Abstract
Exposure of mice to a diet containing 3,5-diethoxycarbonyl-1, 4-dihydrocollidine (DDC) induces porphyrin accumulation, cholestasis, immune response, and hepatobiliary damage mimicking hepatic porphyria and sclerosing cholangitis. Although β-catenin signaling promotes hepatocyte proliferation, and macrophages are a source of Wnts, the role of macrophage-derived Wnts in modulating hepatobiliary injury/repair remains unresolved. We investigated the effect of macrophage-specific deletion of Wntless, a cargo protein critical for cellular Wnt secretion, by feeding macrophage-Wntless-knockout (Mac-KO) and wild-type littermates a DDC diet for 14 days. DDC exposure induced Wnt11 up-regulation in macrophages. Mac-KO mice on DDC showed increased serum alkaline phosphatase, aspartate aminotransferase, direct bilirubin, and histologic evidence of more cell death, inflammation, and ductular reaction. There was impaired hepatocyte proliferation evidenced by Ki-67 immunostaining, which was associated with decreased hepatocyte β-catenin activation and cyclin-D1 in Mac-KO. Mac-KO also showed increased CD45, F4/80, and neutrophil infiltration after DDC diet, along with increased expression of several proinflammatory cytokines and chemokines. Gene expression analyses of bone marrow–derived macrophages from Mac-KO mice and F4/80+ macrophages isolated from DDC-fed Mac-KO livers showed proinflammatory M1 polarization. In conclusion, this study shows that a lack of macrophage Wnt secretion leads to more DDC-induced hepatic injury due to impaired hepatocyte proliferation and increased M1 macrophages, which promotes immune-mediated cell injury.
Chronic liver diseases share a common pathologic mechanism, liver-injury stimulated fibrosis, which, if progressive, can lead to cirrhosis, liver failure, and cancer.1 The inflammatory response to chronic liver injury, mediated by parenchymal and nonparenchymal cells, plays critical roles in fibrosis. Fibrosis is an adaptive response to liver injury that facilitates tissue repair. Clinical observations and experimental models suggest that liver fibrosis is a dynamic, bidirectional process with the capacity for recovery even at advanced stages.2 A better understanding of the mechanisms of fibrosis, repair, and the role of inflammatory cells and mediators is crucial to developing new therapeutic approaches.
In an acute liver injury, hepatocytes contribute by regenerating injured parenchymal cells. In a severe or chronic injury, hepatic progenitor cells, which may arise from cholangiocytes, contribute to hepatocyte regeneration. Activation of hepatic progenitor cells is associated with the development of a ductular reaction and fibrosis in chronic liver diseases.3, 4 Notably, hepatic progenitor cells are believed to coexist in a milieu with leukocytes, including macrophages. It is conceivable that cells such as macrophages may contribute to hepatic progenitor cell activation and differentiation. Macrophages play critical and spatiotemporal roles in liver regeneration, fibrosis development, and resolution of inflammation.5, 6, 7 Macrophages in bone marrow fraction have been shown to induce ductular reaction due to tumor necrosis factor–like weak inducer of apoptosis.8 Another study has shown resident macrophages to be critical in migration of progenitors.9 Macrophage ablation at time of injury led to decreased fibrosis, whereas their ablation during resolution of fibrosis led to persistent fibrosis, suggesting temporal and potentially opposing functions of these cells.5 Last, macrophages, by virtue of being the source of vascular endothelial growth factor, were shown to play a role in regulating endothelial cell function and in turn in extracellular matrix turnover.10 The macrophages, as a source of Wnts, in the context of hepatobiliary injury are currently poorly understood.
The Wnt family contains 19 secreted glycoproteins that can stimulate target cells to regulate cell proliferation, differentiation, and migration via β-catenin and β-catenin–independent pathways.11 Wnt signaling drives hepatocellular differentiation during development,1, 12 and a similar function for Wnts during chronic liver injury has been suggested.13 Multiple studies in rodent models of chronic liver disease have also suggested a role for β-catenin signaling in chronic liver injury, including driving hepatic cellular proliferation and differentiation.14, 15, 16 A loss-of-function mutation in the LRP6 gene, encoding a Wnt coreceptor, was recently associated with human nonalcoholic fatty liver disease.17 Earlier studies have also shown that macrophage- but not epithelial-derived Wnts contribute to liver regeneration after partial hepatectomy.12 When β-catenin conditional knockout mice, that lacked β-catenin in hepatocytes and cholangiocytes, were fed a cholestatic hepatic injury–causing diet containing 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) for a short-term, less progenitor cell response was found. However, long-term DDC feeding led to increased inflammatory cell infiltration and fibrosis.14 It was also recently reported that DDC diet–induced injury increased expression of Wnt7a, Wnt7b, and Wnt10a, which led to hepatobiliary repair observed as increased expression of epithelial cell adhesion molecule and transcription factor SOX-9 (Sox9).18 Recently, Wnt secretion from hepatic stellate cells was shown to be dispensable in causing hepatic fibrosis in two different injury models.19 The role of macrophage-derived Wnt proteins in hepatobiliary repair during cholestatic injury remains unknown.
In the current study, we investigated the contribution of macrophage-derived Wnts to the overall inflammatory milieu, injury, fibrosis, and repair, during DDC diet–induced liver disease by using myeloid-targeted deletion of Wntless (Wls). Wls encodes the chaperone Wls, which is specifically required for all Wnt secretion from a cell.20 Thus, elimination of Wls from myeloid cells prevents secretion of all Wnts from these cells, including monocytes and macrophages. However, although this model falls short on allowing study of a specific Wnt ligand, it allows for elucidating the role of macrophages as source of Wnts during DDC-induced hepatobiliary injury and repair. Interestingly, macrophage-Wntless-knockout (Mac-KO) mice after DDC diet had notably more inflammation compared with control. Some inflammatory cells (eg, CD45- and neutrophil elastase–positive cells) were more abundant in Mac-KO mice after DDC treatment, which may lead to more injury and ductular reaction in the Mac-KO mice. Overall, these studies suggest that a lack of Wnt secretion from macrophages under inflammatory conditions may exacerbate injury by perpetuating inflammation and hindering repair.
Materials and Methods
Mice
Macrophage-specific Wls KO mice (Mac-KO: Wlsloxp/loxp, Lyz2-Cre+/−) were generated by crossing Wlsflox/flox mice with LyzM-Cre+/− mice. Littermates with floxed target genes but without Cre (Wlsflox/flox; Lyz2-Cre−/− and Wlsflox/wt; Lyz2-Cre−/−) were used as controls. Genomic DNA was extracted using QIAamp DNA Micro Kit (Qiagen, Hilden, Germany) from F4/80+ macrophages. For PCR analysis on deleted Wls allele, the following primer sets (forward and reverse, respectively) were used: Wls-P1, 5′-CTTCCCTGCTTCTTTAAGCGTC-3′ (forward); and Wls-P4, 5′-CTCAGAACTCCCTTCTTGAAGC-3′ (reverse).20 The amplification protocol comprised an initial incubation at 95°C for 4 minutes and 30 cycles of 94°C for 30 seconds, 62°C for 30 seconds, and 72°C for 40 seconds, and a final extension of 72°C for 7 minutes. The use of these primers produces the band of 410 bp for the Wls allele. All experiments were performed under strict guidelines of the NIH’s Guide for the Care and Use of Laboratory Animals21 and the Institutional Animal Use and Care Committee at the University of Pittsburgh (Pittsburgh, PA).
DDC-Diet–Induced Liver Injury
Mac-KO and corresponding control mice (male, age 6 weeks) were fed 0.1% DDC diet for 2 weeks, as described in our earlier publication.14, 18 Mice were sacrificed after 2 weeks of DDC diet. Blood and livers were collected for subsequent analyses. Liver was excised, and a portion was fixed in 10% neutral-buffered formalin and processed for paraffin embedding. A portion of liver was frozen in Tissue-Tek OCT compound (Sakura Fintek, Torrance, CA) for frozen sections. The remaining liver was quickly frozen in liquid nitrogen and stored at −80°C.
Serum Biochemistry
Blood samples were collected from the orbital sinus at the time of sacrifice. Serum biochemical measurements for total bilirubin, direct bilirubin, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase were performed by the Biospecimen Biorepository and Processing Core of the Pittsburgh Liver Research Center at the University of Pittsburgh and University of Pittsburgh Medical Center.
Immunohistochemistry and Immunofluorescence
Tissues were fixed in 10% buffered formalin and embedded in paraffin, and sections (4 μm thick) were used for hematoxylin and eosin staining, immunohistochemistry (IHC), or immunofluorescence. Briefly, for IHC, deparaffinized sections were incubated in 3% H2O2 in 1× phosphate-buffered saline for 10 minutes to quench the endogenous peroxidase. Slides were pressure cooked in antigen retrieval buffer (Dako, Glostrup, Denmark) for 20 minutes. Subsequently, slides were washed and incubated with super block buffer (Thermo Fisher Scientific, Pittsburgh, PA) to suppress nonspecific binding, then incubated with primary antibodies in phosphate-buffered saline with 1% bovine serum albumin overnight at 4°C. After washes, the sections were incubated in the appropriate biotin-conjugated secondary antibody (Chemicon, Temecula, CA), for 30 minutes at room temperature. Signal was detected using the Vectastain ABC Elite kit (Vector Laboratories, Inc., Burlingame, CA) and developed using diaminobenzidine (Vector Laboratories, Inc.). For immunofluorescence, sections were incubated in the dark with fluorescein isothiocyanate–conjugated goat anti-rabbit and tetramethylrhodamine-conjugated goat anti-rat secondary antibody (1:500) in 1% bovine serum albumin in phosphate-buffered saline for 1 hour. Then, after another wash with phosphate-buffered saline, sections were counterstained with 1 mg/100 mL DAPI (B2883; MilliporeSigma, Burlington, MA) and mounted using mounting medium containing DAPI. Samples were imaged on a Nikon Eclipse Ti epifluorescence microscope (Nikon Instruments Inc., Melville, NY). Apoptotic nuclei were detected by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining using the ApopTag Peroxidase kit (Intergen Company, Purchase, NY).
Primary antibodies used in the study include anti–glutamine synthase (GS; 1:50 dilution, SC9067, Santa Cruz Biotechnology, Dallas, TX), anti–cyclin D1 (1:50, 9041-po, Lab Vision & Neomarkers, Fremont, CA), anti-CD45 (1:100, SC53665, Santa Cruz Biotechnology), anti–neutrophil elastase (1:1500, AB68672, Abcam Biotechnology, Cambridge, MA), anti-F4/80 (1:100, MCA497GA, Bio-Rad Laboratories, Inc., Hercules, CA), anti–α-smooth muscle actin (1:100, M0851, Dako), anti-Sox9 (1:100; AB5535, MilliporeSigma), and anti–cytokeratin 19 (CK19; 1:10; TRoma III; DSHB Biotechnology, Iowa City, IA).
Western Blot Analysis
Liver lysates were prepared by homogenizing liver tissues in radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors, and 30 to 40 μg of protein was subjected to SDS-PAGE and Western blot analyses.18, 22 The primary antibodies used in the study include anti–β-catenin (BD610154; BD Biosciences, San Jose, CA), anti–active β-catenin that recognizes β-catenin hypophosphorylated at serine-37 and threonine-41 (1:100; Upstate Biotechnology, Lake Placid, NY), anti-GS (1:50 dilution, SC9067, Santa Cruz Biotechnology), and anti-cyclin D1 (1:50, 9041-po, Lab Vision & Neomarkers).
Generation of BMDMs
Bone marrow cells were isolated from femur and tibias of wild-type and Mac-KO mice (n = 3) fed chow diet. Bone marrow cells (5 × 106/plate) were plated in 100-mm petri dishes in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 2 mmol/L l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1× minimal essential medium nonessential amino acids, 1 mmol/L sodium pyruvate, and 20% L929 supernatant (source of macrophage colony-stimulating factor) to generate bone marrow–derived macrophages (BMDMs). Additional medium was added after 3 days, and cells were harvested at day 7. BMDM cells were incubated for 6 hours with 50 ng/mL mouse interferon-γ plus 10 ng/mL lipopolysaccharide or 20 ng/mL mouse IL-4 to induce M1 and M2 macrophages, respectively. BMDMs incubated with medium alone were treated as M0 macrophages.
Cell Separation, RNA Isolation, and Real-Time Quantitative RT-PCR
Total RNA was isolated using either Trizol (Invitrogen, Waltham, MA) or RNeasy Mini Kit (Qiagen).18, 22 After DNase treatment, cDNA was prepared using (0.3 to 1 μg) total RNA and SSOAdvanced iScript kit (Bio-Rad Laboratories, Inc.), according to the manufacturer's instruction. Real-time quantitative RT-PCR was performed on a Bio-Rad CFX96 using SSO-advanced SYBR Green PCR Master Mix (Bio-Rad Laboratories, Inc.). To determine the macrophage-specific elimination of Wls, positive selection of F4/80+ cells from nonparenchymal cells was performed using the QuadroMACS column separation kit (Miltenyi Biotech, Cambridge, MA), as described earlier.18 The purity of F4/80+ cells was verified by real-time quantitative RT-PCR using primers for absence of negative markers albumin (hepatocyte specific) and tyrosine kinase with Ig and EGF homology domains–2 (Tie2; specific for endothelial cells) and for presence of Cd68 (macrophage). In some experiments, macrophages were isolated from DDC-fed mice. Briefly, F4/80+ macrophages were fluorescence-activated cell sorted (FACSAria; BD Biosciences) after staining nonparenchymal cells with F4/80-phosphatidylethanolamine (Biolegend, San Diego, CA). Purity of the F4/80+ macrophages isolated from liver tissue was >98%, and cells were lysed in Trizol and RNA prepared using DirectZol (Zymo Research, Irvine, CA) method. Expression of Nos2 and Arg1 was determined by TaqMan probe using gene-specific primers and probes (Bio-Rad Laboratories, Inc.) in Bio-Rad CFX thermocycler. Two-step PCR with denaturation at 95°C for 15 seconds and annealing and extension at 60°C for 1 minute for 40 cycles was conducted in a CFX96 (Bio-Rad). Expression of target genes was calculated by the ΔΔCt method using threshold cycles for β-actin and β2-microglobulin as normalization references. RNA without reverse transcriptase served as a negative control. The primer sequences for all Wnt primers have been published previously,18 and additional primers used in the current study are listed in Table 1.
Table 1.
Primers and PCR Assays Used
| Gene | Forward primer | Reverse primer | Vendor/assay ID |
|---|---|---|---|
| Arg1 | Primer mix: FAM | Bio-Rad Laboratories, Inc./qMmuCIP0034715 | |
| Arg1 | 5′-CAGAAGAATGGAAGAGTCAG-3′ | 5′-CAGATATGCAGGGAGTCACC-3′ | Integrated DNA Technologies, Inc., Coralville, IA |
| B2M | Primer mix | Bio-Rad Laboratories, Inc./qMmuCID0040553 | |
| B2M | Primer mix: Hex | Bio-Rad Laboratories, Inc./qMmuCIP0042770 | |
| Ccl2 | 5′-TAGGCTGGAGGGCTACAAGA-3′ | 5′-TAGGTTCTGATCTCATTTGGTTCTG-3′ | Integrated DNA Technologies, Inc. |
| Ccl3 | Primer mix | Bio-Rad Laboratories, Inc./qMmuCED0044190 | |
| Ccl4 | Primer mix | Bio-Rad Laboratories, Inc./qMmuCED0044850 | |
| Ccl5 | 5′-CGAAGGAACCGCCAAGTG-3′ | 5′-CTAGAGCAAGCGATGACAGG-3′ | Integrated DNA Technologies, Inc. |
| Cxcl1 | Primer mix | Bio-Rad Laboratories, Inc./qMmuCED0047655 | |
| Cxcl16 | 5′-AGCAAGATGACAGACAGCAAGAAG-3′ | 5′-GCAGTGAGGAAGAAGACAATGGC-3′ | Integrated DNA Technologies, Inc. |
| Sele | 5′-TGCAGTTCTGACGTGTGGTG-3′ | 5′-AACAAGGTAGAGCAATGAGGACGAT-3′ | Integrated DNA Technologies, Inc. |
| Il6 | 5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′ | 5′-TCTGACCACAGTGAGGAATGTCCAC-3′ | Integrated DNA Technologies, Inc. |
| Mgl1 | Primer mix | Bio-Rad Laboratories, Inc./qMmuCIP0036620 | |
| Mrc1 | Primer mix | Bio-Rad Laboratories, Inc./qMmuCID0012670 | |
| Nos2 | Primer mix: Cy5 | Bio-Rad Laboratories, Inc./qMmuCIP0035502 | |
| Nos2 | 5′-CTGGAGGAGCTCCTGCCTCATG-3′ | 5′-GCAGCATCCCCTCTGATGGTG-3′ | Integrated DNA Technologies, Inc. |
| Tnfa | Prime mix Mm.PT.58.12575861 | Integrated DNA Technologies, Inc./Mm.PT.58.12575861 | |
| Vcam1 | 5′-GCTCTGTGGGTTTTGAGGATGA-3′ | 5′-GGATCTTCAGGGAATGAGTAGACC-3′ | Integrated DNA Technologies, Inc. |
| Chil3 | 5′-TCACAGGTCTGGCAATTCTTCTG-3′ | 5′-TTTGTCCTTAGGAGGGCTTCCTC-3′ | Integrated DNA Technologies, Inc. |
Statistical Analysis
All experiments were performed with four to five animals, and representative data are presented. Real-time quantitative RT-PCR analysis was performed on n ≥ 3 mice done in triplicate. All statistics were performed with Prism 6 software version 7.0 (GraphPad Software Inc., La Jolla, CA). Densitometric analysis was performed using Image Pro Plus software version 7 (Media Cybernetics, Rockville, MD). Statistical differences were assessed with Welch's t-test, and P < 0.05 was considered statistically significant.
Results
DDC Diet Causes Altered Wnt Expression in Macrophages
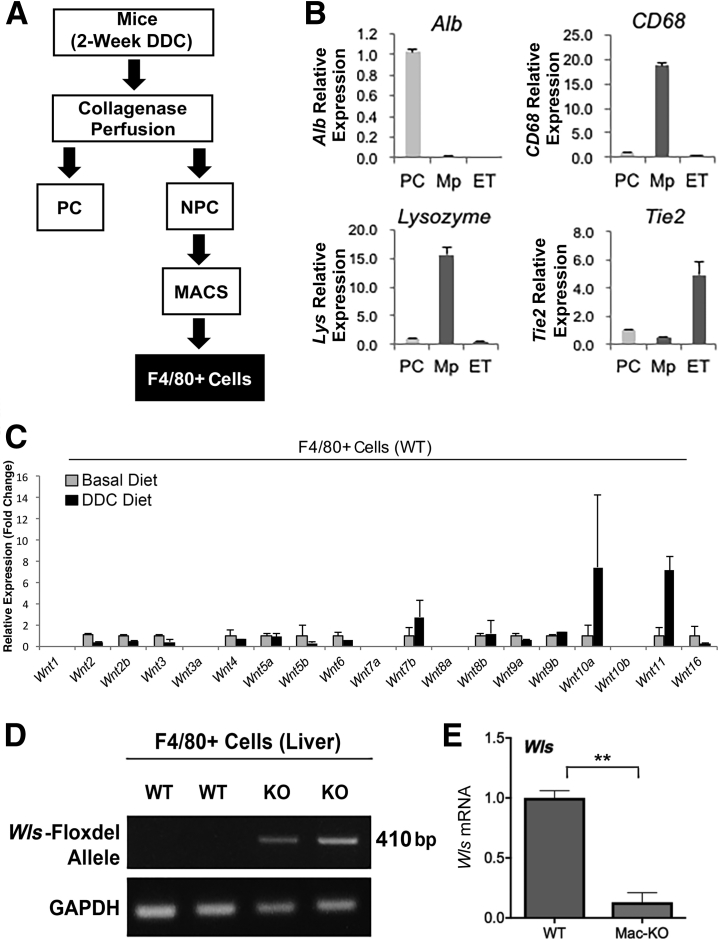
To elucidate the role of macrophage-derived Wnts and their overall impact on DDC-induced cholestatic liver injury, the impact of DDC diet feeding was first examined on Wnt expression. Previously, Wnt7a, Wnt7b, and Wnt10a expression levels were shown to be up-regulated in livers of DDC-fed mice for 14 to 28 days.18 F4/80+ macrophages were isolated using a magnetic bead isolation protocol (F4/80–magnetic-activated cell sorting) from the nonparenchymal cell fractions of livers from normal mice fed either a basal or DDC diet for 14 days (n = 2 mice per condition) (Figure 1A). The purity of the pooled macrophage fractions was confirmed using cell-type–specific gene expression analysis. Using marker genes for hepatocytes (Alb), macrophages (CD68 and lysozyme), and endothelial cells (Tie2), >98% purity of the F4/80+ macrophages was confirmed by both inclusion of positive markers (CD68 and lysozyme) and exclusion of negative markers (albumin and Tie2) via real-time quantitative RT-PCR (Figure 1B). The mRNA expression of several Wnt genes, which revealed a notable up-regulation of Wnt11 in DDC-fed mice, was next assessed; Wnt7b and Wnt10a showed variable increases (Figure 1C). In fact, differential expression was noted in most Wnt genes analyzed in macrophages isolated from mice after DDC diet compared with basal diet (Figure 1C). These data indicate that DDC induced notable alterations in the expression of Wnts in macrophages and that macrophage-derived Wnts may be playing an important role in DDC diet–induced liver pathology.
Figure 1.
Macrophage isolation to characterize DDC-diet–induced changes in Wnt expression and generation of Mac-KO mice. A: Schematic of isolation of various cells after collagenase perfusion, including parenchymal cells (PCs) and nonparenchymal cells (NPCs). NPC fraction was used to isolate macrophages (F4/80) using magnetic-activated cell sorting. B: Purity of cell fractions was verified by both the presence of positive markers in various cell types and simultaneous absence of negative cell-specific markers by real-time quantitative RT-PCR (RT-qPCR). Alb (hepatocytes), CD68, lysozyme [macrophages (Mp)], and Tie2 [endothelial cells (ET)] are seen. Epithelial cell adhesion molecule–positive and CD45–positive cells were separated from nonparenchymal cells after liver perfusion. Two mice fed DDC diet for 2 weeks were used for cell isolation, and isolated cell fractions were pooled for analysis. C: F4/80+ macrophages were isolated and pooled from livers of two mice on normal and two mice on DDC diet for 2 weeks and subjected to RT-qPCR analysis. A notable increase in Wnt11 mRNA expression is observed along with elevation of Wnt7b and Wnt10a in DDC-fed group versus mice on basal diet. Data presented as relative expression, fold change. D: Confirmation of Wls genetic deletion in Mac-KO mice using PCR on DNA from isolated macrophages from two mice from each genotype. Deletion of the floxed Wls exon results in 410-bp band. E: RT-PCR expression analysis of Wls mRNA in isolated macrophages from three WT and three Mac-KO mice shows substantial reduction in Wls expression in Mac-KO mice. Data are expressed as relative expression, fold change. ∗∗P < 0.01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Confirmation of Macrophage-Specific Loss of Wntless Expression
To assess the role of macrophages as the source of the entire Wnt repertoire, all Wnt secretion from macrophages was eliminated by crossing LyzM Cre+/− mice with Wntless/Evi (Wls) Loxp+/+ mice to generate Mac-KO mice. Parenchymal cells and nonparenchymal cells were separated by collagenase perfusion from Mac-KO mice and wild-type (WT) littermate controls. F4/80+ macrophages and CD31+ endothelial cells were then isolated from the nonparenchymal fractions by magnetic bead isolation protocol using F4/80− and CD31–magnetic-activated cell sorting beads. PCR analyses of macrophage DNA from Mac-KO mice showed the deleted allele of Wls, confirming successful recombination in the macrophages (Figure 1D). Furthermore, the enriched macrophage fraction was assessed for mRNA expression of Wls, which was diminished in Mac-KO (Figure 1E), as also described previously.12
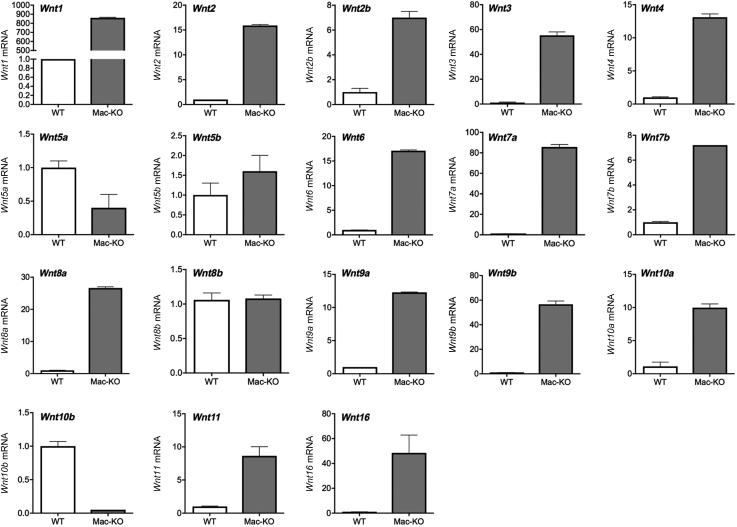
Last, bone marrow progenitor cells were isolated from Mac-KO or WT mice fed chow diet and differentiated into mature M0 macrophages (BMDMs) in vitro, as described in Materials and Methods. Gene expression analysis for Wls and a panel of Wnt ligands showed a significant loss of Wls expression in the Mac-KO, as expected, as well as a remarkably different Wnt gene expression profile compared with WT (Figure 2). All of the Wnt ligands analyzed (total 16) were detected in the BMDMs. Several Wnts (Wnt1, Wnt2, Wnt2b, Wnt3, Wnt4, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt8a, Wnt9a, Wnt9b, Wnt10a, Wnt11, and Wnt16) were up-regulated manyfold in Mac-KO BMDMs compared with WT BMDMs (Figure 2). Wnt5a and Wnt10b were notably down-regulated, whereas there was no change in the expression of Wnt8b (Figure 2). These changes in expression of many Wnt genes, triggered by the loss of Wls, suggest that macrophages are an active and significant source of Wnt ligands, and Wnt secretion by macrophages regulates Wnt gene expression in these same cells through positive and/or negative feedback in an autocrine or paracrine manner.
Figure 2.
Changes in Wnt gene expression in macrophages with Wls deletion. Real-time quantitative RT-PCR for a panel of Wnt ligands on bone marrow–derived macrophages (BMDMs) from WT and Mac-KO mice shows expression of all 16 Wnt genes. However, several Wnt genes (Wnt1, Wnt2, Wnt2b, Wnt3, Wnt4, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt8a, Wnt9a, Wnt9b, Wnt10a, Wnt11, and Wnt16) are up-regulated manyfold in the baseline Mac-KO BMDMs compared with BMDMs from WT mice. Wnt5a and Wnt10b are notably down-regulated, whereas there is no change in the expression of Wnt8b. The expression of each Wnt gene is normalized to glyceraldehyde-3-phosphate dehydrogenase and expressed as fold-change in Mac-KO over WT mice. Three Mac-KO or WT mice were used for isolating and differentiating BMDMs.
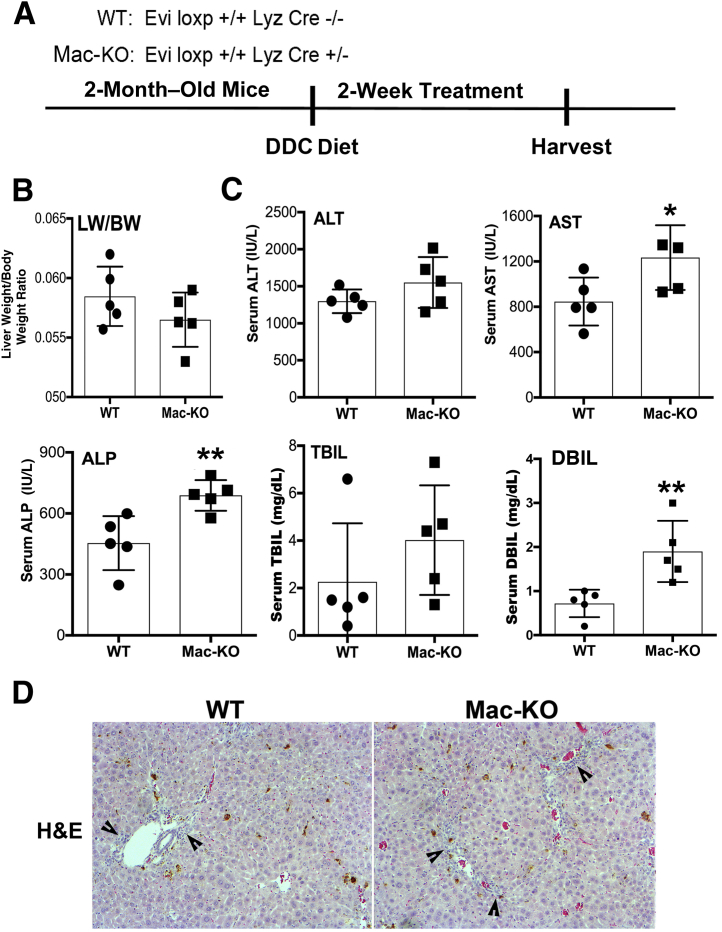
Evidence of Overall Enhanced Hepatic Injury and Decreased Hepatic Repair in the Mac-KO Mice Fed DDC Diet
Two-month–old Mac-KO and WT mice were fed the DDC diet for 14 days (Figure 3). There was no difference in the gross appearance of livers between the two groups, and although the liver weight/body weight ratios were comparable, a trend toward smaller livers was observed in the Mac-KO mice (Figure 3). Serum biochemistry analyses showed significantly higher levels of aspartate aminotransferase, alkaline phosphatase, and direct bilirubin in the Mac-KO mice, whereas total bilirubin and alanine aminotransferase tended to be higher but did not show high significance between Mac-KO and WT mice (Figure 1D). Histologic analysis indicated that, as expected, DDC injury induces prominent ductular reaction and periportal inflammation, which was apparent in both WT and Mac-KO mice by hematoxylin and eosin stain, although a more pronounced ductular reaction, inflammation, and altered hepatic architecture were seen in the Mac-KO mice (Figure 3).
Figure 3.
Evidence of increased hepatobiliary damage in Mac-KO mice after 14 days of DDC diet. A: Schematic depicting DDC diet protocol used in the study. B: No change in liver weight/body weight (LW/BW) ratio between Mac-KO and WT mice after feeding DDC diet for 14 days, but trend toward reduced liver size in Mac-KO mice. C: Significant increases in serum aspartate aminotransferase (AST; normal, 30 to 50 IU/L), alkaline phosphatase (ALP; normal, 20 to 100 IU/L), and direct bilirubin (DBIL; normal, 0 to 0.2 mg/dL) in Mac-KO compared with WT after 14 days of DDC diet. D: Representative hematoxylin and eosin (H&E) images from DDC-treated Mac-KO and WT livers showing evidence of more pronounced ductular reaction and periportal inflammation (arrowheads) in Mac-KO mice. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×100 (D). ALT, alanine aminotransferase; TBIL, total bilirubin.
To verify changes in ductular reaction, which is an indicator of DDC-induced hepatobiliary injury, immunofluorescence staining was performed for CK19 and Sox9. Mac-KO mice showed greater CK19- and Sox9-positive ductular cells in comparison to WT mice, thus confirming histologic findings (Figure 4).
Figure 4.
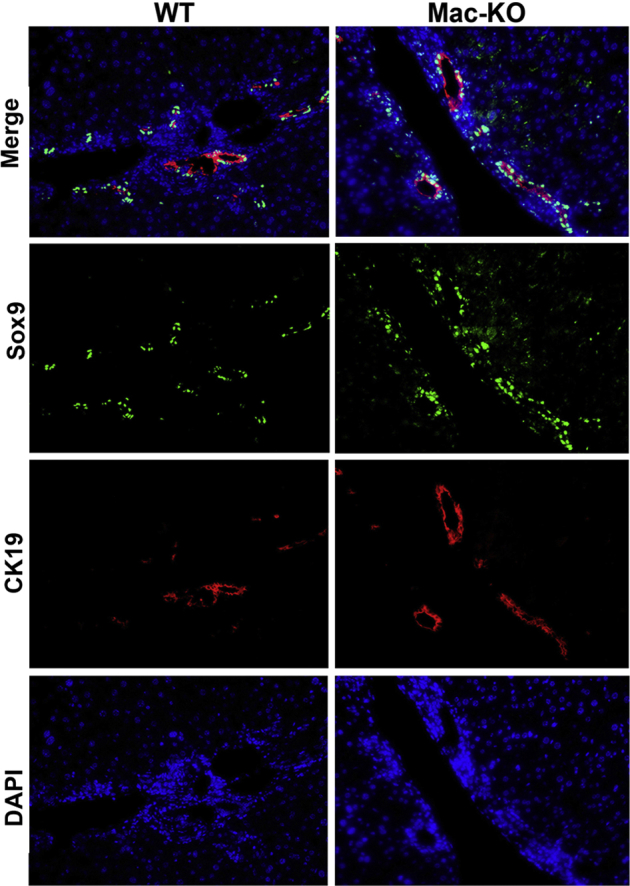
Immunofluorescence analysis verifies increased ductular reaction in the Mac-KO mice after 14 days of DDC diet. Immunofluorescence staining was performed for transcription factor SOX-9 (Sox9) and cytokeratin 19 (CK19) to label ductular reaction, which is enhanced in the Mac-KO mice compared with WT mice after 14 days of DDC diet. DAPI was used as nuclear counterstain. Original magnification, ×200.
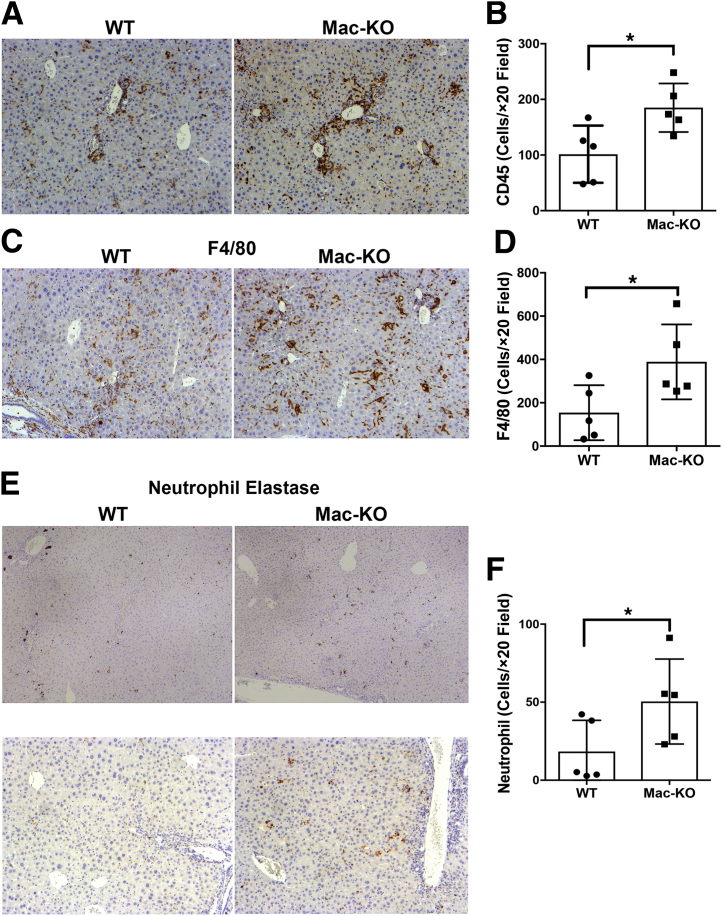
To verify changes in inflammation, it was then investigated whether loss of Wnt secretion from macrophages exacerbated the inflammatory response. DDC-diet–fed Mac-KO mice showed an overall increase in the number of CD45+ leukocytes (Figure 5A). On quantification, this was significantly different from the WT mice (Figure 5B). To specifically examine any differences in the number of macrophages, IHC was performed for F4/80 in the Mac-KO and WT mice after DDC diet (Figure 5C). A significant increase in the number of macrophages was evident in the Mac-KO mice after DDC (Figure 5D). Last, overall neutrophil infiltration was determined in the WT versus Mac-KO mice after 14 days of DDC diet (Figure 5E). A greater and significant increase in neutrophil recruitment was observed in the Mac-KO mice on DDC diet (Figure 5F). These findings suggest that loss of Wnt secretion from macrophages promoted hepatic inflammatory milieu and may be contributing to overall disease pathogenesis after DDC diet.
Figure 5.
Increased immune response in Mac-KO mice compared with WT mice after 14 days of DDC feeding. A: Immunohistochemistry (IHC) for CD45 shows more positive cells in Mac-KO than WT mice, supporting enhanced overall inflammation in this group. B: Quantification of the number of CD45-positive cells per ×20 field shows a significant increase in the number of positive cells in Mac-KO mice after 14 days of DDC compared with WT mice. C: IHC for F4/80 shows more positive cells in Mac-KO than WT mice, depicting greater number of macrophages in this group. D: Quantification of the number of F4/80-positive cells per ×20 field shows a significant increase in the number of positive cells in Mac-KO mice after 14 days of DDC compared with WT mice. E: IHC for neutrophil elastase shows greater number of positive cells in Mac-KO livers than WT livers in response to DDC exposure for 14 days. F: Quantification of the number of neutrophil elastase–positive cells per ×20 field shows a significant increase in the number of positive cells in Mac-KO mice after 14 days of DDC compared with WT mice. Data are expressed as means ± SD. ∗P < 0.05. Original magnifications: ×100 (A, C, and E, bottom panels); ×50 (E, top panels).
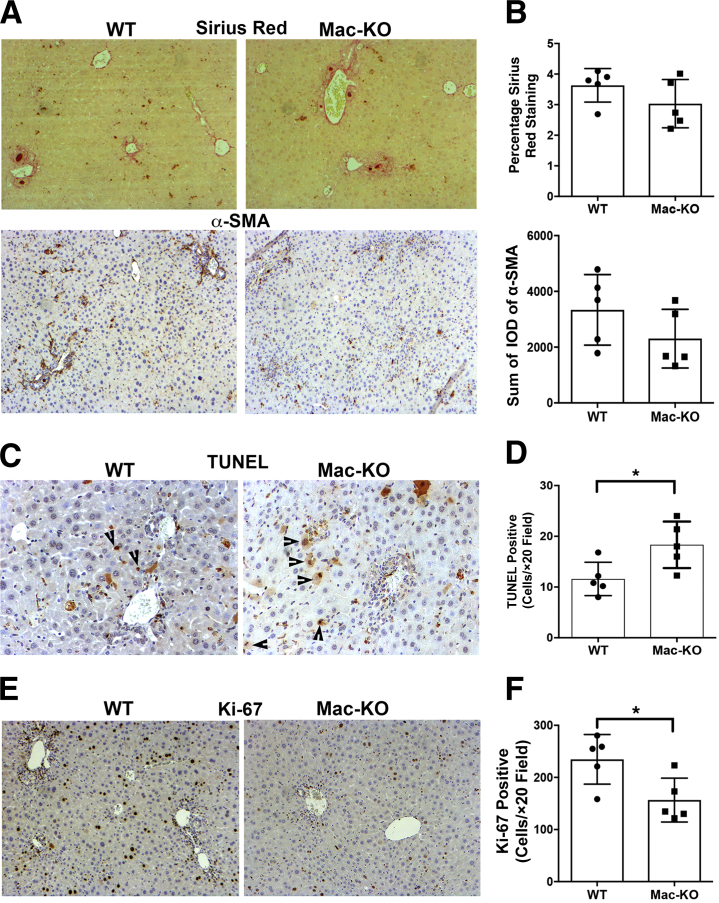
Because increased ductular reaction and inflammation can be associated with increased fibrosis, cell death, and compensatory hepatocyte proliferation, each of these attributes was next examined. Sirius Red staining was used to determine overall collagen deposition in livers of WT and Mac-KO mice after 14 days of DDC. Comparable staining for Sirius Red was evident in the two groups (Figure 6A) and verified quantitatively (Figure 6B). Likewise, staining for the activated myofibroblast marker, α-smooth muscle actin, showed comparable positivity in the two groups (Figure 6A) and was confirmed by densitometric analysis (Figure 6B).
Figure 6.
Increased cell death, decreased proliferation, but comparable fibrosis in Mac-KO versus WT mice after 14 days of DDC diet. A: Staining for Sirius Red (top panels) shows comparable positivity in the Mac-KO and WT mice after 14 days of DDC diet. Immunohistochemistry for α-smooth muscle actin (α-SMA; bottom panels) to identify activated myofibroblasts also shows comparable numbers of positive cells in the Mac-KO and WT mice after 14 days of DDC diet. B: Quantification of both Sirius Red staining and number of α-SMA–positive cells in the Mac-KO and WT mice after 14 days of DDC diet shows insignificant differences between the two groups. C: Increased terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)–positive hepatocytes (arrowheads) in Mac-KO compared with WT mice after 14 days of DDC diet. D: Quantification of the number of TUNEL-positive cells per ×20 field shows a significant increase in the number of positive cells in Mac-KO mice after 14 days of DDC compared with WT mice. E: Decreased Ki-67–positive hepatocytes in Mac-KO compared with WT mice denotes fewer proliferating cells in Mac-KO mice after 14 days of DDC. F: Quantification of the number of Ki-67–positive cells per ×20 field shows a significant increase in the number of positive cells in Mac-KO mice after 14 days of DDC compared with WT mice. Data are expressed as means ± SD. n = 3 mice. ∗P < 0.05. Original magnifications: ×100 (A and E); ×200 (C). IOD, integrated optical density.
DDC diet can lead to cell death due to bile acids and inflammation. To address if hepatocyte cell survival is affected differentially when macrophages cannot secrete Wnts, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining was performed on the livers from Mac-KO and WT mice after 14 days of DDC (Figure 6C). A notable increase in the number of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling–positive hepatocytes was evident in Mac-KO mice, verifying increased hepatocyte death (Figure 6D). Furthermore, in response to DDC-induced injury, the hepatic repair response occurs in the form of enhanced hepatocyte proliferation, which can be detected by IHC for a proliferation marker like Ki-67. As expected, several hepatocytes in WT livers show increased staining for Ki-67; however, relatively fewer hepatocytes show Ki-67 positivity in Mac-KO livers after 14 days of DDC (Figure 6E). On quantification, this difference was statistically significant, suggesting a compromise in hepatic repair in the Mac-KO mice after DDC (Figure 6F).
Decreased β-Catenin Activation in Mac-KO Mice May Compromise Hepatocyte Proliferation after DDC Injury
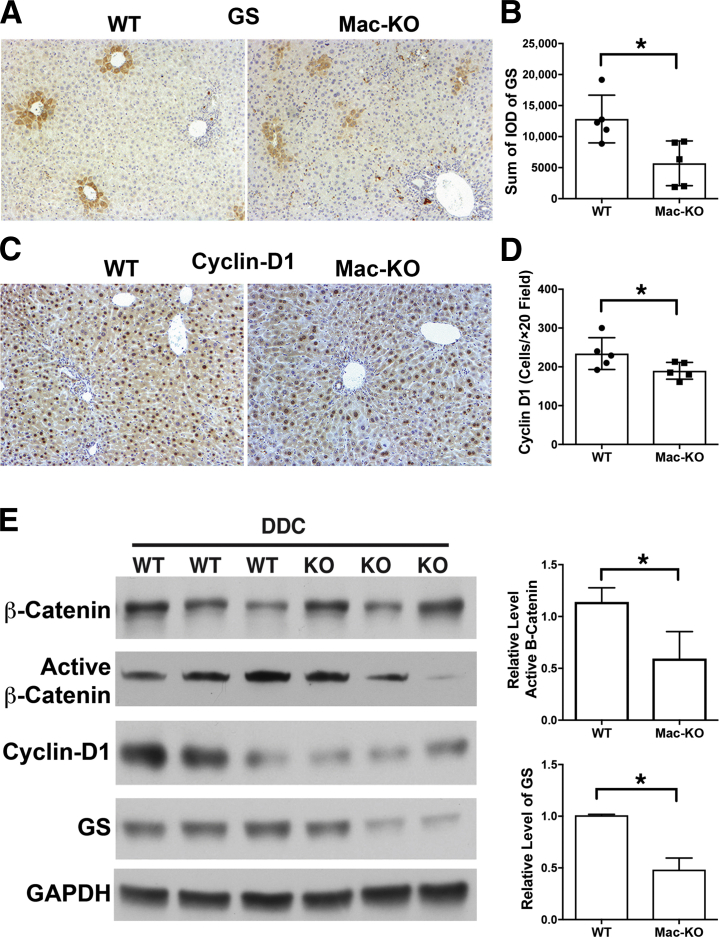
Macrophages play an important role as a source of Wnts for regulating hepatocyte proliferation or fate of progenitors in various forms of hepatobiliary repair, including after partial hepatectomy.12, 13 To investigate whether abolishing Wnt secretion from monocytes and macrophages after DDC diet would affect β-catenin activation in hepatocytes that could result in an impaired regenerative response, IHC and Western blot analyses were performed for β-catenin targets like cyclin-D1 and GS. IHC for GS showed some irregular staining in zone-3 in Mac-KO compared with WT mice after DDC (Figure 7A). Quantification of GS staining showed a significant decrease in overall GS positivity in Mac-KO mice after DDC (Figure 7B). Likewise, number of cyclin-D1–positive hepatocytes appeared to be fewer, albeit marginally, in Mac-KO mice after DDC when compared with WT mice (Figure 7C). Densitometry verified this decrease to be significant (Figure 7D).
Figure 7.
Decreased β-catenin activation in Mac-KO mice after 14 days of DDC diet. A: Immunohistochemistry (IHC) for glutamine synthase (GS) shows positive hepatocytes located at pericentral areas in both WT and Mac-KO mice, but with considerably less regularity and intensity in the Mac-KO mice after 14 days of DDC. B: Quantification of GS staining shows a significant decrease in the number of positive area in the Mac-KO mice after 14 days of DDC compared with WT mice. C: IHC for cyclin-D1 shows relatively fewer, albeit marginally, in the Mac-KO compared with WT mice after 14 days of DDC. D: Quantification of the number of cyclin-D1–positive cells per ×20 field shows a significant decrease in the number of positive cells in Mac-KO mice after 14 days of DDC compared with WT mice. E: Western blot analyses of liver tissue for β-catenin, active β-catenin, GS, and cyclin-D1 shows decreases in active β-catenin and confirms reduced GS and cyclin-D1 after 14 days of DDC diet in Mac-KO mice. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) verified protein loading. Although cyclin-D1 decrease is obvious, densitometry showed a significant decrease in the levels of both active β-catenin as well as GS in Mac-KO compared with WT mice. Liver lysates from three mice per genotype were used for this analysis. Data are expressed as means ± SD. n = 3 mice. ∗P < 0.05. Original magnification, ×100 (A and C). IOD, integrated optical density.
For a more accurate quantitative assessment, Western blot analysis was performed with liver lysates from DDC-diet–fed WT and Mac-KO mice. Although total β-catenin levels were not different between the two groups, active β-catenin levels were reduced in the Mac-KO mice (Figure 7E). Densitometry verified this decrease to be significant. Cyclin-D1 levels were also notably reduced in the Mac-KO livers (Figure 7E). Likewise, GS levels were significantly decreased after DDC in Mac-KO compared with WT mice (Figure 7E). Altogether, these findings suggest that loss of Wnt secretion from macrophages negatively affected proregenerative β-catenin signaling in hepatocytes, and a compromise in repair may also be contributing to overall enhanced hepatobiliary injury after DDC.
Increased Inflammatory Chemokines and Cytokines in Mac-KO Mice after DDC
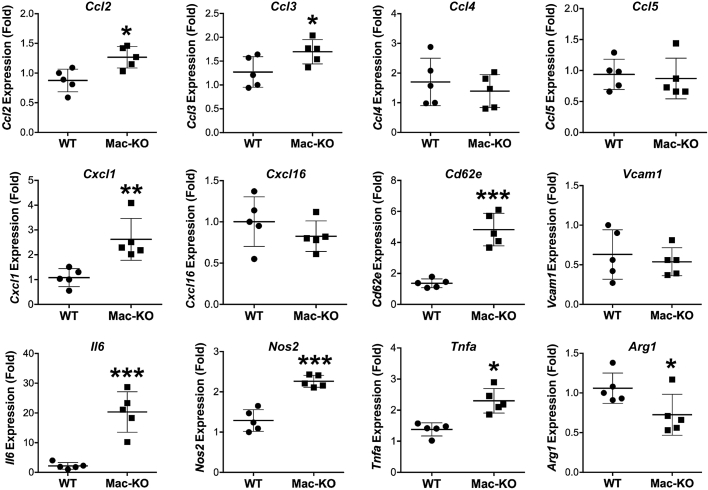
Because DDC diet led to increased CD45+ leukocytes, neutrophils, and F4/80+ macrophages in Mac-KO mice, it was next investigated if expression of chemokines that regulate the recruitment of such inflammatory cells in the liver are altered between the two groups. Gene expression analysis of liver mRNA from the Mac-KO mice after 14 days of DDC diet compared with control diet showed significant increases in chemokines (Ccl2, CCl3, and CxCL1); E-selectin (CD26E), a cell adhesion molecule that regulates inflammatory cell rolling and migration; and inflammatory cytokines (Il6 and Tnfa) (Figure 8). Nos2 (M1 macrophage marker) was also elevated in Mac-KO mice (Figure 8). These findings reveal a coincident increase in proinflammatory cytokines and chemokines in Mac-KO mice after DDC injury, suggesting their contribution to enhanced inflammation and hepatobiliary injury.
Figure 8.
Increased expression of proinflammatory cytokines and chemokines in livers of Mac-KO than WT mice after 14 days of feeding DDC diet. Gene expression analysis using real-time quantitative RT-PCR reveals a significant increase in expression of CCl2, CCl3, Cxcl1, Cd26e, Il6, Nos2, and Tnfa and decreased expression of Arg1 in Mac-KO when compared with WT mice. Data are expressed as relative expression, fold change. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. VCAM, vascular cell adhesion molecule.
Myeloid-Specific Loss of Wnt Secretion Affects Macrophage Plasticity
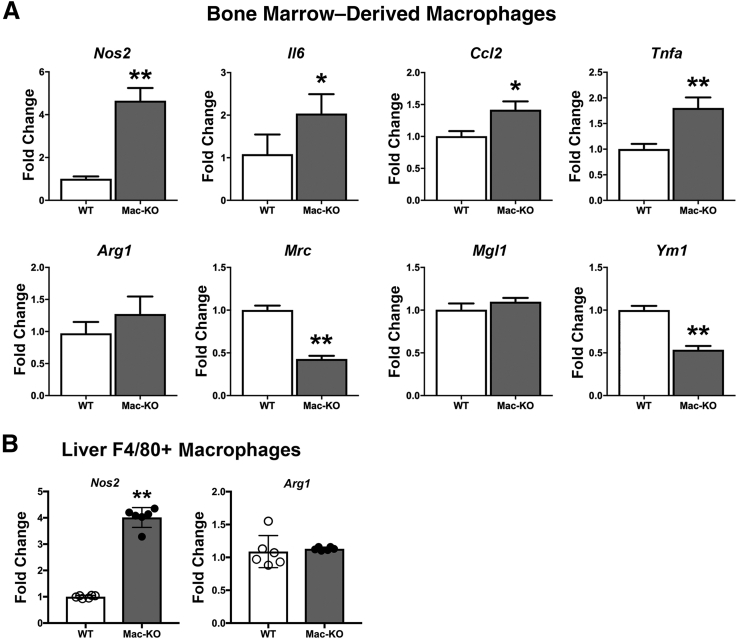
Because an increase was identified in M1 marker Nos2 in Mac-KO mice after DDC diet, and several studies have suggested macrophage polarization to M1 regulates inflammatory and wound healing responses, it was next investigated if loss of Wnt secretion from macrophages showed an effect on basal macrophage plasticity. BMDMs from basal-diet–fed Mac-KO and WT mice were isolated and differentiated in vitro, as above. Gene expression analyses of macrophage polarization marker genes revealed elevated expression of M1 markers (Nos2, IIl1b, Ccl2, and Tnfa), whereas some M2 markers (Mrc and Ym1) were coincidentally decreased in Mac-KO BMDMs (Figure 9A). Next, it was investigated whether macrophages in Mac-KO mice after DDC-diet–induced hepatic injury showed differential macrophage polarization. Using F4/80, macrophages were isolated from DDC-fed WT and Mac-KO mice by fluorescence-activated cell sorting, as described in Materials and Methods. Notably, liver F4/80+ macrophages showed significantly higher expression of Nos2 in Mac-KO mice compared to WT mice, whereas Arg1 levels were comparable (Figure 9B). All together, these findings suggest that macrophage-specific Wls loss, which prevents Wnt secretion from these cells, promotes a proinflammatory M1 macrophage phenotype, both basally and after hepatic injury; hence, Wnt secretion from macrophages may in fact be largely protective and proregenerative in the DDC diet model.
Figure 9.
Myeloid-specific loss of Wnt secretion promotes M1 macrophage phenotype. A: Bone marrow–derived macrophages from Mac-KO mice show significantly higher mRNA expression of Nos2, Il6, Ccl2, and Tnfa (M1 markers) and significantly decreased expression of Mrc and Ym1 (M2 markers) than WT mice, suggesting M1 polarization in Mac-KO mice at baseline without DDC exposure. Data presented as relative expression, fold change. Three mice from each genotype were used. B: Significantly increased expression of M1 marker Nos2 in F4/80-positive macrophages isolated from livers of Mac-KO compared with WT mice fed 14 days of DDC diet. No change in Arg1 expression is observed between the two groups. Data are expressed as relative expression, fold change. ∗P < 0.05, ∗∗P < 0.01.
Discussion
Variable and multifunctional aspects of macrophage biology have revealed that they contribute to acute and chronic inflammation.23, 24 Moreover, macrophages contribute to tissue repair and protection from infection. Wnt secretion by macrophages promotes intestinal crypt regeneration after radiation injury.25 Moreover, macrophages may be one of the sources of stromal Wnts in crypt cells of the small intestine, and macrophage-derived Wnts have been reported to have a significant role in tissue repair and regeneration.26, 27 Recent studies showed that macrophage-derived Wnt7b was crucial for epithelial regeneration in kidney injury.28 Macrophage-derived Wnts have also been shown to effect angiogenesis by regulating vascular endothelial growth factor signaling in vascular endothelial cells.27 Porcupine deletion, that results in loss of Wnt lipidation and subsequent Wnt secretion, has been shown to affect intestinal epithelial regeneration after radiation injury. Thus, there appears to be a paradigm in which macrophage-derived Wnts deliver spatial and temporal signals to promote regeneration after tissue injury. However, the molecular mechanisms regulating macrophage functions in inflammation, tissue repair, and fibrosis are newly emerging.29, 30
Macrophages contribute to liver inflammation, fibrosis, and regeneration via multiple mechanisms. Inflammatory, bone marrow–derived monocytes infiltrate the liver in response to injury, where they are dynamically reprogrammed, depending on the nature and chronicity of the injury.5, 31, 32, 33 Macrophages are among the earliest responders to liver injury, having roles in phagocytosis of apoptotic and necrotic cells and releasing soluble mediators, such as tumor necrosis factor and IL-6, which orchestrate inflammatory and profibrogenic responses to chronic injury. Macrophages are also prominent components of the hepatic progenitor cell niche, which is closely associated with liver fibrogenesis and repair3, 7; the fate relationships among hepatocytes, cholangiocytes, and hepatic progenitor cells and the contribution of hepatic progenitor cells to liver fibrosis and regeneration are controversial,34, 35 and the factors determining their activation and differentiation are not well understood. Wnt proteins, potentially derived from macrophages, have been suggested to promote liver regeneration by driving differentiation of hepatocyte progenitors,13 which could theoretically ameliorate liver fibrosis by promoting repair. Wnt proteins, however, constitute a large family of proteins that have variously attributed profibrogenic and antifibrogenic properties,36, 37 which likely have multiple potential target cells and mechanisms of action during chronic liver disease. Using a choline-deficient–0.5% ethionine-supplemented diet model, liver progenitor cells have also been shown to be dependent on macrophages for their invasiveness and recruitment.9, 38
To further our understanding of the role of macrophages as the source of Wnts in hepatobiliary injury and repair, the DDC diet model and mice lacking the ability to secrete all Wnts from all myeloid cells, by virtue of LysM-Cre knockout of Wls, a protein absolutely essential and specific for all Wnt secretion from a cell,39 were used. In this study, loss of Wnt secretion by macrophages promoted DDC-diet–induced hepatobiliary injury through two major mechanisms. Prevention of Wnt release from macrophages resulted in increased recruitment of inflammatory cells and promoted proinflammatory M1 macrophages and at the same time compromised hepatobiliary repair by diminishing hepatocyte proliferation.
This study suggests that macrophage-derived Wnts facilitate hepatocyte proliferation after DDC-induced injury, which partially explains the increased hepatic damage in Mac-KO mice. Indeed, such a role for macrophage-derived Wnts has been described during acute liver injury, such as hepatectomy, where they promote hepatocyte proliferation.12 Likewise, in chronic liver injury, they promote reprogramming of progenitor cells.13, 40 Overall, our current study suggests that Wnts produced by macrophages may normally be promoting a more proregenerative M2 macrophage phenotype and facilitating repair efforts.
These findings also show that loss of Wnt secretion by myeloid cells, including monocytes and macrophages, promoted proinflammatory M1 macrophage phenotype. Increased injury in Mac-KO mice after DDC was associated with enhanced inflammation, as seen by increased infiltration of macrophages and neutrophils. The current study suggests that preventing Wnt secretion by macrophages makes these cells more prone to exhibit a proinflammatory M1 phenotype and induce recruitment of neutrophils causing more hepatic injury. Macrophage polarization was analyzed, and it was shown that after 2 weeks of DDC diet, the liver macrophages are predominantly M1 rather than M2. The mechanisms contributing to the elevated M1 macrophage response need further investigation.
Which Wnts may be promoting regeneration and repair and/or driving the M2 over M1 macrophage phenotype in the context of DDC injury remains unclear; however, when liver macrophages were isolated by liver perfusion and cell separation techniques, Wnt11 was notably increased after the administration of DDC diet. Wnt11 is known to be induced by transforming growth factor-β and mediates mesenchymal transition of renal epithelial cells via noncanonical Wnt signaling.41 Wnt11 is also reported to inhibit the invasion of bacteria in an enteritis model.42 Wnt7b and Wnt10a were also up-regulated, although there was variability in their expression. Wnt7b secretion by kidney macrophages induces repair after kidney injury.28 A recent study has also shown positive impact of Wnt7a, Wnt7b, and Wnt10a on cholangiocyte proliferation and hepatocyte-to-biliary transdifferentiation after DDC injury.18 In that study, hepatocytes and cholangiocytes were the major source of these Wnts. This study shows that Wnt7b and Wnt10a after DDC injury may also be arising from macrophages, whereas Wnt11 is more exclusive to macrophages. Future genetic studies will be essential to clarify the roles of specific Wnts in DDC and other liver injury models.
In conclusion, Wnt secretion from macrophages is important for hepatobiliary repair in DDC-induced injury, as shown by deletion of Wls in these cells, which promoted an enhanced immune response and M1 polarization of macrophages and simultaneously compromised hepatocyte proliferation.
Footnotes
Supported in part by NIH grants 1R01DK62277, 1R01DK100287, R01CA204586, and R01HL130126 (S.N.); Abbvie Pharmaceuticals research grant I# 0050815 (S.P.M.); an Endowed Chair for Experimental Pathology (S.P.M.); and China Scholarship Council grant 201406285033 (A.J.).
Disclosures: S.P.M. is a consultant for Abbvie and Dicerna and has a research grant from Abbvie. S.G.E. is an employee of Abbvie.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.11.010.
Contributor Information
Shanmugam Nagarajan, Email: shannag@med.unc.edu.
Satdarshan P. Monga, Email: smonga@pitt.edu.
Supplemental Data
References
- 1.Monga S.P. beta-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148:1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iredale J.P., Thompson A., Henderson N.C. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013;1832:876–883. doi: 10.1016/j.bbadis.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Williams M.J., Clouston A.D., Forbes S.J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Gouw A.S., Clouston A.D., Theise N.D. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 5.Duffield J.S., Forbes S.J., Constandinou C.M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzini S., Bird T.G., Boulter L., Bellamy C., Samuel K., Aucott R., Clayton E., Andreone P., Bernardi M., Golding M., Alison M.R., Iredale J.P., Forbes S.J. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59:645–654. doi: 10.1136/gut.2009.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadd V.L., Melino M., Roy S., Horsfall L., O'Rourke P., Williams M.R., Irvine K.M., Sweet M.J., Jonsson J.R., Clouston A.D., Powell E.E. Portal, but not lobular, macrophages express matrix metalloproteinase-9: association with the ductular reaction and fibrosis in chronic hepatitis C. Liver Int. 2013;33:569–579. doi: 10.1111/liv.12050. [DOI] [PubMed] [Google Scholar]
- 8.Bird T.G., Lu W.Y., Boulter L., Gordon-Keylock S., Ridgway R.A., Williams M.J., Taube J., Thomas J.A., Wojtacha D., Gambardella A., Sansom O.J., Iredale J.P., Forbes S.J. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci U S A. 2013;110:6542–6547. doi: 10.1073/pnas.1302168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hul N., Lanthier N., Espanol Suner R., Abarca Quinones J., van Rooijen N., Leclercq I. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am J Pathol. 2011;179:1839–1850. doi: 10.1016/j.ajpath.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantari-Mimoun C., Castells M., Klose R., Meinecke A.K., Lemberger U.J., Rautou P.E., Pinot-Roussel H., Badoual C., Schrodter K., Osterreicher C.H., Fandrey J., Stockmann C. Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology. 2015;61:2042–2055. doi: 10.1002/hep.27635. [DOI] [PubMed] [Google Scholar]
- 11.Nejak-Bowen K.N., Monga S.P. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Mowry L.E., Nejak-Bowen K.N., Okabe H., Diegel C.R., Lang R.A., Williams B.O., Monga S.P. beta-Catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60:964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulter L., Govaere O., Bird T.G., Radulescu S., Ramachandran P., Pellicoro A., Ridgway R.A., Seo S.S., Spee B., Van Rooijen N., Sansom O.J., Iredale J.P., Lowell S., Roskams T., Forbes S.J. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apte U., Thompson M.D., Cui S., Liu B., Cieply B., Monga S.P. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 15.Apte U., Singh S., Zeng G., Cieply B., Virji M.A., Wu T., Monga S.P. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175:1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monga S.P. Role and regulation of beta-catenin signaling during physiological liver growth. Gene Expr. 2014;16:51–62. doi: 10.3727/105221614X13919976902138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S., Song K., Srivastava R., Dong C., Go G.W., Li N., Iwakiri Y., Mani A. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 2015;29:3436–3445. doi: 10.1096/fj.15-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okabe H., Yang J., Sylakowski K., Yovchev M., Miyagawa Y., Nagarajan S., Chikina M., Thompson M., Oertel M., Baba H., Monga S.P., Nejak-Bowen K.N. Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice. Hepatology. 2016;64:1652–1666. doi: 10.1002/hep.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R., Kikuchi A.T., Nakao T., Russell J.O., Preziosi M.E., Poddar M., Singh S., Bell A.W., England S.G., Monga S.P. Elimination of Wnt secretion from stellate cells is dispensable for zonation and development of liver fibrosis following hepatobiliary injury. Gene Expr. 2018 doi: 10.3727/105221618X15373858350141. doi: 10.3727/105221618X15373858350141, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter A.C., Rao S., Wells J.M., Campbell K., Lang R.A. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Academies Press; Washington, DC: 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council: Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 22.Okabe H., Delgado E., Lee J.M., Yang J., Kinoshita H., Hayashi H., Tsung A., Behari J., Beppu T., Baba H., Monga S.P. Role of leukocyte cell-derived chemotaxin 2 as a biomarker in hepatocellular carcinoma. PLoS One. 2014;9:e98817. doi: 10.1371/journal.pone.0098817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glass C.K., Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 24.Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha S., Bhanja P., Kabarriti R., Liu L., Alfieri A.A., Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6:e24072. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefater J.A., 3rd, Ren S., Lang R.A., Duffield J.S. Metchnikoff's policemen: macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S.L., Li B., Rao S., Yeo E.J., Hudson T.E., Nowlin B.T., Pei H., Chen L., Zheng J.J., Carroll T.J., Pollard J.W., McMahon A.P., Lang R.A., Duffield J.S. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clouston A.D., Jonsson J.R., Powell E.E. Hepatic progenitor cell-mediated regeneration and fibrosis: chicken or egg? Hepatology. 2009;49:1424–1426. doi: 10.1002/hep.22893. [DOI] [PubMed] [Google Scholar]
- 30.Wynn T.A., Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandran P., Pellicoro A., Vernon M.A., Boulter L., Aucott R.L., Ali A., Hartland S.N., Snowdon V.K., Cappon A., Gordon-Walker T.T., Williams M.J., Dunbar D.R., Manning J.R., van Rooijen N., Fallowfield J.A., Forbes S.J., Iredale J.P. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dal-Secco D., Wang J., Zeng Z., Kolaczkowska E., Wong C.H., Petri B., Ransohoff R.M., Charo I.F., Jenne C.N., Kubes P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212:447–456. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlmark K.R., Zimmermann H.W., Roderburg C., Gassler N., Wasmuth H.E., Luedde T., Trautwein C., Tacke F. The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52:1769–1782. doi: 10.1002/hep.23894. [DOI] [PubMed] [Google Scholar]
- 34.Michalopoulos G.K., Barua L., Bowen W.C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., Haft A., Vries R.G., Grompe M., Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson W.R., Jr., Chi E.Y., Ye X., Nguyen C., Tien Y.T., Zhou B., Borok Z., Knight D.A., Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P., Horn A., Kireva T., Beyer C., Zwerina J., Schneider H., Sadowski A., Riener M.O., MacDougald O.A., Distler O., Schett G., Distler J.H. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viebahn C.S., Benseler V., Holz L.E., Elsegood C.L., Vo M., Bertolino P., Ganss R., Yeoh G.C. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500–507. doi: 10.1016/j.jhep.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., Basler K., Wntless a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 40.Wu J., Choi T.Y., Shin D. tomm22 Knockdown-mediated hepatocyte damages elicit both the formation of hybrid hepatocytes and biliary conversion to hepatocytes in zebrafish larvae. Gene Expr. 2017;17:237–249. doi: 10.3727/105221617X695195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P., Cai Y., Soofi A., Dressler G.R. Activation of Wnt11 by transforming growth factor-beta drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells. J Biol Chem. 2012;287:21290–21302. doi: 10.1074/jbc.M112.357202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X., Wu S., Xia Y., Li X.E., Xia Y., Zhou Z.D., Sun J. Wingless homolog Wnt11 suppresses bacterial invasion and inflammation in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G992–G1003. doi: 10.1152/ajpgi.00080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.