro A, Nemkov T, Hansen KC, Szczepiorkowski ZM, Dumont LJ. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55(12):2955-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Alessandro A, Reisz JA, Culp-Hill R, Korsten H, van Bruggen R, de Korte D. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion. 2018;58(8):1992-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Alessandro A, Hansen KC, Silliman CC, Moore EE, Kelher M, Banerjee A. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2015;108(2):131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55(6):1155-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion. 2016;56(4):852-862. [DOI] [PubMed] [Google Scholar]
- 23.Pertinhez TA, Casali E, Lindner L, Spisni A, Baricchi R, Berni P. Biochemical assessment of red blood cells during storage by (1)H nuclear magnetic resonance spectroscopy. Identification of a biomarker of their level of protection against oxidative stress. Blood Transfus. 2014;12(4):548-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion. 2016;56(10):2560-2570. [DOI] [PubMed] [Google Scholar]
- 25.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion. 2016;56(10):2571-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Alessandro A, Gray AD, Szczepiorkowski ZM, Hansen K, Herschel LH, Dumont LJ. Red blood cell metabolic responses to refrigerated storage, rejuvenation, and frozen storage. Transfusion. 2017;57(4):1019-1030. [DOI] [PubMed] [Google Scholar]
- 27.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, Yoshida T. CO2 -dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion. 2016;56(2):392-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolfsson Ó, Sigurjonsson ÓE, Magnusdottir M, et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 2017;112(4):326-335. [DOI] [PubMed] [Google Scholar]
- 29.Nemkov T, Sun K, Reisz JA, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med (Lausanne). 2017;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128(12):e32-e42. [DOI] [PubMed] [Google Scholar]
- 31.Paglia G, Sigurjónsson ÓE, Bordbar A, et al. Metabolic fate of adenine in red blood cells during storage in SAGM solution. Transfusion. 2016;56(10):2538-2547. [DOI] [PubMed] [Google Scholar]
- 32.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfus Med Rev. 2014;28(2):41-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paglia G, D’Alessandro A, Rolfsson Ó, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128(13):e43-e50. [DOI] [PubMed] [Google Scholar]
- 34.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. JAMA. 2015;314(23):2514-2523. [DOI] [PubMed] [Google Scholar]
- 35.Lacroix J, Hébert PC, Fergusson DA, et al. ; Canadian Critical Care Trials Group. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372(15):1410-1418. [DOI] [PubMed] [Google Scholar]
- 36.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372(15):1419-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308(14):1443-1451. [DOI] [PubMed] [Google Scholar]
- 38.Belpulsi D, Spitalnik SL, Hod EA. The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus. 2017;15(2):112-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101(5):578-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel R, Johnson DJ, Scott AV, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion. 2016;56(7):1690-1698. [DOI] [PubMed] [Google Scholar]
- 42.Risbano MG, Kanias T, Triulzi D, et al. Effects of aged stored autologous red blood cells on human endothelial function. Am J Respir Crit Care Med. 2015;192(10):1223-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. 2017;127(1):375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1(15):1132-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osei-Hwedieh DO, Kanias T, Croix CS, et al. Sickle cell trait increases red blood cell storage hemolysis and post-transfusion clearance in mice. EBioMedicine. 2016;11:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121(9):1663-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion. 2012;52(7):1388-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donadee C, Raat NJH, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Alessandro A, Culp-Hill R, Reisz JA, et al. ; Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS III–Omics. Transfusion. 2019;59(1):89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Culp-Hill R, Srinivasan AJ, Gehrke S, et al. Effects of red blood cell (RBC) transfusion on sickle cell disease recipient plasma and RBC metabolism. Transfusion. 2018;58(12):2797-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14(9):623-641. [DOI] [PubMed] [Google Scholar]
- 52.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion. 2018;58(12):2978-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31(8):663-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103(2):361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melamud E, Vastag L, Rabinowitz JD. Metabolomic analysis and visualization engine for LC-MS data. Anal Chem. 2010;82(23):9818-9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76(spec no):168-180. [DOI] [PubMed] [Google Scholar]
- 57.de Korte D. New additive solutions for red cells. ISBT Sci Ser. 2016;11(suppl 1):165-170. [Google Scholar]
- 58.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17(1):27-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barber JR, Clarke S. Membrane protein carboxyl methylation increases with human erythrocyte age. Evidence for an increase in the number of methylatable sites. J Biol Chem. 1983;258(2):1189-1196. [PubMed] [Google Scholar]
- 60.Janson CA, Clarke S. Identification of aspartic acid as a site of methylation in human erythrocyte membrane proteins. J Biol Chem. 1980;255(24):11640-11643. [PubMed] [Google Scholar]
- 61.Lou LL, Clarke S. Enzymatic methylation of band 3 anion transporter in intact human erythrocytes. Biochemistry. 1987;26(1):52-59. [DOI] [PubMed] [Google Scholar]
- 62.McFadden PN, Clarke S. Methylation at D-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc Natl Acad Sci USA. 1982;79(8):2460-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galletti P, Ingrosso D, Nappi A, Gragnaniello V, Iolascon A, Pinto L. Increased methyl esterification of membrane proteins in aged red-blood cells. Preferential esterification of ankyrin and band-4.1 cytoskeletal proteins. Eur J Biochem. 1983;135(1):25-31. [DOI] [PubMed] [Google Scholar]
- 64.Galletti P, De Bonis ML, Sorrentino A, et al. Accumulation of altered aspartyl residues in erythrocyte proteins from patients with Down’s syndrome. FEBS J. 2007;274(20):5263-5277. [DOI] [PubMed] [Google Scholar]
- 65.Ingrosso D, D’angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267(14):4397-4405. [DOI] [PubMed] [Google Scholar]
- 66.Ingrosso D, Cimmino A, D’Angelo S, Alfinito F, Zappia V, Galletti P. Protein methylation as a marker of aspartate damage in glucose-6-phosphate dehydrogenase-deficient erythrocytes: role of oxidative stress. Eur J Biochem. 2002;269(8):2032-2039. [DOI] [PubMed] [Google Scholar]
- 67.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu W, Kaneko FT, Zheng S, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18(14):1746-1748. [DOI] [PubMed] [Google Scholar]
- 69.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr. 2015;6(5):513-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freitas Leal JK, Adjobo-Hermans MJW, Brock R, Bosman GJCGM. Acetylcholinesterase provides new insights into red blood cell ageing in vivo and in vitro. Blood Transfus. 2017;15(3):232-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci. 2011;32(1):16-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gräler MH. The role of sphingosine 1-phosphate in immunity and sepsis. Am J Clin Exp Immunol. 2012;1(2):90-100. [PMC free article] [PubMed] [Google Scholar]
- 73.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grant RS, Coggan SE, Smythe GA. The physiological action of picolinic acid in the human brain. Int J Tryptophan Res. 2009;2:71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D’alessandro A, Nemkov T, Hansen KC. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfus. 2016;14(2):140-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp Endocrinol. 2015;219:74-88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.