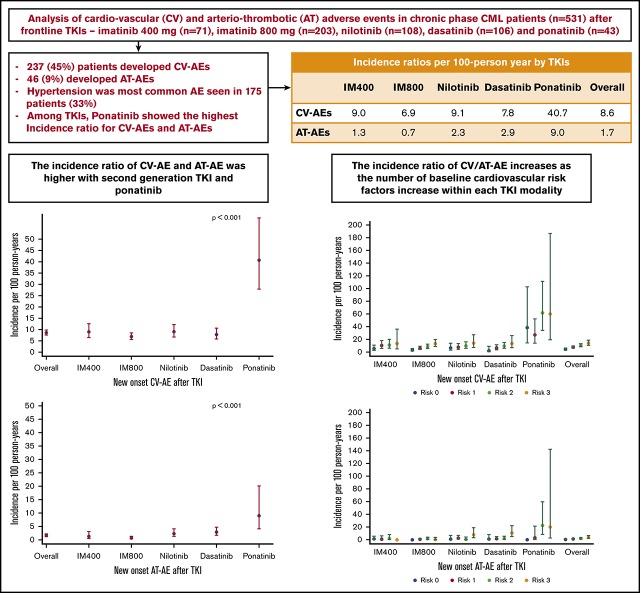
Key Points
TKI therapy is significantly associated with increased IR of CV-AEs and AT-AEs in patients with chronic-phase CML.
Among TKIs, ponatinib has the highest IRR for CV-AEs and AT-AEs.
Abstract
Cardiovascular or arteriothrombotic adverse events (CV- or AT-AEs) are reported in chronic myeloid leukemia (CML) patients treated with tyrosine kinase inhibitors (TKIs). The incidence and characteristics across different TKI have not been systematically analyzed. We analyzed 531 patients treated with frontline TKIs in different prospective trials: imatinib 400 mg (n = 71) and 800 mg (n = 203), nilotinib (n = 108), dasatinib (n = 106), and ponatinib (n = 43). Characteristics and incidence of new-onset CV-AEs and AT-AEs were analyzed. Poisson regression models assessed factors associated with AE incidence. Median follow-up was 94 months (range, 2-195). Overall, 237 patients (45%) developed CV-AEs and 46 (9%) developed AT-AEs. Hypertension was the most common AE seen in 175 patients (33%; grade 3/4 in 17%). CV-AE and AT-AE incidence ratios (IRs) with 95% confidence intervals (CIs) were 8.6 (7.6-9.8) and 1.7 (1.2-2.2) per 100 person-years. Among the TKIs, ponatinib showed the highest IR (95% CI) for CV-AEs and AT-AEs at 40.7 (27.9-59.4) and 9.0 (4.1-20.1). In multivariate analysis, ponatinib therapy was associated with increased incidence rate ratio (IRR) for CV-AEs (4.62; 95% CI, 2.7-7.7; P < .0001) and AT-AEs (6.38; 95% CI, 1.8-21.8; P < .0001) compared with imatinib 400. In summary, there is an increased risk of CV-AEs (except hypertension) and AT-AEs in CML patients treated with newer TKIs, particularly with ponatinib. Patients on TKIs must be informed and closely monitored for vascular AEs. These studies were registered at www.clinicaltrials.gov as #NCT00048672, #NCT00038649, #NCT00050531, #NCT00254423, #NCT00129740, and #NCT01570868.
Visual Abstract
Introduction
Treatment with tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML) is associated with increased risk of cardiovascular and arteriothrombotic adverse events (CV-AEs and AT-AEs).1,2 Ponatinib was temporarily withdrawn from the market in 2014 after a safety warning by the US Food and Drug Administration (FDA) due to the rising incidence of CV-AEs and AT-AEs. Recent reports have highlighted an increased risk (relative to imatinib) with nilotinib 3-5 and to some extent with dasatinib.6-9 In the long-term follow-up of the randomized CML-IV study, the 8-year probability of cardiac and vascular adverse events (AEs) from imatinib (imatinib 400 mg; n = 392, imatinib 800 mg; n = 413, imatinib 400 mg with interferon; n = 323) showed that cardiac or vascular AEs were uncommon with imatinib. The 8-year probability of grade 3-4 cardiac, vascular, or congestive heart failure (CHF) AEs with imatinib was 0.5%, 0.3%, or 3.1%, respectively.10
The relative frequencies of vascular5,11-18 and cardiac19-26 AEs with the different TKIs are difficult to estimate based on the literature because different reports have used different methods for analysis, and what events are included in the general category of “arteriothrombotic events” vary widely, with some reports analyzing a few hundred medical dictionary for regulatory activities (MeDRA) terms while others focus only on specific confirmed diagnosis.
To adjust for these differences, we envisaged this analysis of CV-AEs and AT-AEs occurring during the course of therapy with different TKIs (imatinib 400 mg, imatinib 800 mg, nilotinib, dasatinib, and ponatinib) used as frontline therapy in prospective clinical trials all in the same institution and using the same criteria to define these events.
Patients and methods
We reviewed medical records from 531 patients with newly diagnosed chronic-phase CML (CML-CP) enrolled in consecutive or parallel single-institution clinical trials with TKIs: imatinib 400 mg daily (n = 71), imatinib 800 mg daily (n = 203), nilotinib 400 mg twice daily (n = 108), dasatinib 100 mg daily or 50 mg twice daily (n = 106), and ponatinib starting dose was 45 mg (n = 43). The eligibility criteria, follow-up, monitoring, and end-point definitions were analogous for all trials (see supplemental Key inclusion criteria). Patients were allocated to contemporaneous studies (nilotinib and dasatinib) in an alternating fashion, with no preferential allocated patients based on patient characteristics or cardiovascular risk profile. The therapeutic trials and the chart review study were approved by the institutional review board and performed according to the Declaration of Helsinki. All patients provided written informed consent for participation in the clinical trials.
Patients were monitored for new-onset AEs (including CV-AEs or AT-AEs) after enrollment in the therapeutic trials and until 30 days after discontinuation of study drug. For the present analysis, in addition to the AE logs and serious AE (SAE) reports for each clinical trial, we performed a detailed review of the medical records. Treatment-emergent AEs under MeDRA headings27,28 “cardiovascular,” “vascular,” “artery,” and “thrombosis” were analyzed. This included >1000 individual MeDRA terms. For the purpose of this analysis, CV-AEs included events listed in the Common Terminology Criteria for Adverse Events (CTCAE) under either cardiac or vascular events, including hypertension, arrhythmias, abnormal electrocardiogram or echocardiogram, palpitations, prolongation of QT interval, pericarditis, and peripheral vascular disease (PVD); AT-AEs were grouped into the broad categories of coronary artery disease (CAD), cerebrovascular (CVA) disease (CVD), and peripheral arterial disease (PAD). We considered “new-onset” AE as the first such event that occurred after starting the initial TKI. Patients with hypertension were classified as overall, new-onset, and worsening (in patients with history of hypertension). Baseline demographics and pretreatment risk factors for CV-AEs and AT-AEs were recorded (whichever was available), including body mass index (BMI in kg/m2), history of smoking, hypertension, diabetes, hyperlipidemia, prior vascular events (venous thrombotic disease, CAD, CVD, and peripheral venous disease), and other cardiac conditions (such as cardiac arrhythmias).
With regard to statistical analysis, data were summarized by medians and ranges for continuous variables, and by numbers and percentages for categorical variables. Comparisons between TKI groups were assessed with the Kruskal-Wallis test and Fisher's exact test for continuous and categorical variables, respectively. Person-time incidence ratio (IR) was defined as the number of CV-AEs or AT-AEs during their treatment exposure time. Exposure time was measured from the start of treatment to new event date or last follow-up, whichever occurred first while on their initial TKI, and expressed per 100 person-years while on TKI. Univariate and multivariate (MVA) Poisson regression models were used to analyze the association between the incidence of AEs and clinical/demographic characteristics taking into account the exposure time. Incidence rate ratio (IRR) were calculated using Poisson regression. For continuous variables, we calculated the coefficient (betas) using the Poisson distribution (maximum likelihood) and then took the exponential of the coefficient (β) to get the IRR. Statistical significance was determined at P < .05. Statistical analysis was performed using Stata/SE version 15.1 statistical software (Stata Corporation LP, College Station, TX).
Results
We analyzed 531 patients treated with imatinib 400 (n = 71), imatinib 800 (n = 203), nilotinib (n = 108), dasatinib (n = 106), or ponatinib (n = 43). Baseline patient characteristics, and risk factors for CV-AEs and AT-AEs, are described in supplemental Table 1. The median age at diagnosis was 48 years (15-86 years) and the median follow-up time was 94 months (range, 2-195 months). The median follow-up times were significantly different among TKI cohorts because the corresponding clinical trials were conducted at different times. The median follow-up for the imatinib 400 cohort was 144 months (range, 2-195 months), for imatinib 800 136 months (2-186 months), nilotinib 77 months (3-134 months), dasatinib 85 months (8-132 months), and 42 months (4-55 months) for ponatinib (P < .001).
Prior hypertension was seen in 51%, obesity (BMI ≥ 30 kg/m2) in 32%, hyperlipidemia in 28%, smoking in 23%, diabetes mellitus in 15%, prior history of CAD in 8%, and arrhythmias in 3%. A small percentage of patients were receiving therapy for hyperlipidemia (n = 61; 11%) or receiving aspirin/antiplatelet agents (n = 66; 12%). Distribution of patients according to the number of cardiovascular risk factors at the start of TKI therapy was none (n = 110; 21%), 1 (n = 173; 33%), 2 (n = 160; 30%), and ≥3 (n = 88; 17%).
The distribution of patients with prior history of hypertension and prior smoking was significantly different among the TKI cohorts (P < .001 for both). There was also an imbalance in the frequency of prior history of CAD (P = .06), but no significant difference between cohorts in the frequency of other risk factors including levels of systolic and diastolic blood pressure before the start of therapy (P = .427 and .768, respectively) (supplemental Table 1).
Overall, 237 patients (45%) developed CV-AEs and 46 (9%) developed AT-AEs. The median time to first CV-AE was 26 months (range, 0.1-154 months) and to AT-AE was 46 months (0.3-155 months). A total of 103 of the 237 patients who developed CV-AEs (43%) had a subsequent event while still receiving their initial TKI. Similarly, subsequent AT-AEs were observed in 12 of the 46 patients with AT-AEs (26%). Patients who developed a CV-AE or AT-AE after starting TKI treatment had a higher incidence of known preexisting cardiovascular risk factors (supplemental Table 2).
CV-AEs, AT-AEs by TKI
Among the 237 patients with CV-AEs, hypertension was the most common, seen in 175 of 237 patients (74%). Other frequent CV-AEs included arrhythmias in 57 patients (24%; 23 supraventricular tachyarrhythmia, 32 sinus arrhythmia, and 2 ventricular arrhythmias), palpitations in 18 (8%), peripheral vascular disorders in 4 (2%), prolonged QTc interval in 8 (3%), and pericardial disorders in 7 (3%; including pericarditis and pericardial effusions) (Table 1). The overall occurrence of CV-AEs was highest among patients treated with ponatinib (63%) and similar among other treatment cohorts (P = .13). Hypertension was most frequent among patients taking ponatinib or imatinib 400 (P < .001), whereas arrhythmias were more frequent in patients taking dasatinib or nilotinib (P = .001). Of the 9 patients who developed pulmonary hypertension, 7 (78%) were receiving dasatinib (P = .001; Table 1); CHF was observed in 12 patients, 6 of whom (50%) received imatinib 800.
Table 1.
Frequencies of CV-AEs and AT-AEs across the 5 frontline TKI modalities
| IM400, n = 71 | IM800, n = 203 | Nilotinib, n = 108 | Dasatinib, n = 106 | Ponatinib, n = 43 | P* | Overall, N = 531 | |
|---|---|---|---|---|---|---|---|
| Median follow-up (range), mo | 146 (2-196) | 136 (2-186) | 77 (3-134) | 85 (8-132) | 42 (5-55) | .001 | 94 (2-196) |
| Peak systolic BP, median (range), mm Hg | 148 (110-189) | 145 (97-211) | 144 (103-223) | 146 (106-184) | 146 (102-215) | .88 | 146 (97-223) |
| Peak diastolic BP, median (range), mm Hg | 79 (56-96) | 80 (52-139) | 80 (65-135) | 80 (60-97) | 82 (60-112) | .54 | 80 (52-139) |
| CV-AE,* n (%) | 35 (49) | 90 (44) | 44 (41) | 41 (39) | 27 (63) | .13 | 237 (45) |
| Hypertension | |||||||
| Overall | 34 (48) | 76 (37) | 26 (24) | 16 (15) | 23 (53) | <.001 | 175 (33) |
| New | 22 (31) | 35 (17) | 11 (10) | 1 (1) | 9 (21) | .24 | 78 (15) |
| Worsening | 12 (17) | 41 (20) | 15 (14) | 15 (14) | 14 (33) | .05 | 97 (18) |
| Arrhythmia | 3 (4) | 16 (8) | 16 (15) | 21 (20) | 1 (2) | .001 | 57 (11) |
| Palpitations | 0 (0) | 5 (2) | 4 (4) | 8 (7) | 1 (2) | .08 | 18 (3) |
| Prolonged QT interval | 1 (1) | 1 (1) | 3 (3) | 3 (2) | 1 (2) | .35 | 8 (1) |
| Pericarditis | 1 (1) | 2 (1) | 2 (2) | 1 (1) | 1 (2) | .80 | 7 (1) |
| PVD | 0 (0) | 2 (1) | 1 (1) | 1 (1) | 0 (0) | .99 | 4 (1) |
| AT-AE,* n (%) | 5 (7) | 9 (4) | 11 (10) | 15 (14) | 6 (14) | .03 | 46 (9) |
| CAD | 3 (4) | 5 (3) | 7 (6) | 9 (8) | 2 (5) | .15 | 26 (5) |
| CVA (stroke/TIA) | 1 (1) | 2 (1) | 3 (3) | 3 (3) | 2 (5) | .13 | 11 (2) |
| PAD | 0 (0) | 2 (1) | 0 (0) | 2 (2) | 2 (5) | .11 | 6 (1) |
| Pulmonary hypertension | 0 (0) | 0 (0) | 1 (1) | 7 (7) | 1 (3) | <.001 | 9 (2) |
| CHF | 0 (0) | 6 (3) | 2 (2) | 4 (4) | 0 (0) | .47 | 12 (2) |
| Carotid artery occlusion | 0 (0) | 0 (0) | 0 (0) | 3 (3) | 1 (1) | .03 | 3 (1) |
New-onset hypertension after starting TKI without any prior history of hypertension; worsening is hypertension after TKI in patients with a prior history of hypertension.
BP, blood pressure; TIA, transient ischemic event.
Bold indicates significant P values (P < .05).
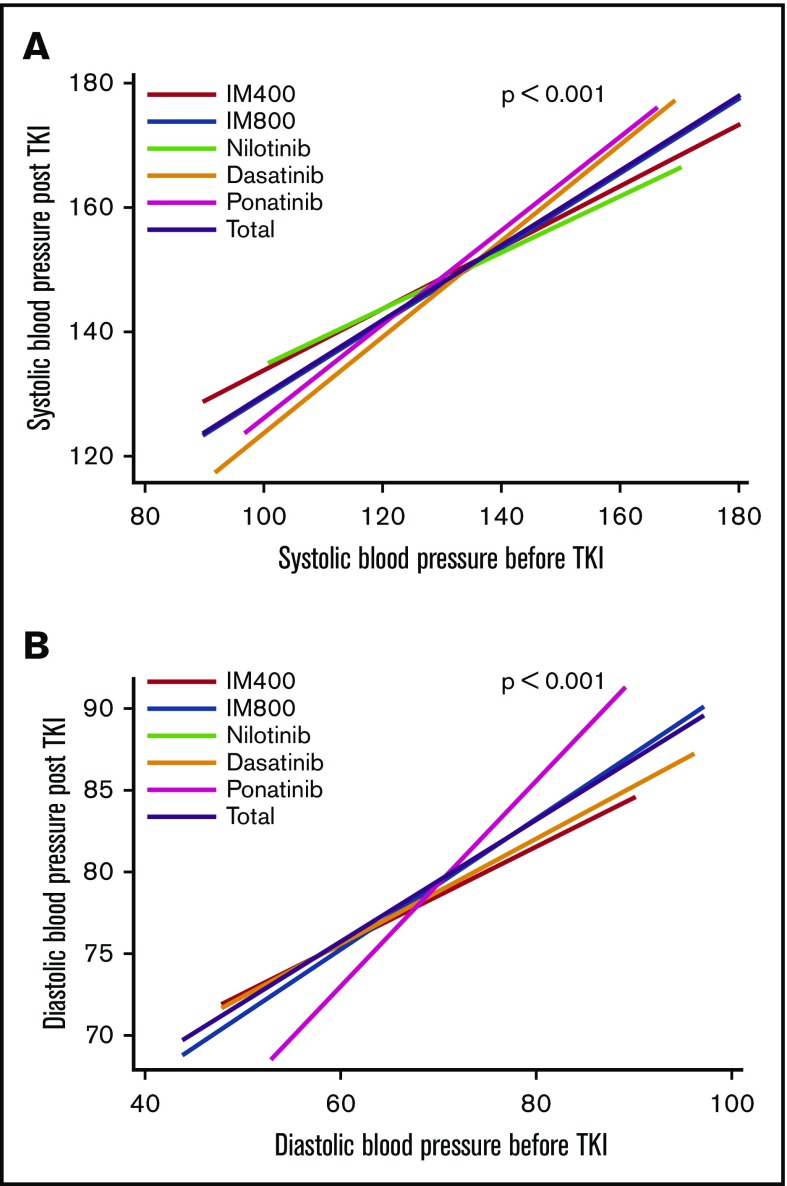
Because hypertension was the commonest CV-AE, we analyzed the pattern of hypertension among TKI cohorts. Figure 1 shows the trends in systolic and diastolic blood pressure before and during TKI therapy. There was a significant increase in blood pressure readings after the start of TKIs (P < .001), with no significant difference in the magnitude of blood pressure change or peak values with different TKIs. The incidence of grade ≥3 hypertension during therapy with TKIs was ponatinib (28%), imatinib 400 (21%), imatinib 800 (20%), nilotinib (16%), and dasatinib (5%) (supplemental Table 3). New-onset hypertension was observed in 78 patients (15%) and worsening hypertension in 97 patients (18%). Of the 78 patients with new-onset hypertension, 28% received imatinib 400, 45% imatinib 800, 14% nilotinib, 1% dasatinib, and 11% ponatinib. Corresponding distribution for worsening hypertension (n = 97) was 12%, 42%, 16%, 15%, and 15%, respectively.
Figure 1.
Changes in blood pressure readings (in mm Hg) before and after TKI treatment: overall and according to the TKI modality. (A) Shows linear prediction plot of the relationship between systolic blood pressure before TKI and systolic blood pressure post-TKI. Systolic blood pressure significantly increased after starting TKIs (P < .001 in all TKIs). (B) Shows linear prediction plot of the relationship between diastolic blood pressure before TKI and diastolic blood pressure post-TKI. Diastolic blood pressure also significantly increased after starting TKIs. There was a significant increase in blood pressure readings after starting TKIs (P < .001), however, there was no significant difference in the magnitude of blood pressure change among different TKIs.
Overall, 46 patients (9%) developed AT-AEs; 56% of these events were CAD, 24% CVA, and 13% PAD (Table 1). The median time to first any AT-AE was 46 months (range, 0.3-155 months), and 42 months for CAD (range, 0.3-155 months), 46 months for CVA (range, 8.4-93 months), and 52 months for PAD (range, 6-103 months). The occurrence rate of AT-AEs was highest among patients treated with ponatinib (14%) and dasatinib (14%), followed by nilotinib (10%), imatinib 400 (7%), and imatinib 800 (4%) (P = .03; Table 1). Among 26 patients with CAD, 9 were taking dasatinib and 7 nilotinib. CVA occurred in 11 patients (3 each on dasatinib and nilotinib, 2 each on ponatinib and imatinib 800). PAD was diagnosed in 6 patients (1%; 2 each on ponatinib, dasatinib, and imatinib 800). Of these 6 patients with PAD, 3 had history of CAD prior to starting TKI therapy. Among the 6 patients who developed PAD, none had ankle brachial index (ABI) performed at baseline and 3 of 6 had a resting ABI performed at the time of onset of symptoms of PAD (0.86, 0.88; 0.33, 0.48; and 0.86, 0.76 in right and left lower extremity for the 3 patients). Carotid artery occlusion was seen in 4 patients (dasatinib 3, ponatinib 1).
IRs of CV-AEs and AT-AEs by TKI
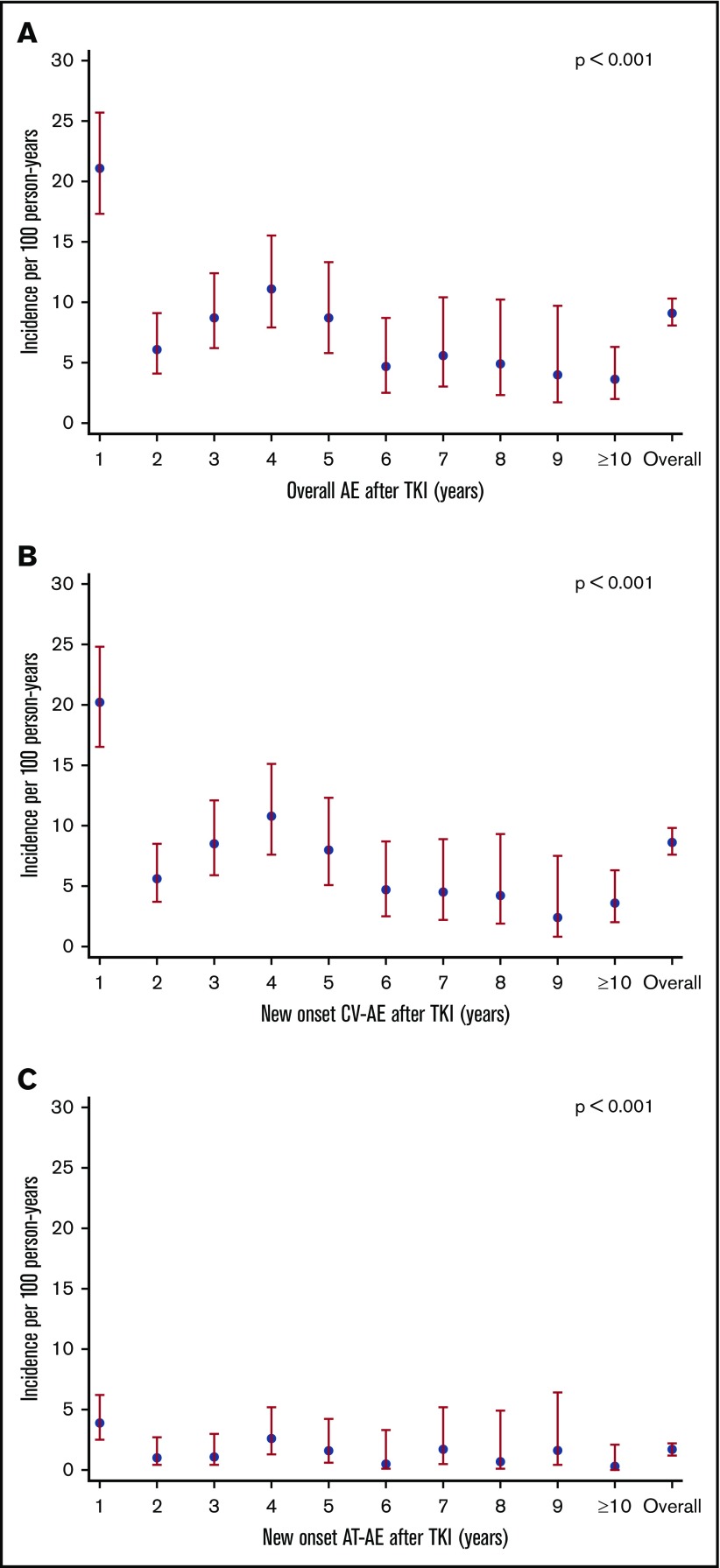
Because the occurrence of CV and AT-AEs is influenced by the exposure time, we calculated the IRs per 100 patients per year. CV-AEs occurred at an overall IR of 8.6 per 100 person-years. The IR of overall AEs and CV-AEs was highest in the first year after start of TKI (Figure 2A-B). The IR of CV-AEs was highest with ponatinib (IR = 40.7 per 100 person-years; P < .001; Table 2; Figure 3A). The IR of AT-AEs for all patients (1.7 per 100 person-years) was also highest in the first year of therapy (Figure 2C). Among TKIs, the IR of AT-AEs was highest with ponatinib (IR = 9.0 per 100 person-years; P < .001); the IR for both nilotinib and dasatinib was also higher compared with imatinib (Table 2; Figure 3B).
Figure 2.
IR of overall and new-onset CV-AEs and AT-AEs per 100 person-years in all patients treated with frontline TKI treatments: trends per year. (A) The IR of patients with overall AEs per 100 person-years shows that the incidence of vascular AEs was highest in the first year of TKI therapy. (B) The IR of patients with new-onset CV-AEs per 100 person-years shows that the incidence of CV-AEs was highest in the first year of TKI therapy. (C) Similarly, the IR of patients with AT-AEs per 100 person-years shows that the incidence of AT-AEs was highest in the first year after TKI therapy and continued to occur at later years. P < .001 in all panels.
Table 2.
IRs of vascular AEs per 100 person-years according to the type of the TKI modality
| IR (95% CI) per 100 person-years by TKIs | ||||||
|---|---|---|---|---|---|---|
| IM400 | IM800 | Nilotinib | Dasatinib | Ponatinib | Overall | |
| Overall AEs | 9.6 (6.9-13.2) | 7.1 (5.8-8.7) | 9.7 (7.3-12.9) | 9.2 (6.9-12.1) | 42.2 (29.1-61.1) | 9.1 (8.1-10.3) |
| CV-AEs | 9.0 (6.5-12.6) | 6.9 (5.6-8.5) | 9.1 (6.7-12.2) | 7.8 (5.8-10.6) | 40.7 (27.9-59.4) | 8.6 (7.6-9.8) |
| CV-AEs without H.T. | 0.0 (0.0-0.2) | 0.1 (0.1-0.2) | 0.3 (0.2-0.5) | 0.4 (0.3-0.6) | 0.5 (0.2-1.3) | 0.2 (0.1-0.2) |
| AT-AEs | 1.3 (0.5-3.1) | 0.7 (0.4-1.3) | 2.3 (1.3-4.1) | 2.9 (1.7-4.7) | 9.0 (4.1-20.1) | 1.7 (1.2-2.2) |
| Overall H.T. | 8.8 (6.3-12.3) | 5.9 (4.7-7.3) | 5.4 (3.6-7.9) | 3.1 (1.9-5.0) | 34.7 (23.0-52.2) | 6.3 (5.5-7.4) |
| New-onset H.T. | 5.7 (3.7-8.6) | 2.7 (1.9-3.8) | 2.3 (1.3-4.1) | 0.2 (0.0-1.4) | 13.6 (7.1-26.1) | 2.8 (2.3-3.5) |
| Worsening H.T. | 3.1 (1.8-5.5) | 3.2 (2.3-4.3) | 3.1 (1.9-5.1) | 2.9 (1.7-4.7) | 21.1 (12.5-35.6) | 3.5 (2.9-4.3) |
| CAD | 0.8 (0.3-2.4) | 0.4 (0.2-0.9) | 1.4 (0.7-3.0) | 1.7 (0.9-3.3) | 3.0 (0.8-12.1) | 0.9 (0.6-1.4) |
| CVA* | 0.3 (0.0-1.8) | 0.2 (0.0-0.6) | 0.6 (0.2-1.9) | 0.6 (0.2-1.8) | 3.0 (0.8-12.1) | 0.4 (0.2-1.7) |
| PAD† | 0 (0) | 0.2 (0.0-0.6) | 0 (0) | 0.4 (0.1-1.5) | 3.0 (0.8-12.1) | 0.1 (0.1-0.5) |
The IRs for overall AEs, new-onset CV-AEs, AT-AEs, hypertension (H.T.), new-onset H.T., CAD, CVA, and PAD.
CI, confidence interval.
Cerebrovascular (CVA) includes stroke/TIA.
P = not significant while P values were significant (<.05) for overall, CV-AEs, CV-AEs without H.T., AT-AEs, hypertension, new-onset AEs, and hypertension and CAD.
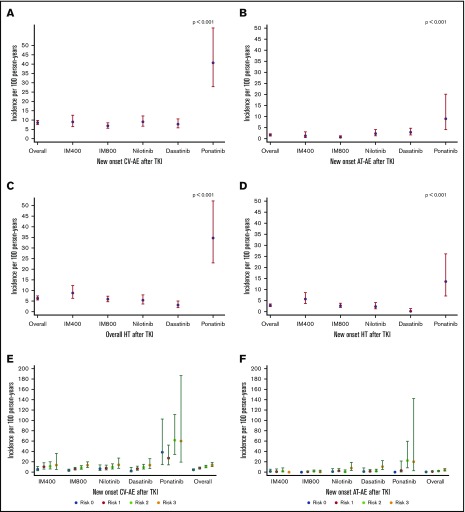
Figure 3.
IRs of new-onset CV-AEs, AT-AEs, overall hypertension, new-onset hypertension, and CV-AEs and AT-AEs according to the number of risk factors per 100 person-years by the frontline TKI modality. (A-B) The IR of patients with new-onset CV-AEs (A) and AT-AEs (B) was significantly higher with ponatinib therapy compared with other TKI modalities; P < .001 in both panels. (C-D) IR of patients with hypertension (overall [C] and new-onset [D]) was highest with ponatinib followed by imatinib 400 mg followed by other TKI modalities; P < .001 in both panels. (E-F) As the number of cardiovascular risk factors increases, the IRs of new-onset CV-AEs (E) and AT-AEs (F) increase within each TKI modality. Patients who received ponatinib had the highest IR (compared with other TKIs) with ≥2 risk factors; P < .001 in both panels.
For hypertension (overall), the IR was 6.3, with the highest IR observed with ponatinib (34.7 per 100 person-years; P < .001) with similar trends for new-onset and worsening hypertension. Excluding hypertension from the CV-AEs, newer TKIs had significantly higher IR compared with imatinib 400.
For patients with CAD, the IR was 0.9 with the highest IR seen with ponatinib (3.0), dasatinib (1.7), and nilotinib (1.4) (P = .03). For CVA, the IR was 0.4 and it was highest with ponatinib 3.0; P = .04). Although the IR was highest with ponatinib (3.0) for PAD, it was of marginal statistical significance compared with other TKIs (P = .054) (Table 2). The IR was highest in each TKI cohort with higher number of baseline cardiovascular risk factors and was highest in patients receiving ponatinib (Figure 3E-F).
MVA Poisson regression model for CV-AEs and AT-AEs
Univariate and MVA Poisson regression models were created to analyze the association between the IRR of different types of vascular AEs and baseline patient characteristics. By MVA analysis, compared with imatinib 400, treatment with ponatinib was associated with a significantly higher IRR for CV-AEs 4.62 (95% CI, 2.74-7.79; P < .0001), but not significantly different to other TKIs (Tables 3 and 4). Other factors associated with a higher IRR (95% CI) for CV-AEs were advanced age (1.03 [1.02-1.04]), low serum albumin (0.57 [0.42-0.78]), BMI ≥ 30 kg/m2 (1.59 [1.21-2.09]), history of hypertension prior to TKIs (1.53 [1.15-2.02]), and higher number of baseline cardiovascular risk factors.
Table 3.
Univariate Poisson regression model for new-onset CV-AEs after starting TKI therapy
| CV-AEs: No | CV-AEs: Yes | IRR (95% CI) | P* | |
|---|---|---|---|---|
| Continuous variables, mean (SD) | ||||
| Age, y | 45 (14) | 53 (15) | 1.04 (1.03-1.05) | <.001 |
| Serum albumin, g/dL | 4.3 (1) | 4 (1) | 0.48 (0.36-0.64) | <.001 |
| BMI, kg/m2 | 28 (7) | 30 (8) | 1.04 (1.03-1.06) | <.001 |
| Categorical variables, n (%) | ||||
| BMI ≥ 30, kg/m2 | ||||
| No | 217 (74) | 142 (60) | ||
| Yes | 77 (26) | 95 (40) | 1.76 (1.36-2.28) | <.001 |
| TKI type | ||||
| IM400 | 36 (12) | 35 (15) | ||
| IM800 | 113 (38) | 90 (38) | 0.77 (0.52-1.13) | .184 |
| Nilotinib | 64 (22) | 44 (19) | 1.00 (0.64-1.56) | .996 |
| Dasatinib | 65 (22) | 41 (17) | 0.86 (0.55-1.36) | .527 |
| Ponatinib | 16 (5) | 27 (11) | 4.50 (2.72-7.44) | <.001 |
| Prior hypertension | ||||
| No | 160 (54) | 101 (43) | ||
| Yes | 134 (45) | 136 (57) | 1.75 (1.35-2.26) | <.001 |
| Prior diabetes | ||||
| No | 257 (87) | 196 (83) | ||
| Yes | 37 (13) | 41 (17) | 1.58 (1.13-2.21) | .008 |
| Prior stroke | ||||
| No | 292 (99) | 232 (98) | ||
| Yes | 2 (0.68) | 5 (2) | 3.30 (1.36-8.00) | .008 |
| Prior CAD | ||||
| No | 279 (95) | 208 (87) | ||
| Yes | 15 (5) | 29 (12) | 2.03 (1.37-2.99) | <.001 |
| Prior arrhythmia | ||||
| No | 286 (97) | 227 (96) | ||
| Yes | 8 (2) | 10 (4) | 1.94 (1.03-3.65) | .041 |
| Prior PVD | ||||
| No | 292 (99) | 233 (98) | ||
| Yes | 2 (1) | 4 (2) | 2.75 (1.02-7.38) | .045 |
| Risk factors | ||||
| 0 | 76 (26) | 34 (14) | ||
| 1 | 102 (35) | 71 (30) | 1.70 (1.13-2.56) | .011 |
| 2 | 78 (26) | 82 (35) | 2.31 (1.55-3.44) | <.001 |
| ≥3 | 38 (13) | 50 (21) | 3.08 (1.99-4.77) | <.001 |
Analysis showing univariate Poisson regression models for new-onset vascular AEs (CV-AEs) after starting TKI therapy.
SD, standard deviation.
Bold indicates significant P values (P < .05).
Table 4.
MVA regression model for CV-AEs and AT-AEs after starting TKI therapy
| Full model | ||
|---|---|---|
| IRR (95% CI) | P* | |
| CV-AEs | ||
| TKI type | ||
| IM400 (reference) | ||
| IM800 | 0.67 (0.44-1.01) | .054 |
| Nilotinib | 0.94 (0.59-1.49) | .786 |
| Dasatinib | 0.76 (0.47-1.22) | .261 |
| Ponatinib | 4.62 (2.74-7.79) | <.0001 |
| Age, y | 1.03 (1.02-1.04) | <.0001 |
| Serum albumin, g/dL | 0.57 (0.42-0.78) | <.0001 |
| Prior hypertension | ||
| No | ||
| Yes | 1.53 (1.15-2.02) | .003 |
| BMI ≥30, kg/m2 | ||
| No | ||
| Yes | 1.59 (1.21-2.09) | .001 |
| Risk factors | ||
| 1 | 1.58 (1.04-2.38) | .030 |
| 2 | 2.23 (1.49-3.35) | <.0001 |
| ≥3 | 3.17 (2.04-4.91) | <.0001 |
| AT-AEs | ||
| TKI type | ||
| IM400 (reference) | ||
| IM800 | 0.33 (0.10-1.03) | .057 |
| Nilotinib | 1.49 (0.48-4.58) | .487 |
| Dasatinib | 2.08 (0.71-6.12) | .184 |
| Ponatinib | 6.38 (1.87-21.80) | .003 |
| Age, y | 1.06 (1.03-1.09) | <.0001 |
| Serum albumin, g/dL | 0.42 (0.20-0.89) | .023 |
| Risk factors | ||
| 1 | 1.95 (0.62-6.11) | .25 |
| 2 | 3.02 (1.00-9.12) | .05 |
| ≥3 | 6.82 (2.23-20.88) | <.0001 |
Analysis showing MVA Poisson regression models for new-onset vascular AEs (CV-AEs and AT-AEs) after starting TKI therapy.
Bold indicates significant P values (P < .05).
Similarly for AT-AEs (Tables 4 and 5), a significantly higher IRR was noted with ponatinib (6.38; 95% CI, 1.87-21.8; P = .003) compared with imatinib 400. There was a higher IRR also for nilotinib (1.49) and dasatinib (2.08) but the difference was not statistically significant. Other factors associated with a significantly higher IRR (95% CI) for AT-AEs were advanced age (1.06 [1.03-1.09]), low albumin (0.42 [0.20-0.89]), and a higher number of baseline risk factors.
Table 5.
Univariate regression model for AT-AEs after starting TKI therapy
| AT-AEs: No | AT-AEs: Yes | IRR (95% CI) | P* | |
|---|---|---|---|---|
| Continuous variables, mean (SD) | ||||
| Age, y | 48 (14) | 58 (14) | 1.06 (1.04-1.09) | <.001 |
| Serum albumin, g/dL | 4 (0.4) | 4 (0.4) | 0.40 (0.22-0.73) | .003 |
| BMI, kg/m2 | 28 (7) | 31 (9) | 1.05 (1.01-1.08) | .007 |
| Categorical variables, n (%) | ||||
| BMI ≥30, kg/m2 | ||||
| No | 330 (68) | 29 (63) | ||
| Yes | 155 (32) | 17 (37) | 1.54 (0.85-2.81) | .156 |
| TKI type | ||||
| IM400 | 66 (14) | 5 (11) | ||
| IM800 | 194 (40) | 9 (19) | 0.54 (0.18-1.60) | .265 |
| Nilotinib | 97 (20) | 11 (24) | 1.75 (0.61-5.04) | .298 |
| Dasatinib | 91 (19) | 15 (33) | 2.21 (0.80-6.09) | .124 |
| Ponatinib | 37 (8) | 6 (13) | 7.00 (2.14-22.94) | .001 |
| Prior hypertension | ||||
| No | 243 (50) | 18 (39) | ||
| Yes | 242 (50) | 28 (61) | 2.02 (1.12-3.65) | .020 |
| Prior diabetes | ||||
| No | 417 (86) | 36 (78) | ||
| Yes | 68 (14) | 10 (22) | 2.09 (1.04-4.22) | .039 |
| Prior smoking | ||||
| No | 377 (78) | 29 (63) | ||
| Yes | 108 (22) | 17 (37) | 2.05 (1.12-3.72) | .019 |
| Prior CAD | ||||
| No | 453 (93) | 34 (74) | ||
| Yes | 32 (7) | 12 (26) | 5.13 (2.66-9.91) | <.001 |
| Risk factors | ||||
| 0 | 106 (22) | 4 (9) | ||
| 1 | 161 (33) | 12 (26) | 2.44 (0.79-7.58) | .122 |
| 2 | 144 (30) | 16 (35) | 3.83 (1.28-11.45) | .016 |
| ≥3 | 74 (15) | 14 (30) | 7.34 (2.41-22.29) | <.001 |
Analysis showing univariate Poisson regression models for new-onset vascular AEs (AT-AEs) after starting TKI therapy.
Bold indicates significant P values (P < .05).
For hypertension, a significantly higher IRR was observed with ponatinib (4.22 ;95% CI, 2.4-7.29; P < .0001). Other factors significantly associated with a higher IRR for hypertension were advanced age, lower albumin, BMI ≥ 30 kg/m2, history of hypertension, and higher number of baseline cardiovascular risk factors. Of note, serum creatinine levels were not significantly associated with hypertension in MVA (not shown). Similarly, for new-onset hypertension, IRR was significantly higher for ponatinib and lower for other TKIs compared with imatinib 400 (not shown); BMI ≥ 30 kg/m2 was also associated with new-onset hypertension. The same results were obtained for worsening hypertension. Excluding hypertension from the CV-AEs (overall or in new-onset CV-AEs), ponatinib, dasatinib, and nilotinib had significantly higher IRR compared with imatinib 400 for other CV-AEs (not shown).
For CAD, although a higher IRR was noted with all TKIs compared with imatinib 400, it was not statistically significant; only advanced age and prior CAD were significantly associated with CAD. Similarly, although a higher IRR of CVA was noted with all TKIs compared with imatinib 400, it was not significant due to the low number of events in each cohort (not shown).
Discussion
Several studies have reported the occurrence of cardiovascular AEs in CML patients receiving TKIs.29,30 Such reports mostly focus on 1 or 2 drugs, and with wide variability in the way these events are investigated and reported. Some reports focus only on very specific diagnoses while others include a wider range of MeDRA terms. This has made the interpretation of the data for the incidence and relative risks with various TKIs difficult and has led to confusion and misconceptions as to the risk associated with each individual agent. Furthermore, the prescriber information for the different drugs present such risks in various formats, which may suggest different risk levels for the different TKIs.31-34 Our analysis, with a median follow-up of 8 years, provides detailed evaluation of the incidence and the factors predisposing for CV-AE and AT-AE in patients with CML-CP who are treated with different TKIs, all analyzed with the same terms and approach.
As expected, patients with preexisting cardiovascular risk factors have a higher risk of developing CV-AEs and AT-AEs, irrespective of the specific TKI used.35,36 The IR of CV-AEs and AT-AEs was highest in the early years of TKI therapy and varied among TKIs. Overall, ponatinib therapy is associated with the highest risk of CV-AEs and AT-AEs. For example, a higher proportion of patients with ponatinib required adjustments in antihypertensive medications and grade 3 hypertension was most frequent with ponatinib and imatinib 400. Among AT-AEs, higher frequencies of CAD were noted with dasatinib or nilotinib, CVA with ponatinib and second-generation TKIs, grade 3 PAD with ponatinib and dasatinib, and carotid artery occlusion with dasatinib and ponatinib. Pulmonary hypertension was most frequent with dasatinib. Notably, the diagnosis was suggested only by echocardiogram in 5 of the 9 patients with this finding; catheterization was only performed (and confirmed the diagnosis) in 4 patients. The overall frequencies of vascular5,11-18,37 and cardiac19-26 AEs varied across various clinical trials depending on the study design, type of TKI, treatment durations, AE definitions, and eligibility criteria. Contrary to some reports,38 we observed that dasatinib was associated with a higher risk of AT-AE relative to imatinib, with a risk that appears similar to that with nilotinib.
Except for the increased risk of hypertension with imatinib, our results are generally consistent with previous studies. A recent metanalysis showed that the risk of vascular occlusive events was higher with newer TKIs compared with imatinib.29 A panel of European LeukemiaNet (ELN) experts39 has recently reviewed the AEs across a large cohort of patients treated with various TKIs in multiple studies, including patients both with frontline and subsequent lines of therapy, and patients in various stages of the disease. Excess risk of PAD was noted with ponatinib and nilotinib. Similarly, ischemic heart disease was reported in ponatinib (12%) and nilotinib studies (1%-10%), but rarely in studies with other TKIs. Also, the 5-year follow-up results from the randomized ENESTnd study40 showed that vascular AEs continue to rise after 5 years, and the frequencies of CAD, CVA, and PAD were higher in nilotinib-treated patients (7.5% vs 2%). The limited number of patients with follow-up beyond year 5 makes it difficult to determine whether the incidence may continue to increase beyond this time point in this study. Similarly, the 5-year follow-up of DASISION41 showed that although CV-AEs were more than twice as common with dasatinib than with imatinib (5% vs 2%), and follow-up events, although uncommon, were also more commonly seen with dasatinib. However, neither of these studies reported a systematic categorization of all CV-AEs and AT-AEs, or adjustment for time of exposure or a Poisson regression model MVA. Importantly, preexisting known cardiovascular risk factors are a major predisposing factor for CV-AEs and AT-AEs after treatment with TKIs.39 However, an analysis of characteristics and incidence rates across second- and third-generation TKIs is unavailable because analyses from different trials have been hampered by differences in eligibility criteria, and different search, analysis, and reporting methods. For example, in the ENESTnd study, patients with a prior unstable angina within 12 months were excluded, whereas DASISION excluded patients with uncontrolled angina within 3 months of the start of therapy. Our analysis is important in trying to shed light on the relative risk with each of the available TKIs by using uniform criteria for reporting, and by performing a MVA analysis to adjust for other risk factors. Unfortunately, we were unable to include bosutinib on this comparison as we did not have a cohort of patients treated with bosutinib as initial therapy. A comprehensive evaluation of the risk of these complications with bosutinib has been recently published.42
Although most of the CV-AEs and AT-AEs were adequately managed after dose reduction of the TKIs, discontinuation of the TKIs, or after management of comorbidities with other medications, in a fraction of patients, SAEs such as arterial occlusion were not reversible. Peripheral arterial occlusion in some patients required stenting and acute myocardial infarction required percutaneous coronary intervention. Continuation of therapy may be associated with recurrence as observed in many of the patients included in this series. Thus, the risk:benefit of continuation of the same TKI, even at lower doses, vs change to an alternative that may offer lower risk, should be carefully evaluated.
Our analysis has some possible limitations as it was conducted retrospectively. It is likely that the small number of patients taking imatinib 400 might influence the difference in proportion of CV-AE as compared with imatinib 800. It would be reasonable to consider the risk for the 2 cohorts of imatinib-treated patients as equivalent. Because the trials were conducted at different time points, there are differences in follow-up duration between various trials; so, although the annual rates were calculated taking into consideration the follow-up of patients, the differences in follow-up duration may have introduced a bias. However, these were all patients treated in prospective clinical trials where AEs were collected and reported prospectively. Compared with the CML-IV study where ∼1100 patients with imatinib were analyzed for AEs showing that CV-AEs are uncommon with imatinib, our study had a smaller number of patients with imatinib and therefore this limitation precludes any definitive conclusions for comparison of CV-AEs with second-generation TKIs.10 In this study, we could not provide a precise estimate of the proportion of the AEs that were definitely related to the drug because these AEs could occur due to multiple risk factors and we have shown that as the number of risk factors increase, the probability of developing CV-AE or AT-AE increases. In addition, we conducted a thorough chart review to further identify symptoms or other findings that could potentially represent CV-AEs or AT-AEs. Also, the analysis involves patients treated in separate trials and at different times spanning more than a decade. However, the studies were all conducted and patients treated at the same single institution minimizing the variability. Despite these limitations, we believe the strategy used for this analysis greatly decreases bias and provides the most comprehensive and uniform approach to explore the incidence and risk factors associated with these important events.
In summary, our results show that the incidence and the risk of CV-AEs and AT-AEs is significantly increased in patients with CML who are taking second- and third-generation TKIs, and in patients with preexisting cardiovascular risk factors. We noted increased incidence and risk of hypertension with all TKIs. The IR for AT-AEs was higher in patients taking ponatinib and to some extent dasatinib and nilotinib. Because most CML patients are long-term survivors who will continue to receive TKIs, newer guidelines for initial workup of CML patients must include a thorough baseline evaluation of cardiovascular risk factors and regular assessment and management of any cardiovascular comorbidity during each visit while on TKI therapy.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by the MD Anderson Cancer Center support grant CA016672 and award number P01 CA049639 from the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: P.J. and J.E.C. designed the study; P.J., P.C.B., G.M.N.-G., and J.E.C. prepared the analysis, collected data, and analyzed results; P.J., P.C.B., P.S., H.A., and J.E.C. wrote the paper; H.K., S.O., F.R., K.S., S.V., G.M.N.-G., E.J., Z.E., T.M.K., G.G.-M., G.B., and J.E.C. managed the patients; and all authors reviewed and gave the final approval for the paper.
Conflict-of-interest disclosure: J.E.C. is a consultant for Bristol-Myers Squibb, Pfizer, Takeda, Fusion Pharma, and Novartis; and receives research support to his institution from Pfizer, Takeda, Sun Pharma, Teva, Bristol-Myers Squibb, and Novartis. F.R. received research funding from Bristol-Myers Squibb, and honoraria from Bristol-Myers Squibb, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Jorge E. Cortes, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: jcortes@mdanderson.org.
References
- 1.Haguet H, Douxfils J, Mullier F, Chatelain C, Graux C, Dogné JM. Risk of arterial and venous occlusive events in chronic myeloid leukemia patients treated with new generation BCR-ABL tyrosine kinase inhibitors: a systematic review and meta-analysis. Expert Opin Drug Saf. 2017;16(1):5-12. [DOI] [PubMed] [Google Scholar]
- 2.Dahlén T, Edgren G, Lambe M, et al. ; Swedish CML Group and the Swedish CML Register Group. Cardiovascular events associated with use of tyrosine kinase inhibitors in chronic myeloid leukemia: a population-based cohort study. Ann Intern Med. 2016;165(3):161-166. [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125(6):901-906. [DOI] [PubMed] [Google Scholar]
- 4.Mayer K, Gielen GH, Willinek W, Müller MC, Wolf D. Fatal progressive cerebral ischemia in CML under third-line treatment with ponatinib. Leukemia. 2014;28(4):976-977. [DOI] [PubMed] [Google Scholar]
- 5.Le Coutre P, Rea D, Abruzzese E, et al. . Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103(17):1347-1348. [DOI] [PubMed] [Google Scholar]
- 6.Minami M, Arita T, Iwasaki H, et al. . Comparative analysis of pulmonary hypertension in patients treated with imatinib, nilotinib and dasatinib. Br J Haematol. 2017;177(4):578-587. [DOI] [PubMed] [Google Scholar]
- 7.Godinas L, Guignabert C, Seferian A, et al. . Tyrosine kinase inhibitors in pulmonary arterial hypertension: a double-edge sword? Semin Respir Crit Care Med. 2013;34(5):714-724. [DOI] [PubMed] [Google Scholar]
- 8.Montani D, Bergot E, Günther S, et al. . Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125(17):2128-2137. [DOI] [PubMed] [Google Scholar]
- 9.Dumitrescu D, Seck C, ten Freyhaus H, Gerhardt F, Erdmann E, Rosenkranz S. Fully reversible pulmonary arterial hypertension associated with dasatinib treatment for chronic myeloid leukaemia. Eur Respir J. 2011;38(1):218-220. [DOI] [PubMed] [Google Scholar]
- 10.Kalmanti L, Saussele S, Lauseker M, et al. . Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29(5):1123-1132. [DOI] [PubMed] [Google Scholar]
- 11.Aichberger KJ, Herndlhofer S, Schernthaner GH, et al. . Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86(7):533-539. [DOI] [PubMed] [Google Scholar]
- 12.Giles FJ, Mauro MJ, Hong F, et al. . Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27(6):1310-1315. [DOI] [PubMed] [Google Scholar]
- 13.Quintás-Cardama A, Kantarjian H, Cortes J. Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk. 2012;12(5):337-340. [DOI] [PubMed] [Google Scholar]
- 14.Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institution study. Eur J Haematol. 2013;90(6):531-532. [DOI] [PubMed] [Google Scholar]
- 15.Kim TD, Rea D, Schwarz M, et al. . Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27(6):1316-1321. [DOI] [PubMed] [Google Scholar]
- 16.Gora-Tybor J, Medras E, Calbecka M, et al. . Real-life comparison of severe vascular events and other non-hematological complications in patients with chronic myeloid leukemia undergoing second-line nilotinib or dasatinib treatment. Leuk Lymphoma. 2015;56(8):2309-2314. [DOI] [PubMed] [Google Scholar]
- 17.Fossard G, Blond E, Balsat M, et al. . Hyperhomocysteinemia and high doses of nilotinib favor cardiovascular events in chronic phase chronic myelogenous leukemia patients. Haematologica. 2016;101(3):e86-e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. . Long-term follow-up of ponatinib efficacy and safety in the phase 2 PACE trial [abstract]. Blood. 2014;124(21). Abstract 3135. [Google Scholar]
- 19.Estabragh ZR, Knight K, Watmough SJ, et al. . A prospective evaluation of cardiac function in patients with chronic myeloid leukaemia treated with imatinib. Leuk Res. 2011;35(1):49-51. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro AL, Marcolino MS, Bittencourt HN, et al. . An evaluation of the cardiotoxicity of imatinib mesylate. Leuk Res. 2008;32(12):1809-1814. [DOI] [PubMed] [Google Scholar]
- 21.Marcolino MS, Ribeiro AL, Clementino NC, et al. . The use of imatinib mesylate has no adverse effects on the heart function. Results of a pilot study in patients with chronic myeloid leukemia. Leuk Res. 2011;35(3):317-322. [DOI] [PubMed] [Google Scholar]
- 22.Abbas R, Hug BA, Leister C, Gaaloul ME, Chalon S, Sonnichsen D. A phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjects. Cancer Chemother Pharmacol. 2012;69(1):221-227. [DOI] [PubMed] [Google Scholar]
- 23.Brümmendorf TH, Cortes JE, de Souza CA, et al. . Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015;168(1):69-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonnichsen D, Dorer DJ, Cortes J, et al. . Analysis of the potential effect of ponatinib on the QTc interval in patients with refractory hematological malignancies. Cancer Chemother Pharmacol. 2013;71(6):1599-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson FM, Agrawal S, Burris H, et al. . Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer. 2010;116(6):1582-1591. [DOI] [PubMed] [Google Scholar]
- 26.Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48(7):964-970. [DOI] [PubMed] [Google Scholar]
- 27.Wood K. The medical dictionary for drug regulatory affairs (MEDDRA). Pharmacoepidemiol Drug Saf. 1994;3(1):7-13. [Google Scholar]
- 28.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109-117. [DOI] [PubMed] [Google Scholar]
- 29.Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016;2(5):625-632. [DOI] [PubMed] [Google Scholar]
- 30.Valent P, Hadzijusufovic E, Hoermann G, et al. . Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leuk Res. 2017;59:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iclusig (ponatinib hydrochloride) [prescribing information]. Cambridge, MA: ARIAD Pharmaceuticals, Inc; December 2017.
- 32.Sprycel (dasatinib) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; November 2017.
- 33.Tasigna (nilotinib) [prescribing information]. East Hanover, NJ: Novartis; March 2018.
- 34.Gleevec (imatinib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals; September 2017.
- 35.Aghel N, Delgado DH, Lipton JH. Cardiovascular events in chronic myeloid leukemia clinical trials. Is it time to reassess and report the events according to cardiology guidelines? Leukemia. 2018;32(10):2095-2104. [DOI] [PubMed] [Google Scholar]
- 36.Assunção PM, Lana TP, Delamain MT, et al. . Cardiovascular risk and cardiovascular events in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Clin Lymphoma Myeloma Leuk. 2019;19(3):162-166. [DOI] [PubMed] [Google Scholar]
- 37.Ross DM, Arthur C, Burbury K, et al. . Chronic myeloid leukaemia and tyrosine kinase inhibitor therapy: assessment and management of cardiovascular risk factors. Intern Med J. 2018;48(suppl 2):5-13. [DOI] [PubMed] [Google Scholar]
- 38.Saglio G, le Coutre P, Cortes J, et al. . Evaluation of cardiovascular ischemic event rates in dasatinib-treated patients using standardized incidence ratios. Ann Hematol. 2017;96(8):1303-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steegmann JL, Baccarani M, Breccia M, et al. . European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30(8):1648-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochhaus A, Saglio G, Hughes TP, et al. . Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortes JE, Saglio G, Kantarjian HM, et al. . Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes JE, Jean Khoury H, Kantarjian H, et al. . Long-term evaluation of cardiac and vascular toxicity in patients with Philadelphia chromosome-positive leukemias treated with bosutinib. Am J Hematol. 2016;91(6):606-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.