Abstract
Aim:
We measured long-term hematopoiesis in continuous bone marrow cultures derived from Toll-like receptor-4 (Tlr4−/−)(C57BL/6J) mice.
Materials and Methods:
We measured hematopoiesis in vitro over 27 weeks in long-term bone marrow cultures from Tlr4−/− and control mice, and irradiation-induced pulmonary fibrosis in mice irradiated to 20 Gy to the thorax.
Results:
There was a significant increase in the duration of hematopoiesis in long-term bone marrow cultures from Tlr4−/− mice in production of total non-adherent cells and day 7 and day 14 multi-lineage colony-forming cells. The histology of bone marrow hematopoietic and stromal cell lines was indistinguishable between different mouse strains. There was no detectable late irradiation pulmonary fibrosis in Tlr4−/− mice.
Conclusion:
Homozygous deletion of both alleles of Tlr4, encoding for an inflammatory mediator receptor, improves the duration of hematopoiesis in vitro and reduces irradiation-induced lung fibrosis.
Keywords: Hematopoiesis, long-term bone marrow cultures, TLR4
Toll-like receptors (TLRs) have been demonstrated to be critically important in the recruitment of inflammatory cells including T-lymphocytes, B-lymphocytes, monocytes, and polymorphonuclear leukocytes. TLRs are involved in both the acute and chronic inflammatory response in a variety of diseases including infections, common autoimmune diseases, and the response of organs and organ systems to inflammatory stimuli. Recent studies have demonstrated that TLRs are a component of the late reaction of the C57BL/6J mouse lung to thoracic irradiation and an increase in Tlr4 has been associated with migration into the lungs of bone marrow-derived progenitors of the fibroblasts representing radiation pulmonary fibrosis (1). In contrast, Tlr1–3, and Tlr 6–9 were not up-regulated in C57BL/6J mouse lung at the time of initiation of pulmonary fibrosis, suggesting that Tlr4 might be a unique member of the TLR family involved in the late inflammatory response.
An organ culture system for bone marrow, long-term bone marrow cultures (LTBMCs), has been utilized to analyze the effect of homologous recombinant deletion of components of the transforming growth factor-beta (TGFβ) signaling pathway also associated with pulmonary fibrosis. Prior studies with LTBMCs from SMAD family member 3 (Smad3−/−) mouse demonstrated improvement in longevity by elimination of the TGFβ response system in vitro. Bone marrow stromal cell lines derived from adherent layers of LTBMCs from Smad3−/− mice (2) demonstrated decreased capacity for migration into the lung of irradiated mice (3). In the present studies, we sought to determine whether marrow derived from Tlr4−/− mice demonstrated altered duration of hematopoiesis and whether the hypothesized increase in duration of hematopoiesis might be associated with stable adherence to the stromal cell layer of the progenitors of the hematopoietic stem cells, and decreased attachment of negative regulatory cells in long-term bone marrow cultures.
Materials and Methods
LTBMCs.
C57BL/6/NHsd and Tlr4−/− mice were obtained from the Jackson Laboratories (B6.B10ScN-Tlr4lps-del/JthJ, stock number 007227; Jackson Laboratories, Bar Harbor, ME, USA). LTBMCs were established from the femur and tibia marrow of mice as described elsewhere (4–8). The contents of a femur and tibia (N=6/genotype) were flushed into McCoy’s 5A medium (Gibco, Gaithersburg, MD, USA) supplemented with 25% horse serum (Cambrex, Rockland, ME, USA), and 10–5 M hydrocortisone sodium hemisuccinate. Cultures were incubated at 33°C in 7% CO2. After four weeks, the horse serum was replaced with 25% fetal bovine serum (FBS) (Thermo Fisher Scientific, Pittsburgh, PA, USA) (6). Cultures were observed weekly for confluence of the adherent layer of the flasks, hematopoietic cell production, and cobblestone island formation. Cobblestone islands of 50 or more cells were scored weekly in each flask (6, 7). A two-sided two-sample t-test was used to compare the number of cobblestone islands between Tlr4−/− and Tlr4+/+ cultures each week. p-Values less than 0.05 were regarded as significant.
Hematopoietic cell colony-forming assays.
A total of 1×105 non-adherent cells from the LTBMCs were removed from the flasks and 5×104 cells/dish were plated in triplicate in semi-solid medium consisting of methylcellulose in Iscove’s modified Dulbecco’s medium (IMDM), FBS, 10% bovine serum albumin, L-glutamine, 3 U/ml erythropoietin, and 2-mercaptoethanol (Stem Cell Technology, Vancouver, BC, Canada) with WEHI-3 cell line-conditioned medium (6) added as a source of interleukin-3. Colony-forming unit granulocyte-macrophage (CFU-GM) of 50 cells or greater were counted on days 7 and 14 after plating. A two-sided two-sample t-test was used as described above. Fresh marrow colonies were subdivided for GFU-GM, burst forming unit erythroid (BFUe) and granulocyte-erythroid-megakaryocyte-macrophage (CFU-GEMM). Data for the weekly cobblestone island numbers, non-adherent cell numbers, percentage of confluence of adherent cells, day 7 colony counts, and day 14 colony counts were collected at week 1 through week 27 (2).
Evaluation of irradiation-induced pulmonary fibrosis in Tlr4−/− mouse lungs.
Lungs from Tlr4−/− C57BL/6J mice irradiated to 20 Gy to the thorax (1, 9, 10) and control C57BL/6J mice were removed at day 200 after irradiation, frozen in Optimum Cutting Temperature (OCT) (Fisher Scientific), sectioned, and stained with hematoxylin and eosin (H & E) for overall histology and Masson’s trichrome for collagen deposition. The histology of 500 high-power fields was reviewed for groups of five lobes of each of five mice per group. Collagen deposition was scored in lungs from thoracic-irradiated and control non-irradiated Tlr4−/− and Tlr4+/+ (C57BL/6J) mice were quantitated by Masson’s trichrome stain as published elsewhere (1, 9, 10).
Statistical methods.
For LTBMC data, weekly cobblestone island numbers, non-adherent cell numbers (×105), percentage confluence of adherent cells, day 7 colony-forming cells, and day 14 colony-forming cells were counted as described elsewhere (8). Data are summarized as the mean±standard deviation, n is the number of mice used, and p-values were calculated with the two-sided two-sample t-test with p-values of <0.05 considered significant. As this was an exploratory study, p-values were not adjusted for multiple comparisons. Similar calculations and tests were performed for the non-adherent cell numbers and percentage of confluence of adherent cells. Day 7 colony count data were compared between Tlr4−/− and C57BL/6 at each week with the two-sided two sample t-test. Similar to day 7 data, day 14 data were analyzed as published elsewhere (11).
Results
Longevity of hematopoiesis in continuous bone marrow cultures is increased with Tlr4−/− marrow.
We quantitated parameters of longevity of hematopoiesis in both the adherent layer of LTBMCs and in the production of non-adherent cells and their capacity for colony formation over 27 weeks. Figure 1A demonstrates confluence of the adherent layer in marrow cultures. Figure 1B demonstrates cobblestone island formation in long-term cultures. Figure 1C demonstrates cumulative cobblestone islands. Figure 1D shows weekly cell production, and Figure 1E shows cumulative total cell production. The longevity of production of hematopoietic cells, which form weekly day-7 colony-forming unit progenitors, and cumulative production are shown in Figure 1F and G, respectively. Weekly continuous production of day-14 CFU-GEMM, which are more primitive multi-lineage hematopoietic progenitor cell colonies is shown in Figure 1H and cumulative production in Figure 1I. Statistical analysis of day-7 CFU progenitors and day-14 CFU-GEMM are shown in Tables I and II. Tlr4−/− mouse marrow generated significantly more hematopoietic cells for a longer duration in LTBMCs.
Figure 1.
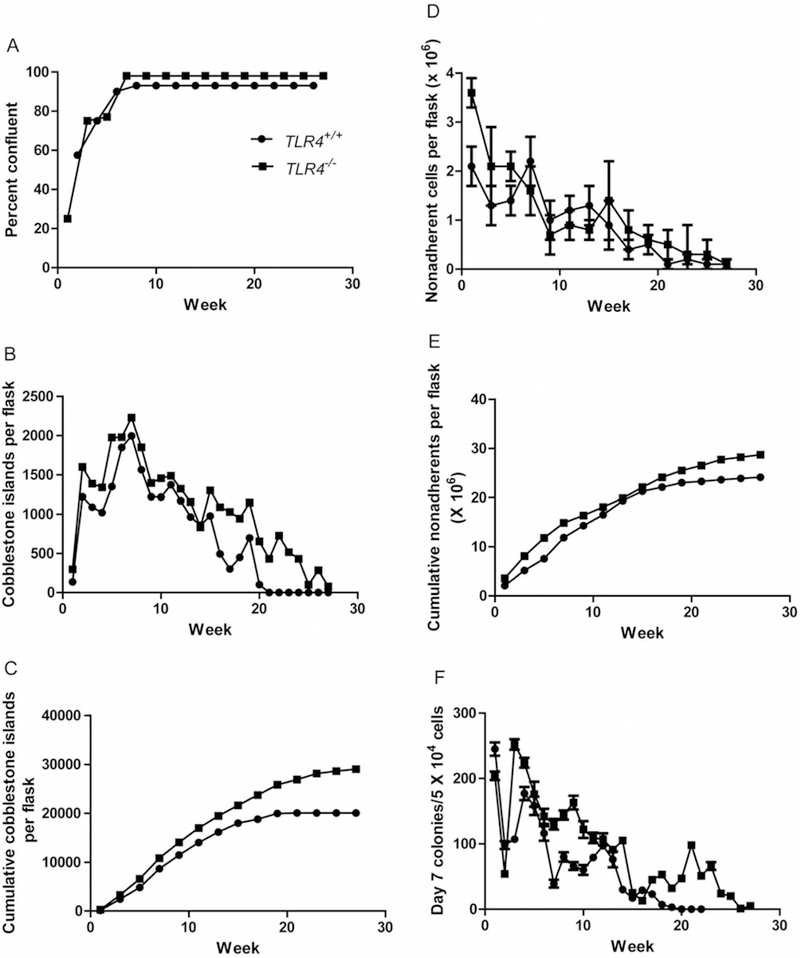
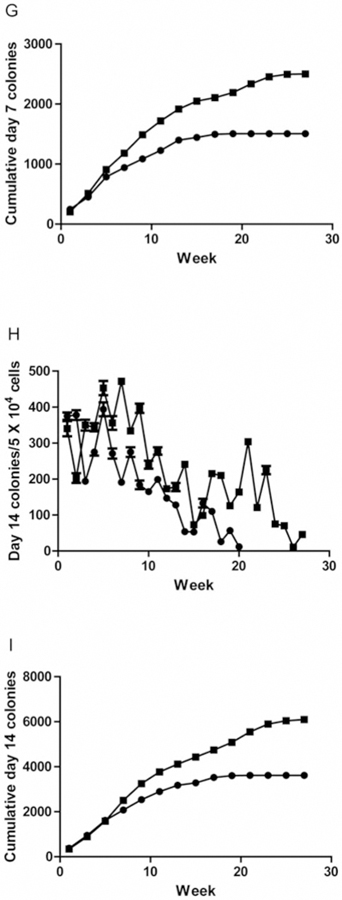
Hematopoiesis in long-term bone marrow cultures from Toll-like Receptor 4 (TLR4−/−) compared to TLR4+/+ mice. A) Confluence of adherent layer; B) Cobblestone islands weekly; C) Cumulative cobblestone islands; D) Weekly production of non-adherent cells; E) Cumulative production of non-adherent cells, F) Weekly CFU-GEMM scored at day 7; G) Cumulative day 7 CFU-GEMM; H) Weekly Day 14 CFU-GEMM; i) Cumulative day 14 CFU-GEMM.
Table I.
Analysis of hematopoiesis in long term bone marrow cultures (LTBMCs) from toll-like receptor 4−/− (TLR4−/− mice): Weekly production of “day 7” colony forming unit granulocycte, erythrocyte, macrophage, megakaryocyte CFU-GEMM.
| Group | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|
| TLR4−/− | 204.3±6.5 (n=3) | 53.7±5.0 (n=3) | 251.7±8.0 (n=3) | 223.7±7.5 (n=3) | 176.0±19.1 (n=3) |
| C57BL/6 | 245.3±10.1 (n=3) | 98.0±6.0 (n=3) | 107.3±5.5 (n=3) | 176.7±10.1 (n=3) | 157.7±14.0 (n=3) |
| p-Value | 0.0041 | 0.0006 | <0.0001 | 0.0029 | 0.2512 |
| Group | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 |
| TLR4−/− | 142.0±11.5 (n=3) | 129.7±8.1 (n=3) | 144.3±7.0 (n=3) | 163.0±10.5 (n=3) | 122.0±12.8 (n=3) |
| C57BL/6 | 116.0±11.1 (n=3) | 39.0±6.0 (n=3) | 79.7±7.1 (n=3) | 66.0±6.0 (n=3) | 59.7±7.5 (n=3) |
| p-Value | 0.0484 | 0.0001 | 0.0004 | 0.0002 | 0.0019 |
| Group | Week 11 | Week 12 | Week 13 | Week14 | Week 15 |
| TLR4−/− | 109.0±8.0 (n=3) | 107.3±9.0 (n=3) | 90.7±3.5 (n=3) | 105.3±4.2 (n=3) | 25.3±3.5 (n=3) |
| C57BL/6 | 79.3±4.5 (n=3) | 96.7±5.0 (n=3) | 76.3±12.0 (n=3) | 29.7±4.0 (n=3) | 17.0±3.0 (n=3) |
| p-Value | 0.0050 | 0.1481 | 0.1183 | <0.0001 | 0.0354 |
| Group | Week 16 | Week 17 | Week 18 | Week 19 | Week 20 |
| TLR4−/− | 12.7±2.5 (n=3) | 45.0±5.0 (n=3) | 53.0±5.0 (n=3) | 32.0±4.6 (n=3) | 46.7±5.5 (n=3) |
| C57BL/6 | 29.3±4.2 (n=3) | 23.0±5.0 (n=3) | 6.7±3.1 (n=3) | 2.7±2.5 (n=3) | 0.0±0.0 (n=3) |
| p-Value | 0.0040 | 0.0057 | 0.0002 | 0.0006 | 0.0046 |
| Group | Week 21 | Week 22 | Week 23 | Week 24 | Week 25 |
| TLR4−/− | 98.0±5.6 (n=3) | 51.3±3.5 (n=3) | 66.0±6.0 (n=3) | 23.7±4.0 (n=3) | 19.7±3.5 (n=3) |
| C57BL/6 | |||||
| p-Value | |||||
| Group | Week 26 | Week 27 | Week 28 | Week 29 | Week 30 |
| TLR4−/− | 0.7±0.6 (n=3) | 5.0±1.0 (n=3) | |||
| C57BL/6 | |||||
| p-Value | |||||
The “day 7” colony counts at each week, where data are summarized as mean±standard deviation, n is the sample size, and p is the p-Value for comparison to C57BL/6 group with the two-sided two sample t-test. Significant p-Values of less than 0.05 are shown in bold.
Table II.
Analysis of hematopoiesis in long-term bone marrow cultures (LTBMCs) from toll-like receptor 4−/− (TLR4−/−) mice: Weekly production of “day 14” colony forming unit granulocycte, erythrocyte, macrophage, megakaryocyte CFU-GEMM.
| Group | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|
| TLR4−/− | 340.0±21.0 (n=3) | 202.7±13.5 (n=3) | 349.7±15.2 (n=3) | 344.0±11.5 (n=3) | 453.0±19.7 (n=3) |
| C57BL/6 | 374.7±10.1 (n=3) | 377.7±13.5 (n=3) | 194.3±8.0 (n=3) | 274.7±9.7 (n=3) | 394.0±19.1 (n=3) |
| p-Value | 0.0614 | 0.0001 | 0.0001 | 0.0013 | 0.0203 |
| Group | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 |
| TLR4−/− | 356.3±19.1 (n=3) | 472.0±9.0 (n=3) | 334.0±7.0 (n=3) | 397.3±12.9 (n=3) | 240.0±11.0 (n=3) |
| C57BL/6 | 271.0±14.1 (n=3) | 190.7±7.5 (n=3) | 274.7±13.5 (n=3) | 184.0±12.0 (n=3) | 164.7±6.0 (n=3) |
| p-Value | 0.0034 | <0.0001 | 0.0025 | <0.0001 | 0.0005 |
| Group | Week 11 | Week 12 | Week 13 | Week14 | Week 15 |
| TLR4−/− | 278.0±10.5 (n=3) | 173.3±8.0 (n=3) | 177.7±11.5 (n=3) | 240.7±8.5 (n=3) | 73.3±4.5 (n=3) |
| C57BL/6 | 198.7±9.5 (n=3) | 146.7±8.0 (n=3) | 128.3±8.1 (n=3) | 54.0±5.6 (n=3) | 52.7±6.0 (n=3) |
| p-Value | 0.0006 | 0.0152 | 0.0037 | <0.0001 | 0.0089 |
| Group | Week 16 | Week 17 | Week 18 | Week 19 | Week 20 |
| TLR4−/− | 98.7±7.0 (n=3) | 215.0±9.0 (n=3) | 210.0±4.0 (n=3) | 125.7±6.5 (n=3) | 164.0±7.0 (n=3) |
| C57BL/6 | 133.3±12.0 (n=3) | 109.7±4.0 (n=3) | 25.7±5.5 (n=3) | 57.0±7.5 (n=3) | 11.7±2.1 (n=3) |
| p-Value | 0.0125 | 0.0001 | <0.0001 | 0.0003 | <0.0001 |
| Group | Week 21 | Week 22 | Week 23 | Week 24 | Week 25 |
| TLR4−/− | 303.7±7.1 (n=3) | 121.3±8.3 (n=3) | 224.7±11.5 (n=3) | 75.0±4.6 (n=3) | 70.3±6.0 (n=3) |
| C57BL/6 | |||||
| p-Value | |||||
| Group | Week 26 | Week 27 | Week 28 | Week 29 | Week 30 |
| TLR4−/− | 11.0±3.0 (n=3) | 46.0±4.6 (n=3) | |||
| C57BL/6 | |||||
| p-Value | |||||
The “day 14” colony counts at each week, where data are summarized as mean±standard deviation, n is the sample size, and p is the p-Value for comparison to C57BL/6 group with the two-sided two sample t-test. Significant p-Values of less than 0.05 are shown in bold.
Radiation-induced pulmonary fibrosis in mice irradiated to 20 Gy to the thorax.
No fibrosis was detected in lungs of unirradiated controls. In contrast, large aggregates of lymphoid infiltrates were seen in 2 out of 5 mice (Figure 2). Sheets of monophorphic cells of lymphoid origin in aggregates of unusually large size not limited to perivascular areas (Figure 2D–E). Smaller lymphoid infiltrates were consistently seen perivascularly. Increased numbers of macrophages were present in the airways diffusely throughout the lung in both irradiated C57BL/6J mice and Tlr4−/− mice. Nine macrophages were seen per ×40 power field (average). Macrophages with iron pigments were also seen. Quantitative analysis of the fibrosis is shown in Figure 3.
Figure 2.
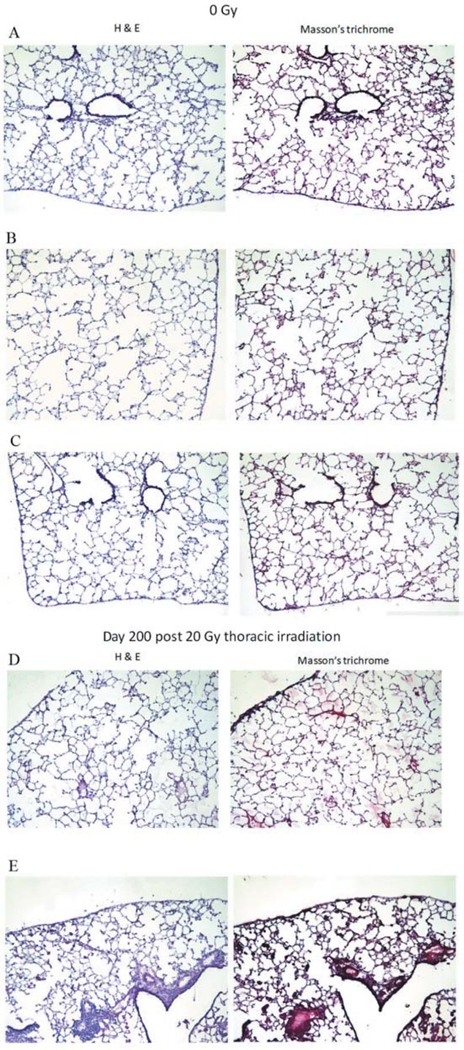
Histopathological analysis of lungs from Toll-Like Receptor 4 (TLR4−/−) mice irradiated to 20 Gy to the thorax. Histopathology (left H&E; right Masson’s trichrome) of three unirradiated TLR4−/− mouse lungs (A-C), and two 20-Gy irradiated, day-200 mouse lungs with infiltrates of inflammatory cells (D-E) (original magnification, ×20).
Figure 3.
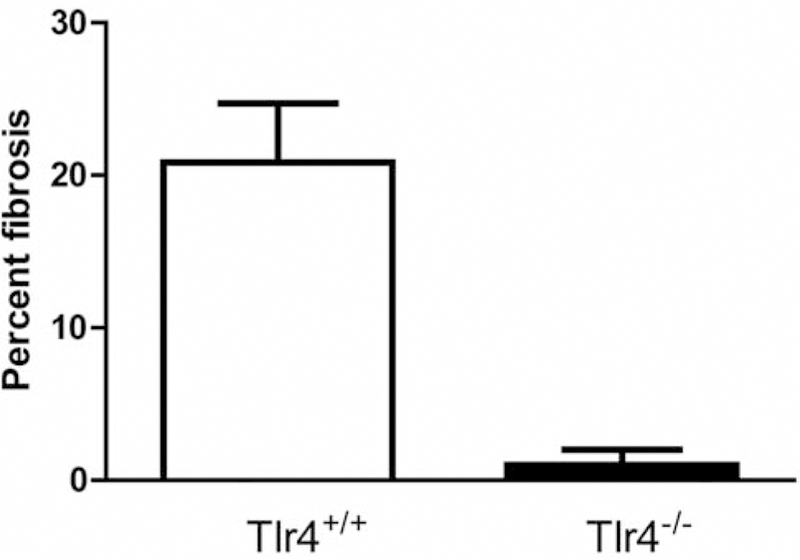
Quantitation of pulmonary fibrosis in the lungs of Toll-like Receptor 4 (TLR4−/−) mice irradiated with 20 Gy to the thorax. C57BL/6NHsd and TLR4−/− mice were irradiated to 20 Gy to the pulmonary cavity. The mice were sacrificed 200 days after irradiation. Lungs were removed, frozen in OCT, sectioned, stained with hematoxylin and eosin, and the percentage of fibrosis was determined as described in the methods. N=10 per group.
Discussion
TLRs are involved in many aspects of normal tissue responses to inflammation, infection, and oxidative stress (12, 13). TLRs are also involved in adhesion of migrating cells, both normal immunoreactive and cancer cells, to endothelial cells and other components of tissues (14–16). Recent literature has linked inflammatory response to cancer progression, both invasion and metastasis. A question has arisen as to whether TLRs initiate oxidative stress or are a response to it (14–16).
In a recent publication, TLRs were shown to be differentially expressed in mouse strains prone to radiation pulmonary fibrosis and those resistant to it (17). A bi-phasic response to irradiation of the lung has been demonstrated with C57BL/6/NHsd mice, which exhibit initial acute radiation pneumonitis or inflammatory response, followed by a latent period, during which lung histology returns to normal and pulmonary gene transcripts associated with the inflammatory response return to baseline levels (17). The initiation of late radiation fibrosis in the lungs of C75BL/6NHsd mice has been associated with a second up-regulation of RNA transcripts associated with acute radiation injury, although the mechanism of initiation of the late response remains unknown. In contrast, mice genetically resistant to radiation fibrosis, such as the C3H/HeNHsd mouse, demonstrate an acute radiation pneumonitis response, but no decrease in RNA transcripts and no latent period. Comparative analysis of RNA transcripts in the lungs of these two mouse strains have shown elevation of RNA and protein for Tlr4 in the fibrosis-prone mouse which are absent in the fibrosis-resistant mouse. Whether Tlr4 elevation represents a component of the response to oxidative stress or initiation of oxidative stress events is unknown.
A system that may elucidate stem cell and supportive cell events during continuous oxidative stress of an organ system is the LTBMC system (4–7). During incubation of whole bone marrow organ cultures in a high-humidity incubator, weekly medium change has been associated with stimulation of cycling of hematopoietic stem cells in the adherent layer and the release of differentiated and proliferating cells into the non-adherent layer. The culture system responds to stimuli of oxidative stress, including ionizing irradiation, cytotoxic agents, and some classes of infectious agents, by altering multiple parameters of hematopoiesis.
In the present studies, we tested whether absence of Tlr4 affected the multiple parameters of hematopoiesis that are easily tested in a LTBMC system. We hypothesized that up-regulation of Tlr4 in response to oxidative stress might limit certain parameters of hematopoiesis since up-regulation of Tlr4 in the lung of C57BL/6NHsd mice was associated with initiation of fibrosis and decrease in function of the mouse lung. LTBMCs were established from Tlr4−/− mice on the C57BL/6 background and the duration of hematopoiesis compared to that in wild-type mice from the same background strain. Results demonstrated that absence of Tlr4 increased multiple parameters of hematopoiesis in long-term culture. Thus, absence of Tlr4 adds yet another component to the list of genetic markers associated with increased longevity of hematopoiesis in continuous marrow culture including absence of caspase-1 (18), Tgf-β signaling pathway by removal of Smad3 (2), and absence of mitochondrial/neuronal nitric oxide synthase known to be necessary for generation of the cytotoxic peroxynitrate radical (11).
In contrast, homologous recombinant deletion of other genes hypothesized to be critically important in the oxidative stress response, including nuclear factor (erythroid-derived 2)-like 2 (Nrf2), does not significantly alter hematopoiesis in long-term culture with marrow from those mice (19).
The improved duration of hematopoiesis in Tlr4−/− mouse marrow cultures, now provides a system by which to dissect the different components of the marrow microenvironment and their involvement in the oxidative stress and inflammatory responses that can be associated with long-term culture of each component of a functioning explanted organ system in vitro.
Acknowledgements
This article was supported by the following grants: NIH R01-CA119927-11, R01 HL094488-02, and NIH/NIAID 1U19A168021-06. This project used the UPCI Animal Facility that is supported in part by award P30CA047904.
References
- 1.Kalash R, Epperly MW, Goff J, Dixon T, Sprachman MM, Zhang X, Shields D, Cao S, Franicola D, Wipf P, Berhane H, Wang H, Au J and Greenberger JS: Amelioration of irradiation pulmonary fibrosis by a water-soluble bifunctional sulfoxide radiation mitigator (MMS350). Radiat Res 180(5): 474–490, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epperly MW, Cao S, Goff J, Shields D, Zhou S, Glowacki J and Greenberger J: Increased longevity of hematopoiesis in continuous bone marrow cultures and adipocytogenesis in marrow stromal cells derived from Smad3–/– mice. Exp Hematol 33: 353–362, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Epperly MW, Franicola D, Zhang X, Nie S, Wang H, Bahnson AB, Shields DS, Goff JP, Shen H and Greenberger JS: Reduced irradiation pulmonary fibrosis and stromal cell migration in SMAD3–/– marrow chimeric mice. In Vivo 20(5): 573–582, 2006. [PubMed] [Google Scholar]
- 4.Greenberger JS: Sensitivity of corticosteroid-dependent, insulin-resistant lipogenesis in marrow preadipocytes of mutation diabetic-obese mice. Nature 275: 752–754, 1978. [DOI] [PubMed] [Google Scholar]
- 5.Mauch P, Greenberger JS, Botnick LE, Hannon EC and Hellman S: Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. Proc Natl Acad Sci USA 77: 2927–2930, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakakeeny MA and Greenberger JS: Granulopoiesis longevity in continuous bone marrow cultures and factor dependent cell line generation: Significant variation among 28 inbred mouse strains and outbred stocks. J Natl Cancer Inst 68: 305–317, 1982. [PubMed] [Google Scholar]
- 7.Neben S, Anklesaria P, Greenberger JS and Mauch P: Quantitation of murine hematopoietic stem cells in vitro by limiting dilution analysis of cobblestone area formation on a clonal stromal cell line. Exp Hematol 21: 438–444, 1993. [PubMed] [Google Scholar]
- 8.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, Zhang X, Shields D, Houghton F, Wang H, Wipf P, Parmar K and Greenberger JS: Radiobiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi anemia (Fancd2–/–) mice. Radiat Res 181: 76–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epperly MW, Travis EL, Sikora C and Greenberger JS: Magnesium superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL1, TNF-α, and TGF-β correlates with delay of organizing alveolitis/fibrosis. Biol Blood Bone Marrow Transplant 5: 204–214, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Carlos T, Guo HL and Greenberger JS: Pulmonary irradiation-induced expression of VCAM-1 and ICAM-1 is decreased by MnSOD-PL gene therapy. Biol Blood Bone Marrow Transplant 8(4): 175–187, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Epperly MW, Cao S, Zhang X, Franicola D, Shen H, Greenberger EE, Epperly LD and Greenberger JS: Increased longevity of hematopoiesis in continuous bone marrow cultures derived from NOS1 (nNOS, mtNos–/–) homozygous recombinant negative mice correlates with radioresistance of hematopoietic and bone marrow stromal cells. Exp Hematol 35: 137–145, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Paun A, Fox J, Balloy V, Chignard M, Qureshi ST and Haston CK: Combined Tlr2 and Tlr4 deficiency increases radiation-induced pulmonary fibrosis in mice. Int J Radiat Oncol Biol Phys 7(4): 1198–1205, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK, van der Poll T, Florquin S and Leemans JC: TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol 21(8): 1299–1308, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Z, Zhu Y and Jiang H: Inhibiting toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study. Respir Res 10: 126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imado T, Iwasaki T, Kitano S, Satake A, Kuroiwa T, Tsunemi S and Sano H: The protective role of host toll-like receptor-4 in acute graft-versus-host disease. Transplantation 90(10): 1063–1070, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Margaritopoulos GA, Antoniou KM, Karagiannis K, Samara KD, Lasithiotaki I, Vassalou E, Lymbouridou R, Koutala H and Siafakas NM: Investigation of toll-like receptors in the pathogenesis of fibrotic and granulomatous disorders: a bronchoalveolar lavage study. Fibrogenesis Tissue Repair 2010; 3:20 Published online Oct.11, 2010 Doi: 10.1186/1755-1536-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalash R, Berhane H, Au J, Rhieu BH, Epperly MW, Goff J, Dixon T, Wang H, Zhang X, Franicola D, Shinde A and Greenberger JS: Differences in irradiated lung gene transcription between fibrosis-prone C56BL/6NHsd and fibrosis-resistant C3H/HeNHsd mice. In Vivo 28(2): 147–171, 2014. [PMC free article] [PubMed] [Google Scholar]
- 18.Epperly M, Cao S, Shields D, Franicola D, Zhang X, Wang H, Friedlander R and Greenberger JS: Increased hematopoiesis in continuous marrow cultures and radiation resistance of marrow stromal cells form caspase-1 knockout mice. In Vivo 27: 419–430, 2013. [PMC free article] [PubMed] [Google Scholar]
- 19.Berhane H, Epperly M, Cao S, Goff J, Franicola D, Wang H and and Greenberger JS: Radioresistance of bone marrow stromal and hematopoietic progenitor cell lines derived from Nrf2−/− homozygous deletion recombinant negative mice. In Vivo 27: 571–582, 2013. [PMC free article] [PubMed] [Google Scholar]


