Abstract
Background: This study aims to compare the seventh and eighth editions of the American Joint Commission on Cancer/Union for International Cancer Control (AJCC/UICC) tumor, node, metastasis staging system for patients with papillary thyroid cancer (PTC) in two national patient cohorts.
Methods: Adult PTC patients undergoing surgery were selected from the Surveillance, Epidemiology and End Results (SEER) program (2004–2012) and the National Cancer Database (2004–2012). Staging criteria for the seventh and eighth AJCC/UICC editions were applied separately to each cohort. Survival probabilities were estimated using the Kaplan–Meier method. Multivariable Cox proportional hazards models were used to estimate the association of stage with survival in both settings. The Akaike information criterion was used to assess model performance.
Results: About 23% of patients were downstaged from the seventh to the eighth edition in SEER, while 24% were downstaged in the National Cancer Database. Disease-specific survival (DSS) and overall survival (OS) were significantly related to stage at diagnosis when using both the seventh and eighth editions of the AJCC/UICC staging system (p < 0.001). Patients classified into higher stages (III and IV) in the eighth edition showed a worse prognosis than those classified into similar stages in the seventh edition. After adjustment, PTC stages as defined by both editions were significantly associated with DSS and OS. With respect to both DSS and OS, the eighth edition PTC model appeared to be a better fit to the data (smaller Akaike information criterion values) compared to the seventh edition.
Conclusion: Based on these large contemporary national cohorts, the eighth edition AJCC/UICC tumor, node, metastasis classification for PTC is superior to the seventh edition for predicting survival.
Keywords: : papillary thyroid cancer, AJCC/UICC staging system, National Cancer Database, Surveillance, Epidemiology and End Results, overall survival, disease-specific survival
Introduction
Over time, many different systems have been employed to stage papillary thyroid cancer (PTC), including the American Joint Commission on Cancer/Union for International Cancer Control's tumor, nodes, metastases (AJCC/UICC TNM) (1); age, grade, extent, size (AGES) (2); age, metastases, extent of disease, size (AMES) (3); metastases, age, completeness of resection, invasion locally, size (MACIS) (4–6); the European Organization for Research and Treatment of Cancer (EORTC) (7); and the National Thyroid Cancer Treatment Cooperative Study (NTCTCS) (8). The AJCC/UICC TNM system for tumor staging has become the most widely used to anticipate patients' survival prognosis, and has undergone several iterations over time.
A new edition of the AJCC/UICC TNM staging system (the eighth edition) has been released, and it is anticipated to be introduced into clinical practice in January 2018 (9). In previous editions, an age cut point of 45 years was employed. Patients who were <45 years could only be assigned to stage I (absence of distant/extra-cervical metastases) or stage II (presence of distant/extra-cervical metastases), while patients ≥45 years could be assigned to stages I–IV, based on local tumor characteristics and the presence/absence of cervical lymph node or distant metastases (10). A retrospective study of >31,000 patients in the Surveillance, Epidemiology, and End Results (SEER) program by Adam et al. assessed the relationship between age and cancer-specific survival in PTC (11), and found that patient age was associated with mortality from PTC in a linear manner, without an apparent age cut point demarcating a sharp survival difference. Another study by Nixon et al. used a single institution database to determine that the age of 55 years served as a superior cut point than that of 45 years for PTC staging (12). The proposed eighth edition has moved the cut point to age 55 years. It has also reclassified patients with unknown lymph node status more specifically, and removed “minimal” extrathyroidal extension from staging, designating patients as having either disease limited to the thyroid or gross extrathyroidal extension.
The primary aim of this study was to compare the seventh and eighth editions of PTC staging according to the AJCC/UICC TNM system based on two national cohorts, and to determine if the eighth edition provides better delineation for disease-specific survival (DSS) and overall survival (OS) based on disease stages compared to the seventh edition.
Methods
Data
The SEER program of the National Cancer Institute (NCI) collects and publishes data on cancer incidence and survival from 14 population-based cancer registries and three supplemental registries covering approximately 28% of the U.S. population (13,14). The National Cancer Database (NCDB) is a nationwide, facility-based, comprehensive clinical surveillance resource oncology data set that currently captures 70% of all newly diagnosed malignancies in the United States annually. The NCDB, established in 1989, culls information from >1500 Commission on Cancer–accredited facilities, and is jointly sponsored by the American College of Surgeons and the American Cancer Society (15–17).
These two data sets were examined in tandem in order to describe fully the staging criteria changes from the seventh to eighth editions of the AJCC/UICC TNM staging system. SEER contains a more limited sample of patients and reports DSS in addition to OS. The NCDB contains more patients and collects more variables. However, it only includes overall survival (OS). Data on DSS were obtained from the SEER data set, and the NCDB was used as the source of data for OS for the purposes of comparing the seventh and eighth editions of the AJCC/UICC staging systems for PTC.
Study population
Both SEER and the NCDB were queried for all adult patients (≥18 years) diagnosed with PTC who underwent thyroid surgery of any extent between 2004 and 2012. A PTC diagnosis was identified with the ICD-O, third edition codes: 8050/3, 8260/3, 8340/3, 8341/3, 8342/3, 8343/3, and 8344/3 (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy). Demographic variables included patient age at diagnosis, sex, race/ethnicity, and year of diagnosis. Treatment variables included extent of surgery and radioactive iodine (RAI). Pathologic characteristics included presence/absence of multifocal disease, extrathyroidal extension, and pathologic T, N, and M stages. Patients with missing data on pathologic T, N, and M stages or survival information were excluded (Supplementary Fig. S1). Because both SEER and NCDB data files contain de-identified records, this study was granted exempt status by the Institutional Review Board.
Changes from seventh to eighth editions of the AJCC/UICC TNM staging system
In the eighth edition of the AJCC/UICC TNM staging system, most of the critical elements of the seventh edition were retained, but many changes were made (Supplementary Tables S2 and S3). The explicit inclusion of pNX among the staging criteria in the eighth edition is a departure from the seventh edition staging system. Patients for whom lymph nodes were not resected at the time of surgery, and therefore had no lymph node tissue made available to pathology for evaluation, are designated pNX. Information about the number of lymph nodes found to contain metastases pathologically was used to confirm pNX classification (Supplementary Fig. S2). For the purposes of this study, it was assumed that patients classified as pNX had clinically negative lymph nodes with likely no significant lymph node metastasis, and therefore they were treated in the analysis for the seventh edition classification as having cN0 status.
Statistical analyses
The seventh and eighth editions of the AJCC/UICC staging system for PTC were applied to the NCDB and SEER cohorts. Unadjusted survival estimates were calculated using the Kaplan–Meier method, and log-rank tests were used to test for differences in survival among the four staging groups. Cox proportional hazard modeling was used to estimate the effect of stage on survival after adjustment for known covariates, including patient sex, race/ethnicity, year of diagnosis, insurance status, margins status, tumor multifocality, RAI use, extrathyroidal extension, and extent of surgery (Supplementary Table S4). Statistical performance of the adjusted models fit under both AJCC/UICC editions was assessed using the Akaike information criteria (AIC) (18). The AIC measures the relative quality of statistical models for a given set of data, thereby providing a means for model selection. Among many possible models, the model with the smallest AIC has the least information lost and is chosen as the best model to represent the true model. The proportional hazards assumptions were assessed by examination of the Martingale residuals (19). Martingale residuals are useful in assessing model adequacy with respect to the proportional hazards assumptions and the functional form of the influence of a covariate in a model while adjusting for others. If the proportional hazards assumption was violated for a given covariate, interpretation was still made, and should be considered the average effect, as suggested in statistical literature (20).
A two-sided significance level of 0.05 was used for all statistical tests. No adjustments were made for multiple comparisons. Analyses were performed using SAS v9.4 (SAS Institute, Cary, NC) and R v3.3.0 (Vienna, Austria).
Results
Patient characteristics
A total of 64,342 patients met the inclusion criteria in the SEER cohort. Of these, 79% were female, and 82% were white. The median age was 48 years (interquartile range [IQR] = 38–58 years), and the median follow-up time was 53 months (range 0–119 months; Table 1). Nearly 41% were classified as <45 years of age in the seventh edition compared to 67% who were <55 years of age in the eighth edition. There were fewer stage I patients in the seventh edition than there were in the eighth edition (76% vs. 90%, respectively), and there were more stage IV patients in the seventh edition compared to the eighth edition (6% vs. 1%; Table 2).
Table 1.
Patient Demographic and Clinicopathologic Characteristics of Papillary Thyroid Cancer Patients in the SEER Program (2004–2012) and the NCDB (2004–2012)
| Patient characteristics | SEER (N = 64,342) | NCDB (N = 179,698) |
|---|---|---|
| Age, median (IQR) | 48 (38–58) | 49 (39–60) |
| Sex | ||
| Female | 50,736 (78.9%) | 139,778 (77.8%) |
| Race/ethnicity | ||
| White | 52,714 (81.9%) | 153,508 (87.0%) |
| Black | 3957 (6.2%) | 12,300 (7.0%) |
| Other | 7671 (11.9%) | 10,554 (5.9%) |
| Insurance status | ||
| Insured | 45,579 (70.8%) | 172,453 (95.9%) |
| Not insured | 1318 (2.1%) | 4652 (2.6%) |
| Margin statusa | ||
| Negative | — | 154,991 (86.3%) |
| Positive | — | 19,122 (10.6%) |
| Tumor focality | ||
| Multifocal | 26,356 (41.0%) | 71,144 (39.6%) |
| Unifocal | 37,199 (57.8%) | 104,772 (58.3%) |
| Extrathyroidal extension | ||
| Present | 10,764 (16.7%) | 27,982 (15.6%) |
| Absent | 53,578 (83.3%) | 151,539 (84.3%) |
| Surgery | ||
| Total | 55,057 (85.6%) | 153,302 (85.3%) |
| Partial | 9285 (14.4%) | 26,396 (14.7%) |
| Radioactive iodine | ||
| Yes | 32040 (49.8%) | 87,268 (48.6%) |
| No | 31,028 (48.2%) | 84,709 (47.1%) |
| Pathologic T stage | ||
| 1 | 40,420 (62.8%) | 117,129 (65.2%) |
| 2 | 10,196 (15.9%) | 27,937 (15.6%) |
| 3 | 11,461 (17.8%) | 29,093 (16.2%) |
| 4 | 2265 (3.5%) | 5539 (3.0%) |
| Pathologic M stage | ||
| 0 | 63,805 (99.2%) | 178,595 (99.4%) |
| 1 | 537 (0.8%) | 1103 (0.6%) |
Percentages may not add up to 100% due to missing data.
Margin status not captured in SEER.
SEER, Surveillance, Epidemiology, and End Results; NCDB, National Cancer Database; IQR, interquartile range.
Table 2.
Comparison of Characteristics of PTC Patients Between Seventh and Eighth Editions of the AJCC/UICC Staging System Based on TNM Stage, Age Group, and Pathologic N Stage in NCDB and SEER (2004–2012)
| SEER (N = 64,342) | NCDB (N = 179,698) | |||
|---|---|---|---|---|
| Patient characteristics | 7th edition | 8th edition | 7th edition | 8th edition |
| Stage | ||||
| I | 48,815 (75.9%) | 57,769 (89.8%) | 135,574 (75.4%) | 160,538 (89.3%) |
| II | 4355 (6.8%) | 5245 (8.2%) | 12,927 (7.2%) | 15,744 (8.8%) |
| III | 7530 (11.7%) | 668 (1.0%) | 21,302 (11.9%) | 2367 (1.3%) |
| IV | 3642 (5.7%) | 660 (1.0%) | 9895 (5.5%) | 1049 (0.6%) |
| Age groupa | ||||
| Young | 26,662 (41.4%) | 43,346 (67.4%) | 67,120 (37.4%) | 112,911 (62.8%) |
| Old | 37,680 (58.6%) | 20,996(32.6%) | 112,578 (62.6%) | 66,787 (37.2%) |
| Pathologic N stage | ||||
| 0 | 51,182 (79.6%) | 50,443 (78.4%) | 146,539 (81.5%) | 105,553 (58.7%) |
| 1 | — | 13,160 (20.4%) | — | 33,189 (18.5%) |
| 1a | 8191 (12.7%) | — | 20,554 (11.4%) | — |
| 1b | 4969 (7.7%) | — | 12,635 (7.1%) | — |
| X | — | 739 (1.2%) | — | 40,974 (22.8%) |
7th edition: young (<45 years), old (≥45 years); 8th edition: young (<55 years), old (≥55 years).
AJCC/UICC, American Joint Commission on Cancer/Union for International Cancer Control; TNM, tumor, node, metastasis.
Among the 179,698 patients who met the inclusion criteria in the NCDB cohort, 78% were female, and 87% were white. The median age was 49 years (IQR = 39–60 years), and median follow-up time was 50 months (range 0–132 months; Table 1). There were 37% classified as <45 years of age in the seventh edition compared to 63% who were <55 years of age in the eighth edition. Fewer patients (75% vs. 89%) were grouped into stage I in the seventh edition compared to the eighth edition, and more patients (6% vs. 1%) were assigned to stage IV in the seventh edition compared to the eighth edition (Table 2).
Patient stage migration
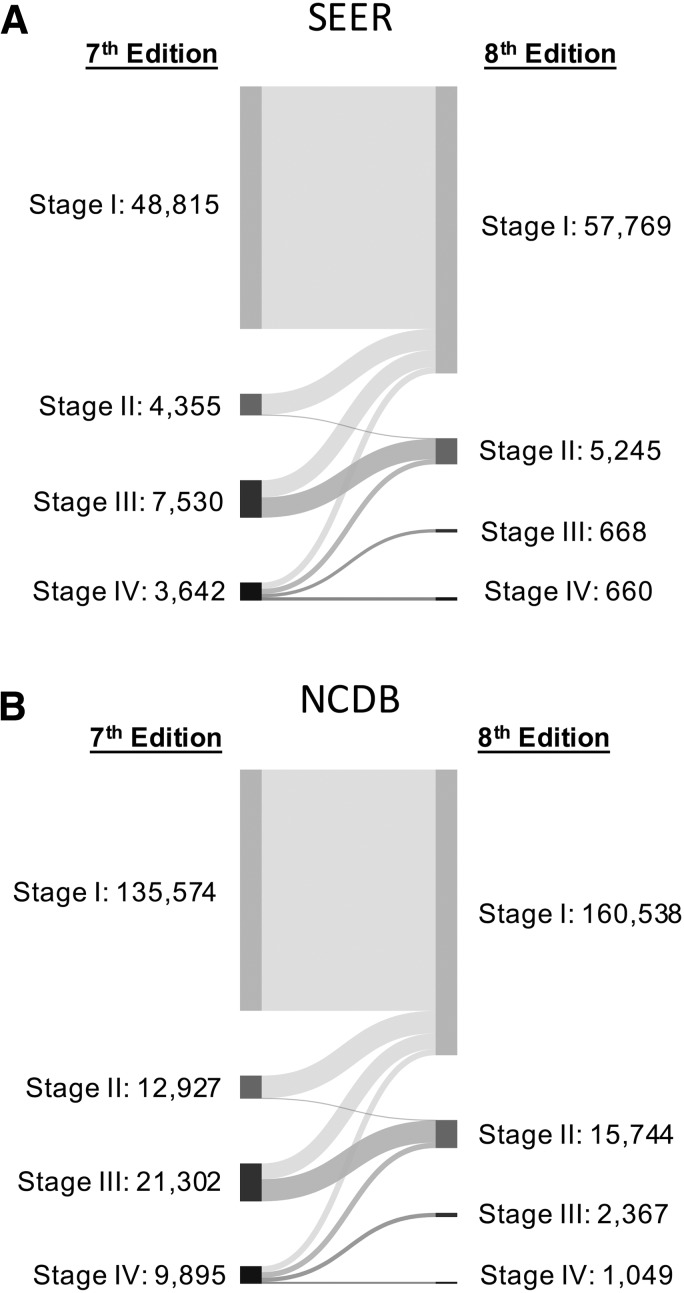
After classifying patients across all stages from the seventh to the eighth editions in SEER, 77% of patients remained in their previous stage, and the remaining 23% of patients were reclassified to a lower stage. All patients with stage I disease in the seventh edition remained in stage I in the eighth edition; about 3% retained stage II classification, and 18% retained stage IV classification. All stage III patients in the seventh edition were downstaged in the eighth edition (Fig. 1 and Table 3). In the NCDB, 76% of patients remained in their previous stage, while most of the remaining patients were reclassified to lower stages. Again, all stage III patients in the seventh edition were downstaged in the eighth edition (Fig. 1 and Table 3). Changes in T, N, or M accounted for more patient movement into lower stages of disease than the change in age alone (24% vs. 17%) in both SEER and NCDB.
FIG. 1.
Alluvial flow diagram representing the restaging of patient cohorts from the seventh to the eighth edition of the American Joint Commission on Cancer/Union for International Cancer Control (AJCC/UICC) tumor, node, metastasis (TNM) staging system in (A) the Surveillance, Epidemiology, and End Results (SEER) program and (B) the National Cancer Database (NCDB). Numbers represent the absolute number of patients within each stage, with flow line width proportional to the number of patients moving to a new stage classification. For the percentage of patients moving to each new stage, see Table 3.
Table 3.
Restaging from the Seventh Edition to the Eighth Edition of the AJCC/UICC TNM Staging System in SEER (2004–2012) and NCDB (2004–2012)
| Eighth edition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SEER (N = 64,342) | NCDB (N = 179,698) | |||||||||
| Seventh edition | I | II | III | IV | Total | I | II | III | IV | Total |
| I | 48,815 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48,815 | 135,574 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 135,574 |
| II | 4224 (97.0%) | 131 (2.5%) | 0 (0.0%) | 0 (0.0%) | 4355 | 12,694 (98.2%) | 233 (1.8%) | 0 (0.0%) | 0 (0.0%) | 12,927 |
| III | 3448 (45.8%) | 4082 (54.2%) | 0 (0.0%) | 0 (0.0%) | 7530 | 8862 (41.6%) | 12,440 (58.4%) | 0 (0.0%) | 0 (0.0%) | 21,302 |
| IV | 1282 (35.2%) | 1032 (28.3%) | 668 (18.3%) | 660 (18.1%) | 3642 | 3408 (34.4%) | 3071 (31.0%) | 2367 (23.9%) | 1049 (10.6%) | 9895 |
| Total | 57,759 | 5245 | 668 | 660 | 64,342 | 160,538 | 15,744 | 2367 | 1,049 | 179,698 |
Percentages are based on row totals.
Unadjusted and adjusted survival analyses
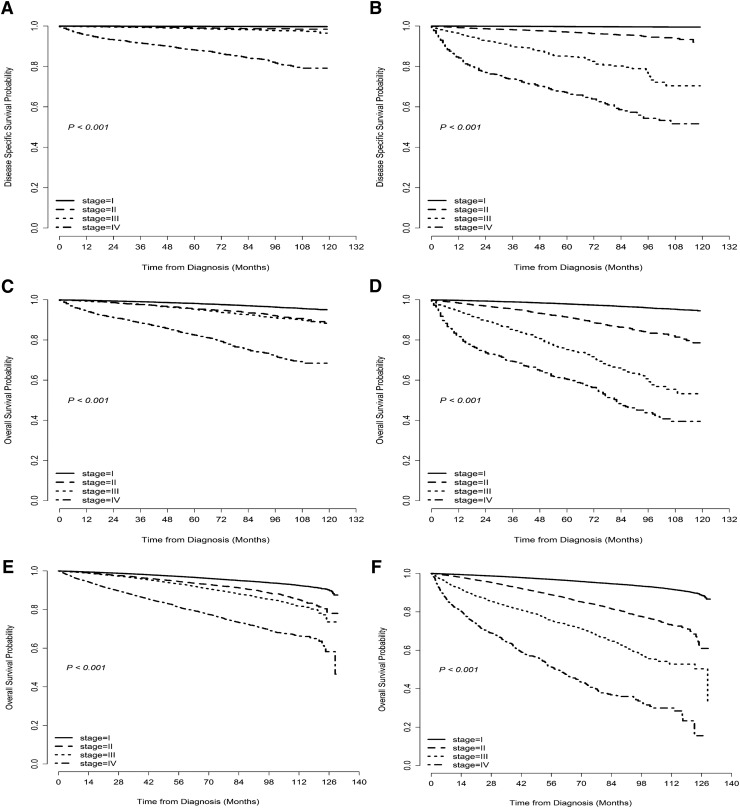
Due to insufficient follow-up for the SEER cohort, only five-year DSS and five-year OS could be prepared for the seventh and eighth editions. DSS was significantly related to stage at diagnosis when using both seventh and eighth editions of the AJCC/UICC staging system (p < 0.001), and there was more differentiation between stages I–IV with regard to five-year DSS in the eighth edition compared to the seventh edition (Fig. 2A and B). Similar results were seen for OS in both SEER and NCDB cohorts (Fig. 2C–F). Overall, patients assigned to higher stages (III and IV) in the eighth edition demonstrated a worse prognosis than those classified into similar stages in the seventh edition (Table 4).
FIG. 2.
Unadjusted disease-specific survival (DSS) curves for patients with papillary thyroid cancer (PTC) in the SEER program using the AJCC/UICC TNM staging (A) seventh and (B) eighth edition models. Unadjusted overall survival (OS) curves for patients with PTC in the SEER database using (C) the seventh and (D) the eighth edition models. Similarly, unadjusted OS curves for patients with PTC in the NCDB database using (E) the seventh and (F) the eighth edition models.
Table 4.
Comparison of the Five-Year Disease-Specific Survival and Overall Survival in SEER and 5- and 10-Year Overall Survival in NCDB Between the Current Seventh and Proposed Eighth Editions of AJCC/UICC Staging System for TNM Stage
| SEERa | NCDB | |||||||
|---|---|---|---|---|---|---|---|---|
| 5-year DSS rate [CI] | 5-year OS rate [CI] | 5-year OS rate [CI] | 10-year OS rate [CI] | |||||
| Stage | 7th edition | 8th edition | 7th edition | 8th edition | 7th edition | 8th edition | 7th edition | 8th edition |
| I | 99.8 [99.7–99.9] | 99.7 [99.6–99.8] | 98.1 [97.9–98.2] | 97.8 [97.7–98.0] | 96.9 [96.7–97.0] | 96.6 [96.5–96.7] | 91.5 [90.9–91.9] | 90.6 [90.1–91.0] |
| II | 99.1 [98.7–99.4] | 96.7 [95.9–97.3] | 95.1 [94.2–95.8] | 90.7 [98.7–91.6] | 94.1 [93.6–94.5] | 88.0 [87.4–88.7] | 82.3 [80.0–84.3] | 70.9 [68.5–73.2] |
| III | 98.8 [98.4–99.1] | 85.2 [81.7–87.9] | 94.9 [94.3–95.6] | 72.9 [68.9–76.4] | 92.7 [92.3–93.1] | 74.3 [72.2–76.2] | 79.9 [78.1–81.7] | 52.8 (48.9, 56.5) |
| IV | 88.2 [86.9–89.3] | 66.9 [62.7–70.8] | 79.9 [78.4–81.3] | 55.3 [51.2–59.1] | 80.1 [79.2–81.1] | 49.5 [45.8–52.9] | 64.9 [62.7–67.2] | 23.3 [15.8–31.5] |
10-year DSS in SEER could not be calculated due to insufficient patient follow-up.
DSS, disease-specific survival; OS, overall survival; CI, confidence interval.
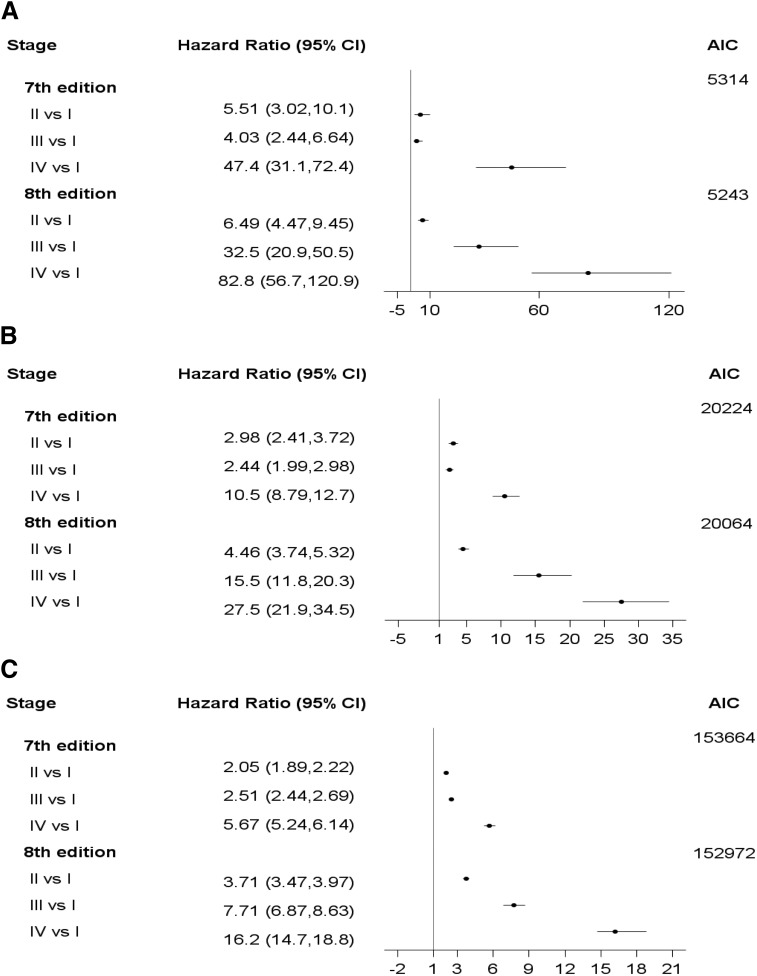
After adjustment, PTC stages as defined by both editions were significantly associated with OS and DSS. With respect to DSS in SEER, the AJCC eighth edition PTC model appeared to be a better fit to the data with an AIC of 5243 compared to the seventh edition model's AIC of 5314. Similar results were observed for OS in SEER (Fig. 3). With a smaller AIC of 152,972, the eighth edition model was a better fit to the data than the seventh edition model with an AIC of 153,664 for OS in the NCDB (Fig. 3).
FIG. 3.
Comparison of hazard ratios for stage groupings and model performance in the seventh and eighth editions of AJCC/UICC TNM staging system for PTC using (A) DSS in SEER, (B) SO in SEER, and (C) OS in NCDB.
Discussion
The seventh edition of the AJCC/UICC TNM staging system for PTC has served as the major set of criteria for predicting survival in patients with PTC since 2010. Based on evidence that has emerged since then, the eighth edition staging system was crafted to improve predictions for prognosis in order to tailor PTC management better (9). This study compared head-to-head the prognostic ability of the seventh and eighth editions of the AJCC/UICC TNM classification for PTC with regard to DSS and OS in the largest patient cohorts available in the United States, and found that the eighth edition was a significantly better predictor of survival.
One of the biggest changes made in the eighth edition was changing the cut point for patient age from 45 to 55 years. In the second edition of the AJCC/UICC staging system published in 1983, an age cut point of 45 years was introduced, and this remained as a major determinant of survival going forward with every subsequent revision. In recent years, multiple studies have found that PTC aggressiveness appears to increase with advancing patient age in a linear fashion, and that no single age cut point can readily place patients into dichotomous low- and high-risk categories driven primarily by patient age (21–23). As a result, some thought-leaders have suggested that age should be removed from the PTC staging system altogether. Several recommendations have been made, from using mathematical models to account for age (24) to using multiple age category cut points (21).
Most recently, the AJCC/UICC eighth edition endorsed an increase in the single age cut point from 45 years (in the seventh edition) to 55 years. This recommendation was largely based on a study by Nixon et al. (12), which attempted to find the optimal age cut point for predicting DSS related to DTC. A total of 1807 patients were included in this single institution database study, with a median follow-up of 109 months. Recursive partitioning was employed to identify the factors most predictive of DSS. For patients with M0 disease, age was the second most powerful predictor of DSS, and a cut point of 54 years was optimal. Among patients with M1 disease, a cut point of 56 years was best. The 10-year DSS with a cut point of 45 years was 99.6% for stage I and 81% for stage IV. Changing the age cut point to 55 years, 10-year DSS was 99.2% for stage I and 74% for stage IV, and 17% of patients were downstaged to a lower risk category in the eighth edition according to the present results.
This study also found that patients were primarily downstaged from the seventh to the eighth editions, according to analyses of both the SEER and NCDB databases. There also was superior separation of unadjusted survival between stages I, II, III, and IV, as well as improved statistical fitness on adjusted multivariable analyses. This improved fit of the data after adjustment for known covariates was maintained for both SEER and NCDB databases (in relation to DSS and OS), and is perhaps the most important finding from the current study. It was found that the AIC values were lower in the eighth edition models compared to the seventh edition models for both OS and DSS.
Overall, the data show that the eighth edition has a more discriminating classification than the seventh edition with regard to predicting patient survival. Additional changes made to the eighth edition included collapsing the N1a and N1b lymph node categories describing the central and lateral compartments, respectively, into a single N1 metric. While this change appears to have improved the accuracy of prognosis for patients with PTC within the TNM staging system, this system is still limited to factors such as tumor size, extrathyroidal extension, capsular invasion, vascular invasion, regional lymph node involvement, and distant metastases (1). Recent research has demonstrated the importance of other factors outside of demographics and pathologic characteristics, including molecular markers, to afford better prediction of patient survival (25). A future model that includes all of these factors may be better for determining more accurate prognosis and thus for guiding management strategies. The current American Thyroid Association guidelines state that the initial treatment for patient with differentiated thyroid cancer “are to improve overall and disease-specific survival, reduce the risk of persistent/recurrent disease and associated morbidity, and permit accurate disease staging and risk stratification, while minimizing treatment-related morbidity and unnecessary therapy” (26). The eighth edition changes will impact a significant number of patients, especially those who will be downstaged. According to the guidelines, these patients will be viewed as lower risk overall, and this will likely lead to less aggressive adjuvant RAI administration for these patients, and will decrease overtreatment.
The limitations of this study include factors that pertain to all large database studies. There is potential for coding errors, but the NCDB and SEER databases are standardized and highly audited. Neither the NCDB nor SEER data sets include novel predictors of outcomes, such as molecular markers. Neither data set includes information about disease recurrence, which is a more clinically relevant outcome for PTC. However, AJCC/UICC staging is intended only to predict survival not recurrence. Ten-year DSS is not yet available from the SEER data set, as enough time has not elapsed from the start of TNM reporting in 2004 to the last year of follow-up of this database of 2013. A strength of this study is the very large number of contemporary patients who were evaluated.
Conclusion
This comprehensive study identified patients who were upstaged or downstaged based on evaluation using the seventh versus the eighth AJCC/UICC staging systems. It was found that there was greater separation of survival curves based on disease stage in the eighth edition. After adjustment for covariates, it was further demonstrated that the eighth edition model was a better fit to the data than the seventh edition model using AIC. With these results, the AJCC/UICC eighth edition indeed appears to be superior to the seventh edition for predicting patient survival.
Supplementary Material
Acknowledgments
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. S.M.T. is supported by NIH grant P30CA014236, PI: Michael Kastan MD, PhD, as part of the Duke Cancer Institute.
The data used in the study were derived from a de-identified National Cancer Database (NCDB) file and Surveillance, Epidemiology, and End Results Program (SEER) file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodologies employed, or the conclusions drawn from these data by the investigators.
A portion of these data will be presented at the American College of Surgeons meeting, October 22–26, 2017, San Diego, CA.
Author Disclosure Statement
Dr. Sosa is a member of the Data Monitoring Committee for the Medullary Thyroid Cancer Consortium Registry, funded by Novo Nordisk, Astra Zeneca, GlaxoSmithKline, and Eli Lilly. None of the other authors has any conflict of interest to disclose.
References
- 1.Edge SB, Compton CC. 2010. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474 [DOI] [PubMed] [Google Scholar]
- 2.Hay ID, Grant CS, Taylor WF, McConahey WM. 1987. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 102:1088–1095 [PubMed] [Google Scholar]
- 3.Cady B, Rossi R. 1988. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104:947–953 [PubMed] [Google Scholar]
- 4.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058. [PubMed] [Google Scholar]
- 5.Dean DS, Hay ID. 2000. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control 7:229–239 [DOI] [PubMed] [Google Scholar]
- 6.Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, Hyslop T, Roman SA, Sosa JA. 2015. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol 33:2370–2375 [DOI] [PubMed] [Google Scholar]
- 7.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, Mayer M, Sylvester RJ, van Glabbeke M. 1979. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer 15:1033–1041 [DOI] [PubMed] [Google Scholar]
- 8.Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, Haugen BR, Ho M, Klein I, Ladenson PW, Robbins J, Ross DS, Specker B, Taylor T, Maxon HR 3rd 1998. Prospective multicenter study of thyroiscarcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer 83:1012–1021 [DOI] [PubMed] [Google Scholar]
- 9.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. (eds) 2017. AJCC Cancer Staging Manual. Eighth edition. Springer International Publishing, New York, NY, pp 872–927 [Google Scholar]
- 10.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. (eds) 2010. AJCC Cancer Staging Handbook. Seventh edition. Springer-Verlag, New York [Google Scholar]
- 11.Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. 2016. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol 34:4415–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gonen M, Shah JP. 2016. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol 23:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available at: https://seer.cancer.gov/ (accessed May8, 2017)
- 14.Centers for Disease Control and Prevention. United States Cancer Statistics (USCS): Surveillance, Epidemiology, and End Results (SEER) Program. Available at: https://www.cdc.gov/cancer/npcr/uscs/technical_notes/contributors/seer.htm (accessed May8, 2017)
- 15.American College of Surgeons. National Cancer Database. Available at: https://www.facs.org/quality-programs/cancer/ncdb (accessed May7, 2017)
- 16.American Colledge of Surgeons. Getting started—a user's guide. Available at: http://ncdbpuf.facs.org/node/274 (accessed May12, 2017)
- 17.Winchester DP, Stewart AK, Bura C, Jones RS. 2004. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol 85:1–3 [DOI] [PubMed] [Google Scholar]
- 18.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans Automat Control 19:716–723 [Google Scholar]
- 19.Lin DY, Wei LJ, Ying Z. 1993. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 80:557–572 [Google Scholar]
- 20.Allison PD. 2010. Survival Analysis Using SAS: A Practical Guide. Second edition. Sas Institute, Inc., Cary, NC [Google Scholar]
- 21.Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha A, Tuttle RM, Shah JP, Patel SG. 2015. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid 25:1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. 2015. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid 25:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyer SL, Smith VA, Lentsch EJ. 2012. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg 147:221–226 [DOI] [PubMed] [Google Scholar]
- 24.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI. 2012. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 97:E878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Q, Li X, Acharya CR, Hyslop T, Sosa JA. 2017. A novel integrative risk index of papillary thyroid cancer progression combining genomic alterations and clinical factors. Oncotarget 8:16690–16703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.