Abstract
Experimental models of neuropathic pain (NP) typically rely on withdrawal responses to assess the presence of pain. Reflexive withdrawal responses to a stimulus are used to evaluate evoked pain and, as such, do not include the assessment of spontaneous NP nor evaluation of the affective and emotional consequences of pain in animal models. Additionally, withdrawal responses can be mediated by spinal cord reflexes and may not accurately indicate supraspinal pain sensation. This is especially true in models of traumatic spinal cord injury (SCI), wherein spastic syndrome, a motor disorder characterized by exaggeration of the stretch reflex that is secondary to hyperexcitability of the spinal reflex, can cause paroxysmal withdrawals not associated with NP sensation. Consequently, the aim of this study was to utilize an assessment of supraspinal pain sensation, the Rat Grimace Scale (RGS), to measure both spontaneous and evoked NP after a contusion SCI at cervical level 5 in adult male rats. Spontaneous and evoked pain were assessed using the RGS to score facial action units before and after the application of a stimulus, respectively. Rodents exhibited significantly higher RGS scores at week 5 post-injury as compared to baseline and laminectomy controls before the application of the stimulus, suggesting the presence of spontaneous NP. Additionally, there was a significant increase in RGS scores after the application of the acetone. These data suggest that the RGS can be used to assess spontaneous NP and determine the presence of evoked supraspinal pain sensation after experimental cervical SCI.
Keywords: : cold allodynia, cold hypersensitivity, neuropathic pain, pain sensation
Introduction
Spinal cord injury (SCI) is a traumatic event affecting 238,000–332,000 people in the United States with 12,000 new cases annually.1 Chronic neuropathic pain (NP) can be a debilitating consequence of SCI, affecting an average of 65% of patients.2 Evoked NP occurs in response to peripheral stimuli and can be categorized as hyperalgesia, in which sensitivity to noxious stimuli is increased, or allodynia, in which innocuous stimuli become noxious. Spontaneous NP occurs independently of external stimuli and is described by patients as an intermittent, burning or stabbing sensation commonly rated as severe.2,3 The presence and severity of NP are associated with greater impairments in physical, emotional, and social functioning,4 and alleviation of NP is one of the highest priorities for the patient.5
Numerous animal models of NP have been developed to elucidate mechanisms as well as determine the analgesic potential of pharmacological interventions. The most commonly used models involve compression injury or axotomy of peripheral nerves innervating the rodent hindpaw, which result in both hyperalgesia and allodynia (see Campbell and Meyer6 for a review). To emulate NP post-SCI, several pre-clinical models have been developed, including spinal cord contusion models.7–9 Previous studies have found hypersensitivity to mechanical,10–14 cold,10,14,15 and thermal (heat) stimuli11,13,16 after thoracic or cervical contusion injuries in rodents. However, these studies did not evaluate spontaneous pain. Given that spontaneous pain is more prevalent among SCI patients, increases over time, and is rated more problematic than evoked pain,3,17 there remains an ongoing discussion as to the importance of evaluating spontaneous pain in pre-clinical models of NP. For example, Mogil and Crager reviewed studies published in the journal Pain and found that only 10% (26 of 259 studies) included assessments of spontaneous pain.18 Others have placed less emphasis on measures of spontaneous pain sensation19 with regard to the clinical relevance of NP models. Evaluation of spontaneous pain measures in the SCI literature is also rare and restricted to conditioned place preference, avoidance tests,20 or overgrooming.21,22 Unfortunately, these assessments all have a large motor component and therefore may not be optimal for study of SCI-induced spontaneous NP. Additionally, place preference and overgrooming behavior can be confounded by learning, memory, and other affective states (e.g., anxiety)18 and are thus multi-faceted behavioral outcome measures with complex interpretation, particularly in injured animals.
SCI-induced changes in pain sensation to evoked stimuli are traditionally assessed by determining changes in withdrawal responses to a stimulus applied to the paw or torso of the animal. A limitation to the classic reflexive withdrawal measures as indicators of SCI-induced pain sensation under much discussion is the concept of hyper-reflexive versus hypersensitive states post-SCI. Some research suggests that withdrawal responses indicate hyper-reflexia, not hypersensitivity, and, as such, are not reliable indicators of pain sensation.18,19 This interpretation of paw withdrawal behavior is based on two central ideas. First, paw withdrawal responses are mediated by activation of spinal reflex pathways without supraspinal activation. Second, hyper-reflexia is demonstrated in thoracic and cervical contusion models of SCI, and spasms can be elicited by various stimuli.23–25 This question as to whether paw withdrawal responses indicates SCI-induced NP was raised in a thoracic contusion SCI model by Baastrup and colleagues.26 They found decreased thresholds for withdrawal responses to von Frey and acetone (cold) stimuli, but did not observe a substantial change in stimulus thresholds required to elicit spinal/brainstem responses, including guarding and licking of the affected paw. These data suggest some dissociation between spinally mediated withdrawal responses and supraspinally mediated behaviors.26 Similarly, van Gorp and colleagues reported that stimulus thresholds to elicit an escape response were lower than thresholds required to elicit a withdrawal response, again suggesting divergence between withdrawal responses and supraspinally mediated pain-like behaviors.27 Interestingly, van Gorp and colleagues did not find a relationship between withdrawal responses and spasticity. Alternatively, other research indicates that there are increased brainstem and cerebral responses from below-level mechanical stimulus.15,28 Taken together, these studies raise the important point that the relationship between paw withdrawal responses and supraspinal responses requires further investigation. We posit that evaluation of the relationship between paw withdrawal responses and supraspinal responses in a cervical SCI model will further advance our understanding of these behavioral outcomes.
To that end, we selected the Rat Grimace Scale (RGS) to evaluate the presence of spontaneous pain sensation and a supraspinal component of evoked pain sensation in our rat model of cervical level 5 (C5) hemicontusion SCI. The RGS offers several advantages compared to other supraspinal pain measurements for SCI models, including: 1) It evaluates brain-mediated responses; 2) requires no pre-training of the rodents; 3) is observational without invasive monitoring; and 4) and is not affected by motor impairments. The grimace scale also has translational validity because it is based on successful use of facial coding to interpret pain in young children and infants.29,30 In terms of rodents, the grimace scale was first developed in mice using a 0.9% acetic acid abdominal constriction pain model31 and was then shown to reliably quantify spontaneous pain post-laparotomy.32 The grimace scale has since been adapted to assess post-procedural pain in rabbits33,34 and horses35 and was translated to the rat by Sotocinal and colleagues.36 They demonstrated that in addition to being highly reliable in assessing spontaneous pain post-laparotomy and induced inflammation in rats, the RGS scores accurately differentiated between rodents with “pain” and with “no pain.”36 In addition to being a reliable outcome measure in different species, Oliver and colleagues demonstrated that the RGS is also highly reliable with different models, raters, and environments pertaining to pain.37 Moreover, the RGS has been utilized to examine pain mechanisms in multiple pain models, including laparotomy,38 plantar incision,39 induced inflammatory pain,40 and experimental tooth movement20 as well as to test the efficacy of analgesics post-laparotomy41,42 and implantation surgery.37 Interestingly, only one study has previously used the grimace scale in an SCI model. Wu and colleagues used the Mouse Grimace Scale to assess the effect of cell-cycle inhibition treatment on spontaneous pain after a T10 contusion.43 Here, we extend these findings to evaluate both evoked and spontaneous supraspinal pain in the rat. Although the use of rats is widespread throughout the pre-clinical SCI literature, the grimace scale has yet to be characterized in a rat model of SCI. Therefore, we hypothesized that the RGS can be used to assess supraspinal pain sensation occurring both spontaneously and in response to a stimulus after cervical SCI as well as to better elucidate the relationship between supraspinal responses and paw withdrawal.
Methods
Animals
Adult male Sprague-Dawley rats (275–300 g) were obtained from Charles River Laboratories (Hartford, CT) and group-housed 2–3 per cage with access to standard rat chow and water ad libitum. The room was maintained under a 12/12-h light/dark cycle (6:00 am/6:00 pm) at a temperature of 25°C. Animals were divided into two groups: 1) uninjured laminectomy control (LAM; n = 5) and 2) cervical SCI (n = 10). All animals were allowed to acclimate to the facility and investigators for 5 days after arrival, followed by 1 week of acclimation to behavioral testing equipment. Baseline assessments were performed 1 week pre-injury. Previous data from our lab using this model suggest that at week 5 post-SCI, animals exhibit NP-like behaviors, including increased paw withdrawal responses to acetone and overgrooming. Thus, for this study, all assessments were performed at week 5 post-injury. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (Birmingham, AL).
Induction of cervical level 5 hemicontusion spinal cord injury
The dominant paw of each animal was determined before surgery using the Paw Placement test. Animals received a hemicontusion at C5 on the side of the spinal cord ipsilateral to the dominant paw. In this model, the dominant side is injured to standardize the effect of use-dependent plasticity in functional recovery. The Infinite Horizon SCI Impactor device (Precision Systems and Instrumentation, Lexington, KY) was used, as previously described, to induce a severe hemicontusion SCI.9 Briefly, each animal was anesthetized with 4% isoflurane (Piramal Critical Care, Bethlehem, PA) in oxygen for 4 min and maintained on 2% isoflurane in oxygen for the duration of the procedure. Body temperature was maintained at 37°C throughout surgery with the use of a rectal thermometer and heating pad. The surgical site was shaved, aseptically prepared for surgery, and an incision was made from the C2 to T2 vertebrae. Muscles were retracted to reveal the vertebral column, and the underlying paravertebral muscles of C4–C6 were removed. The lamina covering the C5 vertebrae was removed bilaterally and animal stabilized on the impactor with clamps on the dorsal processes of C2 and T2. The impactor tip (0.8 mm in diameter) was aligned above the exposed dorsal aspect of the C5 spinal cord in order to cover the side ipsilateral to the dominant paw. A hemicontusion injury was delivered to all animals in the SCI group by the impactor device at a force of 300 kdyn. Animals in the LAM control group received the full surgical procedure through the C5 bilateral laminectomy omitting the contusion injury. Subsequent to impact (SCI) or laminectomy, the musculature and skin were sutured in layers and animals remained in a heated recovery cage until ambulant. Immediately post-surgery and twice a day (am and pm) for 5 days thereafter, animals received subcutaneous injections of sterile saline (3 mL; Hospira Inc., Lake Forest, IL) and carprofen (5 mg/kg; Rimadyl; Pfizer, New York, NY) as a fluid replacement and analgesic. Post-surgery and once a day (am) for 5 days thereafter, injections included enrofloxacin (2.5 mg/kg; Baytril; Bayer HealthCare LLC, Shawnee Mission, KS) as a prophylactic antibiotic.
Assessment of cold hypersensitivity
Hypersensitivity to cold was assessed by applying acetone to the hindpaw, which has been shown to elicit a significant increase in withdrawal responses after spinal cord contusion injuries in rats.10,14,15 We utilized methods previously described by Choi and colleagues,44 with slight adaptations. Animals were placed in a clear Plexiglas box (20 × 9 × 10 cm) on an elevated platform with a wire mesh bottom to allow access to the plantar surface of the hindpaws. After a 5-min acclimation period, a single drop of acetone (Sigma-Aldrich, St. Louis, MO) was produced at the end of a 1-mL syringe. Avoiding contact of the syringe with the paw, the acetone drop was applied to the plantar surface of the contralateral hindlimb 1 cm posterior to the footpad of the third and fourth phalanges. There is a short time delay as acetone evaporates before eliciting the sensation of coolness44; therefore, a withdrawal response occurring within 20 sec of application was recorded along with the time of withdrawal. Acetone application was performed using three trials for each animal with at least 5 min between each trial. The average time in seconds to withdrawal of the three trials was used to determine withdrawal time. Importantly, for the comparison to the RGS, if the animal had a withdrawal response in any of the three trials, the animal was placed in the “withdrawal” group for data analysis.
Assessment of supraspinal pain
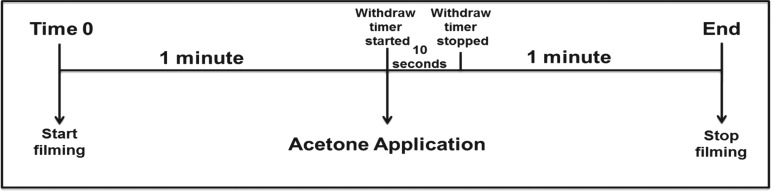
Supraspinal sensation of pain was assessed using an adaptation of the RGS methodology from Sotocinal and colleagues.36 Filming for the RGS was performed simultaneously with the acetone test using a high-speed digital camera (Exilim EX-F0; Casio America Inc., Dover, NJ). The animal was filmed in high definition at an operating speed of 60 frames per second with autofocus and backlight correction to produce images in which the animal's face (including nose/cheek, whiskers, eyes, and ears) were clearly visible. The animal's face was filmed 1 min before the application of acetone, and this comprised the “before” acetone period and was used to assess spontaneous pain (i.e., before the application of the stimulus). Filming continued as acetone was applied and for 1 min thereafter, which represented the “after” acetone period and assessment of evoked pain (Fig. 1). This was repeated three times during the testing session during each application of acetone. The camera was held by the investigator for the entirety of filming in order to maintain focus on the animal's face as the animal moved.
FIG. 1.
Rat Grimace Scale (RGS) data collection scheme. Recording began 1 min before the application of acetone, representing the Before Acetone Period. Acetone was applied and the withdrawal timer started. A withdrawal response within 10 sec of application was recorded. Recording was continuous and ended 1 min after acetone was applied. The minute after acetone comprised the After Acetone Period.
To prepare for scoring, one screen shot per second was extracted from the video recording for the 20 sec before and 20 sec after the application of acetone by an investigator naïve to the experimental group. The screen shots for all three tests were given a unique coding number, combined into a PowerPoint (Microsoft, Redmond, WA) slide presentation, and randomized using a PowerPoint macro code. Two additional investigators naïve to the screen shot code scored the animal's face on each PowerPoint slide (representing 1 sec of the test) using the RGS. The scale consists of four action units (AUs): orbital tightening; nose and cheek flattening; ear position; and whisker position. Descriptions of each action unit during pain are provided in Table 1. Each AU received a “0” if signs of pain were absent, “1” if there were moderate signs present, and “2” if there were obvious signs of pain (Fig. 2). If the AU was not clearly visible, it was labeled as “not assessable” and excluded from further analysis. After scoring, the PowerPoint slide deck was derandomized and the scores for each AU were averaged for each slide. Slide scores were then averaged across three raters, decoded, and categorized into “before” acetone and “after” acetone bins for analysis (Fig. 3). This resulted in two average RGS scores for each animal: one represents the time period before and the other representing the time period after the stimulus. Evaluation of inter-rater reliability was conducted by having all scorers independently evaluate an identical set of eight slide decks from injured and uninjured animals not evaluated in the current study. The percentage agreement was calculated as 93% ± 11%.
Table 1.
Action Units and Their Description During Pain Sensation
| RGS action units | Description during pain |
|---|---|
| Orbital tightening | The area of the eye decreases from wide to squinted. |
| Nose and cheek flattening | The nose and cheek display a flatter and longer appearance. |
| Ear position | Ears appear curled or pointed and often appear spread out. |
| Whisker position | Whiskers appear tense and bunched together or angled down. |
RGS, Rat Grimace Scale.
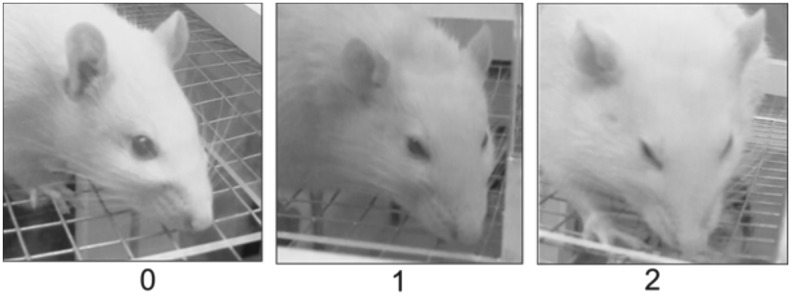
FIG. 2.
Representative images and corresponding Rat Grimace Scale scores. A score of “0” indicated the absence of signs of pain, “1” indicated moderate signs of pain, and “2” indicated obvious signs of pain.
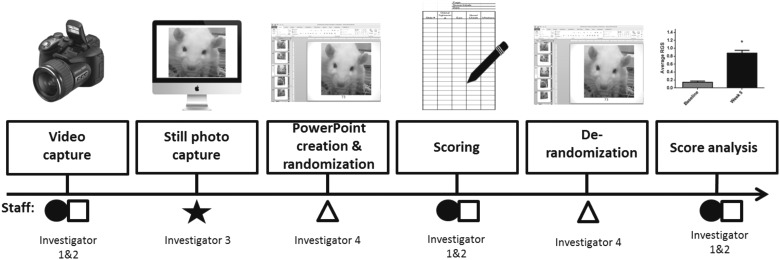
FIG. 3.
Rat Grimace Scale analysis procedure. After video capture, an investigator naïve to the experimental groups captured screen shots from the digital recording. Another investigator naïve to the experimental groups placed each screen shot onto a PowerPoint slide, gave each slide a unique coding number, and randomized the slides using a randomization matrix. Two investigators naïve to the experimental groups scored each slide, and the scores were averaged. Derandomization was conducted by a separate investigator as was final data analysis. Thus, a minimum of four investigators were involved to maintain anonymity of experimental conditions (i.e., injury or control and before or after acetone).
Tissue preparation for histopathology
On day 35 post-SCI, animals were euthanized with 4% isoflurane (Piramal Critical Care, Bethlehem, PA) for 8 min, then transcardially perfused with cold 0.1 M of phosphate-buffered saline (PBS; pH 7.4) for 5 min, followed by the fixative, Excell Plus (American Mastertech, Lodi, CA), for 20 min. The epicenter was marked with tissue dye (Triangle Biomedical Sciences, Inc., Durham, NC), and the spinal cord from C2 to T2 was removed. Spinal cord tissue was post-fixed in Excell Plus for 24 h at 4°C and cryoprotected with 10% sucrose/PBS for 1 hour at 4°C, followed by 30% sucrose for 48 h. A 3-mm section was blocked from the epicenter and embedded into Tissue Tek® optimum cutting temperature compound (Scigen Scientific, Gardena, CA) and stored at −80°C until sectioning. Serial 30-μm transverse sections were sliced using a cryostat (Leica Microsystems, Wetzlar, Germany) and mounted onto 1% gelatin-coated slides for subsequent evaluation of spinal cord pathology. Tissue sections were collected, such that 10 sets of adjacent sections were obtained, resulting in a 300-μm intersection interval. From these 10 sets of similarly spaced serial sections, one set was randomly selected for histological assessment of pathology, as described below.
Cresyl violet histology
To visualize neurons in the gray matter and lesion volume, cresyl violet acetate (Sigma-Aldrich) histology was performed as previously described.9 Briefly, tissue sections were dehydrated using increasing ethanol concentrations from 70% to 100%, followed by clearing with xylene, rehydrated in ethanol, and processed with 0.1% cresyl violet acetate for 5 min. Tissue was rinsed with double-distilled water, followed by 95% ethanol with acetic acid for 2 minutes, dehydrated with alcohol, washed with xylene, and, finally, cover-slipped using Permount (Thermo Fisher Scientific, Walthan, MA) mounting medium.
Quantification of dorsal and ventral horn neurons using stereology
Tissue sections were visualized on an Olympus IX-73 microscope with an Olympus DP-73 digital camera (Olympus, Tokyo, Japan) using with Visiopharm newCAST™ stereology software (Visiopharm, Broomfield, CO). The optical fractionator probe was used to estimate the total number of neurons in the dorsal and ventral horns at the lesion epicenter, as previously described.45–47 Briefly, a separate contour of the ipsilateral dorsal horn and the ipsilateral ventral horn was traced, and a counting frame (60 × 60 μm2) was superimposed on the micrograph at a random location within each contour. The counting frame was then moved by the optical fractionator probe macro to nonoverlapping locations along the x- and y-axes. Neuronal cell counts were evaluated at 400 × magnification with the following inclusion criteria: 1) an ovoid, triangular, or multipolar cellular profile; 2) soma diameter greater than 10 μm; and 3) an intact cell membrane with a clearly defined nucleus. Eight sections per animal were evaluated, for a total tissue sampling volume of approximately 2940 μm3.
Quantification of percentage of lesion area
Lesion area at the epicenter was determined using an Olympus IX-73 microscope attached to an Olympus DP-73 digital camera with Visiopharm newCAST™ stereology software. Lesion area was obtained at 40 × magnification by placing a contour around the entire spinal cord and second contour around the injured area, including both gray and white matter. The percentage of the lesioned area was calculated for each section by taking the value of the injured area / total spinal cord area × 100. Eight sections per animal were evaluated, for a total tissue sampling volume of approximately 2940 μm.3 Given that the injury is a hemicontusion, the percentage lesion area is always less than 50%.
Statistical analyses
All data were analyzed using GraphPad Prism 7 Statistical Software (GraphPad Software Inc., San Diego, CA) with significance set at a p value of p ≤ 0.05. All data are presented as mean ± standard error of the mean. Pre- and post-injury differences in RGS scores were analyzed using a one-way analysis of variance, followed by Holm-Sidak post-hoc analysis. Differences in RGS score between trials with and without a withdraw response were analyzed using Student's t-test.
Results
Rat Grimace Scale detects the presence of spontaneous neuropathic pain sensation after spinal cord injury
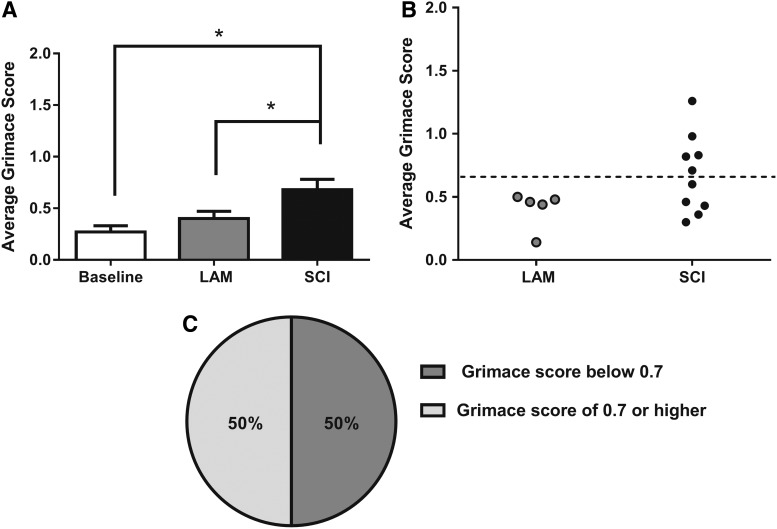
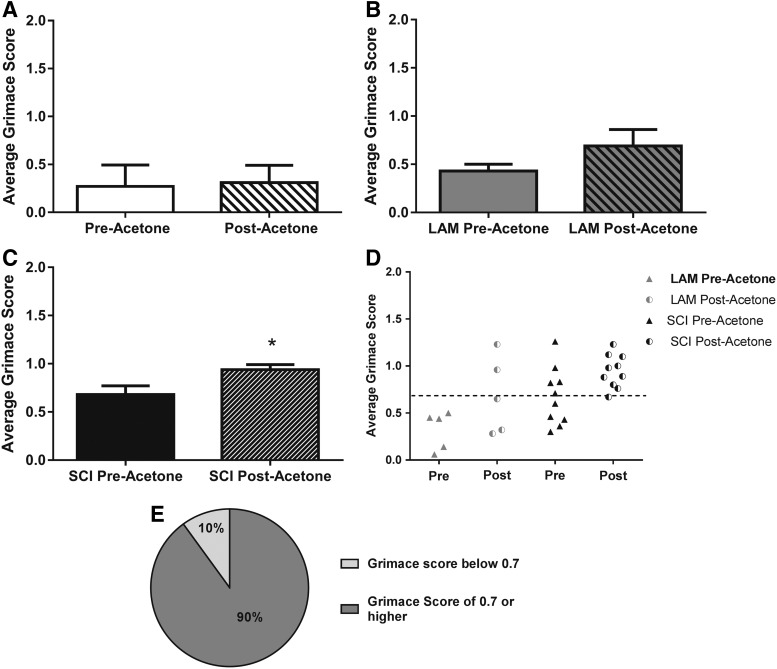
We utilized the RGS to evaluate the presence of spontaneous NP in the rat C5 hemicontusion model of SCI. As seen in Figure 4A, we found a significant increase in average grimace score at week 5 post-injury (0.68 ± 0.10) compared to pre-surgery baseline (0.28 ± 0.06) and week 5 LAM controls (0.44 ± 0.06; p ≤ 0.05). Based on Oliver and colleagues,37 we utilized a threshold score (analgesic intervention score, rounded to 0.7) to categorize SCI animals into “pain” and “no-pain” subsets.37 As seen in Figure 4B, no animals in the LAM group reached the analgesic intervention score of 0.7 (dotted line), suggesting that none were exhibiting spontaneous pain. In comparison, a subset of animals in the SCI group (50%) had a grimace score at or above the analgesic intervention score, suggesting that these animals were experiencing spontaneous pain (Fig. 4B,C). We concluded from these data that the RGS can also be used to distinguish between animals exhibiting spontaneous pain from those with no spontaneous pain post-SCI, and that only animals in the SCI group exhibited spontaneous NP.
FIG. 4.
SCI induced significant elevation in Rat Grimace Scale (RGS). (A) Grimace scores increase significantly after SCI compared to baseline and LAM controls (*p ≤ 0.05, one-way analysis of variance). (B) Individual scores subdivided by group indicate that the animals in the LAM group have a grimace score below 0.7, whereas a subset of animals from the SCI group have a grimace score of 0.7 or higher, indicating spontaneous pain sensations. (C) Fifty percent of animals in the SCI group demonstrate spontaneous pain sensations according to the RGS by having a score of 0.7 of higher. SCI, spinal cord injury; LAM, laminectomy.
Rat Grimace Scale detects supraspinal pain sensation related to cold hypersensitivity after spinal cord injury
We next examined the hypothesis that the RGS could be used to indicate supraspinal pain sensation to evoked stimuli. Specifically, we tested this by assessing the RGS in conjunction with cold hypersensitivity using the application of acetone to the contralateral hindpaw, as previously described.44 We first evaluated the effect of acetone application on the average grimace score in animals before surgical manipulation (baseline). As seen in Figure 5A, we found no significant differences in average grimace score when comparing in the 20 sec before the application of acetone (0.25 ± 0.06 pre-acetone) with the 20 sec after the acetone application (0.29 ± 0.04 post-acetone) in nonsurgical animals. Next, we assessed the effect of acetone application on average grimace score in the uninjured laminectomy group at week 5 post-surgery. Similar to the baseline data, we found no significant differences in the average grimace score in the 20 sec before the application of acetone (0.43 ± 0.07 pre-acetone) as compared to the 20 sec after the acetone application (0.75 ± 0.13 post-acetone; Fig. 5B). Notably, a nonsignificant trend toward a higher average grimace score was observed in the LAM group following acetone application. In contrast, when the average grimace score before and after the application of acetone was compared in the animals that received SCI at week 5 post-injury, we observed a significant increase in RGS score following the application of acetone (0.68 ± 0.10 pre-acetone; 0.88 ± 0.07 post-acetone; p ≤ 0.05; Fig. 5C). As we did for the spontaneous pain, we also evaluated the effect of group on the percentage of animals with average grimace scores below versus at/above the analgesic intervention score as a separator into “pain” and “no-pain” groups related to evoked pain (Fig. 5D). We found that 90% of the animals in the SCI group exhibited an average grimace score at or above the analgesic intervention score after the acetone stimulus (Fig. 5E). Taken together, these data indicate that the grimace score can be used as an indicator of supraspinal sensation of evoked cold hypersensitivity and that a large percentage of animals in the SCI group exhibited hypersensitivity to a cold stimulus, as indicated by this supraspinal pain response.
FIG. 5.
SCI induces increases in average Rat Grimace Scale post-acetone. (A) No significant difference in average grimace scores between pre- and post-acetone at baseline. (B) No significant difference in average grimace scores between pre- and post-acetone in the LAM group. (C) Animals in the SCI group exhibited a significant increase in average grimace score after acetone (*p ≤ 0.05, Student's t-test). (D) Individual scores subdivided by group. A subset of animals in the LAM group exhibited a grimace score above 0.7 post-acetone. Contrastingly, a majority of animals in the SCI group exhibited a grimace score above 0.7 post-acetone. (E) For the SCI group, 90% of the animals exhibited a grimace score of 0.7 or higher post-acetone. SCI, spinal cord injury; LAM, laminectomy.
The Rat Grimace Scale parallels the reflexive withdrawal response in animals with spinal cord injury
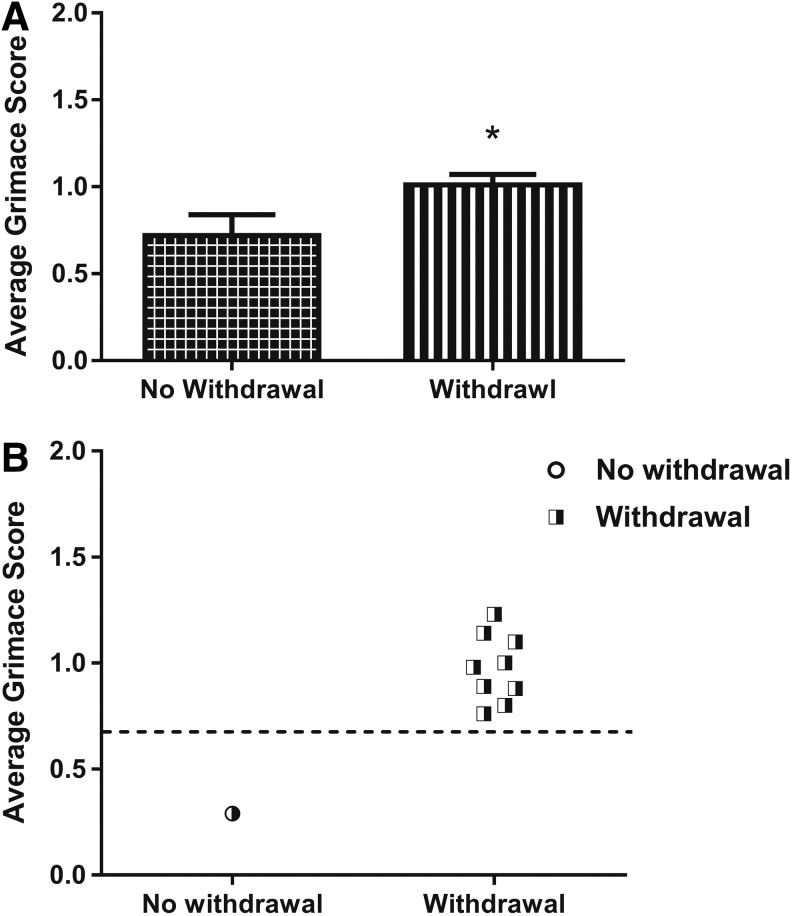
Given that we found that the RGS can be used to indicate spontaneous pain and a supraspinal response after an evoked stimulus, we next asked whether there is a correspondence between elevated RGS and paw withdrawal responses. An important aspect of the relationship between spontaneous pain and the reflexive withdrawal response is that, although mechanistically different,48 supraspinal pain indicators and reflex responses are not always mutually exclusive depending on injury magnitude and SCI model. First, we analyzed all the acetone trials of every animal in the SCI group. We found that the average grimace score after acetone was significantly higher in the trials in which there was a paw withdrawal response as compared to trials without a withdrawal response (0.69 ± 0.10 without withdrawal; 1.12 ± 0.08 with withdrawal; p ≤ 0.05; Fig. 6A). Next, we evaluated the individual animals' average grimace score in instances with and without a paw withdrawal to acetone (Fig. 6B). We found that most (8 of 10) animals that exhibited paw withdrawals also exhibited an average grimace score greater than the analgesic intervention score (0.7). Additionally, we observed that the 2 animals that did not exhibit paw withdrawal had a corresponding average grimace score below 0.7 (Fig. 6B). These data suggest that although the paw withdrawal reflex is spinally mediated and grimace is supraspinally mediated, the vast majority of trials in which a paw withdrawal occurred corresponded to an elevated grimace, suggesting that cold hypersensitivity is sufficient to elicit reflexive paw withdrawal and an accompanying supraspinal pain response, albeit by activation of different sensory pathways.
FIG. 6.
Average grimace score is increased in trials with corresponding paw withdrawal. (A) Average Rat Grimace Scale (RGS) score in animals in the spinal cord injury group that had a withdrawal response was significantly higher than the average RGS score in animals with no corresponding withdrawal (*p ≤ 0.01, Student's t-test). (B) Individual scores subdivided by withdrawal response post-acetone indicate that all animals with a withdrawal response exhibited a grimace score of 0.7. Contrastingly, the 1 animal that did not exhibit a withdrawal response had a corresponding grimace score below 0.7.
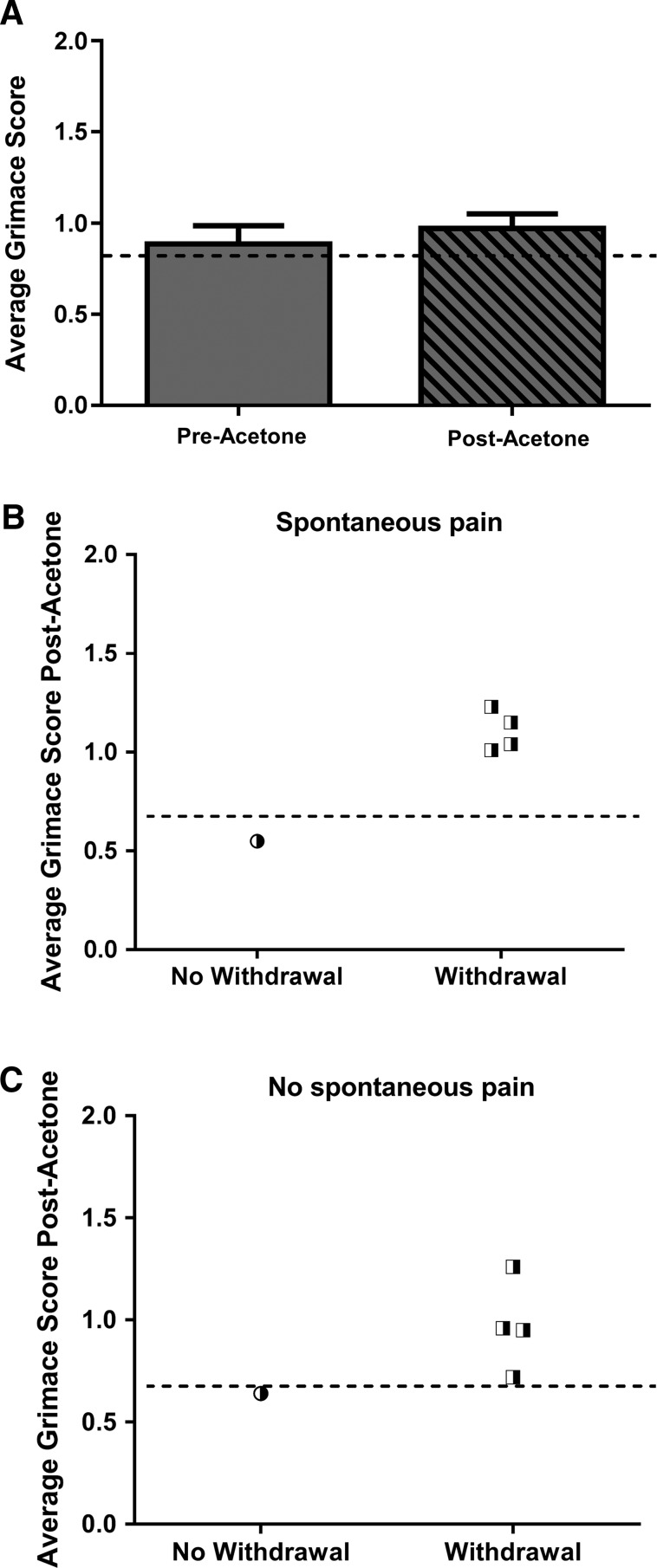
We next considered only the animals in the SCI group that exhibited spontaneous NP (at or above the analgesic intervention score). In animals with spontaneous pain, we observed no statistical differences between the average grimace score before or after the acetone stimulus (Fig. 7A), which suggests that the pain sensation from the evoked stimulus (cold hypersensitivity) is not additive to the spontaneous pain. Interestingly, as shown in Figure 7B, the subset of animals with spontaneous NP exhibited a paw withdrawal response to acetone in 80% of the trials (Fig. 7B). Last, we evaluated the subset of animals that did not exhibit spontaneous NP (below threshold of 0.7) to determine the relationship between average grimace score and paw withdrawal. We found that in animals not exhibiting spontaneous NP, 80% of the animals in which a paw withdrawal occurred corresponded to an average grimace score at or above the analgesic intervention score (Fig. 7C). Taken together, these data suggest that there is a strong correspondence between paw withdrawal and average grimace score, with higher RGS scores associated with withdrawal.
FIG. 7.
Average grimace score is increased in trials with corresponding paw withdrawal responses independent of spontaneous pain status. (A) Animals in the SCI group with a spontaneous Rat Grimace Scale (RGS) score above the analgesic intervention score did not exhibit a significant difference in RGS post-acetone. (B) Considering only the animals in the spinal cord injury (SCI) group with spontaneous RGS score above the analgesic intervention score, all animals exhibiting a withdrawal response post-acetone had a corresponding RGS above 0.7. (C) Considering only the animals in the SCI group with a spontaneous RGS below the analgesic intervention score, all animals exhibiting a withdrawal response had a grimace score above 0.7.
No changes in lesion area or dorsal horn neuron counts between pain and no-pain animals
We compared injury induction characteristics and gross pathological features of the lesions between animals classified in the pain versus no-pain categories as indicated by an RGS at/above versus below 0.7 in the spontaneous pain assessment. The average impact force for animals that received SCI and were later classified in the pain category was 306 ± 3.1 versus 307 ± 7.5 kDA for those animals in the no-pain category. The average tissue displacement for animals that received SCI and were subsequently classified into the pain category was 1750 ± 28.9 versus 1730 ± 20.0 μm for those in the no-pain category. These data indicated that there were not significant differences in the injury induction parameters between animals in the pain and no-pain categories.
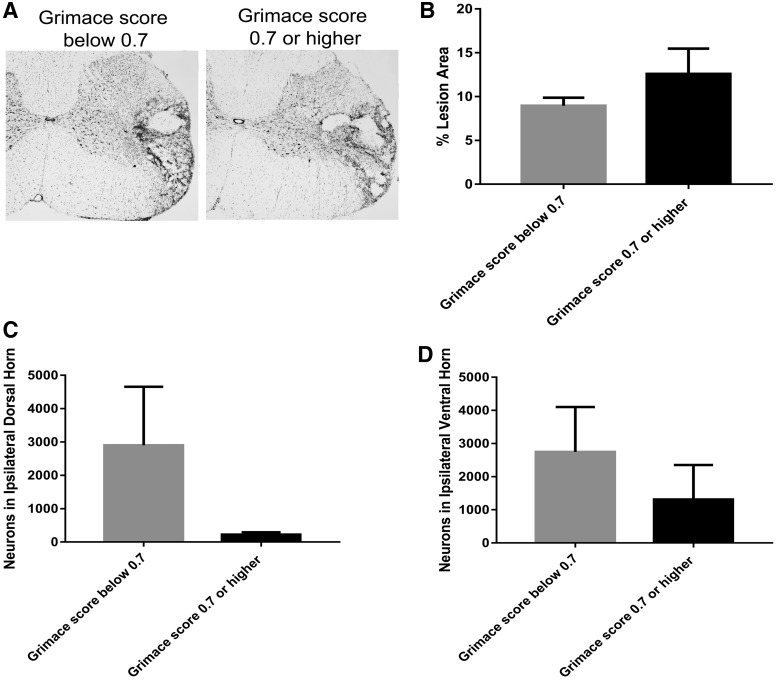
Next, we compared the lesion area in animals that received an SCI and were classified in the pain versus no-pain categories. When we evaluated the single-tissue section at the lesion epicenter (defined as the section with the greatest lesion area), we found an average lesion area of 19.1 ± 6.3% for animals in the pain category and an average lesion area of 13.1 + 1.5% for animals in the no-pain category. Thus, we did not observe a significant difference between these injury groups (p = 0.52). We also evaluated the average percentage lesion area across the entire injury area (approximately 2940 μm3 in eight serial sections); as seen in Figure 8A,B, we found no significant differences (p = 0.41) in percent lesion area between pain and no-pain categories. Taken together, these data suggest that lesion area was similar for animals in both the pain and no-pain categories.
FIG. 8.
Representative micrographs from lesion epicenter from animals in pain and no pain groups. (A) Representative micrographs of cresyl violet histochemistry of the ipsilateral spinal cord are shown at 4 × magnification (scale bar = 200 μm for all images) from an animal that received SCI and was below (left) or above (right) the analgesic intervention score. (B) Average percentage lesion area in animals that received a spinal cord injury (SCI) and exhibited a Rat Grimace Scale (RGS) score below and above the analgesic intervention score. (C) Neuron counts from the ipsilateral dorsal horn in animals that received an SCI and exhibited an RGS score below and above the analgesic intervention score. (D) Neuron counts from the ipsilateral ventral horn in animals that received an SCI and exhibited an RGS score below and above the analgesic intervention score.
Next, we evaluated the number of surviving neurons in the dorsal horn and ventral horn using unbiased stereology in SCI animals as divided into pain and no-pain groups. We observed a nonsignificant trend (p = 0.13) toward reduction of dorsal horn neurons in the pain group as compared to the no-pain group (Fig. 8B). Similarly, we observed a nonsignificant trend (p = 0.46) toward reduction in the number of neurons in the ventral horn in the pain group as compared to the no-pain group. These data suggest, although preliminary, that there may be a trend toward differences in spinal cord pathology between pain categories.
Discussion
We found that the RGS can be used to determine the presence of spontaneous NP sensation in a rat cervical hemicontusion model of SCI. In addition, we found that the RGS can also detect evoked pain from cold stimuli. We did not observe a heightened pain response, as indicated by RGS score from an evoked stimulus, in the cases when the animal exhibited spontaneous pain before the stimulus, which suggests that the supraspinal sensation of spontaneous and evoked pain are not additive with regard to intensity. Importantly, these findings are in a cervical model of SCI, which models the most common in injury classification in human SCI population. We also found a strong correspondence between a high grimace score (supraspinally mediated) and a paw withdrawal (spinal reflex mediated) response to a cold stimulus, suggesting that the presence of both high grimace score and paw withdrawal is likely a robust indicator of pain.
Spontaneous pain after spinal cord injury
Persons with SCI indicate that spontaneous pain is more severe and more disruptive of everyday life than evoked pain3,17; yet, the experimental emphasis in pre-clinical models often does not include evaluation of spontaneous pain.18 This is, in part, attributed to the difficulty of evaluating spontaneous pain in animal models. The RGS has been developed to measure spontaneous supraspinal pain sensation36 in animal models of NP. This technique is based on facial coding in nonverbal or infant humans29,30 and has been applied in rodents,31,36 rabbits,33,34 and horses.35 Here, we demonstrate that rats with a C5-hemicontusion SCI exhibit elevated spontaneous grimace scores as compared to LAM controls. Our finding is similar to that of Wu and colleagues,43 who recently demonstrated that SCI induces elevated grimace scores in mice, which can be attenuated by a pharmacological manipulation that reduces NP. Thus, these studies indicate that in mice and now in rats, grimace scores indicate spontaneous NP post-SCI.
Analgesic intervention score to delineate pain/no-pain groups
Recently, Oliver and colleagues identified a delineation point within the RGS, which can be used to categorize animals into those with spontaneous pain versus no pain, termed the analgesic intervention score.37 They determined that the analgesic intervention score was 0.67 by assessing the RGS in conjunction with expert pain/no-pain classification to perioperative pain. Further, Oliver and colleagues applied this analgesic intervention score analysis to data published by Sotocinal and colleagues36 and determined that the analgesic intervention score correctly identified time points associated with peaks in the RGS after Freund's adjuvant, intraarticular kaolin/carrageenan, and laparotomy.36,37 In addition, the analgesic intervention score correctly identified the morphine-treated animals versus the controls in Freund's adjuvant–treated rats. Intriguingly in the only study to date to use grimace to evaluate spontaneous pain post-SCI, mice in the SCI group receiving the vehicle also exhibited grimace scores above 0.7 whereas the sham groups did not.43 Based on these findings, we used the RGS score of 0.7 as the analgesic intervention score to categorize rats that exhibit spontaneous pain from those with no spontaneous pain. We found that animals that received only the laminectomy procedure did not exhibit an RGS above the analgesic intervention score when we measured spontaneous pain. Additionally, we found that the average RGS for the SCI group was above the analgesic intervention score, and when each animal was considered individually, we observed that 50% of the SCI animals (as compared to 0% of the LAM animals) exhibited RGS scores for spontaneous pain above the analgesic intervention score. Taken together, our data provide further support that the analgesic intervention score accurately delineates animals into pain and no-pain phenotype groups, which suggest that spontaneous pain, as indicated by the RGS, could be used as a criterion to subdivide animals in models of SCI-induced NP, particularly those with heterogeneity.
It is important to consider that not all animals from this model develop NP pain according to our data. Our data are similar to that reported by Detloff and colleagues, who found that 40% of rats injured by hemicontusion at the C5 level exhibited hypersensitivity to evoked stimuli.49 Additionally, heterogeneity has also been observed in other models of SCI, including the thoracic spinal stenosis model in which 59% of animals (13 of 22) develop hyperalgesia post-SCI.48,50 With regard to analytical simplicity, it is advantageous for all animals in a study to develop pain. Yet, heterogeneity can also be used to address clinically relevant questions related to mechanisms of SCI-NP, especially when a reliable method of segregation of groups such as the RGS is utilized. This lays the groundwork for further studies in which comparison between pain and no-pain animals for determination of mechanisms of pain formation is a key component.
Evoked supraspinal pain after spinal cord injury
We found that acetone application results in a grimace score above the analgesic intervention score in 90% animals with SCI, which indicates that this contusion SCI model induces evoked SCI-induced NP. Several methods are utilized to evaluate supraspinal pain, including operant escape tasks,50 place escape/avoidance,26 and the escape response test.27 However, there are some limitations to these methodologies for evaluating supraspinal response in terms of SCI studies. For example, tasks such as operant escape and place escape avoidance require pre-training of the animal and are dependent on motor function, which is often compromised in SCI animals. Additionally, the escape response task requires extensive animal handling in performing the task, potentially increasing fear or stress and thereby exacerbating the affective component of pain.51,52 Given that the rodent grimace involves noninvasive and observational recording, we posit that this test is highly useful for evaluation of supraspinally mediated behaviors related to SCI-induced NP. Importantly, any evaluation method should be carefully selected to consider the SCI model, experimental design, and research question.
Evoked supraspinal pain and correspondence to paw withdrawal
One important contribution of this study is the evaluation of supraspinal-evoked pain response and its correspondence to reflexive paw withdrawal response in a cervical hemicontusion SCI model. We found that post-SCI, the average RGS score associated with the evoked stimulus was above the analgesic intervention score in the trials wherein a paw withdrawal occurred. Moreover, in the instances in which there was not a paw withdrawal, the average RGS score was below the analgesic intervention score. When considering the individual responses, all of the rats that exhibited a paw withdrawal also had an associated RGS score above the analgesic intervention score. Conversely, in the 2 rats that did not exhibit paw withdrawal, the associated RGS score was below the analgesic intervention score. Taken together, our data suggest that there is a correspondence between paw withdrawal and a painful RGS in the cervical hemicontusion model. Given that paw withdrawal is a spinally mediated reflex and grimace is a supraspinally mediated response, our data suggest that evaluation of both RGS and paw withdrawal afford a robust indicator of evoked pain response, which includes both spinal and supraspinal responses. However, the mechanisms that underlie these responses were not addressed in the current study.
Classically, research has utilized withdrawal responses as an indicator of pain. Recent studies have questioned the exclusive reliance on paw withdrawal,26,27,50 as well as question the sufficiency of reflexive responses to measure the presence of pain. The use of paw withdrawal in the study of SCI-induced NP is even further complicated by hyper-reflexia and spastic syndrome post-SCI.19,53 Importantly, Baastrup and colleagues26 and van Gorp and colleagues27 reported divergence between withdrawal responses and supraspinally mediated behaviors, which differs from our findings wherein we see a correspondence. There are many methodological differences between our study and the previous work. The foremost of these is the consideration of the type of supraspinal response used for comparison. For example, the Baastrap and von Gorp studies evaluated escape/attack responses, including jumping, licking, guarding, vocalizing, struggling, and biting, but did not assess grimace. It is possible that the grimace response is an intermediary response that precedes the more-pronounced escape-/attack-based responses in terms of pain intensity. This idea is supported by assessment of pain severity in veterinary medicine, wherein escape/attack behaviors and vocalization are generally accepted as indicators of more-extreme pain.54 Additional studies are needed to evaluate this premise. A second methodological consideration that varies between our study and the previous work is the probability that different models and magnitudes of SCI may yield different pathobiological underpinnings and, consequently, differing pain phenotypes. Although we do not know the mechanism, this study highlights the difference in pain evaluation outcomes between cervical and thoracic SCI models.
Our finding of correspondence between supraspinal and reflexive responses is important because it has been noted that below-level pain post-SCI in humans is highly likely to have a cerebral component, 26,55 as well as a reflexive element. Thus, it is clinically relevant to evaluate both supraspinal measures and reflexive withdrawal responses in pre-clinical models. In fact, in a recent review by Vierck and Yezierski, it was noted that in order to determine whether pain is involved in the spinal reflexive response from a given stimulus, it is necessary to assess those responses to supraspinal pain measures.48
Lesion analysis
We performed standard assessments of lesion area and dorsal and ventral horn neuronal counts to evaluate whether there are differences in injury pathology post-SCI that may contribute to pain in SCI rats. Our assessments found no significant differences in lesion size between animals in no-pain versus pain categories, as based on the analgesic intervention score. Interestingly, the presence of pain appears to be independent of lesion size, suggesting that other pathological changes may be contributing to pain. In addition, we found no significant differences between dorsal and ventral horn neuron counts between animals with no pain compared to those with pain, although nonsignificant trends were observed. These studies are limited by a small sample size and cursory pathological evaluation; thus, future studies will more directly and completely address this important question.
Study caveats
Although our study highlights the need to evaluate both supraspinal and reflexive withdrawal measures in assessment of SCI-induced NP, we recognize a number of experimental caveats. The first is that we evaluated RGS and withdrawal responses at only one time point post-SCI. Our previous work with the C5-hemicontusion model indicates that by week 5 post-SCI, the animals have reached a plateau in functional recovery.45 Additionally, other studies indicate that by week 5 post-SCI, SCI-induced NP is present in rodents.26,27 However, our study is limited, in that only one time point was assessed. Future work should evaluate the RGS and paw withdrawal across a more-extensive time course post-SCI. This limitation is especially important when considering the use of the RGS in evaluating therapeutic strategies, and further experiments are required in this cervical model to determine the therapeutic window for pain development to optimize analgesic effects of pharmacotherapy.
Another caveat of this study is we did not perform sensory threshold testing. Our preliminary data indicated that this model is most sensitive to acetone using reflexive withdrawal response. The acetone test does not afford threshold testing; however, we recognize the need to follow up our current findings using experimental paradigms that evaluate increasing levels of cold stimulus to determine at what stimulus threshold pain becomes apparent using the RGS. Future studies will also extend threshold testing to mechanical (Von Frey) and thermal (heat) stimuli to model human pain responses.
Another important consideration is that affective changes, such as fear and anxiety, could contribute to the behavioral changes observed in the RGS. Our experimental design sought to minimize these possibilities through rigorous acclimation before each set of experiments; however, this parameter was not formally evaluated. Other possible contributing factors to the RGS may also be numbness, paresthesia, or dysesthesias, but our experiments did not account for these possible effects. Future experiments are required to understand whether there is a specific effect of these altered biological states on behavior and the RGS post-SCI.
An interesting, yet unexpected, result from our study is that 2 of 5 of the animals in the uninjured LAM group exhibited a grimace score above the analgesic intervention score, suggesting that some animals in the LAM group experienced evoked NP. Although the LAM group is typically used in pre-clinical SCI studies as a procedural control, this is an invasive procedure. It is recognized that surgery can induce chronic neuropathic pain,38 and in the clinical arena, complications and post-operative pain post-laminectomy are a frequent concern.56,57 Indeed, laminectomy is often used in rats to model failed back syndrome,58 a condition associated with NP. Alternatively, this trend toward a heightened response post-laminectomy may be attributed to introduction of a new stimulus, and future studies should include a control substance, such as water, to evaluate the effect of a liquid stimulus alone.
Conclusion
NP is a serious consequence of SCI with limited currently available treatment options.59 Improved modeling of SCI-induced NP is likely to improve understanding of the pathobiology and therapeutic targets. Importantly, given that most patients with SCI have multiple concomitant pain types, including both spontaneous and evoked pain, a pre-clinical model that measures multiple pain types is valuable. The results from the current study emphasize the need to assess both supraspinal and reflexive responses in pre-clinical models of SCI-induced NP, given that we found a correspondence between the RGS and paw withdrawal responses. Moreover, this study illustrates the utility of the RGS in studies designed to assess heterogeneity of SCI-induced NP, as related to both pathobiology and development of therapeutic interventions.
Acknowledgments
This research was supported by Department of Defense Congressionally Directed Medical Research Program grant W8IXWH-13-1-0482 (to C.L.F) and the University of Alabama at Birmingham Center for Exercise Medicine Interdisciplinary Training in Pathobiology and Rehabilitation Medicine grant 1T32HD071866 sponsored by the National Institutes of Health (to K.Y.H). The authors also acknowledge the work of John Ness, Sarah Owens, and Indya Woods in the filming and still photo capture. They additionally thank Robert Sorge, PhD, and Tammie Quinn for technical assistance with the RGS.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Spinal Cord Injury Statistical Center (NSCISC). (2013, March). Spinal Cord Injury Facts and Figures at a Glance. University of Alabama at Birmingham (ed). National Spinal Cord Injury Statistical Center: Birmingham, AL [Google Scholar]
- 2.Siddall P.J., and Loeser J.D. (2001). Pain following spinal cord injury. Spinal Cord 39, 63–73 [DOI] [PubMed] [Google Scholar]
- 3.Siddall P.J., McClelland J.M., Rutkowski S.B., and Cousins M.J. (2003). A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257 [DOI] [PubMed] [Google Scholar]
- 4.Jensen M.P., Chodroff M.J., and Dworkin R.H. (2007). The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 68, 1178–1182 [DOI] [PubMed] [Google Scholar]
- 5.Hammell K.R. (2010). Spinal cord injury rehabilitation research: patient priorities, current deficiencies and potential directions. Disabil. Rehabil. 32, 1209–1218 [DOI] [PubMed] [Google Scholar]
- 6.Campbell J.N., and Meyer R.A. (2006). Mechanisms of neuropathic pain. Neuron 52, 77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulsebosch C.E., Hains B.C., Crown E.D., and Carlton SM. (2009). Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 60, 202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunge R.P., Puckett W.R., Becerra J.L., Marcillo A., and Quencer R.M. (1993). Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–89 [PubMed] [Google Scholar]
- 9.Dunham K.A., Siriphorn A., Chompoopong S., and Floyd C.L. (2010). Characterization of a graded cervical hemicontusion spinal cord injury model in adult male rats. J. Neurotrauma 27, 2091–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsey A.E., LoVerso R.L., Tovar C.A., Hill C.E., Beattie M.S., and Bresnahan J.C. (2000). An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil. Neural Repair 14, 287–300 [DOI] [PubMed] [Google Scholar]
- 11.Carlton S.M., Du J., Tan H.Y., Nesic O., Hargett G.L., Bopp A.C., Yamani A., Lin Q., Willis W.D., and Hulsebosch C.E. (2009). Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwak Y.S., Hassler S.E., and Hulsebosch C.E. (2013). Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain 154, 1699–1708 [DOI] [PubMed] [Google Scholar]
- 13.Nees T.A., Tappe-Theodor A., Sliwinski C., Motsch M., Rupp R., Kuner R., Weidner N., and Blesch A. (2016). Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 157, 687–697 [DOI] [PubMed] [Google Scholar]
- 14.Yoon Y.W., Dong H., Arends J.J., and Jacquin M.F. (2004). Mechanical and cold allodynia in a rat spinal cord contusion model. Somatosens. Mot. Res. 21, 25–31 [DOI] [PubMed] [Google Scholar]
- 15.Jung J.I., Kim J., Hong S.K., and Yoon Y.W. (2008). Long-term follow-up of cutaneous hypersensitivity in rats with a spinal cord contusion. Korean J. Physiol. Pharmacol. 12, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putatunda R., Hala T.J., Chin J., and Lepore A.C. (2014). Chronic at-level thermal hyperalgesia following rat cervical contusion spinal cord injury is accompanied by neuronal and astrocyte activation and loss of the astrocyte glutamate transporter, GLT1, in superficial dorsal horn. Brain Res. 1581, 64–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finnerup N.B., Norrbrink C., Trok K., Piehl F., Johannesen I.L., Sorensen J.C., Jensen T.S., and Werhagen L. (2014). Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J. Pain 15, 40–48 [DOI] [PubMed] [Google Scholar]
- 18.Mogil J.S., and Crager S.E. (2004). What should we be measuring in behavioral studies of chronic pain in animals? Pain 112, 12–15 [DOI] [PubMed] [Google Scholar]
- 19.Vierck C.J., Hansson P.T., and Yezierski R.P. (2008). Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain 135, 7–10 [DOI] [PubMed] [Google Scholar]
- 20.Liao L., Long H., Zhang L., Chen H., Zhou Y., Ye N., and Lai W. (2014). Evaluation of pain in rats through facial expression following experimental tooth movement. Eur. J. Oral Sci. 122, 121–124 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K., Nakaji S., Yamada M., Totsuka M., Sato K., and Sugawara K. (2002). Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 8, 6–48 [PubMed] [Google Scholar]
- 22.Abraham K.E., McGinty J.F., and Brewer K.L. (2001). The role of kainic acid/AMPA and metabotropic glutamate receptors in the regulation of opioid mRNA expression and the onset of pain-related behavior following excitotoxic spinal cord injury. Neuroscience 104, 863–874 [DOI] [PubMed] [Google Scholar]
- 23.Benz E.N., Hornby T.G., Bode R.K., Scheidt R.A., and Schmit B.D. (2005). A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch. Phys. Med. Rehabil. 86, 52–59 [DOI] [PubMed] [Google Scholar]
- 24.Bravo-Esteban E., Taylor J., Abian-Vicen J., Albu S., Simon-Martinez C., Torricelli D., and Gomez-Soriano J. (2013). Impact of specific symptoms of spasticity on voluntary lower limb muscle function, gait and daily activities during subacute and chronic spinal cord injury. NeuroRehabilitation 33, 531–543 [DOI] [PubMed] [Google Scholar]
- 25.Ganzer P.D., Meyers E.C., Sloan A.M., Maliakkal R., Ruiz A., Kilgard M.P., Robert L.R. 2nd. (2016). Awake behaving electrophysiological correlates of forelimb hyperreflexia, weakness and disrupted muscular synchronization following cervical spinal cord injury in the rat. Behav. Brain Res. 307, 100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baastrup C., Maersk-Moller C.C., Nyengaard J.R., Jensen T.S., and Finnerup N.B. (2010). Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 151, 670–679 [DOI] [PubMed] [Google Scholar]
- 27.van Gorp S., Deumens R., Leerink M., Nguyen S., Joosten E.A., and Marsala M. (2014). Translation of the rat thoracic contusion model; part 1-supraspinally versus spinally mediated pain-like responses and spasticity. Spinal Cord 52, 524–528 [DOI] [PubMed] [Google Scholar]
- 28.Hulsebosch C.E., Xu G.Y., Perez-Polo J.R., Westlund K.N., Taylor C.P., and McAdoo D.J. (2000). Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma 17, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 29.Gilbert C.A., Lilley C.M., Craig K.D., McGrath P.J., Court C.A., Bennett S.M., and Montgomery C.J. (1999). Postoperative pain expression in preschool children: validation of the child facial coding system. Clin. J. Pain 15, 192–200 [DOI] [PubMed] [Google Scholar]
- 30.Grunau R.E., Oberlander T., Holsti L., and Whitfield M.F. (1998). Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain 76, 277–286 [DOI] [PubMed] [Google Scholar]
- 31.Langford D.J., Bailey A.L., Chanda M.L., Clarke S.E., Drummond T.E., Echols S., Glick S., Ingrao J., Klassen-Ross T., Lacroix-Fralish M.L., Matsumiya L., Sorge R.E., Sotocinal S.G., Tabaka J.M., Wong D., van den Maagdenberg A.M., Ferrari M.D., Craig K.D., and Mogil J.S. (2010). Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 7, 447–449 [DOI] [PubMed] [Google Scholar]
- 32.Matsumiya L.C., Sorge R.E., Sotocinal S.G., Tabaka J.M., Wieskopf J.S., Zaloum A., King O.D., and Mogil J.S. (2012). Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 51, 42–49 [PMC free article] [PubMed] [Google Scholar]
- 33.Keating S.C., Thomas A.A., Flecknell P.A., and Leach M.C. (2012). Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS One 7, e44437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampshire V., and Robertson S. (2015). Using the facial grimace scale to evaluate rabbit wellness in post-procedural monitoring. Lab. Anim. (N.Y.) 44, 259–260 [DOI] [PubMed] [Google Scholar]
- 35.Dalla Costa E., Minero M., Lebelt D., Stucke D., Canali E., and Leach M.C. (2014). Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS One 9, e92281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotocinal S.G., Sorge R.E., Zaloum A., Tuttle A.H., Martin L.J., Wieskopf J.S., Mapplebeck J.C., Wei P., Zhan S., Zhang S., McDougall J.J., King O.D., and Mogil J.S. (2011). The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver V., De Rantere D., Ritchie R., Chisholm J., Hecker K.G., and Pang D.S. (2014). Psychometric assessment of the Rat Grimace Scale and development of an analgesic intervention score. PLoS One 9, e97882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borsook D., Kussman B.D., George E., Becerra L.R., and Burke D.W. (2013). Surgically induced neuropathic pain: understanding the perioperative process. Ann. Surg. 257, 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawano T., Eguchi S., Iwata H., Yamanaka D., Tateiwa H., Locatelli F.M., and Yokoyama M. (2016). Effects and underlying mechanisms of endotoxemia on post-incisional pain in rats. Life Sci. 148, 145–153 [DOI] [PubMed] [Google Scholar]
- 40.Asgar J., Zhang Y., Saloman J.L., Wang S., Chung M.K., and Ro J.Y. (2015). The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience 310, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawano T., Takahashi T., Iwata H., Morikawa A., Imori S., Waki S., Tamura T., Yamazaki F., Eguchi S., Kumagai N., and Yokoyama M. (2014). Effects of ketoprofen for prevention of postoperative cognitive dysfunction in aged rats. J. Anesth. 28, 932–936 [DOI] [PubMed] [Google Scholar]
- 42.Waite M.E., Tomkovich A., Quinn T.L., Schumann A.P., Dewberry L.S., Totsch S.K., and Sorge R.E. (2015). Efficacy of common analgesics for postsurgical pain in rats. J. Am. Assoc. Lab. Anim. Sci. 54, 420–425 [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J., Zhao Z., Zhu X., Renn C.L., Dorsey S.G., and Faden A.I. (2016). Cell cycle inhibition limits development and maintenance of neuropathic pain following spinal cord injury. Pain 157, 488–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi Y., Yoon Y.W., Na H.S., Kim S.H., and Chung J.M. (1994). Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59, 369–376 [DOI] [PubMed] [Google Scholar]
- 45.Siriphorn A., Dunham K.A., Chompoopong S., and Floyd C.L. (2012). Postinjury administration of 17beta-estradiol induces protection in the gray and white matter with associated functional recovery after cervical spinal cord injury in male rats. J. Comp. Neurol. 520, 2630–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kachadroka S., Hall A.M., Niedzielko T.L., Chongthammakun S., and Floyd C.L. (2010). Effect of endogenous androgens on 17beta-estradiol-mediated protection after spinal cord injury in male rats. J. Neurotrauma 27, 611–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaovipoch P., Jelks K.A., Gerhold L.M., West E.J., Chongthammakun S., and Floyd C.L. (2006). 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J. Neurotrauma 23, 830–852 [DOI] [PubMed] [Google Scholar]
- 48.Vierck C.J., and Yezierski R.P. (2015). Comparison of operant escape and reflex tests of nociceptive sensitivity. Neurosci. Biobehav. Rev. 51, 223–242 [DOI] [PubMed] [Google Scholar]
- 49.Detloff M.R., Wade R.E., Jr., and Houle J.D. (2013). Chronic at- and below-level pain after moderate unilateral cervical spinal cord contusion in rats. J. Neurotrauma 30, 884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vierck C.J., Baastrup C., Maersk-Moller C., Roth M., Cannon R.L., Finnerup N.B., and Yezierski R.P. (2015). A preclinical model of hyperalgesia following spinal stenosis/compression. Eur. J. Pain 19, 1158–1167 [DOI] [PubMed] [Google Scholar]
- 51.Mogil J.S. (2015). Social modulation of and by pain in humans and rodents. Pain 156, Suppl. 1, S35–S41 [DOI] [PubMed] [Google Scholar]
- 52.Langford D.J., Tuttle A.H., Briscoe C., Harvey-Lewis C., Baran I., Gleeson P., Fischer D.B., Buonora M., Sternberg W.F., and Mogil J.S. (2011). Varying perceived social threat modulates pain behavior in male mice. J. Pain 12, 125–132 [DOI] [PubMed] [Google Scholar]
- 53.Shahani B.T., and Young R.R. (1971). Human flexor reflexes. J. Neurol. Neurosurg. Psychiatry 34, 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morton D.B., and Griffiths P.H. (1985). Guidelines on the recognition of pain, distress and discomfort in exerimental animals and an hypothesis for assesment. Vet. Rec. 116, 431–436 [DOI] [PubMed] [Google Scholar]
- 55.Yezierski R.P., and Vierck C.J. (2010). Reflex and pain behaviors are not equivalent: lessons from spinal cord injury. Pain 151, 569–570 [DOI] [PubMed] [Google Scholar]
- 56.Vasigh A., Najafi F., Khajavikhan J., Jaafarpour M., and Khani A. (2016). Comparing gabapentin and celecoxib in pain management and complications after laminectomy: a randomized double-blind clinical trial. Iran. Red Crescent Med. J. 18, e34559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasigh A., Jaafarpour M., Khajavikhan J., and Khani A. (2016). The effect of gabapentin plus celecoxib on pain and associated complications after laminectomy. J. Clin. Diagn. Res. 10, Uc04–Uc08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C.Y., Huang Y.H., Lee J.S., Tai T.W., Wu P.T., and Jou I.M. (2016). Efficacy of topical cross-linked hyaluronic acid hydrogel in preventing post laminectomy/laminotomy fibrosis in a rat model. J. Orthop. Res. 34, 299–306 [DOI] [PubMed] [Google Scholar]
- 59.Watson J.C., and Sandroni P. (2016). Central neuropathic pain syndromes. Mayo Clin. Proc. 91, 372–385 [DOI] [PubMed] [Google Scholar]