Abstract
There remains a need for large animal models to evaluate tissue-engineered vascular grafts (TEVGs) under arterial pressure to provide preclinical data for future potential human clinical trials. We present a comprehensive method for the interrogation of TEVGs, using an ovine bilateral arteriovenous (AV) shunt implantation model. Our results demonstrate that this method can be performed safely without complications, specifically acute heart failure, steal syndrome, and hypoxic brain injury, and it is a viable experimental paradigm. Our method allows for a non-invasive evaluation of TEVGs in terms of graft flow, graft diameter, and graft patency, while also allowing for graft needle puncture under ultrasound guidance. In addition, traditional pathological analysis, histology, and immunohistochemistry may be performed with the contralateral side providing paired control data to eliminate inter-subject variability while reducing the total number of animals. Further, we present a review of existing literature of preclinical evaluation of TEVGs in large animal models as AV conduits.
Keywords: : arteriovenous shunts, tissue-engineered vascular grafts, vascular surgery, large animal models, nanofibers, electrospinning
Introduction
There is a constant need for technological innovation and advancement to combat the rising global burden of cardiovascular disease1 and end-stage renal disease (ESRD).2,3 One such innovation that is beginning to revolutionize the field of cardiovascular surgery is tissue-engineered vascular grafts (TEVGs).4–6 The need for TEVGs is becoming increasingly evident in reconstructive arterial surgery and bypass procedures, as the gold standard autologous vessels are limited in availability and existing synthetic grafts such as expanded polytetrafluoroethylene (ePTFE) and polyethylene terephthalate (Dacron®) are thrombogenic and exhibit compliance mismatch.7 In addition, in the natural history of arteriovenous (AV) grafts in ESRD patients,8 at 2 years postimplantation, 96% of AV grafts require at least one salvage procedure (thrombectomy, angioplasty, or surgical revision) and 49% of AV grafts experience permanent graft failure. There remains an unmet clinical need for AV grafts with good long-term patency rates. As TEVGs can undergo endothelialization and remodeling to form functional vascular neotissue,9 there is potential for TEVGs to increase existing long-term patency rates of AV grafts for ESRD patients.
Before TEVGs can be used clinically, the grafts must be preclinically evaluated in large animal models. Several TEVG clinical trials have been conducted and many are currently underway, prompted by the results from large animal studies. A clinical trial has been completed for large-diameter vessels, involving the implantation of both bone marrow cell-seeded poly(glycolic) acid (PGA) and poly(lactide-co-caprolactone) (PLCL) grafts as extracardiac cavopulmonary conduits.10,11 Shinoka et al. and Hibino et al. demonstrated that the biodegradable conduits exhibited endothelialization and were 100% patent at 1 year of follow-up and 84% patent at 6 years of follow-up, citing seeding techniques and previous preclinical results from canine and ovine models.10–14 There have also been clinical trials for small-diameter TEVGs built on the polymer synthesis and implantation techniques previously established by using ovine, porcine, and canine models.11,15,16 Olausson et al. described a case of using decellularized donor iliac vein as a venous bypass around an obstructed extrahepatic portal vein that remained patent at 1 year of follow-up.17 Two other clinical trials tested the potential of the Lifeline graft, consisting of endothelial cell-seeded fibroblast sheets rolled into a tube, as AV grafts in patients with ESRD.2,3 However, these Lifeline grafts had low patency rates and were prone to dilation and delamination. Therefore, although preliminary human trials have yielded some success, it is evident that novel TEVGs and more extensive preclinical testing are required.
TEVGs have classically been tested in interposition and bypass models in large animals.18 In these models, the animals are divided into a control group and an experiment group, such that the control and test grafts are placed in different individuals with potentially different vascular microenvironments. As studies involving large animals often involve few subjects, the inter-subject differences could confound the results. Further, the ability to implant both the control graft and the experimental TEVG in the same animal will reduce the cost of large animal models and overall, TEVG testing, thus allowing for more TEVGs to enter clinical trials.
In addition, interposition and bypass models do not recapitulate the mechanical microenvironment of shunted AV circulation and, thus, are not ideal for the evaluation of their potential use in AV grafts for hemodialysis access in patients with ESRD. A review of existing literature revealed a paucity of studies exploring AV conduit grafts in large animal models; previous studies will be outlined in the Discussion section. Here, we present a comprehensive protocol for the interrogation of TEVGs, specifically PGA/PLCL nanofiber grafts, using a bilateral AV conduit model in the ovine system that allows us to better control for confounding factors that are attributed to inter-subject differences and to more accurately simulate AV hemodynamics.
Materials and Methods
TEVG creation
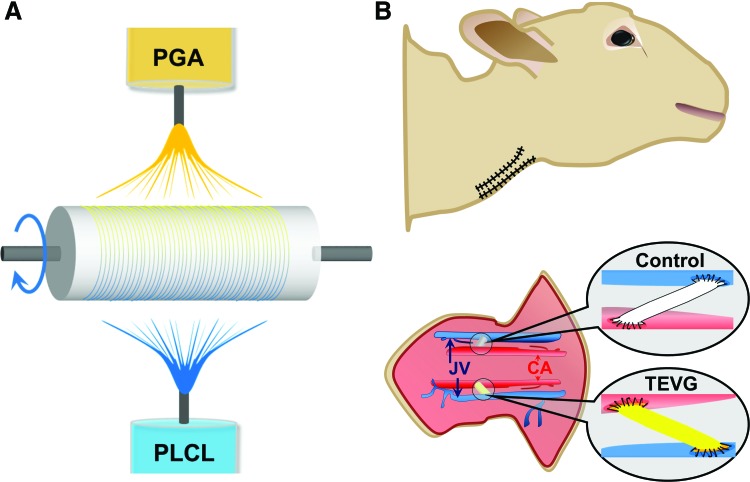
PGA/PLCL scaffolds are created by co-electrospinning as previously described,19 with the differences being a customized graft length of 5 cm and a customized inner diameter of 5 mm (Fig. 1A). First, 10 wt% PGA and 5 wt% PLCL were separately dissolved in hexafluoroisopropanol and stirred with a magnetic stir bar for a minimum of 3 h at room temperature. Using separate syringes for each solution, both solutions were simultaneously electrospun onto a rotating grounded mandrel (30 revolutions/min) that was positioned 20 cm from the syringe tips, with +25 kV charge applied. The PGA solution (10 wt%) was dispensed at 2.5 mL/h, whereas the PLCL solution (5 wt%) was dispensed at 5.0 mL/h, to create co-electrospun PGA and PLCL scaffolds with a PGA:PLCL weight ratio of 1:1. After electrospun nanofibers were deposited onto the grounded mandrel, the electrospun scaffold was removed from the mandrel. The wall thickness was then measured with a digital snap gauge by placing the scaffold between two glass slides to ensure a desired wall thickness of 0.4 mm was achieved. The scaffolds were sectioned into 5 cm lengths, packed into foil pouches that were then placed in Tyvek pouches, and received 25 kGy of gamma irradiation for sterilization.
FIG. 1.
Bilateral AV shunts as a method for evaluating TEVGs in large animal models. (A) Schematic illustration of electrospinning process (PGA/PLCL) and scaffold creation. (B) Schematic illustration of bilateral carotid artery-EJV AV shunt creation in the ovine model. AV, arteriovenous; EJV, external jugular vein; TEVGs, tissue-engineered vascular grafts. Color images available online at www.liebertpub.com/tec
Graft implantation
All sheep experiments received prior approval from the Animal Care and Use Committee at QTest Labs (Columbus, OH). On the day of the implantation, animals were premedicated in their home cages with tiletamine-zolazepam administered via an intramuscular injection. After sternal recumbency was achieved, anesthesia was induced with intravenous fentanyl, ketamine, and supplemental tiletamine-zolazepam. Anesthesia was maintained with isoflurane (1–2% in 100% O2) and a continuous fentanyl infusion. Sheep were positioned in dorsal recumbency for the duration of the implantation procedure. The common carotid artery (CCA) and external jugular vein (EJV) were exposed bilaterally. Animals were then anti-coagulated with intravenous administration of heparin (100 IU/kg).
Vascular grafts were implanted as a CCA to ipsilateral EJV AV grafts in sheep (n = 6; 5 female and 1 castrated male, body weight: 51.4 ± 3.9 kg), with nanofiber PGA/PLCL TEVGs on the right and ePTFE (Bard IMPRA) on the left. Standard vascular anastomosis was performed with running 7-0 Prolene (Ethicon, Inc.) sutures, and the ePTFE graft and nanofiber PGA/PLCL TEVG (both 5 cm in length) were implanted deep into the respective ipsilateral sternocephalicus muscle, as a straight horizontal vascular conduit, due to a propensity to kink when tunneled in the subcutaneous tissue (Figs. 1B and 2C, D, sternocephalicus muscle reflected out of view for graft visualization).
FIG. 2.
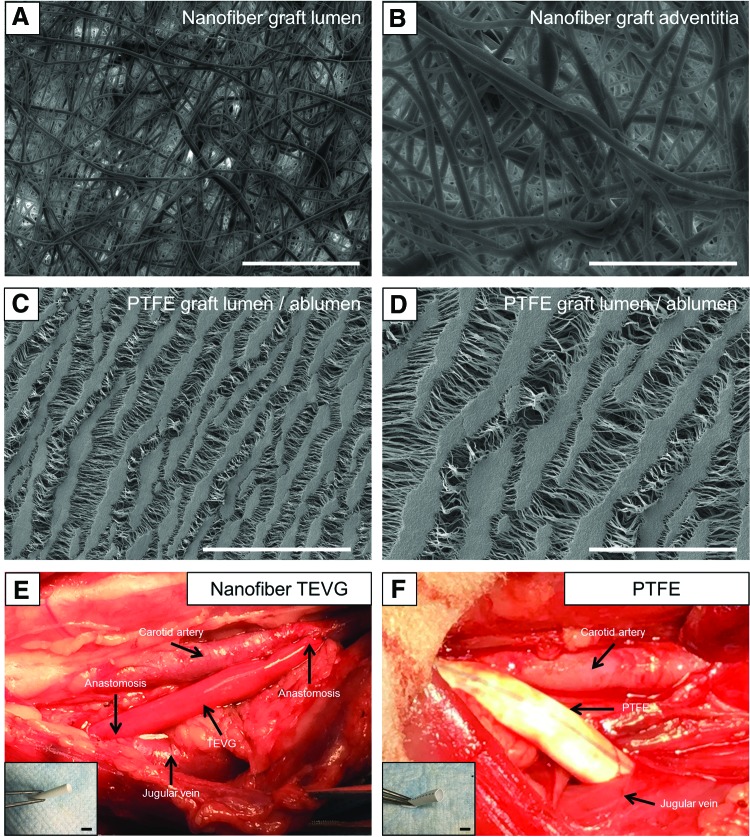
In vivo implantation of biodegradable nanofiber scaffolds in an ovine model. SEM of PGA/PLCL (1:1) nanofiber graft luminal (A) and adventitia surface (B). (C, D) SEM of ePTFE graft luminal/abluminal surfaces. In vivo implantation of PGA/PLCL (1:1) nanofiber TEVG (E) in the ovine model as a right-sided carotid artery-EJV AV shunt, with ePTFE control on the left side (F). Scale bar: 100 μm (A, C), 50 μm (B, D), 1 cm (E, F). ePTFE, expanded polytetrafluoroethylene; SEM, scanning electron microscopy. Color images available online at www.liebertpub.com/tec
After hemostasis, the muscle, subcutaneous tissue, and skin were closed in layers. Antibiotics (cefazolin) were administered intra-operatively, until the 7th postoperative day. Postoperative analgesia was provided in the form of a fentanyl patch applied to lateral thorax, intramuscular buprenorphine, and intravenous flunixin meglumine at the completion of surgery. Flunixin meglumine was continued for at least 3 days, and the fentanyl patch was removed after day 3. A compressive bandage was applied around the neck to protect the surgical site from contamination. All sheep were given oral aspirin (325 mg/day) and clopidogrel (225 mg twice daily) until the end of the study.
Color Doppler ultrasound was performed weekly beginning at least 7 days after implantation to determine graft patency and to measure lumen diameter. In addition, both nanofiber TEVGs and ePTFE grafts were punctured under ultrasound guidance at 4 weeks. To facilitate imaging and graft punctures, sheep were sedated with tiletamine-zolazepam (4 mg/kg IM). Before graft puncture, the overlying skin was prepped with a chlorhexidine scrub and alcohol to minimize the risk of infection. Ultrasound images were collected by using a Philips HD11 XE ultrasound and a probe of adequate frequency (Philips L15-7io) to assess the vascular structures.
Animals were sacrificed by using pentobarbital sodium at 4 weeks after surgery, and grafts were explanted.
Histology and immunohistochemistry
Explanted TEVG and ePTFE samples were fixed in formalin (10% at 4°C for 24 h); embedded in paraffin for standard histology with hematoxylin and eosin (H&E), Masson's trichrome, Verhoeff-Van Gieson (VVG), and von Kossa (VK) staining. For immunohistochemistry, tissue sections were deparaffinized, rehydrated, and blocked for endogenous peroxidase activity and nonspecific staining, before incubation with von Willebrand factor (vWF) primary antibodies (Dako; 1:2000). Biotinylated secondary antibodies and streptavidinated HRP were then used before color development by chromogenic reaction with 3,3-diaminobenzidine (Vector). Nuclei counterstaining was performed with hematoxylin (Gill's formula; Vector).
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to visualize pore size and fiber diameter in the tissue samples, using a Hitachi S-4800 Scanning Electron Microscope (5.0–15.0 kV), after dehydration with hexamethyldisilazane and gold sputter coating. ImageJ (NIH) was used to quantify pore size and fiber diameter (15 measurements from ≥5 random images of either the luminal surface or a transverse section).
Statistical analysis
All data were presented as mean ± standard deviation and statistical significance was determined by Student's t-test by using GraphPad Prism (version 6; GraphPad Software, Inc., CA), with a p-value <0.05 considered statistically significant. A paired t-test was used to compare ePTFE with TEVG samples.
Results
Bilateral AV grafts can be successfully implanted, evaluated, and assessed in large animals
The fiber diameter and pore size of the TEVGs were determined to be 860.81 ± 292.51 nm and 9.84 ± 5.19 μm, respectively, by SEM (Fig. 2A, B). We noted that the blood seeping through the needle holes was less in nanofiber TEVGs than in ePTFE grafts.
All sheep successfully underwent bilateral CCA-EJV AV graft implantation procedures and recovered from anesthesia. Follow-up examinations were performed the next day and revealed a palpable thrill in all AV grafts. Only one sheep had a positional graft that caused a weakened thrill depending on head carriage. Three sheep had trace facial edema, and only two sheep had marked facial edema. Two sheep had prolapsed and erythematous nictitating membranes. All facial edema and swelling had resolved by day 2 in all animals. Active hemorrhage was present in only one sheep after removal of the compressive bandage (ePTFE graft side), which resolved after the bandage was replaced. Stertorous respiration was present in two sheep. A dull mentation was only noted in one sheep after surgery, which resolved within 24 h. No other neurologic deficits were noted. Although each sheep had bilateral distention of the EJVs, there were no symptoms of right-sided congestive heart failure. Only one animal revealed dyspnea due to pharyngeal edema, which ultimately resulted in the death of the sheep due to asphyxia after unsuccessful rescue attempts on the day after implantation. Two sheep were lost during the first week of the study due to TEVG graft rupture (days 4 and 7). The remaining three sheep survived until the end of the study. On necropsy, TEVGs appeared to rupture as if torn longitudinally from neck bending and stretching, rather than rupturing from exceeding the TEVG burst pressure.
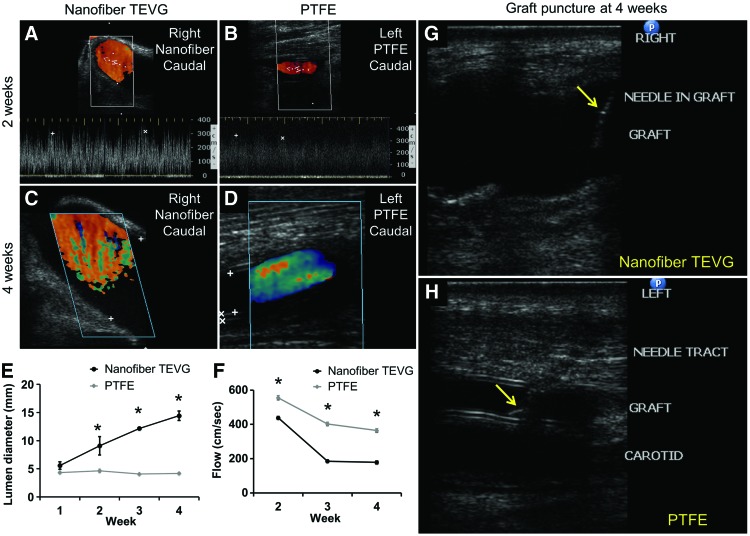
Beginning 1 week after implantation, the three sheep that survived to the 4-week end-point underwent weekly ultrasound examination of the grafts. For nanofiber TEVGs, two were patent at 4 weeks (Fig. 3A, C), with one occlusion at 2 weeks. For ePTFE, all grafts were patent (Fig. 3B, D). Weekly evaluation of lumen diameter, graft wall thickness, and blood flow by ultrasound was feasible.
FIG. 3.
Graft diameter, blood flow rate, and puncture under ultrasound guidance. Ultrasound images of nanofiber TEVGs (A, C) and ePTFE grafts (B, D) at 2 weeks (A, B) and 4 weeks (C, D) after surgery. (E, F) Lumen diameter and blood flow rate of TEVGs versus ePTFE grafts. Asterisk indicates statistical significance between the two groups (p < 0.05). (G, H) Successful needle puncture of grafts at 4 weeks after AV shunt surgery under ultrasound guidance. Arrows refer to the needle within the grafts. Color images available online at www.liebertpub.com/tec
Nanofiber TEVGs had increasing lumen diameter over 4 weeks postimplantation, as compared with the control ePTFE grafts that had an unchanging lumen diameter, with statistically significant differences between control and experimental TEVG lumen diameter at 2 weeks (p = 0.038), 3 weeks (p < 0.001), and 4 weeks (p < 0.001) after implantation (Fig. 3E). In addition, nanofiber TEVGs had a higher flow rate, compared with control ePTFE grafts, at 2, 3, and 4 weeks (p < 0.001).
Finally, both TEVG and ePTFE grafts were punctured at 4 weeks under ultrasound guidance without complications such as hemorrhage and nervous injuries (Fig. 3G, H).
Explantation of vascular grafts
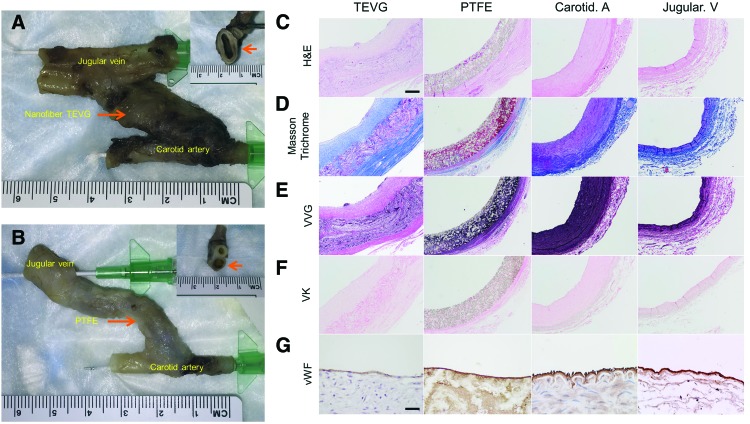
All tissue samples (nanofiber TEVGs, ePTFE grafts, CCAs, EJVs) were harvested without tissue damage (Fig. 4A, B), and they were evaluated by representative staining (Fig. 4C–F). Even though in the nanofiber TEVGs, scaffolds remained (Fig. 4C) at 4 weeks, they are in the process of remodeling (Fig. 4D, E) without vascular calcification (Fig. 4F). At 4 weeks, endothelialization of nanofiber TEVGs was satisfactory as demonstrated by vWF staining (Fig. 4G). There were no monolayer cells in the internal lumen of the ePTFE graft on H&E staining, suggesting that positive staining for vWF showed pseudointimal layer formation (Fig. 4G).
FIG. 4.
Macrographs, histology, and immunohistochemistry of explanted vascular grafts. Photographs of TEVG AV shunt (A) and ePTFE AV shunt (B) 4 weeks after surgery. H&E (C), Masson's trichrome (D), VVG (E), VK staining (F), and vWF immunostaining (G) of explanted vascular grafts. Scale bar (C–F) 200 μm, (G) 50 μm. H&E, hematoxylin and eosin; VK, von Kossa; VVG, Verhoeff-Van Gieson; vWF, von Willebrand factor. Color images available online at www.liebertpub.com/tec
Discussion
In developing a method for bilateral AV implantation of vascular grafts in ovine models, we have established a new protocol for the preclinical evaluation of TEVGs. Although there are many pre-existing testing methods, few have addressed the need for an experimental control that considers inter-subject differences in vascular physiology. Our model allows for the implantation of both the ePTFE control graft and the experimental TEVG into the same animal at the same anatomic location, essentially eliminating any confounding factors derived from variations in vascular microenvironments.
Here, we report the first use of a bilateral AV shunt as a testing algorithm for evaluation of TEVGs in ovine models. This procedure did not result in acute heart failure, steal syndrome, or hypoxic brain injury, providing support for the use of bilateral AV shunts as a viable experimental paradigm. We also punctured our grafts with a 16-gauge dialysis needle and found that our method allows for preclinical simulation of actual puncture of AVGs/AVFs for hemodialysis access.
Our experimental TEVGs exhibited dilation and significant longitudinal strain from lateral flexion of the neck, predisposing the grafts to rupture (n = 2), and suboptimal flow of blood through the venous system, increasing the risk of stenosis. Accordingly, of the three surviving animals, one experienced eventual occlusion of the graft within 2 weeks postimplantation as determined by ultrasonography, whereas two animals had patent grafts at 1 month postimplantation. As a result of these findings, the TEVG material is currently being modified to address the shortcomings identified in this study. Graft remodeling was also imperfect as a histological examination revealed elastin layers that were not native like. However, this is likely attributed to the fact that the short implantation period did not allow enough time for comprehensive graft remodeling.
Regarding the vessels for graft anastomosis, we chose to implant a cervical AV graft, instead of an extremity AV graft, as the extremity vessels are too small in diameter. The only other site where we might technically be able to implant an AV graft in the sheep is between the femoral artery and the femoral vein, but both vessels are also too small to keep patency in the sheep (∼3 mm in diameter). In addition, it is hard to keep a femoral AV graft in position when the sheep recovers from anesthesia and becomes mobile, which may lead to graft tension.
As TEVGs remodel in vivo after implantation, it is possible for the vascular neotissue to be weak and for punctures to cause excessive bleeding and create wounds that do not heal. To assess the strength of the vascular neotissue, we punctured our grafts with a 16-gauge dialysis needle, without bleeding or wound complications. Other possible complications, including nervous injuries, AV graft stenosis, thrombosis, aneurysm formation, and rupture, can be assessed by physical examination (such as in cases of nervous injuries), direct visualization (such as in cases of large aneurysm formation), and ultrasound imaging (Fig. 3). We suggest that our model, by allowing for these assessments, will aid in the long-term goal of creating optimized TEVGs, which are amenable to hemodialysis access.
The small animal models will not be able to provide necessary preclinical data with human-scale hemodynamic flow dynamics and appropriately sized vascular graft characteristics. Puncturing these grafts in small animal models will also be technically difficult and clinically less relevant than in large animal models. Further, our model could also be used to assess grafts that are designed for bypasses, vessel replacements, and congenital heart defects. Our model can also be customized to modify the way the graft is implanted, for example, straight graft between two vessels, a U-shape graft, side-to-side anastomosis, end-to-side anastomosis, etc. Stability and patency in an AV implantation model could translate to greater clinical success as the grafts are able to tolerate more adverse conditions than the physiological settings that they were intended for.
There has been a dearth of literature on the study of AV grafts in large animals (Table 1). The commonly used control ePTFE graft has been evaluated in the sheep model and displayed neointima hyperplasia, thrombosis, and only 25% patency at 12 weeks postimplantation, highlighting the massive room for improvement in vascular graft technology.20 There have been two general approaches in the development of vascular grafts tested as AV conduits in large animal models: treated ePTFE grafts and vascular grafts made of biodegradable polymers. ePTFE grafts treated with factors to recruit host endothelial cells have been largely successful in increasing endothelialization compared with untreated ePTFE grafts that are currently the mainstay of prosthetic grafts in AV grafts, but there have been conflicting data on the degree of intimal hyperplasia.21,22 Success of the biopolymer AV grafts study has been inconsistent, as one group demonstrated positive results while using collagen matrix tubes in baboons whereas another group experienced stenosis in all grafts.23,24 The only difference between the two studies was that the latter group seeded their grafts before implantation but no further conclusions can be drawn at this time, due to the paucity of literature.
Table 1.
Preclinical Evaluation of TEVGs in Large Animal Models as Arteriovenous Conduits
| Authors and year | Large animal model | Vascular graft | Site of implantation | Result |
|---|---|---|---|---|
| Kohler et al. (1999)20 | Sheep | PTFE | Unilateral carotid artery to EJV | Venous stenosis reliably produced within 4 weeks |
| Rotmans et al. (2005)22 | Pig | Anti-CD34-coated ePTFE grafts; uncoated ePTFE grafts as control | Bilateral carotid artery to IJV | Increased endothelialization and intimal hyperplasia at 4 weeks after implantation compared with controls |
| Li et al. (2005)21 | Sheep | P15 cell-binding peptide-treated ePTFE grafts; untreated ePTFE as control | Unilateral carotid artery to jugular vein | Reduced intimal hyperplasia and higher degree of endothelialization compared with controls |
| Dahl et al. (2011)23 | Baboon | Decellularized bioreactor vessel derived from cadaveric allogenic smooth muscle cells seeded onto PGA | Unilateral axillary artery to brachial vein | 88% of grafts were patent at 6 months and did not display dilatation, calcification, and intimal hyperplasia |
| Tillman et al. (2012)24 | Sheep | Decellularized arterial scaffold seeded with ovine endothelial cells | Unilateral carotid artery to EJV | All grafts developed venous outflow stenosis |
| Present study | Sheep | PGA/PLCL 1:1 nanofiber | Bilateral carotid artery to EJV | Remodeling and endothelialization of TEVG noted without calcification or stenosis |
EJV, external jugular vein; ePTFE, expanded polytetrafluoroethylene; IJV, internal jugular vein; TEVGs, tissue-engineered vascular grafts.
Although different animal species have been used as large animal models of cardiovascular disease,25,26 including pigs, sheep, cattle, horses, and non-human primates, there are limited data directly comparing these large animal species with each other, and comparing these large animal models with humans. Comparing the present ovine study with the previous porcine study,22 there is less intimal hyperplasia in the ovine model than in the porcine model. In addition, there are anatomical differences in between the ovine jugular vein and the porcine jugular vein. The ovine internal jugular vein (IJV) is deep, small, and often absent,27 as compared with the porcine jugular vein.28 Thus, although we anastomosed the CCA to the EJV in the ovine model, the previous porcine study22 used the IJV to create the AV graft implantation model.
The clinical importance of developing TEVGs that recapitulate the properties of autologous vessels is particularly salient in the growing population of patients with ESRD requiring vascular access for hemodialysis.29 Where autologous grafts are unavailable, acute hemodialysis catheters and prosthetic grafts serve as less-than-ideal alternatives; acute hemodialysis catheters are associated with a high risk of infection and mortality; and synthetic grafts are prone to stenosis and thrombosis.30,31 Many TEVGs are currently in the translational stage of development whereby they are evaluated in large animal models for possible use for hemodialysis access in ESRD.
There remains a need for an animal model that will simulate the supraphysiological flow rates and turbulence in AV fistulas. The implantation of grafts as AV shunts would allow for TEVGs to undergo more rigorous testing under conditions of increased mechanical forces generated by turbulent flow, akin to AV grafts currently approved for clinical use in ESRD.
Conclusion
Bilateral AV implantation allows for the direct comparison of the experimental graft and the control graft as confounding factors derived from inter-subject differences in vascular physiology are eliminated. Although there has been concern that bilateral implantation may exert increased strain on the animal and lead to complications such as heart failure and tissue hypoxia, our study has provided preliminary data abating this concern. Therefore, bilateral AV shunts constitute a good method for the preclinical evaluation of vascular grafts and TEVGs for use in hemodialysis and even in broader applications in the vascular surgery field, such as bypasses and vessel replacements.
Acknowledgment
The authors thank Chen Yu Huang, PhD, for her illustration of their animal model in Figure 1.
Disclosure Statement
Nanofiber Solutions, Inc. provided the graft materials.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation 133, e38, 2016 [DOI] [PubMed] [Google Scholar]
- 2.McAllister T.N., Maruszewski M., Garrido S.A., et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet (London, England) 373, 1440, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Wystrychowski W., McAllister T.N., Zagalski K., Dusserre N., Cierpka L., and L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg 60, 1353, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Benrashid E., McCoy C.C., Youngwirth L.M., et al. Tissue engineered vascular grafts: origins, development, and current strategies for clinical application. Methods (San Diego, Calif.) 99, 13, 2016 [DOI] [PubMed] [Google Scholar]
- 5.G N, Tan A., Gundogan B., et al. Tissue engineering vascular grafts a fortiori: looking back and going forward. Expert Opin Biol Ther 15, 231, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Ong C.S., Zhou X., Huang C.Y., Fukunishi T., Zhang H., and Hibino N. Tissue engineered vascular grafts: current state of the field. Expert Rev Med Devices 14, 383, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Ravi S., and Chaikof E.L. Biomaterials for vascular tissue engineering. Regen Med 5, 107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller P.E., Carlton D., Deierhoi M.H., Redden D.T., and Allon M. Natural history of arteriovenous grafts in hemodialysis patients. Am J Kidney Dis 36, 68, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Fukunishi T., Best C.A., Sugiura T., et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One 11, e0158555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibino N., McGillicuddy E., Matsumura G., et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139, 431, 436.e431–432, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Shinoka T., Shum-Tim D., Ma P.X., et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg 115, 536, discussion 545–536, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Watanabe M., Shin'oka T., Tohyama S., et al. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng 7, 429, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Matsumura G., Miyagawa-Tomita S., Shin'oka T., Ikada Y., and Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation 108, 1729, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Shin'oka T., Matsumura G., Hibino N., et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 129, 1330, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumi H., Miyawaki F., Arakawa H., Tsuji T., and Tanigawa M. Experience of vein grafting in Gottingen minipigs. Exp Anim 50, 191, 2001 [DOI] [PubMed] [Google Scholar]
- 16.L'Heureux N., Dusserre N., Konig G., et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 12, 361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olausson M., Patil P.B., Kuna V.K., et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet 380, 230, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Swartz D.D., and Andreadis S.T. Animal models for vascular tissue-engineering. Curr Opin Biotechnol 24, 916, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukunishi T., Best C.A., Sugiura T., et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg 153, 924, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler T.R., and Kirkman T.R. Dialysis access failure: a sheep model of rapid stenosis. J Vasc Surg 30, 744, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Li C., Hill A., and Imran M. In vitro and in vivo studies of ePTFE vascular grafts treated with P15 peptide. J Biomater Sci Polym Ed 16, 875, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Rotmans J.I., Heyligers J.M., Verhagen H.J., et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation 112, 12, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Dahl S.L., Kypson A.P., Lawson J.H., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 3, 68ra69, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Tillman B.W., Yazdani S.K., Neff L.P., et al. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J Vasc Surg 56, 783, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Tsang H.G., Rashdan N.A., Whitelaw C.B.A., Corcoran B.M., Summers K.M., and MacRae V.E. Large animal models of cardiovascular disease. Cell Biochem Funct 34, 113, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilahur G., Padro T., and Badimon L. Atherosclerosis and thrombosis: insights from large animal models. J Biomed Biotechnol 2011, 12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W., Park W., Uchida B., et al. The ovine jugular vein as a model for interventional radiology procedures. Radiol Oncol 42, 59, 2008 [Google Scholar]
- 28.Habib C.A., Utriainen D., Peduzzi-Nelson J., et al. MR imaging of the yucatan pig head and neck vasculature. J Magn Reson Imaging 38, 641, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Feldman H.I., Kobrin S., and Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol 7, 523, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Wasse H. Catheter-related mortality among ESRD patients. Semin Dial 21, 547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allon M. Current management of vascular access. Clin J Am Soc Nephrol 2, 786, 2007 [DOI] [PubMed] [Google Scholar]