Abstract
Genomic medicine has greatly matured in terms of its technical capabilities, but the diffusion of genomic innovations worldwide faces significant barriers beyond mere access to technology. New global development strategies are sorely needed for biotechnologies such as genomics and their applications toward precision medicine without borders. Moreover, diffusion of genomic medicine globally cannot adhere to a “one-size-fits-all-countries” development strategy, in the same way that drug treatments should be customized. This begs a timely, difficult but crucial question: How should developing countries, and the resource-limited regions of developed countries, invest in genomic medicine? Although a full-scale investment in infrastructure from discovery to the translational implementation of genomic science is ideal, this may not always be feasible in all countries at all times. A simple “transplantation of genomics” from developed to developing countries is unlikely to be feasible. Nor should developing countries be seen as simple recipients and beneficiaries of genomic medicine developed elsewhere because important advances in genomic medicine have materialized in developing countries as well. There are several noteworthy examples of genomic medicine success stories involving resource-limited settings that are contextualized and described in this global genomic medicine innovation analysis. In addition, we outline here a new long-term development strategy for global genomic medicine in a way that recognizes the individual country's pressing public health priorities and disease burdens. We term this approach the “Fast-Second Winner” model of innovation that supports innovation commencing not only “upstream” of discovery science but also “mid-stream,” building on emerging highly promising biomarker and diagnostic candidates from the global science discovery pipeline, based on the unique needs of each country. A mid-stream entry into innovation can enhance collective learning from other innovators' mistakes upstream in discovery science and boost the probability of success for translation and implementation when resources are limited. This à la carte model of global innovation and development strategy offers multiple entry points into the global genomics innovation ecosystem for developing countries, whether or not extensive and expensive discovery infrastructures are already in place. Ultimately, broadening our thinking beyond the linear model of innovation will help us to enable the vision and practice of genomics without borders in both developed and resource-limited settings.
Keywords: : Strategy for global biotechnology development, precision medicine, developing countries, public health genomic, Fast-Second Winner
Introduction
Genomic medicine is inherently a “science without borders,” aiming at advancing therapeutic and diagnostic medicine worldwide. The field has greatly matured over the past decade in terms of its technical capabilities. On the other hand, the vision and practice of genomics without borders face formidable challenges and opportunities beyond simply access to technology (McCarthy et al., 2013). For example, an astute global and yet customized strategy for the diffusion of global genomics across nation state borders is lacking.
Diffusion of genomic medicine globally cannot adhere to a “one-size-fits-all” model, in the same way as drug treatments should be customized. This begs a timely, difficult but crucial question: How should developing countries, and the resource-limited regions of the developed countries, invest in genomic medicine?
One might argue that a full-scale infrastructure investment from discovery to the translational implementation of genomic science is ideal and should be in place in all countries worldwide. However, this may not always be feasible, with resource limitations; or, if achieved, scientific capacity building may not be real time and commensurate with global innovative breakthroughs, thereby resulting in time lags and inequities in global science.
A simple transfer of genomic services from developed to developing countries is unlikely to be feasible. Developing countries should not be seen simply as recipients and beneficiaries of genomic medicine developed elsewhere because important advances in genomic medicine have been (and will continue to be) materializing in developing countries as well.
There are several noteworthy examples of genomic medicine success stories involving resource-limited settings, some of which will be contextualized and described in this global genomic medicine innovation analysis. In addition, we outline here a new long-term development strategy for global genomic medicine in a way that recognizes the pressing public health priorities and disease burdens of the individual countries concerned. We term this approach “Fast-Second Winner” model of biotechnology innovation without borders, which broadens our approach to global innovation beyond a narrow linear model that invariably commences upstream from discovery science.
Where Do We Stand Currently in Global Genomics?
Recent advances in genomics, other related multi-omics biotechnologies (transcriptomics, proteomics, metabolomics, glycomics) and study designs such as genome-wide association studies, next-generation sequencing, and the companion big-data technologies for genomics data translation have served to change our past perceptions of traditional medical practice and have served to herald a new era, that of Genomic Medicine. These new technologies are gradually being adopted by diagnostic laboratories and hospitals in the United States and Western Europe, whereas several regulatory bodies, such as the U.S. Food and Drug Administration (http://www.fda.gov) and the European Medicines Agency (http://www.ema.europa.eu), are issuing guidelines to facilitate and, at the same time, rationalize the translation of genomic and precision medicine interventions into the clinic. In principle, genomic and precision medicine are two separate but highly complementary disciplines, the former comprising an integral part of the latter. In other words, genomic medicine guides customization of healthcare, with medical decisions, practices, or products being tailored to the individual patient mainly by means of molecular and cellular-based approaches but also in conjunction with imaging and other analytical approaches.
However, the pace of implementation of genomic medicine varies from country to country, depending on a number of different parameters. These parameters include barriers, arising mainly from the lack of resources and infrastructure, poor technology and knowledge transfer, the limited genomics education of healthcare professionals, and occasionally misplaced skepticism regarding the potential benefits that this new discipline has to offer medicine (Forero et al., 2016). In addition, most advances in human and medical genomics stem from resource-rich developed countries. Therefore, they can generally not be translated directly into the settings frequently found in resource-limited emerging countries. As such, the potential of genomic medicine is often not fully appreciated, whether by biomedical scientists and healthcare professionals or by the general public and policymakers. When one takes into account that around 85% of the world's population lives in developing/resource-limited countries, it becomes apparent that the issue of providing access to genomic medicine in these settings is crucial.
Despite these barriers, there have been several notable examples of the successful implementation of genomic medicine projects in resource-limited settings across different continents that pertain to either population pharmacogenomics or public health genomics. Moreover, there have been various successful efforts to build research networks with common interests in genomic medicine. Some of these are summarized later, and we believe that they can serve as model cases for the implementation of genomic medicine in other resource-limited environments. Herein, the notions “developing country” and “resource-limited settings” will be used to denote an environment in which: (1) resources assigned for genomics research are scarce, (2) access to genomics knowledge and information is restricted, (3) genomic implementation is limited, (4) genomics education is relatively poor, and/or (5) collaborative opportunities for genomics research with renowned institutions are rare for various reasons, such as geographical, societal, economic, or political.
Capacity building in genomic medicine
One of the most important reasons that genomics research is lagging behind in resource-limited settings, as compared with developed countries, is the lack of dedicated resources and research centers that help to spearhead the generation of new knowledge by training and educating young scientists, medical students, clinicians, etc.
Research in Africa has revealed insufficient genetic knowledge at the level of medical education, whereas international genetics research on Africa has consistently failed to build capacity in the two-decade post-initiation of the Human Genome Project (Matovu et al., 2014; Wonkam et al., 2006, 2011a). Since then, the latter trend is rapidly changing with initiatives such as the H3Africa Consortium that is fully engaging young Africa Sciences in Genomics Research (Consortium, 2014; Matovu et al., 2014) and providing new hope to accelerate Precision Medicine in Africa. For example, the Department of Science and Technology, South Africa, has recently launched the South African Precision Medicine Initiative.
Also, in Latin America, there are very few research centers that have the capability to facilitate genomics research and education. Yachay Tech University (http://www.yachaytech.edu.ec/en) is the first research-intensive University in Ecuador. It was inaugurated in March 2014 in one of the most biodiverse countries to be found anywhere in the world, as part of a long-term national plan toward a knowledge-based economy. As part of this initiative, Yachay Tech is planning to establish the Ecuadorian Center for Genomics and Bioinformatics. Such a research center would act as a magnet not only within Ecuador but also for the entire South American region. The new Institute will be established in cooperation with the Golden Helix® Foundation (www.goldenhelix.org), a UK-based non-profit research organization active in research and education in genomics and translational research, with a special interest in disseminating an understanding of human genetics and genomics and promoting, in tandem with research organizations, the clinical application of pharmacogenomics in medical practice.
The two institutions are committed to carrying out research in the area of Genomics and Bioinformatics related particularly to the following fields: Systems Biology, Bioinformatics, Biotechnology, Biodiversity, Conservation Biology, Ecology, Evolutionary Biology, and, last but not least Population Genetics, Pharmacogenomics and Genomics and Translational Medicine. In the field of human genomics education, the new Institute, in conjunction with the Golden Helix Academy, will organize graduate courses in human genomics, genome informatics, and public health genomics, whereas it will also provide academic certificates and diplomas in the aforementioned disciplines. Overall, the envisaged Ecuadorian Institute of Genomics and Bioinformatics at Yachay Tech aspires to be a recognized center of reference for Genomics in South America for the analysis of data on genetic disease incidence and to guide research and public health policies of relevance to various populations and ethnic groups. In addition, this Institute promises to be a key partner in research projects addressing the tremendous wealth of the continent's biodiversity and its sustainable management.
Apart from Latin America, there are also similar efforts in other parts of the world. In the United Arab Emirates, the Zayed Bin Sultan Center for Health Sciences (http://www.cmhs.uaeu.ac.ae/en/zchs) has been established in the United Arab Emirates University, with the generous support of the Zayed Foundation, and it aims at becoming the flagship research center of the College of Medicine and Health Sciences for population health. The center was established to support and contribute to the United Arab Emirates University mission of becoming a world-class center for applied research, with national and international outreach and innovation while preserving the unique values of the United Arab Emirates. The center intends to forge formal links with other universities and institutions through equitable memoranda of understanding. In the field of Genomic Medicine, the first project focusing on Public Health Genomics was designed and already started, in an effort to canvass the genomic medicine environment in the United Arab Emirates with the prospect of extending this project into neighboring countries in the region (see Genomic Medicine and Public Health section).
Population pharmacogenomics
Pharmacogenomics is steadily being implemented in the world's health systems, although at a rather heterogeneous pace. The pace of implementation is significantly faster in the United States and western European countries, whereas other countries in Europe and elsewhere are currently lagging behind. The landscape is heterogeneous even within Europe mostly due to the lack of harmonization of the national guidelines in Europe and most importantly, differences in resource availability (Mitropoulos et al., 2011). Another important parameter is the variable prevalence of pharmacogenomic biomarkers in different ethnicities around the world; these have scarcely been documented. Given that the cost-effectiveness of genome-guided interventions relies on our knowledge of the prevalence of certain biomarkers in different ethnic groups, it becomes challenging to define those actionable pharmacogenomic biomarkers on which drug-dose recommendations will be set.
The Euro-PGx project, launched in 2010 by the Golden Helix Foundation and forming an integral part of the Pharmacogenomics for Every Nation Initiative (http://www.goldenhelix.org/activities/research/pharmacogenomics), aims at determining the spectrum and the variation of pharmacogenomic biomarker allele frequencies across a large number of European countries, many of which are characterized by limited resources, in an effort to provide the basis for deriving country-specific drug-dose recommendations. In addition, a project that aims at raising pharmacogenomics awareness among biomedical scientists and healthcare professionals has been initiated by organizing several single-day educational events (the Golden Helix Pharmacogenomics days; http://pharmacogenomicsdays.goldenhelix.org) in various European countries.
As expected, the findings from this large-scale genotyping effort in 18 European countries, using the DMET+ microarray (Affymetrix, Santa Clara, CA) and conventional genotyping methods, suggested that the prevalence of individual pharmacogenomic biomarkers varies significantly, despite the strong Caucasian genetic component in all of the European populations analyzed (Mizzi et al., 2016). In particular, data from the analysis of 36 clinically actionable pharmacogenomic variants in all 18 European populations revealed significant differences in the prevalence of 7 pharmacogenomic biomarkers in at least 7 European populations, which are implicated in the efficacy and toxicity of 51 drugs. As far as warfarin dose recommendation is concerned, it is evident that there are marked differences in the predicted average warfarin dose in different European populations analyzed (Mizzi et al., 2016). These data may help in amending existing treatment recommendations, and in rationalizing treatment modalities for these drugs in the respective countries.
As of mid-2017, this project is being replicated, with a special focus on South East Asian populations, under the umbrella of the Global Genomic Medicine Collaborative (G2MC) Pharmacogenomics Working group and includes eight South East Asian populations, mostly from developing countries (Table 1). The 100 Pharmacogene Resequencing Project will focus on the resequencing of 100 pharmacogenes in a large number of healthy donors of South East Asian descent. Genotyping will be performed at the RIKEN Institute in Japan, using the whole pharmacogene resequencing panel and will include genotyping of the HLA genes and CYP2D6 gene amplification. Healthy donors from Europe and the Gulf Cooperation Council (GCC) countries will be analyzed as controls. This project is anticipated to yield important benefits for the participating countries, including (1) capacity building, (2) harmonizing education activities, (3) performing economic evaluations to determine whether certain genome-guided treatment modalities are cost-effective, (4) motivating the replication of these studies in these populations using a much larger population sample, and (5) making people aware of the clinical value of pharmacogenomics.
Table 1.
Features of the Two Large Population Pharmacogenomics Projects, Conducted in Developing Countries
| Study population | No. of populations | No. of samples | Reference |
|---|---|---|---|
| Euro-PGx project | |||
| European | 18 | 1105 | Mizzi et al. (2016) |
| Saudi Arabian | 1 | 499 | |
| South African | 3a | 106 | |
| 100 Pharmacogene Resequencing Projectb | |||
| Southeast Asian | 8 | 800 | Ongoing |
| European | 3 | 300 | |
| Latin American | 3 | 300 | |
| Middle Eastern | 2 | 200 | |
Refers to three South African sub-populations.
Sample collection is expected to begin in fall 2017.
In addition to the burden of communicable disease such as HIV and malaria, nearly ¾ of newborns with sickle cell disease (SCD) are born in Africa (Piel et al., 2013), indicating the urgent need in this setting to further accelerate research on pharmacogenomics, which could ultimately improve the prevention of disease and patient care. Fortunately, emerging genomics research involving African scientists are improving our knowledge of genomic modifiers of SCD, which will be useful for anticipatory guidance in clinical practice (Geard et al., 2017; Makani et al., 2011; Wonkam and Hurst, 2014).
Rethinking the Linear Model of Innovation for Genomics Without Borders
A Fast-Second Winner development strategy
The linear model of innovation has been a dominant framework that has guided and shaped scientists' and innovators' approach to scientific breakthroughs. The linear innovation model commences from discovery science and proceeds in a linear fashion toward translational research, in its various phases, then on to implementation science followed by innovation diffusion and capacity building. This model does not take into account that innovations may, sometimes if not always, arise out of user needs. More importantly, the linear model creates a tendency to commence innovations from discovery science, which, in some cases, may not be feasible with limited resources. Innovations, by definition, are unprecedented practices and products and, as such, innovators often fail and their mistakes can inform other innovators who may have fewer resources or who cannot afford to fail because they have limited financial or technical resources.
Building on the earlier context, and the fact that innovations and innovators can commence “innovating” not only upstream but also “midstream,” we propose here a new long-term development strategy termed the “Fast-Second Winner” mode of innovation (Fig. 1).
FIG. 1.
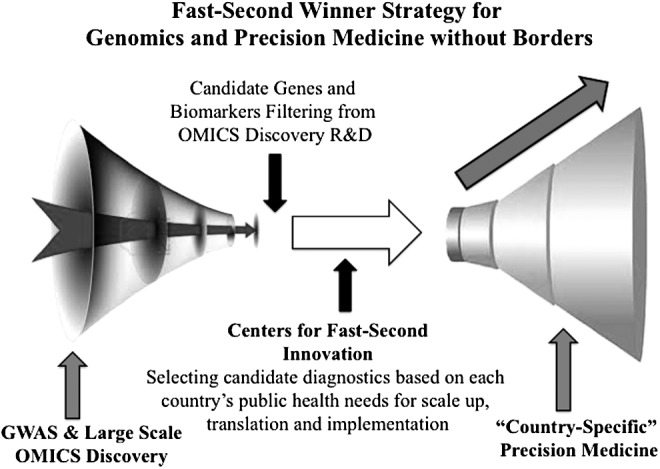
Outline of the Fast-Second Winner model, as an à la carte innovation model to tailor global genomic and personalized medicine development in a country-specific manner. Please note that this model is not only based on wet-lab scientific aspects but also, and most importantly, applies to other public health-related process of implementing genomics in clinical practices that involve many different aspects (cost-effectiveness analysis, education, local health priorities, etc. GWAS, Genome-Wide Association Studies; R&D, Research and Development).
The Fast-Second Winner model recognizes the pressing public health priorities and disease burdens of individual countries and supports midstream innovations that build on emerging highly promising biomarker and diagnostic candidates from global science. Importantly, a mid-stream entry into innovation can enhance collective learning from other innovators' mistakes upstream in discovery science and boost the probability of success for translational and implementation science when resources are limited. Hence, this à la carte model of global innovation offers multiple entry points into global genomics innovation ecosystem for developing countries, whether or not extensive discovery infrastructure is already in place.
This is contrary to investing in ALL aspects of innovation horizontally from discovery infrastructure to translation to implementation science in a given country, which is not only expensive but also does not serve well the unique needs of each country. Lastly, to be able to apply a second rapid model of innovation, one needs some kind of an innovation observatory, which can best identify the emerging candidate markers or drug and marker combinations and suggest them to various countries, filtering and distilling them through a given country's own public health needs.
Other Capacities Necessary for Genomics Without Borders
Raising genomics awareness among healthcare professionals
One of the key elements to ensure efficient integration of genomics into clinical practice is to enrich health professionals' genomics knowledge. At present, a typical practicing physician has only very limited training in genetics. Healthcare professionals' lack of genetics knowledge, coupled with the lack of awareness on the part of patients and the general public about the benefits of genetic information, leaves individuals confused as to which test might be beneficial in their own particular case. Participation in genomics conferences would contribute to improving healthcare professionals' genomics education.
To this end, there are a plethora of genomics conferences that are organized in various countries worldwide. However, registration and tuition fees to attend these conferences, which are mostly organized in the United States and Western Europe, are prohibitively expensive for participants originating from developing countries and low-resource environments, such as South East Asia, the Middle East, South and Central Europe, Africa, and Latin America. There has, thus, been an urgent need to establish a series of Conferences in Genomic Medicine and Pharmacogenomics, where high scientific quality coupled with affordable registration fees would attract participants from the developing world.
Ideally, if high profile academics and researchers were invited as plenary and keynote speakers to genomics conferences organized in these regions, this would, in turn, attract corporate and charitable entities as sponsors of these scientific events, which would reciprocally reduce the overall registration fees, thereby making these meetings more affordable for participants from developing countries. Moreover, organizing such conferences in different venues and with different themes related to Genomic Medicine would reduce the logistical burden and make it easier and more cost-effective for participants from developing countries to attend.
The advances and considerations mentioned earlier gave rise to the concept of the Golden Helix Conferences, as high-level scientific summits focusing on themes pertaining to the fields of genomics, pharmacogenomics, and personalized medicine. In brief, the Golden Helix Conferences aimed at advancing genomics worldwide under three interlinked themes of interest to precision medicine without borders:
• The Golden Helix Symposia (http://symposia.goldenhelix.org) range in length from 2 to 4 days, with an average attendance of 450 participants (ranging from 250 to 900 participants). Conferences are usually held in environments that are conducive to multidisciplinary thinking and collaboration.
• The Golden Helix Pharmacogenomics Days (http://pharmacogenomicsdays.goldenhelix.org) are international educational event series organized in major cities with tertiary academic healthcare facilities. The aim of this event is to provide timely updates on the field of pharmacogenomics to local biomedical scientists and healthcare providers. Contrary to the Golden Helix Symposia, the Golden Helix Pharmacogenomics Days have a more educational ethos, span between a half day and a full day in length, and address on average 150 participants. Registration is free of charge, attracting mostly not only local scholars but also participants from neighboring countries and regions.
• The Golden Helix Summer Schools (http://summerschools.goldenhelix.org) are a new forum in the Golden Helix Conferences series. Established conceptually in 2013, and jointly organized under the umbrella of the Golden Helix Foundation and the Genomic Medicine Alliance (GMA), the Golden Helix Summer Schools are 5-day-long international educational activities, usually organized in the summer period to allow attendance by scholars in training and others, that aim at providing a unique academic program, catalyzing collaborations, and opening up future training opportunities for participants.
Since November 2008, when the 1st Golden Helix Symposium® was organized in Athens, Greece, there have been 32 Golden Helix Conferences that have been organized in 27 major cities in 18 different countries in Europe, the Americas, the Middle East, Africa, and South East Asia, attracting more than 6000 participants from more than 100 countries from all five continents, from students to senior academics and researchers; several participants have registered to attend recurrent events, an indication of the impact of these gatherings. The concept of the Golden Helix Conferences represents a paradigm of a well-designed and coordinated effort to improve the genomics education of healthcare professionals, specifically addressing the needs of participants from developing countries.
Further, to enable smoother integration of genomics into medicine, there is a need to raise genomics awareness of patients and the general public. To this end, Genome British Columbia coordinates such a dedicated initiative, known as Geneskool (https://www.genomebc.ca/education/outreach-programs). This program focuses on providing basic genomic education and awareness to children from 9th to 12th grade, including classrooms and full-day campus workshops, traveling suitcase exhibits, and summer science programs for teens. Similar initiatives were also developed in Mexico, in an effort to engage the young Mexican public on issues of genomic medicine, where the National Institute of Genomic Medicine (INMEGEN; http://www.inmegen.gob.mx) has developed a comic book series on genomic medicine.
At the same time, the seamless incorporation of genomic medicine into clinical practice is hindered by insufficient genomics education, which is not uniformly provided in the various academic institutions worldwide (Mai et al., 2014; Mitropoulou et al., 2014; Pisanu et al., 2014). By analyzing a large number of post-graduate and undergraduate curricula in 98 universities in 11 South Eastern European countries, Pisanu et al. (2014) have shown that there are major discrepancies in the provision of pharmacogenomics education, compared with the situation in North Western Europe. Such studies might provide the basis to harmonize pharmacogenomics education in South East European countries with those of North West European countries with a view to bringing about a smoother integration of pharmacogenomics into mainstream medical practice.
Genomic Medicine and Public Health
Integration of genomics research findings into medicine is a multistep approach that depends on several disciplines mostly related to public health. Such disciplines include addressing the ethical, social, and legal issues pertaining to genomic medicine, assessing the educational needs of healthcare professionals, at both the undergraduate and postgraduate levels, as far as genomic medicine is concerned, and precisely mapping the environment of the stakeholders and decision makers who are involved in genomic medicine. In this latter case, there are several key players and stakeholders, whose genomics awareness and views vary significantly; hence, performing a systematic mapping of these views and different genomics awareness levels should positively impact our better understanding of the policy environment as well as the role of the relevant key stakeholders in the field.
Previously, Mitropoulou et al. (2014) assessed the level of support (or opposition) to pharmacogenomics and genomic medicine in Greece. These authors have shown that the majority of the stakeholders, namely academic and research institutions, biomedical scientists, physicians, geneticists, biotechnology, and pharmaceutical companies, to name a few, have a positive attitude to genomic medicine. It is, however, noteworthy that the Ministry of Health and the Public Health insurance funds are more skeptical as far as implementation of genomic medicine is concerned, most likely due to the large initial investment and reimbursement requirements.
A similar project is currently under way in the United Arab Emirates. In particular, the main aims of this project are to: (1) investigate the state of genomics education in the United Arab Emirates, by assessing the pharmacogenomics educational environment in undergraduate and post-graduate university and college curricula; (2) assess the level of genomics education of undergraduate students, residents, and specialized physicians; (3) assess the level of awareness of healthcare professionals and the general public as far as genomic medicine is concerned; and (4) survey the currently available provisions for implementing genomic medicine and pharmacogenomics. To this end, there is a productive partnership between the Zayed Bin Sultan Center for Health Sciences (United Arab Emirates University) and the Golden Helix Foundation (see Capacity building in genomic medicine section). This is the first time that such a project will have been implemented in the United Arab Emirates and the GCC countries in general, allowing the canvasing of the genomic medicine environment in the United Arab Emirates and, ultimately, extending this project into neighboring GCC countries with the aim of harmonizing genomics education and the provision of genomic medicine in the United Arab Emirates and the GCC countries in general.
Similarly, a Public Health Genomics project is planned, in parallel to the 100 Pharmacogene Resequencing Project in South East Asia (see Population and Pharmacogenomics section), and in partnership with the Golden Helix Foundation, so that the benefits of the pharmacogenomic analysis in these populations can be maximized. As with the related project already running in the United Arab Emirates, this parallel project will include: (1) mapping the pharmacogenomics educational environment in the participating countries in South East Asia, and (2) raising pharmacogenomics awareness among healthcare professionals by organizing scientific conferences and other educational activities, similar to those described earlier.
Regrettably, in Africa, the proportion of medical professionals with a thorough understanding of human and medical genomics is still relatively low. An attempt in sub-Saharan Africa to triangulate the views of multiple stakeholders related to prenatal diagnosis of SCD revealed several discrepancies that usefully inform future policy actions (Wonkam and Hurst, 2014). In fact, scientists originating from Africa are participating in studies focusing on the genomics of monogenic diseases (Mercier et al., 2013; Mtatiro et al., 2014; Wonkam, 2015; Wonkam et al., 2014) and multifactorial conditions (Tekola Ayele et al., 2012), with findings that are expected to assist the effective practice of genomic medicine, that is well established in South Africa (Beighton et al., 2012), in some Northern African countries (Chaabouni-Bouhamed, 2008; El-Beshlawy et al., 2012) and recently initiated in Central Africa (Wonkam et al., 2011b).
In addition, the knowledge of the nature of pathogenic variants of various monogenic conditions among people of African Ancestry needs to be accelerated, for the purpose of molecular diagnosis in a clinical setting, taking advantage of the power of next-generation sequencing technologies. For example, recent data have shown that GJB2 and GJB6 pathogenic variants that account for nearly 50% of causes on Non-Syndromic Hearing Impairment in populations of European and Asian descent are virtually nonexistent among Africans (Wonkam, 2015). In a small group of 10 families, the use of targeted panel sequencing, including 116 hearing impairment genes, have identified mostly novel pathogenic variants in 7/10 families in Cameroon, indicating the opportunity for novel variants and gene discovery in Africa (Lebeko et al., 2016).
Economics in Genomic Medicine
Genome-guided drug treatment modalities are expected to reduce national healthcare expenditure (Snyder et al., 2014). To date, the scientific discipline of economic evaluation in genomic medicine, pharmacogenomics, and public health genomics is still poorly developed. However, there are several studies that have demonstrated that genotype-guided therapy not only can be cost-effective but also may help to improve the quality of life of the patients. The most remarkable example related to resource-limited countries comes from the Thai population, where it has been previously shown that HLA-B*15:02-guided carbamazepine treatment is cost-effective compared with conventional treatment and can reduce the incidence of Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), a severe carbamazepine-induced adverse drug reaction (Grosse, 2008; Rattanavipapong et al., 2013). Similar results were also obtained for the Singaporean population (Dong et al., 2012).
A few studies have aimed at assessing the cost-effectiveness of genome-guided interventions in cardiology. In particular, Mitropoulou et al. (2015) attempted prospectively to evaluate the cost-effectiveness of warfarin treatment in Croatian elderly ischemic stroke patients with atrial fibrillation. These authors have shown that pharmacogenomics-guided warfarin treatment represented a cost-effective therapeutic option for the management of these patients, with an estimated incremental cost-effectiveness ratio of the pharmacogenomics-guided versus the control groups of €31,225/QALY (Quality-Adjusted Life Year). In addition, a retrospective economic analysis of genome-guided clopidogrel treatment in Serbia indicated that pharmacogenomics-guided clopidogrel treatment may represent a cost-saving approach for the management of myocardial infarction patients undergoing primary percutaneous coronary intervention (Mitropoulou et al., 2016). Such analyses would also be facilitated by the development of standardized methodologies for the economic evaluation of genome-guided interventions (Fragoulakis et al., 2016, 2017), which, in addition to the already existing economic evaluation models in genomic medicine, will be of the utmost importance as innovative tools for performing cost-effectiveness analyses in such a rapidly evolving discipline.
Lastly, the issue of pricing and reimbursement in genomic medicine is a cornerstone for the incorporation of genomics into clinical medicine, given the lack of harmonization between pricing and reimbursement policies between European countries, contrary to the situation pertaining in the United States (Logue, 2003). In Europe, very few countries (e.g., the Netherlands, the United Kingdom, and Switzerland) reimburse genetic testing services, whereas other countries clearly lag behind. As such, the general strategy toward pricing and reimbursement for genomic medicine should first be outlined, providing an overview of the rationale and basic principles guiding the governance of genomic testing services, clarifying their objectives, and allocating and defining responsibilities among and between stakeholders, focusing on different European countries' healthcare systems.
Particular attention should be paid to issues pertaining to pricing and reimbursement policies, the availability of essential genomic tests that differ between various countries owing to differences in disease prevalence and public health relevance, the prescribing and use of genomic testing services according to existing or new guidelines, budgetary and fiscal control, the balance between price and access to innovative testing, monitoring and evaluation for cost-effectiveness and safety, and the development of research capacity (Vozikis et al., 2016). Subsequently, it is hoped that a more technical analysis will lead to the introduction of a robust policy in relation to pricing and reimbursement in genomic medicine, thereby contributing to an effective and sustainable healthcare system with benefits to the economy at large.
Building regional and global genomic medicine research networks
Synergizing efforts among researchers with common research interests from various parts of the world could significantly expedite efforts to bring genomic medicine, as with every other discipline, closer to fruition (Manolio et al., 2015).
The GMA (http://www.genomicmedicinealliance.org) was established in mid-2013 with the primary goal of developing a global network focusing on the translation of genomic knowledge into clinical use, with a special focus on the participation of developing countries and of resource-limited settings. The GMA has several unique features as a research network, in which it differs from existing consortia and initiatives in this field (Cooper et al., 2014). First, membership is free of charge, which is important to attract members from developing countries. Second, it has a flat governance structure, comprising the Scientific Advisory and the Steering Committees. Third, this network has a stated goal and commitment to bring together genomics research institutions from developing countries with those from developed countries (Cooper et al., 2014).
The GMA aims at: (1) encouraging and catalyzing multidisciplinary collaborative research between partner institutions and/or scientists, with at least one partner affiliated with an institute in a developing country; (2) liaising between research organizations, clinical entities, and regulatory agencies on topics related to genomic medicine; (3) facilitating the introduction of pharmacogenomics and advanced “omics” technologies and analyses into mainstream clinical practice; (4) proposing international guidelines and draw up recommendations for activities pertaining to genomic medicine, in close collaboration with other scientific academic entities, agencies, and regulatory bodies; and (5) developing independently and coordinate, in close collaboration with partner institutions, educational activities in the sphere of genomic medicine (Cooper et al., 2014). GMA research activities span seven different Working Groups, namely Genome Informatics, Pharmacogenomics, Cancer Genomics, Rare Diseases, Public Health Genomics, Genethics, and Economic Evaluation in Genomic Medicine. Each of the Working Groups' activities are coordinated by the corresponding Working Group and Activity leaders.
Consistent with one of the stated goals of the GMA, namely the fruitful engagement between research groups from both developing and developed countries to study families with rare diseases or unique clinical features (especially countries with a higher incidence of consanguinity and/or well-defined founder populations), the GMA actively participates in the DRIFT (Discovery Research Investigating Founder Population Traits) Consortium. In early 2016, a call for research collaboration was made by the Regeneron Genetics Center and the GMA, aimed specifically at developing countries. DRIFT aims at understanding the genetic architecture of founder populations throughout the world, with a direct impact on human health and disease. The DRIFT Consortium aims at cataloging population-specific allelic architecture, with a view to understanding the biological and functional consequences of specific genomic variants identified, and to sharing best-practice approaches to relieve disease burden in these populations. Ever since its establishment, the expansion of the GMA membership base has progressed at a very rapid pace, currently comprising more than 1300 members from >70 countries worldwide, from academia as well as from corporate and regulatory sectors, including developing countries in the Middle East, Asia, and Latin America.
The Human Variome Project (HVP; http://www.humanvariomeproject.org) is an international non-governmental organization that is working to ensure that all information on genetic variation and its effect on human health can be collected, curated, interpreted, and shared freely and openly (Kaput et al., 2009). The HVP provides a central coordinating function for national and international efforts to integrate the collection, curation, interpretation, and sharing of information on variation in the human genome into routine clinical practice and research. HVP members number more than 1100 individual researchers, healthcare professionals, policy makers, and organizations coming from 81 countries that collaborate to develop and maintain the necessary standards, systems, and infrastructure to support global-scale genomic knowledge sharing.
The HVP aims at: (1) collaboratively developing technical standards and harmonized common approaches so that data from different sources can be easily shared in an interoperable manner that is sensitive to the ethical, legal, and social requirements of both the data sources and consumers; (2) coordinating an international platform to facilitate discussion of genomics in global health with the aim of fostering necessary professional interaction and debate in the area of genomics, global health, service delivery, and safety; (3) linking world-leading professionals and institutions with genomics professionals, researchers, and academics in all parts of the world, facilitating knowledge exchange and interactive debate; and (4) establishing a global evidence base for knowledge sharing in medical genetics and genomics and bringing relevant issues to the attention of Ministries of Health, Science and Technology and Education.
At the same time, the SEAPharm Consortium (http://www.pharmagct.org) is a regional pharmacogenomics network in South East Asia, consisting of leading researchers from Japan, Korea, Taiwan, Malaysia, Indonesian, and Thailand who share knowledge and experience in pharmacogenomics research. The network organizes its annual meeting each year and envisages the achievement of sustainable pharmacogenomics research, so that South East Asian countries can liaise and work together in conducting clinical studies for various drugs, which can be effective and safe for the 560 million South East Asians.
There are also other important international efforts, such as the Global Alliance for Genomics and Health (GA4GH; http://www.ga4gh.org), the Human Genome Organization (http://www.hugo-international.org), and the G2MC (http://www.g2mc.org). These organizations have various activities related to genomic and precision medicine, such as genomic data sharing (GA4GH), implementation of genomic medicine (G2MC), with activities that are also relevant to resource-limited countries. The activities of these organizations have been outlined elsewhere and, as such, lie outside the scope of this article.
Discussion
The implementation of genomic medicine does not always progress at a uniform pace in all parts of the world. There are countries, such as the United States and countries in Northern Europe, where genomic medicine is being implemented at a more rapid pace, depending on different factors, such as the sufficient genetic literacy of healthcare professionals, state-of-the-art infrastructure, and established reimbursement rules for genetic testing and legislation governing the use of genetic tests. Nevertheless, there are several examples of the successful implementation of genomic medicine applications in countries that do not fall into the previous category and can be considered as developing or low-resourced countries from South East Asia, the Middle East, and Latin America. In these countries, the genomic medicine applications are mostly confined to those genetic conditions that are prevalent in the region and, hence, may constitute a significant health and/or economic burden for these countries.
Carriers of the HLA-B*1502 allele receiving carbamazepine treatment may develop SJS/TEN (Borchers et al., 2008; Chung et al., 2004) and the same is true for carriers of the HLA-B*5801 allele who may develop severe cutaneous adverse reactions (Hung et al., 2005), when treated with allopurinol. In Taiwan, the value of pharmacogenomics in tailor-made therapeutics has been acknowledged and, after a large prospective clinical study that confirmed the benefit of HLA-B*1502 genotyping to identify subjects at genetic risk for the conditions mentioned earlier (Chen et al., 2011), the Taiwanese government began to reimburse the genotyping costs as of 2010.
In South East Asia and in Thailand in particular (Ramathibodi Hospital, Bangkok), a pharmacogenomics card was adopted to summarize patients' HLA gene variant information to predict the risk of developing SJS/TEN. Such pharmacogenomics cards have also been used in other settings and clinical studies, such as the PREPARE study of the Ubiquitous Pharmacogenomics (U-PGx) Consortium (www.upgx.eu), and could be readily applied in the context of tailor-made therapeutics.
In the United Arab Emirates, after pharmacogenomics research into the role of variants in the G6PD gene and their association with drug-induced hemolytic anemia (Bayoumi et al., 1996) and the prevalence of NAT2, CYP2D6, CYP2C9, and VKORC1 pharmacogenomic variant allele frequencies in the Emirati population (Al-Gazali et al., 2016; Fortina et al., 2014; Qumsieh et al., 2011; Woolhouse et al., 1997), Dubai Hospital was prompted to integrate pharmacogenomics information for chemotherapeutic agents; whereas the United Arab Emirates Health Authority policy of reporting adverse drug reactions in the United Arab Emirates currently requires expert pharmacogenomics recommendation within the first 24 h related to each adverse drug reaction reported (http://www.haad.ae). Similarly, raising pharmacogenomics awareness among healthcare professionals is currently gathering momentum in other Middle Eastern countries, such as Oman (Pathare et al., 2012), Saudi Arabia (Abu-Elmagd et al., 2015), Lebanon (Ossaily and Zgheib, 2014), and Qatar (Elewa et al., 2015).
In Latin America, the INMEGEN (http://www.inmegen.gob.mx) was built in Mexico as a capacity-building effort to establish infrastructure for research and educational activities for Genomic Medicine, not only for Latin America but also for the wider Latin American region. In Africa, the burden of HIV/AIDS, tuberculosis, and malaria is disproportionately high compared with other countries/regions, and genomic data have been used to show that several genetic variants can provide increased resistance or susceptibility to HIV infections (Sirugo et al., 2008); whereas the variability observed in the patterns of genomic variations in the CYP genes among African populations has also been used to rationalize drug use (Dandara et al., 2014). What has been mentioned earlier constitute some of the most successful examples of the implementation of genomic medicine in resource-limited countries, whereas other examples have been also reported with significant impact on the provision of medical services in developing countries (Lopez-Correa and Patrinos, unpublished data; Mitropoulos et al., 2015).
Conclusions and Outlook
The implementation of genomic medicine in resource-limited environments can be a difficult and lengthy process, which could deprive less privileged populations of the benefits that genome-guided interventions have to offer. Given the scarcity or even lack of resources in developing countries, the implementation of genomic medicine can only be realized in these environments under certain conditions. First of all, generation of primary data in under-equipped research institutions may be a challenging task. As such, and as far as advancement of genomics research is concerned, strategic partnerships should be established with research entities from developed countries, which are likely to create benefits for all parties involved. Not only will developing countries benefit from the various training opportunities that will be created in addition to knowledge transfer, but also developed countries may benefit too, through participation in multicenter projects to study cases and individuals with unique clinical features and/or rare diseases (Cooper et al., 2014; Manolio et al., 2015).
On the implementation front, it would be highly beneficial for developing countries to prioritize their efforts toward implementing actionable genomic medicine interventions, including, but not limited to, pharmacogenomic tests, genetic testing for the highly prevalent inherited conditions, which differ dramatically around the world. Therefore, country-, population-, and region-specific data and research are needed. At the same time, it seems reasonable for decision makers and policymakers to invest an equal amount of effort into expanding the public health aspects of genomic medicine interventions, such as (1) mapping the stakeholders' environment and their opinions and stance related to genomics; (2) enacting new legislation and measures to set out a legal framework for the provision of genetic services, so that quality is ensured and the general public and the patients are safeguarded; and (3) adopting guidelines for the reimbursement of genetic tests, based on related cost-effectiveness analyses.
Lastly, if for some countries, the acquisition of expensive genomic technology and equipment may seem unrealistic, investment in continuous genomics education of healthcare professionals and biomedical scientists would be far easier and with a short- to medium-term return on such investment. Continuous genetics education can be provided to both graduate professionals who should be encouraged to attend, from which certificates/diplomas will be obtained, and also to undergraduate students, by harmonizing the university curriculum with those of other developed countries, which could be used as a paradigm for this purpose. In all that has been stated, synergies should be sought not only with renowned academics and institutions from developed countries but also with entities from other developing countries that have successfully implemented genomic medicine interventions.
Human genomics promises to transform medical practice around the world. Much attention has been focused on developed countries where most research leading to Genomic and Precision Medicine is performed. However, resource-poor developing countries also have the potential to adopt genomic medicine. Therefore, we strongly believe that the examples described in this article could be readily replicated by other countries with a view to expediting their transition to the genomic medicine era, by harmonizing their strategies and policies with those of various other national healthcare systems that already enjoy the tangible benefits of genomics. Clearly, the inclusion of other omics into Precision Medicine will further contribute to these developments.
Ultimately, broadening our thinking beyond the linear model of innovation, to encompass such models as the Fast-Second Winner model of knowledge-based innovation, should help enable the vision and practice of genomics without borders in both developed and resource-limited settings.
Abbreviations Used
- DRIFT
Discovery Research Investigating Founder Population Traits
- G2MC
Global Genomic Medicine Collaborative
- GA4GH
Global Alliance for Genomics and Health
- GCC
Gulf Cooperation Council
- GMA
Genomic Medicine Alliance
- GWAS
Genome-Wide Association Studies
- HVP
Human Variome Project
- INMEGEN
National Institute of Genomic Medicine
- SCD
Sickle cell disease
Acknowledgments
This article was informed, in part, by discussions with the Public Health Genomics Working Group of the Genomic Medicine Alliance. No funding was received in support of the present innovation analysis. The views expressed are the personal opinions of the authors and do not necessarily represent the views of their affiliated institutions. The authors thank the editors of OMICS: A Journal of Integrative Biology for discussions on the Fast-Second Innovation model for global genomics development strategy. Work at Yachay Tech was supported by internal grant 39-2017. B.R.A. is supported by UAEU grant 31R091.
Author Disclosure Statement
C.M. and G.P.P. are members of the Golden Helix Foundation. J.K.V.R. and G.P.P. are members of the Scientific Advisory Council of the Golden Helix Foundation. D.N.C. and G.P.P. are members of the International Scientific Advisory Committee of the Genomic Medicine Alliance.
References
- Abu-Elmagd M, Assidi M, Schulten H-J, et al. (2015). Individualized medicine enabled by genomics in Saudi Arabia. BMC Med Genomics 8, S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gazali L, Al-Jaibeji HS, John A, et al. (2016). Allele and genotype frequencies of the two single nucleotide polymorphisms in the VKORC1 gene that are most important for warfarin treatment among Emiratis. Hamdan Med J 9, 75–84 [Google Scholar]
- Bayoumi R, Nur-E-Kamal M, Tadayyon M, et al. (1996). Molecular characterization of erythrocyte glucose-6-phosphate dehydrogenase deficiency in Al-Ain District, United Arab Emirates. Hum Hered 46, 136–141 [DOI] [PubMed] [Google Scholar]
- Beighton P, Fieggen K, Wonkam A, Ramesar R, and Greenberg J. (2012). The University of Cape Town's contribution to medical genetics in Africa: From the past into the future. SAMJ 102, 446–448 [DOI] [PubMed] [Google Scholar]
- Borchers AT, Lee JL, Naguwa SM, Cheema GS, and Gershwin ME. (2008). Stevens–Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev 7, 598–605 [DOI] [PubMed] [Google Scholar]
- Chaabouni-Bouhamed H. (2008). Tunisia: Communities and community genetics. Public Health Genomics 11, 313–323 [DOI] [PubMed] [Google Scholar]
- Chen P, Lin J-J, Lu C-S, et al. (2011). Carbamazepine-induced toxic effects and HLA-B* 1502 screening in Taiwan. N Engl J Med 364, 1126–1133 [DOI] [PubMed] [Google Scholar]
- Chung W-H, Hung S-I, Hong H-S, et al. (2004). Medical genetics: A marker for Stevens–Johnson syndrome. Nature 428, 486. [DOI] [PubMed] [Google Scholar]
- Consortium HA. (2014). Enabling the genomic revolution in Africa. Science 344, 1346–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DN, Brand A, Dolzan V, et al. (2014). Bridging genomics research between developed and developing countries: The Genomic Medicine Alliance. Pers Med 11, 615–623 [DOI] [PubMed] [Google Scholar]
- Dandara C, Swart M, Mpeta B, Wonkam A, and Masimirembwa C. (2014). Cytochrome P450 pharmacogenetics in African populations: Implications for public health. Expert Opin Drug Metab Toxicol 10, 769–785 [DOI] [PubMed] [Google Scholar]
- Dong D, Sung C, and Finkelstein EA. (2012). Cost-effectiveness of HLA-B* 1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 79, 1259–1267 [DOI] [PubMed] [Google Scholar]
- El-Beshlawy A, El-Shekha A, Momtaz M, et al. (2012). Prenatal diagnosis for thalassaemia in Egypt: What changed parents' attitude? Prenat Diagn 32, 777–782 [DOI] [PubMed] [Google Scholar]
- Elewa H, Alkhiyami D, Alsahan D, and Abdel-Aziz A. (2015). A survey on the awareness and attitude of pharmacists and doctors towards the application of pharmacogenomics and its challenges in Qatar. J Eval Clin Pract 21, 703–709 [DOI] [PubMed] [Google Scholar]
- Forero DA, Wonkam A, Wang W, et al. (2016). Current needs for human and medical genomics research infrastructure in low and middle income countries. J Med Genet 53, 438–440 [DOI] [PubMed] [Google Scholar]
- Fortina P, Khaja NA, Ali MTA, et al. (2014). Genomics into healthcare: The 5th Pan Aarab Human Genetics conference and 2013 Golden Helix symposium. Hum Mutat 35, 637–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoulakis V, Mitropoulou C, Katelidou D, van Schaik RH, Maniadakis N, and Patrinos GP. (2017). Performance ratio based resource allocation decision-making in genomic medicine. OMICS 21, 67–73 [DOI] [PubMed] [Google Scholar]
- Fragoulakis V, Mitropoulou C, van Schaik RH, Maniadakis N, and Patrinos GP. (2016). An alternative methodological approach for cost-effectiveness analysis and decision making in genomic medicine. OMICS 20, 274–282 [DOI] [PubMed] [Google Scholar]
- Geard A, Pule GD, Chetcha Chemegni B, et al. (2017). Clinical and genetic predictors of renal dysfunctions in sickle cell anaemia in Cameroon. Br J Haematol 178, 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse SD. (2008). Assessing cost-effectiveness in healthcare: History of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 8, 165–178 [DOI] [PubMed] [Google Scholar]
- Hung S-I, Chung W-H, Liou L-B, et al. (2005). HLA-B* 5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 102, 4134–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaput J, Cotton RG, Hardman L, et al. (2009). Planning the Human Variome Project: The Spain report. Hum Mutat 30, 496–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeko K, Sloan-Heggen C, Noubiap J, et al. (2016). Targeted genomic enrichment and massively parallel sequencing identifies novel nonsyndromic hearing impairment pathogenic variants in Cameroonian families. Clin Genet 90, 288–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue L. (2003). Genetic testing coverage and reimbursement: A provider's dilemma. Clin Leadersh Manag Rev 17, 346–350 [PubMed] [Google Scholar]
- Mai Y, Mitropoulou C, Papadopoulou XE, et al. (2014). Critical appraisal of the views of healthcare professionals with respect to pharmacogenomics and personalized medicine in Greece. Pers Med 11, 15–26 [DOI] [PubMed] [Google Scholar]
- Makani J, Menzel S, Nkya S, et al. (2011). Genetics of fetal hemoglobin in Tanzanian and British patients with sickle cell anemia. Blood 117, 1390–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Abramowicz M, Al-Mulla F, et al. (2015). Global implementation of genomic medicine: We are not alone. Sci Transl Med 7, 290ps213–290ps213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matovu E, Bucheton B, Chisi J, et al. (2014). Research capacity: Enabling African scientists to engage fully in the genomic revolution. Science 344, 1346–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, McLeod HL, and Ginsburg GS. (2013). Genomic medicine: A decade of successes, challenges, and opportunities. Sci Transl Med 5, 189sr184–189sr184 [DOI] [PubMed] [Google Scholar]
- Mercier S, Küry S, Shaboodien G, et al. (2013). Mutations in FAM111B cause hereditary fibrosing poikiloderma with tendon contracture, myopathy, and pulmonary fibrosis. Am J Hum Genet 93, 1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K, Al Jaibeji H, Forero DA, et al. (2015). Success stories in genomic medicine from resource-limited countries. Hum Genomics 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K, Johnson L, Vozikis A, and Patrinos GP. (2011). Relevance of pharmacogenomics for developing countries in Europe. Drug Metab Drug Interact 26, 143–146 [DOI] [PubMed] [Google Scholar]
- Mitropoulou C, Fragoulakis V, Bozina N, et al. (2015). Economic evaluation of pharmacogenomic-guided warfarin treatment for elderly Croatian atrial fibrillation patients with ischemic stroke. Pharmacogenomics 16, 137–148 [DOI] [PubMed] [Google Scholar]
- Mitropoulou C, Fragoulakis V, Rakicevic LB, et al. (2016). Economic analysis of pharmacogenomic-guided clopidogrel treatment in Serbian patients with myocardial infarction undergoing primary percutaneous coronary intervention. Pharmacogenomics 17, 1775–1784 [DOI] [PubMed] [Google Scholar]
- Mitropoulou C, Mai Y, Van Schaik RH, Vozikis A, and Patrinos GP. (2014). Stakeholder analysis in pharmacogenomics and genomic medicine in Greece. Public Health Genomics 17, 280–286 [DOI] [PubMed] [Google Scholar]
- Mizzi C, Dalabira E, Kumuthini J, et al. (2016). A european spectrum of pharmacogenomic biomarkers: Implications for clinical pharmacogenomics. PLoS One 11, e0162866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtatiro SN, Singh T, Rooks H, et al. (2014). Genome wide association study of fetal hemoglobin in sickle cell anemia in Tanzania. PLoS One 9, e111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossaily S. and Zgheib NK. (2014). The pharmacogenetics of drug metabolizing enzymes in the Lebanese population. Drug Metab Drug Interact 29, 81–90 [DOI] [PubMed] [Google Scholar]
- Pathare AV, Zadjali SA, Misquith R, et al. (2012). Warfarin pharmacogenetics: Polymorphisms of the CYP2C9, CYP4F2, and VKORC1 loci in a genetically admixed Omani population. Hum Biol 84, 67–77 [DOI] [PubMed] [Google Scholar]
- Piel FB, Patil AP, Howes RE, et al. (2013). Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 381, 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanu C, Tsermpini E-E, Mavroidi E, Katsila T, Patrinos GP, and Squassina A. (2014). Assessment of the pharmacogenomics educational environment in Southeast Europe. Public Health Genomics 17, 272–279 [DOI] [PubMed] [Google Scholar]
- Qumsieh RY, Ali BR, Abdulrazzaq YM, Osman O, Akawi NA, and Bastaki SM. (2011) Identification of new alleles and the determination of alleles and genotypes frequencies at the CYP2D6 gene in Emiratis. PLoS One 6, e28943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanavipapong W, Koopitakkajorn T, Praditsitthikorn N, Mahasirimongkol S, and Teerawattananon Y. (2013). Economic evaluation of HLA-B* 15: 02 screening for carbamazepine-induced severe adverse drug reactions in Thailand. Epilepsia 54, 1628–1638 [DOI] [PubMed] [Google Scholar]
- Sirugo G, Hennig BJ, Adeyemo AA, et al. (2008). Genetic studies of African populations: An overview on disease susceptibility and response to vaccines and therapeutics. Hum Genet 123, 557. [DOI] [PubMed] [Google Scholar]
- Snyder SR, Mitropoulou C, Patrinos GP, and Williams MS. (2014). Economic evaluation of pharmacogenomics: A value-based approach to pragmatic decision making in the face of complexity. Public Health Genomics 17, 256–264 [DOI] [PubMed] [Google Scholar]
- Tekola Ayele F, Adeyemo A, et al. (2012). HLA class II locus and susceptibility to podoconiosis. N Engl J Med 366, 1200–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozikis A, Cooper DN, Mitropoulou C, et al. (2016). Test pricing and reimbursement in genomic medicine: Towards a general strategy. Public Health Genomics 19, 352–363 [DOI] [PubMed] [Google Scholar]
- Wonkam A. (2015). Letter to the editor regarding “GJB2, GJB6 or GJA1 genes should not be investigated in routine in non syndromic deafness in people of sub-Saharan African descent.” Int J Pediatr Otorhinolaryngol 79, 632–633 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Bitoungui VJN, Vorster AA, et al. (2014). Association of variants at BCL11A and HBS1L-MYB with hemoglobin F and hospitalization rates among sickle cell patients in Cameroon. PLoS One 9, e92506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonkam A, and Hurst S. (2014). A call for policy action in sub-Saharan Africa to rethink diagnostics for pregnancy affected by sickle cell disease: Differential views of medical doctors, parents and adult patients predict value conflicts in Cameroon. OMICS 18, 472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonkam A, Kenfack MA, Muna WF, and Ouwe-Missi-Oukem-Boyer O. (2011a). Ethics of human genetic studies in sub-saharan Africa: The case of cameroon through a bibliometric analysis. Dev World Bioeth 11, 120–127 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Njamnshi AK, and Angwafo FF. (2006). Knowledge and attitudes concerning medical genetics amongst physicians and medical students in Cameroon (sub-Saharan Africa). Genet Med 8, 331–338 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Tekendo CN, Sama DJ, et al. (2011b). Initiation of a medical genetics service in sub-Saharan Africa: Experience of prenatal diagnosis in Cameroon. Eur J Med Genet 54, e399–e404 [DOI] [PubMed] [Google Scholar]
- Woolhouse NM, Qureshi MM, Bastaki SM, Patel M, Abdulrazzaq Y, and Bayoumi RA. (1997). Polymorphic N-acetyltransferase (NAT2) genotyping of Emiratis. Pharmacogen Genomics 7, 73–82 [DOI] [PubMed] [Google Scholar]


