Abstract
Immunoglobulin heavy chain (IGH) clonality testing by next-generation sequencing (NGS) offers unique advantages over current low-throughput methods in the assessment of B-cell lineage neoplasms. Clinical use remains limited because assays are not standardized and validation/implementation guidelines are not yet developed. Herein, we describe our clinical validation and implementation of NGS IGH clonality testing and summarize our experience based on extensive routine use. NGS-based clonality testing targeting IGH FR1, FR2, FR3, and the conserved leader sequence upstream of FR1 was validated using commercially available kits. Data were analyzed by commercial and in-house–developed bioinformatics pipelines. Performance characteristics were evaluated directly comparing with capillary electrophoresis (CE) assays (BIOMED-2 primers). Assays were monitored after implementation (>1.5 years), concurrently testing by CE methods. A total of 1189 clinical samples were studied (94 validation, 1095 postimplementation). NGS showed superior performance compared with CE assays. For initial assessment, clonality detection rate was >97% for all malignancy types. Concordance with CE was 96%; discordances were related to higher sensitivity/resolution of NGS and improved detection in cases with high somatic hypermutation. Routine NGS clonality assessment is feasible and superior to existing assays, enabling accurate and specific index clone assessment and future tracking of all rearrangements in a patient sample. Successful implementation requires new standardization, validation, and implementation processes, which should be performed as a multicenter and multidisciplinary collaboration.
B-cell neoplasms are a heterogeneous group of disorders that vary widely in clinical behavior. In current practice, the diagnosis and stratification into specific subtypes is based on a combination of clinical characteristics, morphology, immunophenotype, and the identification of well-defined genetic alterations. Clonality assessment, although not strictly required to establish a diagnosis in many cases, is pivotal in the overall diagnostic assessment of the most challenging cases.
Ig heavy chain (IGH) gene rearrangements are the most widely applied targets for clonality assessment in B-cell lymphoid proliferations. Rearrangements start at the earliest stages of B-cell development with recombination events between one of the many V, (D), and J genes, which result in the formation of unique V(D)J sequences that ultimately encode the antigen-binding region of the Ig chain.1, 2 Variable addition or subtraction of nucleotides at the junctional regions in the process of recombination, and additional changes associated with somatic hypermutation later in development add further diversity to the antigen-binding regions. Because of the wide range of possible rearrangements, the ultimate attained diversity of Ig molecules is estimated to be in the order of 1012. Each rearrangement is independent and unique, with negligible probability that the same sequence would be shared by two unrelated cells.3 Therefore, when identical rearrangements are seen in a group of cells, they reflect the clonal nature of the population. Establishing the relationship of the rearrangements in a lymphoid population, as homogeneous versus heterogeneous, constitutes the basis of clonality testing.
For the past decade, PCR-based analysis of Ig/T-cell receptor rearrangements has been the gold standard method for clonality assessment.4 Standardized, low-throughput, multiplex PCR assays for nearly all Ig/T-cell receptor targets, established by the EuroClonality/BIOMED-2 consortium, have collectively shown an unprecedentedly high rate of detection in the most common B- and T-cell malignancies.3, 5, 6, 7, 8 The assays have been further optimized and widely commercialized by Invivoscribe, Inc. (IVS; San Diego, CA) to become the most broadly used assays for clonality testing in clinical laboratories. Current PCR-based assays identify clonality based on overrepresentation of amplified V-J or D-J products as separated by fragment size, using gel or capillary electrophoresis (CE). But despite their robustness, high reliability, and reproducibility, limitations for broader clinical use arise because of their relatively low sensitivity (approximately 5%). Also, because the electrophoresis-based assays cannot readily differentiate between clonal populations that have the same PCR product size but different sequences, the assay's utility in tracking an index clone at low level and in the setting of minimal residual disease is limited.
With the advent of next-generation sequencing (NGS) technology, the landscape of clonality testing is rapidly changing. NGS-based clonality assessment targeting IGH goes beyond the simple answer of clonal versus not clonal to establish a high-resolution image of all rearrangement events in a sample, enabling an accurate evaluation of the clonal relationship of multiple lesions, delineating intraclonal diversity in mature B-cell malignancies that undergo somatic hypermutation, and defining the stable and dynamic aspects of the immune repertoire. After treatment, residual disease can be better defined by tracking the behavior of specific clonal tumor populations, including their suppression, reemergence, and evolution, as well as the concurrent changes in the background repertoire.9, 10, 11 Compared with other targets of clonality assessment in B-cell malignancies, such as multigene mutation analysis,12, 13, 14 IGH rearrangements can be detected in >95% of all B-cell neoplasms, each unique to the patient and specific to the B-cell population, providing a highly specific and sensitive way of tracking a disease-associated clone.
Although several publications using NGS-based methods for clonality testing and minimal residual disease monitoring already exist, most remain confined to a proof of principle.9, 10, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Assay design, protocols, and data interpretation are highly heterogeneous, and in many cases proprietary, precluding their use as a meaningful guide for clinical validation and broad adoption into routine clinical practice. With the recent availability of commercial, off-the-shelf assays, and the impending transition of many clinical laboratories to NGS platforms, more institutions are considering the implementation of NGS-based clonality testing. The successful transition, however, will entirely rely on further optimization of existing assays, extensive clinical validation, standardization, and the generation of new guidelines that can be broadly used by clinical laboratories.
The fundamental aim of this study is to describe our validation/implementation of NGS-based B-cell clonality assessment in routine clinical practice, concentrating primarily on the initial characterization of an index clone that can be confidently used for monitoring purposes. We describe our extended clinical experience with the assay with direct comparison with a well-established standardized electrophoresis-based method (BIOMED-2) and outline some of the lessons learned in the process.
Materials and Methods
Tissue Procurement and Standard Electrophoresis–Based Clonality Testing
Clinical cases received for routine assessment of IGH clonality were identified. The lymphoma classification (World Health Organization 2016 revision),29 disease status, and results of ancillary studies (morphologic assessment, immunohistochemical analysis, flow cytometry, and molecular studies) were recorded. Genomic DNA was extracted from blood, bone marrow, and fresh or formalin-fixed, paraffin-embedded (FFPE) tissue, depending on the sample submitted for analysis; and IGH clonality testing was performed using commercial BIOMED-2 multiplex PCR master mixes and controls (IGH clonality assay tubes A, B, and C; Invivoscribe, Inc.), following manufacturer's protocol. Fragment length of the fluorescently labeled PCR products was analyzed by capillary electrophoresis on an ABI 3730 DNA analyzer (Thermo Fisher Scientific, Life Technologies Corporation, Carlsbad, CA). Results were interpreted according to latest EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/T-cell receptor clonality testing in suspected lymphoproliferations.3
Clonality Testing by Next-Generation Sequencing
NGS-based clonality testing was performed using commercially available LymphoTrack assays (Invivoscribe, Inc.) targeting IGH (FR1, FR2, FR3, and the conserved leader sequence upstream of FR1) and IgK, following manufacturer's protocols. Briefly, amplification by PCR was performed using one or multiple master mixes containing primers designed with barcoded sequence adaptors. After purification and quantification, libraries were sequenced on an Illumina MiSeq instrument (Illumina, San Diego, CA).
Data Analysis
Sequencing results were analyzed using the LymphoTrack MiSeq Software version 2.3.1 (Invivoscribe, Inc.) and an in-house–developed analysis pipeline30 for streamlined evaluation and visualization.
Our bioinformatics pipeline integrates demultiplexing, adaptor trimming, and FASTQ file generation from the output of the MiSeq sequencer. Paired-end reads are combined, and FASTA files are generated. Primer sequences are identified and trimmed, and identical sequences are removed. Each unique sequence is blasted using IgBLAST to search local IGH databases. IGH reference data are downloaded from the international ImMunoGeneTics information system (IMGT) website (http://www.imgt.org). The IgBLAST output is grouped on the basis of different VJ combinations, and the generated output is loaded into a MySQL database, along with hit details for each sequence. Visualization tools were generated for easy and streamlined review of results.
Clonality Determination
Empirical clonality calling rules for the validation followed the provided manufacturer's recommendations: >20,000 total reads, based on final LymphoTrack MiSeq Software output, was considered optimal coverage; a clonal call required the top merged reads (one or two) to be ≥2.5% and ≥3 times the percentage reads of the third most prevalent unique sequence (beginning of the background, after merging). Clonality rules were later adjusted on the basis of the validation data and experience developed during our implementation phase and monitoring of the assay (Table 1).
Table 1.
Empirical, Single-Center Clonality Rules
| Dominant sequences | ||||
|---|---|---|---|---|
| 1–2 | 3–4 | >4 | 0 | |
| Category 1: optimal results >100,000 total reads for all cases (>50,000 suitable in selected cases)∗ | ||||
| If dominant sequences are >2.5% of the total reads and >10× the polyclonal background |
Same VJ use Align and merge if related If 1–2 dominant clones (after additional merging) >2.5% of total reads and >10× the polyclonal background |
Different VJ uses >2.5% of total reads and >10× the polyclonal background |
Different VJ uses >2.5% of total reads and >10× the polyclonal background |
All merged sequences <2.5% |
| Monoclonal (monoallelic/biallelic) or biclonal | Monoclonal (monoallelic/biallelic) or biclonal | Oligoclonal | Oligoclonal | Not clonal |
| Category 2: qualified results (30,000–50,000 reads) | ||||
| If dominant sequences >5% of total reads and >20× polyclonal background |
Same VJ use Align and merge if related 1–2 dominant (after additional merging) >5% of total reads and >20× the polyclonal background |
Different VJ uses >5% of total reads and >20× polyclonal background |
Different VJ uses >5% of total reads and >20× polyclonal background |
All merged sequences <2.5% |
| Monoclonal (monoallelic/biallelic) or biclonal (confirm with other primer sets) |
Monoclonal (monoallelic/biallelic) or biclonal (confirm with other primer sets) |
Oligoclonal (confirm with other primer sets) |
Oligoclonal (confirm with other primer sets) |
Not clonal |
| Category 3: failure (<30,000) total reads | ||||
Qualified results should be interpreted with caution and should only be reported if the same clone is confirmed by another primer set.
A total of 50,000 to 100,000 reads suitable for analysis of a clonal sample provided that the polyclonal background is highly variable.
Initial Validation
To assess the performance characteristics of the NGS assays, a well-defined set of archival diagnostic samples for which clonality assessment by fragment analysis (FR1 tube A, FR2 tube B, and FR3 tube C primers) had already been performed was selected. Cases were preselected to encompass a broad range of sample types and tumor content. Dilution studies to assess the limit of detection of a clone for initial characterization were performed using a commercially available sensitivity panel set (Invivoscribe, Inc.) containing a well-characterized clonal control DNA (IVS-0019) diluted into a standard polyclonal negative control DNA from tonsil (IVS-0000) at 100% (undiluted sample), 30%, 20%, 10%, 5%, and 1%. Separate, noncommercial dilutions of 5%, 2.5%, and 1% and further dilutions of 0.1%, 0.01%, and 0.001% were prepared to assess low-level detection of the index clone within a polyclonal background.
Interassay and intra-assay reproducibility were assessed using patient samples, all tested in triplicate, in the same run and on three separate runs, on different days and different instruments by two different technologists. To avoid confounding due to use of common barcodes, interassay replicates were assigned unique barcodes.
Minimum input requirements for detection of a clone at low level were assessed using a previously characterized patient sample containing two clones and sequentially decreasing amounts of total DNA template from 500 to 25 ng. Dilutions of lymphoid-derived DNA (IVS-0013) into nonlymphoid DNA (epithelial cell line NCI H1975; ATCC, Manassas, VA) were also prepared using a total DNA input of 250 ng with the lymphoid DNA component sequentially decreasing from 125 to 0.0025 ng.
Implementation and Monitoring of the Assay
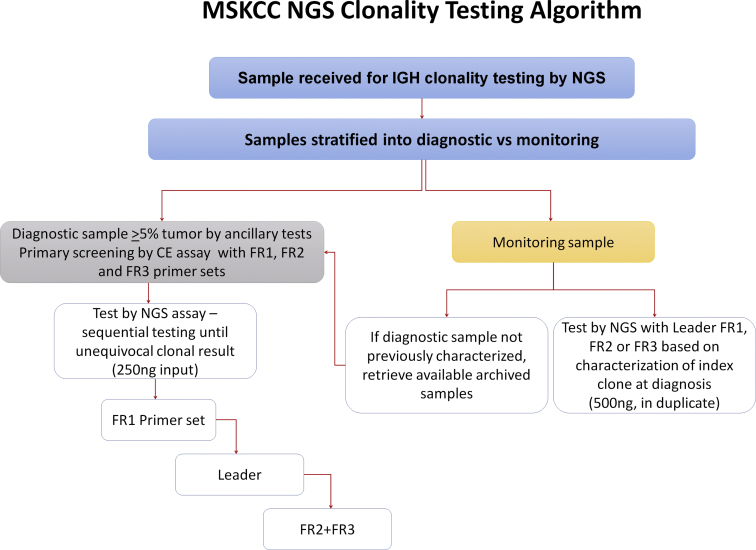
After initial validation, the assay performance was assessed for 1.5 years. All samples submitted for initial characterization were concurrently tested with routine clonality CE and NGS assays. Sample criteria for initial characterization included a formal diagnosis of a lymphoid or plasma cell neoplasm and at least 5% tumor content based on concurrent flow cytometry and/or morphologic assessment. Samples submitted for monitoring were tested by NGS only, in duplicate, and results were compared with flow cytometry results, performed according to established protocols31 and other ancillary studies, including morphology, immunohistochemistry, and molecular tests, when available. For monitoring samples without prior characterization of the diagnostic clone, an archival sample with high tumor content was evaluated to characterize the clonal sequence associated with the neoplasm. The algorithm for testing during the monitoring phase of the assay is outlined in Supplemental Figure S1.
Results
Initial Validation Study for Characterization
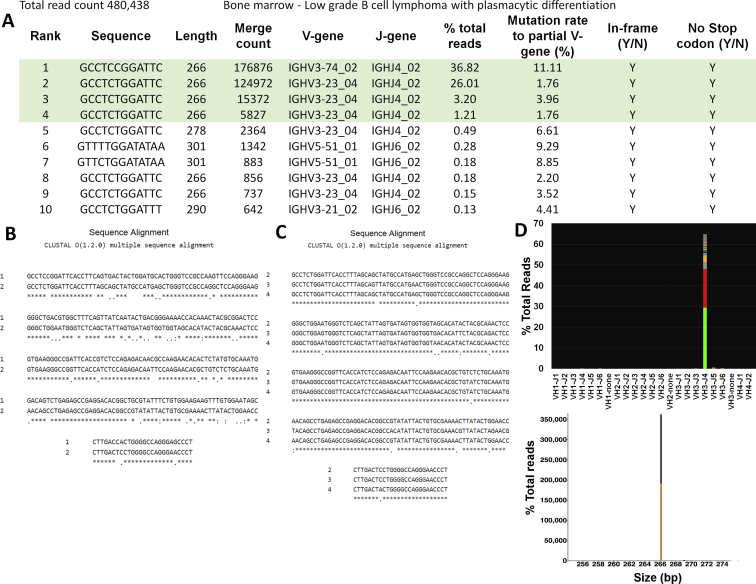
A total of 94 samples were tested as part of the initial NGS validation study. All 94 were tested with FR1, and subsets were tested with leader, FR2, and FR3. Representative results are presented in Figure 1, A–C, and Supplemental Table S1. Total read counts averaged 475,650 (range, 45,326 to 2,829,232). In this preselected set, the overall concordance between assays was 96% (90/94) (Figure 1D). Discrepant results were found in four cases (three plasma cell neoplasms, one diffuse large B-cell lymphoma), all clonal by NGS and polyclonal by fragment analysis. Retrospective review of the CE tracings showed slightly prominent peaks within a high polyclonal background in all four cases, whereas the NGS assay uncovered a clear dominant sequence, high above the background sequences (>50 times higher than the third most prevalent sequence after merging). Details of each sample are included in Supplemental Table S2.
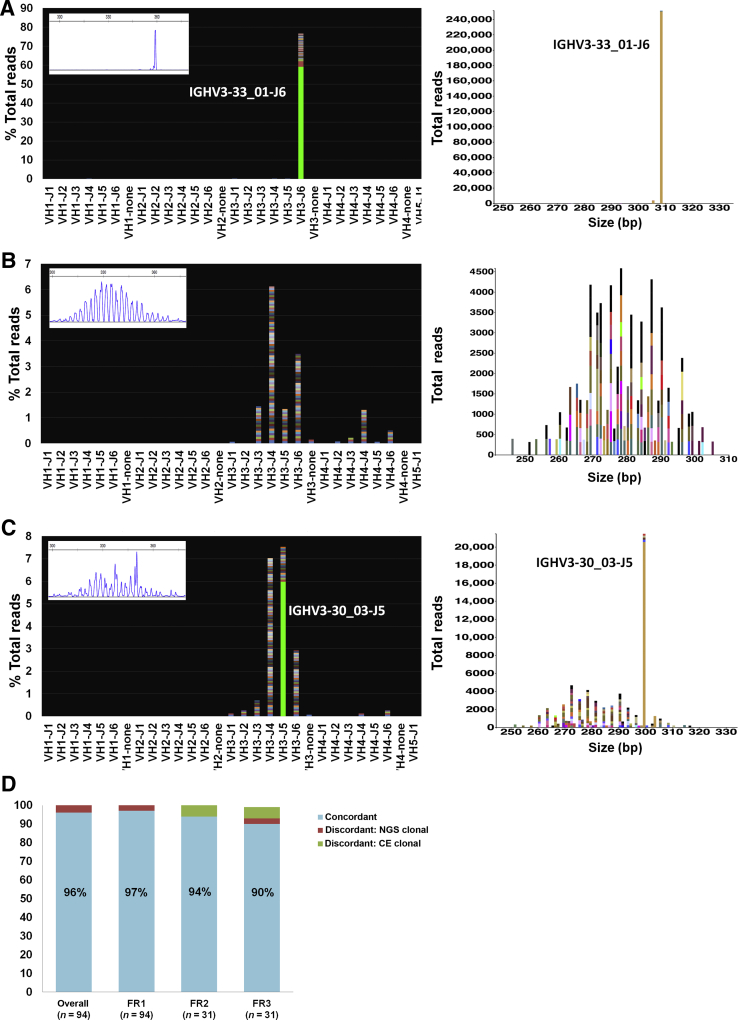
Figure 1.
Representative examples from the validation set. A: Clonal case (Sample 16, diffuse large B-cell lymphoma, formalin-fixed, paraffin-embedded tissue; 330,591 total reads). Left panel: Snapshot of the V-J sequence frequency graph showing the distribution of reads stratified by sequence and family. Most reads are restricted to a rearrangement using V3-33 and J6 family and share an identical sequence, as exemplified by the solid green of the bar, whereas reads with slightly different sequences are stacked on top in various colors. Inset: Corresponding capillary electrophoresis (CE) results (FR1 BIOMED-2 primers). Right panel: Sequences plotted by the Memorial Sloan Kettering (MSK) LymphoClone viewer graphically display the clonal reads based on size and V-J use. B: Polyclonal case (Sample 63, no disease, BM aspirate; 470,767 total reads). Left panel: Snapshot of the V-J sequence frequency graph shows multiple, multicolored bars with a variable distribution of VJ use. Each colored band on a bar denotes a different sequence. Inset: Corresponding CE results (FR1 BIOMED-2 primers). Right panel: MSK LymphoClone viewer shows multicolored bars arranged by size and VJ use in a gaussian distribution typical of a polyclonal pattern. C: Discrepant case (Sample 48, plasma cell neoplasm involving BM, 4% by aspirate differential count; 353,964 total reads). Left panel: V-J sequence frequency graph shows a dominant clonal sequence (solid green bar) within a polyclonal background (multicolored bars). Inset: Corresponding CE results (FR1 primers) show a polyclonal pattern with a slight prominent peak at approximately 350 bp without a definitive clonal Ig heavy chain (IGH) rearrangement. Right panel: Corresponding MSK LymphoClone viewer plot shows a solid yellow bar depicting the clone within a polyclonal background. D: Validation accuracy study for the FR1, FR2, and FR3 primer sets. The overall concordance for a clonality call based on all primer sets targeting IGH was 96%, with superior clonal detection using next-generation sequencing (NGS). Results stratified by individual primer sets show higher discordance rate for FR3 primers.
Comparing the individual primer sets side by side, the concordance for NGS versus CE was 97% (91/94) for FR1, 94% (29/31) for FR2, and 90% (28/31) for FR3 (Figure 1D). Comparison of FR1 and leader NGS primers showed 98% concordance (41/42), with one case not clonal by leader but clonal with FR1 primers.
Limit of Detection and Linearity
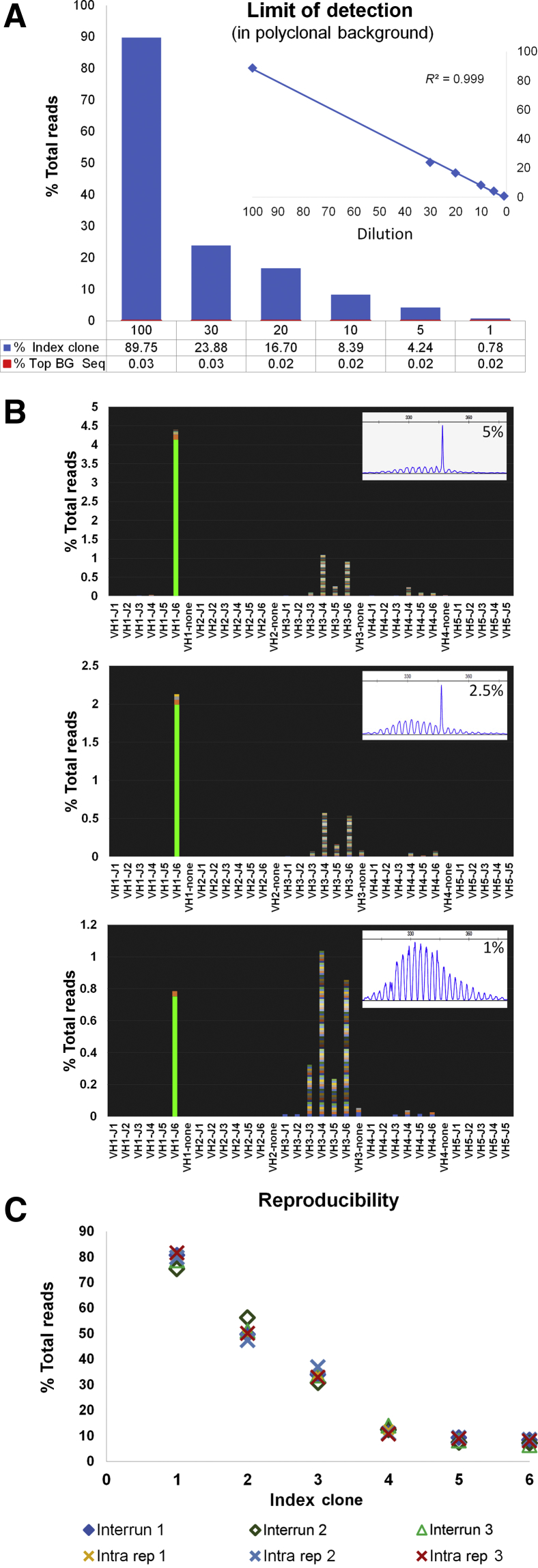
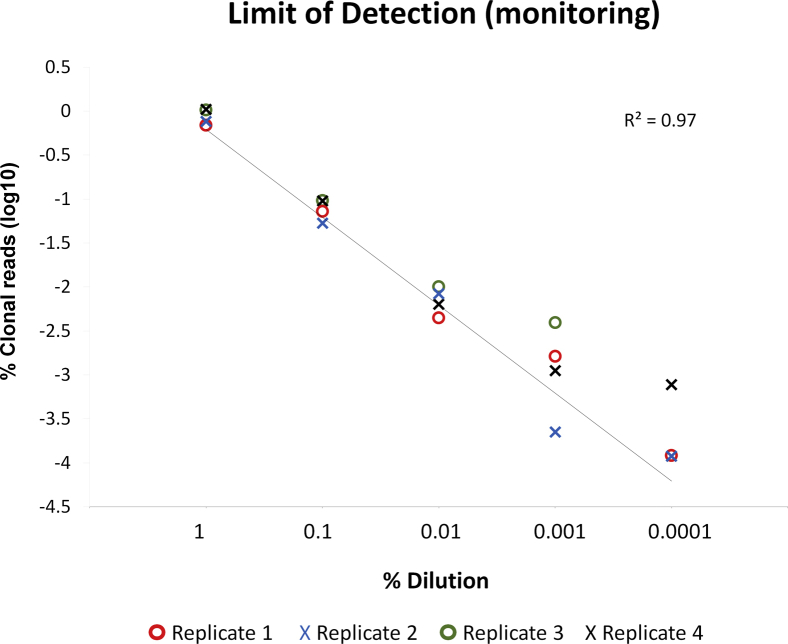
Results of the dilution series to establish the limit of detection of a clone for initial clonal characterization are summarized in Figure 2, A and B. Using an input of 250 ng in all reactions, the index clonal sequence could be detected as a dominant sequence high above the background at all dilution levels with good linearity. Concordance with the fragment analysis assay was seen down to the 2.5% dilution. Results of the serial dilution to determine detection of a known clone hidden in a polyclonal background are summarized in Supplemental Figure S2. Using an input of 2 μg distributed in four replicate reactions (500 ng each), the index clonal sequence could be detected in all replicates down to the 0.001% dilution.
Figure 2.
Limit of detection and reproducibility studies. A: Limit of detection for characterization: clonal control DNA (IVS-0019) diluted into a polyclonal DNA (tonsil, IVS-0000) at 100% (undiluted sample), 30%, 20%, 10%, 5%, and 1%. The index clone is detected as a dominant sequence at all dilution levels. Details of the index clone detection (expressed as a percentage of the total reads) and the level of the polyclonal background [expressed as the percentage of the top background (BG) sequence (Seq)] under each bar. Inset: Linearity across the entire dilution series. B: Snapshots of the V-J sequence frequency graph at the 5%, 2.5%, and 1% dilutions show the index clone as the dominant sequence, high above the polyclonal background (220×, 104×, and 39×, respectively). Insets: Corresponding capillary electrophoresis results (FR1). C: Interassay and intra-assay reproducibility studies tracking six clones in three patient samples. Samples tested in triplicate in the same run (intra-assay rep 1, 2, and 3) and on different runs (interassay runs 1, 2, and 3). Clonal calls were 100% reproducible with minimal variability in the percentage of total reads. IVS, Invivoscribe; rep, replicated.
Precision and Reproducibility Studies
Interassay and intra-assay reproducibility studies were performed on the basis of three patient samples: two clonal sequences were tracked for each (total six clonal sequences). Results are summarized in Figure 2C and Supplemental Table S3. Clonal calls were 100% reproducible, with low variability in the percentage of total reads supporting the rearrangement (SD, 0.8% to 3.3%).
DNA Input Studies
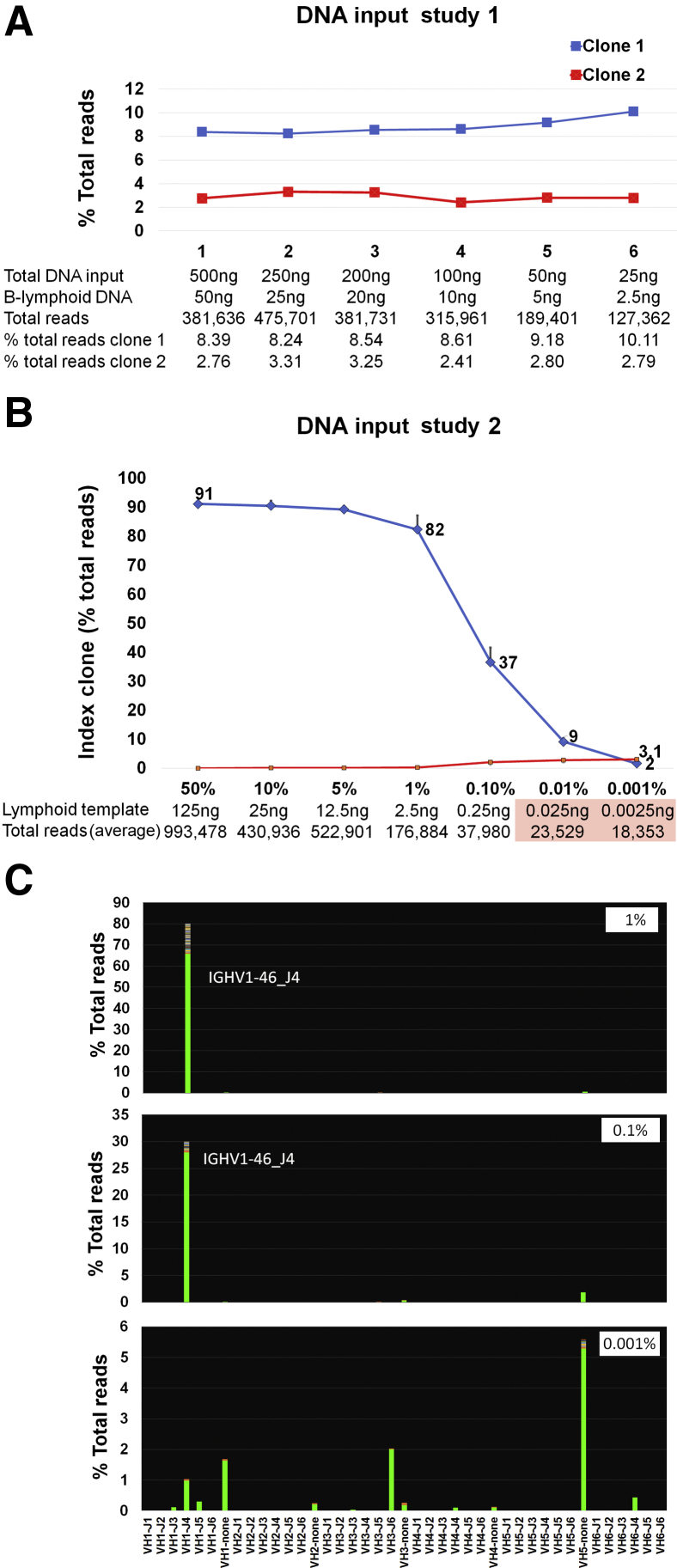
Minimum input requirements for detection of a clone when present at low level are summarized in Figure 3A. For this study, a previously characterized clinical sample known to have two clones was used and specifically selected for the presence of the clones at low level. On the basis of flow cytometry, the B-cell component on this sample was approximately 10%. Decreasing the total DNA input down to 25 ng has no significant effect on clone detection.
Figure 3.
DNA input studies. A: DNA input study using decreasing amounts of total DNA from a patient sample containing approximately 10% B-lymphoid cells. Total DNA input and estimated B-cell–derived DNA detailed at each level. Decreasing the total DNA input from 500 to 25 ng has a moderate effect on the total number of reads but no significant impact on clone detection. B: DNA input study using decreasing amounts of clonal lymphoid DNA into epithelial DNA. Total DNA input is fixed at 250 ng. Graph shows the level of detection of the index clone in a dilution series with sequentially decreasing amounts of clonal lymphoid DNA diluted into epithelial DNA (four replicate reactions performed for each dilution). On the x axis, seven dilutions are shown (50%, 10%, 5%, 1%, 0.1%, 0.01%, and 0.001% lymphoid component in a total DNA input of 250 ng). Details of the estimated lymphoid component at each level and total number of reads generated are seen below. In the undiluted characterized lymphoid sample, the clonal sequence encompassed 90% of the total rearranged Ig heavy chain reads. This clone, and its relative proportion, remained constant down to the 12.5-ng input. Further decreases in the lymphoid DNA input lead to a sequential decrease in the overall proportion of the index clone and preferential amplification of nonspecific (pseudoclonal) sequences. Further decreases in the lymphoid DNA input lead to a sequential decrease in the overall proportion of the index clone and preferential amplification of nonspecific (pseudoclonal) sequences, ultimately leading to a failure pattern as total reads decrease below 30,000 (pink box). C: Representative views of the V-J sequence frequency graphs at 1%, 0.1%, and 0.001%. The 0.001% dilution shows a failure pattern, with multiple bars depicting nonspecific sequences with no J segment; the index clone is not detected.
Effective DNA Input Assessment
Given that IGH primers only amplify DNA from cells in which IGH is rearranged and not from cells with IGH in germline configuration, measuring total DNA does not adequately reflect the available effective template in mixed samples. To further assess the performance of the assay in samples with low effective template, a dilution study of sequentially decreasing amounts of clonal lymphoid DNA diluted in epithelial DNA was performed (fixed total DNA input of 250 ng), and results are summarized in Figure 3, B and C. Four replicates of each dilution support consistent detection of the clone at the expected level down to the 1% dilution (2.5 ng lymphoid-derived DNA). Beyond this level, clonal detection is compromised and total clonal reads decrease below 50,000. Inputs generating <30,000 reads show a failure pattern.
Implementation and Monitoring of the Assay
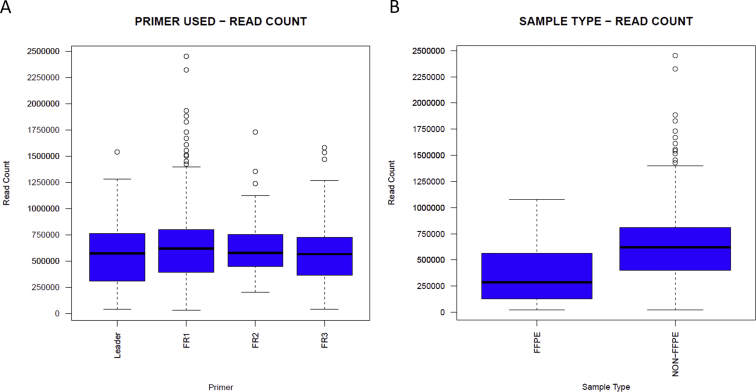
After initial validation, the performance of the NGS assays was closely monitored for 1.5 years. During this time, 1095 IGH rearrangement studies by NGS were performed: 775 for initial characterization (11 lymph node/soft tissue aspirates, 8 fresh tissue biopsy specimens, 267 blood, 438 bone marrow aspirates, 51 FFPE tissue) and 320 for monitoring (302 bone marrow, 16 blood, 2 fresh tissue). Characterization samples were unique (single sample per patient at the time of initial diagnosis at our institution). Monitoring cases encompassed multiple follow-up samples from the patients previously characterized (range, two to six per patient). All characterization cases were confirmed to represent a B-cell or plasma cell neoplasm by a combination of morphology, flow cytometry, and molecular findings, with at least 5% tumor content. The overall distribution of the samples by disease type is summarized in Supplemental Table S4. The failure rate for characterization samples was 1.2% (9/775), defined as low-sequencing read count of <30,000 reads (based on adjusted parameters established after initial validation). Common reasons for failure included insufficient amplification in multiple attempts despite adequate starting total DNA input but relatively low tumor content, <8% (six bone marrow samples: four plasma cell neoplasms, one hairy cell leukemia, one lymphoplasmacytic lymphoma); and low starting total DNA, <50 ng (two FFPE samples and one soft tissue aspirate). For successfully sequenced samples, total read counts averaged 622,242 (range, 31,684 to 2,451,851) (Supplemental Figure S3A for stratification by primer sets). A total of 96% (736/766) of cases had total read counts >100,000. FFPE samples generally showed lower total number of reads and a higher failure rate compared with non-FFPE samples [4% (2/51) versus 0.8% (6/724), respectively] (Supplemental Figure S3B).
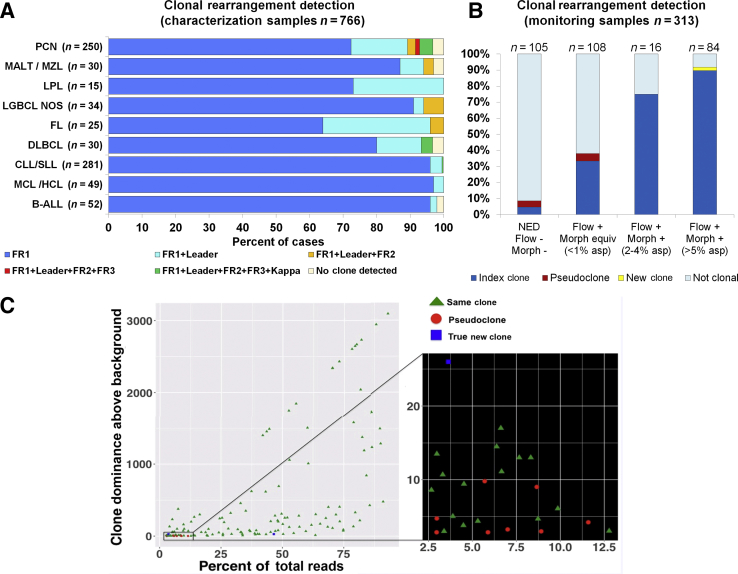
Initial characterization was performed using a sequential approach (Supplemental Figure S1). Of the 766 characterization samples successfully sequenced, 709 (92.6%) were clonal by our standard BIOMED-2 CE assays (FR1, FR2, and FR3 primers). The NGS assays detected a clone in all 709 samples and in 34 additional cases (4.4%, 34/766), for an overall clonality detection of 97% (743/766). Retrospective review of the CE tracings in discrepant cases showed prominent peaks in 24 cases, which did not meet criteria for clonality by CE assay, but findings by NGS methods allowed unequivocal clonal calling because of the higher resolution of the background. Four cases were clonal with leader primers only. Twenty-three cases (3%, 23/766) remained nonclonal despite adequate tumor content of ≥5% (range, 5% to 68%, based on combination of flow cytometry, morphology, and immunohistochemistry). With the additional use of Ig κ primers (investigational use only, not included as part of this validation study), 12 additional cases were classified as clonal. Eleven cases (1.4%, 11/766) remained polyclonal after all testing. Results are summarized in Figure 4A and Supplemental Table S4.
Figure 4.
Summary of clonal characterization results in cases during the first year of assay implementation. A: Characterization cohort after implementation. Using a sequential testing approach (FR1 > leader > FR2 + FR3), overall clonality detection was 97%. Most disease categories (95%) can be successfully characterized with FR1 and leader primers only. Disease categories susceptible to somatic hypermutation show a greater number of cases requiring several primer sets for adequate characterization. With the use of κ primers (investigational), the overall detection rate would increase slightly to 98%. See details in Supplemental Table S4. B: Primary clonality assessment using monitoring samples. Samples were stratified into four categories: i) no evidence of disease (NED), ii) disease by flow only, iii) disease by flow and morphology at 2% to 4%, and iv) disease by flow and morphology at ≥5%. A clone was detected in 44% of all cases (9%, 38%, 75%, and 92% in categories 1 to 4, respectively). In 92% of the clonal cases (129/140), the detected clone was identical to the index clone of the original diagnostic sample. Ten cases had a different clone (eight deemed to represent pseudoclones and two new clones). C: The details of clones detected in the monitoring samples: scatter plot shows the relationship between the level of the detected clone (x axis, clone percentage of total reads) and its dominance above the background sequences (y axis). All sequences determined to represent a pseudoclone were detected in the context of a high polyclonal background (dominance of clone over the background, <10×), as seen in the expanded section to the right. All other clones were detected at >10×. Raising the cutoff to >10× for all samples would increase the specificity to 100% at the expense of some sensitivity (sensitivity would decline to 91% based on similar analysis using a receiver operating characteristic curve). This would affect primarily samples with tumor content <2%. n = 766 (A); n = 313 (B). asp, aspirate; B-ALL, B-cell acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; equiv, equivalent; FL, follicular lymphoma; HCL, hairy cell leukemia; LGBCL, low-grade B-cell lymphoma; LPL, lymphoplasmacytic lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma; MCL, mantle cell lymphoma; morph, morphology; MZL, marginal zone lymphoma; NOS, not otherwise specified; PCN, plasma cell neoplasm; SLL, small lymphocytic lymphoma.
Among 320 monitoring samples, 313 (from 218 patients) were successfully studied. Seven samples failed because of inadequate amplification (<30,000 reads) in the context of low or virtually no B-cell component based on concurrent assessment by flow cytometry. All monitoring samples were tested with one primer set only, based on results of the initial characterization.
Monitoring samples in this study were specifically used to assess our ability to detect a clone in cases with low tumor content (<5%), fine-tune our criteria for clonality assessment, and confirm the accuracy of the initially characterized clone. Samples were first stratified into four categories based on the presence or absence of disease and degree of involvement by a combination of flow cytometry and morphology. Each sample was independently analyzed for evidence of clonality while blinded to the information of the previously characterized index clone. Results were then compared with the initial sample and with all other monitoring samples from the same patient. A clone was detected in 44% (139/313) of the samples. Each clone was annotated with the percentage clonality (percentage of total reads), its dominance above the background (percentage total reads for the clone divided by the percentage total reads for the third most prevalent clonal sequence after merging), and the relationship to the index clone (same versus different). A total of 93% (129/139) were identical to the clone previously characterized, whereas 7% (10/139) harbored a different clone. Two were confirmed to be true emerging new clones based on increasing and persistent presence in subsequent samples with higher disease. Both still harbored the original index clone at low level. The remaining clones (eight) were deemed to represent pseudoclones based on equivocal evidence or absence of morphologic disease and the lack of the clones in prior or subsequent samples from the same patient. No evidence of contamination was identified on the basis of a search for the same clone in other samples in the same run. All pseudoclones were detected at low level, between 2.9% and 11% of the total reads, and in the context of low dominance above the polyclonal background, <10×. The polyclonal background showed low variability. Results are summarized in Figure 4, B and C.
Discussion
In the present study, we described our validation and extended clinical experience with the commercial NGS-based clonality assays from Invivoscribe. We specifically concentrated on the use of the assays for the assessment and characterization of an index clone that could be confidently used for monitoring and in staging samples. For this purpose, we developed stringent criteria for clonality calling, which took into consideration the original guidance from the product developer, validation results, and lessons learned from our extended experience with the assay of >1.5 years. Every case in this article was reviewed by at least two pathologists who are dual trained and board certified in hematopathology and molecular genetic pathology, interpreting the information in the context of all clinical and pathologic information available. The interpretation rules and some of the technical and biological pitfalls encountered, along with potential solutions, have been consolidated in Tables 1 and 2 and associated Supplemental Figures S4–S6, which other users may find helpful in their own validations.
Table 2.
Pitfalls and Technical Considerations Based on Our Experience with the Assay
| Pitfalls | Potential reasons | Suggestions and potential solutions |
|---|---|---|
| 1–2 dominant sequences <2.5% but well above the polyclonal background, >20× | True clone but low tumor content True clone but inefficient PCR because of improper annealing of primers Targeted immune response with dominant specificity |
Repeated testing with additional primers or test a separate sample with higher tumor content If similar results but not meeting criteria for clonality, recommend calling dominant clonal sequence and suggest additional testing in other samples to confirm this represents the index clone. |
| Multiple dominant sequences (same VJ use) after merging—individually do not meet criteria for clonality (>100,000 reads) | Usually related sequences Several single-base changes and clonal drift associated with ongoing SHM Small insertions/deletions in repetitive regions of the genome—technical or biological |
Align sequences and establish relationship If closely related, add percentages and reassess Individual clonal sequences will need to be tracked separately in future samples (Supplemental Figures S4–S6) |
| Multiple dominant sequences (different VJ uses) and low number of total reads <100,000 | Low specific template or inefficient PCR because of improper annealing of primers | Repeated testing with higher DNA input and additional primer sets |
| Multiple dominant sequences of different VJ use, high total reads (>100,000 reads), and absent or low polyclonal background | Multiple populations—consider biclonal and biallelic rearrangements | Review the clonal frequencies and prediction of productive versus nonproductive to help predict if biclonal and biallelic subsets. Biallelic clones: generally same frequency, 1 productive and 1 nonproductive. Biclonal: widely different frequency >10% both productive or nonproductive Interpret in the context of other ancillary studies. If flow shows several populations, consider retesting on segregated populations. |
| Low read count despite adequate total DNA input | Low specific template or inefficient PCR because of improper annealing of primers | Check B-cell/plasma cell content by histology and flow cytometry. Repeat with multiple primer sets and adjust DNA content to ensure at least 10 ng of DNA is from lymphoid or plasma cell component |
| Clonal products with no V or J segment (specified as none) | Short V or J segment precludes family assignment or miss-priming events | Align the sequence to IMGT or BLAT and confirm it represents a true rearrangement. Repeat with additional primer sets to confirm If miss-priming event, do not call clonal If true rearrangement but J segment is too short for family name assignment, can call clonal and use the clone for future tracking Cases with low number of reads have several miss-priming events—very short truncated segments or long products with repetitive regions that do not constitute a true rearrangement. Many of these may be automatically filtered out by the Invivoscribe software and would only be seen if data are reanalyzed with other software (Supplemental Figure S6). Cases with more than one miss-priming event should be interpreted with caution—repeat with other primer sets. |
| Other technical considerations |
|---|
| Establishing the background: In a true polyclonal background, each sequence is generally <0.5% of all of the rearranged reads. In most clonal cases, background starts at sequence rank 3. In cases with ongoing SHM, multiple dominant sequences of same use can be seen and require additional merging. The polyclonal background may be shifted beyond rank 4. For an adequate clonal determination, the polyclonal background should be highly variable. In cases with low read count, multiple clonal signals, and a background with low variability (oligoclonal pattern), recommend retesting with higher DNA input and additional primer sets. Additional merging: Refer to examples in Supplemental Figures S4–S6. In cases with ongoing somatic hypermutation, differences among reads may exceed two bases and may require additional merging for final clonal determination
|
BLAST, basic local alignment search tool; BLAT, BLAST-like alignment tool; IMGT, international ImMunoGeneTics information system; SHM, somatic hypermutation.
Because the intended use for the NGS assay was to establish the identity of a disease-associated clone for future monitoring, the initial sample acceptance criteria for characterization were set out to include only samples with unequivocal evidence of disease and tumor content of at least 5%. This cutoff was initially established on the basis of our validation because it provided the closest concordance with the fragment analysis methods. In our review of all characterization samples in this article, this cutoff provides high resolution of a clone across most sample types. At this level, even in the presence of a predominant polyclonal B-lymphoid component, a clone can be detected at >2.5% of the total clonal reads while still remaining high above the polyclonal background (generally >20×). Through the analysis of the monitoring samples, however, it was demonstrated that the index clone could still be characterized in many cases with lower tumor content, depending on the composition of the nonneoplastic component, but with significantly lower overall success rate. Importantly, as tumor content decreases, there is a higher risk of overcalling clonal sequences that are not the disease-associated clone (pseudoclones) when the original criteria for clonal calling are used (threshold of 3× the background). This is related to low effective template and low background variability. Applying more stringent rules for the dominance of the clone over the background to 10× maximizes specificity at the expense of sensitivity, primarily affecting low tumor content cases (<2%) but would have no significant impact in cases with higher tumor content. In the characterization cohort, cases not meeting this more stringent criteria were rare (0.5% of the cases; 4/745), all associated with relatively low tumor content (range, 5% to 20%) and with high somatic hypermutation. On the basis of these findings, final clonality rules were adjusted to this more stringent threshold for all samples. For initial characterization, it is still recommended that assessment be performed in samples with unequivocal morphologic disease of at least 5% for maximal success rate.
An important consideration for test implementation, given the amplicon-based approach of the NGS assays, was to assess PCR bias and potential impact on clonality calling. Extensive work has already been performed by Invivoscribe, Inc., to optimize the consensus primer sets and ensure appropriate balance and functionality in a multiplex setting. Through the validation, it was demonstrated that the primer sets perform similar to the corresponding standardized fragment analysis assays. Accuracy studies in the validation cohort were performed using at least two primer sets on most cases and in different PCRs. In all cases, identical clonal sequences were detected despite the use of completely different primers, confirming that the clone detected was a true clone and not due to PCR bias of a specific primer set. Consistent detection of the clone was also confirmed over a wide dilution range and DNA template inputs, at the expected levels and with excellent linearity when all other nonclonal rearrangements were also present in the background.
In cases with low specific template, selective amplification of short and nonspecific fragments may compromise the detection of the clone and, occasionally, lead to overcalls if stringent rules for clonality calling are not established. Defining minimum input requirements for clonality assessment is challenging because the effective template is not defined by the total DNA input but the proportion of DNA derived from cells of B lineage. On the basis of results of the minimal input studies and overall experience working with the assay, when the lymphoid-derived template decreases below 10 to 15 ng, clonal detection and characterization is compromised by several factors, including a marked decrease in the number of supporting total reads, a decline in the expected frequency of the index clone, and a concurrent increase in nonspecific sequences that do not represent true rearrangements (generally, fragments with no characterized V or J segment and decreased V coverage). Adjustments to the original clonality rules were made to minimize errors, requiring at least 50,000 total reads as the minimum cutoff for assessment of clonality (>100,000 preferred) in combination with stringent rules for clonal calling, as described in Table 1. Occasionally, a cutoff of 30,000 could be used, but this should only be considered in cases with high tumor content if no other sample is available and if the same results are supported by two or more primer sets. Our recommendations for DNA input are summarized in Supplemental Table S5.
A technical aspect intrinsic to NGS technology that is important to mention is the issue of sample crossover contamination and its potential impact in the assessment of clonality and characterization. Similar to findings by other authors,32 the analysis of the NGS data for this study has identified low, but clearly detectable, levels of sample misalignment or barcode contamination within individual runs. When specifically searched for, the index sequence of a characterization sample could be identified in other samples within the same run. These sequences consistently remain <0.05% of the total reads and hidden within the polyclonal background. As such, this has no impact on the accurate detection and characterization of an index clone but would have important implications for monitoring samples if a sample from the same patient with high level of disease is tested at the same time. To prevent this, staging or monitoring samples are never run together with the diagnostic sample from the same patient in the same run. Similarly, low-level run-to-run carry over can also be detected when standard washing protocols are used. Accordingly, more stringent washing protocols, as recommended by Invivoscribe, Inc., and Illumina are followed for highly sensitive applications (using dilute sodium hypochlorite solution), which eradicates the problem. As an additional precaution, a rotating MiSeq instrument schedule is used so that samples from the same patient are not run in the same MiSeq instrument within three runs. As part of the quality control process, the positive control sequence and the detected index sequence of each characterization sample in all other samples of the same run (including the negative control) were searched to confirm the diagnostic sequences do remain at low level even in samples with lower coverage.
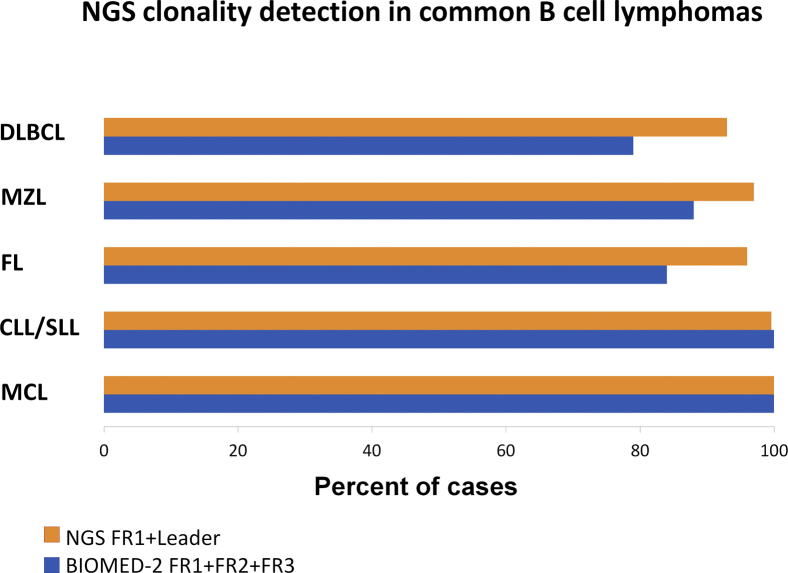
Overall, we find that the commercial Invivoscribe NGS assays for IGH clonality assessment collectively show an unprecedentedly high rate of clonal detection in B-cell and plasma cell neoplasms, averaging 97% and 93%, respectively. Compared with existing standardized BIOMED-2 CE assays targeting FR1, FR2, and FR3, the NGS assays show superior detection in cases with lower tumor burden because of sequence specificity, allowing higher resolution of prominent peaks partially hidden in a polyclonal background. The addition of the leader primers also provides higher clonal detection rate in cases with high somatic hypermutation. Using the stepwise testing approach, which was primarily dictated by the order of commercial availability of the primer sets, it was shown that the combination of leader and FR1 primers enables clonal detection in >95% of all B-cell malignancies tested. This is similar, and in most disease settings higher, to the detection rate of standardized assays using FR1, FR2, and FR3 together, based on published data (Supplemental Figure S7).7, 8 Subsequent use of the FR2 and FR3 primers did not significantly increase the clonal detection rate in most tumor types in our cohort, but they were found to be particularly useful in challenging cases with significant somatic hypermutation and in some FFPE samples in which DNA was highly fragmented. However, the performance of specific primer sets is highly dependent on the integrity of the DNA used. Our described experience is based on clinical samples of high quality, uniformly processed at our institution with optimized and standardized protocols and undergoing minimal storage time. Because DNA fragment sizes and the proportion of amplifiable DNA from FFPE samples are critically affected by the fixation method, processing protocols, and length of storage, it can be expected that in more degraded samples, the success rates would favor FR2 and FR3 primers rather than FR1. In our broader experience with nonclinical FFPE samples, high failure rates of >25% have been seen for leader primers and between 6% and 10% for FR1, FR2, and FR3 primer sets.
Similar to previously published observations for the standard assays, approximately 6% to 7% of diffuse large B-cell lymphoma and plasma cell neoplasms remain without a detectable clone when only IGH is targeted and would benefit from IGK testing to maximize detection.7, 8 Details of the validation and experience using the IGK NGS assay will be included in a separate article. In our limited experience so far, IGK testing would boost the detection rate on the most challenging cases to >97%, which is similar to what is reported using the full spectrum of BIOMED-2 primers.33
In conclusion, the assessment of clonality by NGS methods in routine clinical practice is feasible and provides distinct advantages over previous low-throughput methods. The successful implementation of the new technology comes with many challenges because it still requires the development of new standardization, validation, and implementation guidelines through the concerted effort of multiple institutions and various specialty fields: immunobiology, immunoinformatics, molecular pathology, and hematopathology, to name a few. To our knowledge, this study constitutes one of the first large reports of the use of NGS-based clonality testing as a routine clinical assay, describing the validation and implementation processes and documenting extended assay monitoring with direct comparison to established robust standard methods. We summarize our single-institution experience, emphasizing that the reliable assessment of clonality by NGS methods for future use in disease monitoring is not solely dependent on the quality and reproducibility of the assay and protocols. Extended experience in the interpretation of results and the integration of such results with the clinical, morphologic, and immunophenotypic assessment of each patient sample represents the basis for exploiting the full potential of this powerful new technology.
Acknowledgments
We thank Invivoscribe, Inc., for technical support with next-generation sequencing assays; and the members of our fragment analysis team for overall support.
Footnotes
Supported by the NIH Comprehensive Cancer Center Core grant P30 CA008748 (Memorial Sloan Kettering Cancer Center).
Disclosures: M.E.A. has received honoraria from Invivoscribe, Inc.
Supplemental material for this article can be found at https://doi.org/10.1016/j.jmoldx.2018.10.008.
Supplemental Data
Supplemental Figure S1.
Algorithm for clonality testing at Memorial Sloan Kettering Cancer Center (MSKCC). CE, capillary electrophoresis; IGH, Ig heavy chain; NGS, next-generation sequencing.
Supplemental Figure S2.
Limit of detection for tracking of a known clone at low level. Dilutions of 1%, 0.1%, 0.01%, 0.001%, and 0.0001%. Four replicates are shown for each to assess reproducibility of detection. The known clonal sequence could be consistently detected in all replicates down to the 0.001% dilution. Higher variability with respect to the percentage of the index clone is seen at levels beyond 0.01%. At the 0.0001% dilution, the clonal sequence is detected in only three of four replicates.
Supplemental Figure S3.
Comparison of total read counts by primer set and sample type. A: Comparison of total read counts in the characterization subset stratified by primer set. No significant difference is seen among primer sets. Higher number of outliers in the FR1 subset is related to the higher number of samples sequenced with FR1. B: Comparison of final read counts of formalin-fixed, paraffin-embedded (FFPE) samples compared with non-FFPE samples (blood, bone marrow aspirates). The FFPE samples often generate lower read counts but remain >100,000 in most cases. n = 775 (A); n = 51 (B, FFPE samples); n = 724 (B, non-FFPE samples).
Supplemental Figure S4.
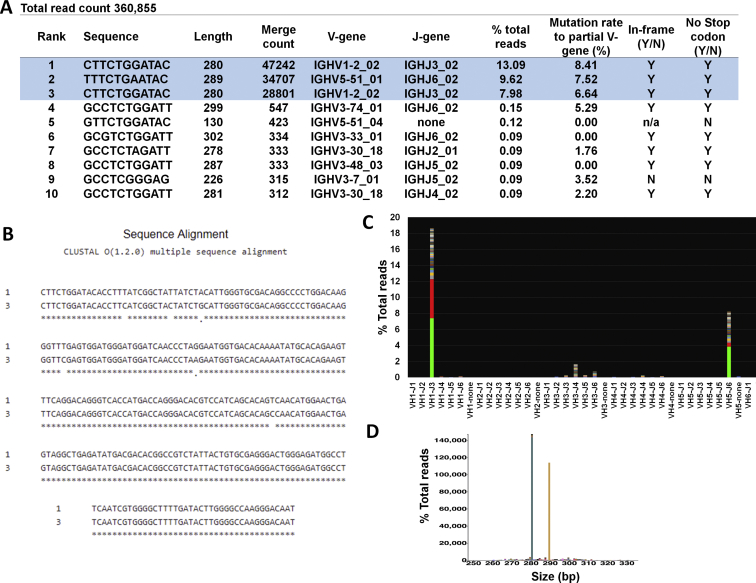
Representative example requiring additional merging in the final analysis. A: On the basis of the merged read summary output, there are three dominant sequences (highlighted in blue). Sequences ranked 1 and 3 have the same length and VJ use. B: When aligned, sequences are related, differing by six bases, C to T and G to A substitutions typical of somatic hypermutation events. After additional merging, there are two separate clones: clone 1: 280 bp, V1-2-02;J3, 21.07% of total reads (13.09 + 7.98); and clone 2: 289 bp, V5-51_01;J6, 9.62% of total reads. The two sequences are 140× and 64× of the background, respectively (21.07/0.15 and 9.62/0.15, respectively). In this case, the background starts at the fourth sequence. Both clones meet criteria for clonality. C: Snapshot of the V-J sequence frequency graph of LymphoTrack MiSeq Software version 2.3.1 shows two clonal sequences with same use (VH1-J3) stacked and the third sequence at V5;J6. D: Sequences plotted by the Memorial Sloan Kettering LymphoClone viewer show sequences merged by VJ family use and size. Correlation with corresponding flow cytometry shows the bone marrow sample is involved by both chronic lymphocytic leukemia and plasma cell neoplasm. N, no; n/a, not applicable; Y, yes.
Supplemental Figure S5.
Representative example requiring additional merging in the final analysis. Not all sequences of the same size and family use can be merged as a single sequence. A: On the basis of the merged read summary output, there are two dominant sequences (rank, 1 to 2), but it is difficult to know where the background starts. The four top sequences (highlighted in green) show the same size but different VJ families. Sequences 1 and 2 are unrelated despite the same size and family (different subfamily), whereas sequences ranked 2, 3, 4, 7, and 8 are related and constitute the same clone with ongoing somatic hypermutation. B and C: Alignment of sequences 1 and 2 shows the sequences are widely different, whereas sequences 2 to 4 are related. D: Top panel: Snapshot of the V-J sequence frequency graph of LymphoTrack MiSeq Software version 2.3.1 shows a single bar with multiple colors (red and green are different subfamily). Sequences plotted by the Memorial Sloan Kettering LymphoClone viewer (middle) show sequences merged by VJ family use and size (single peak, two distinct colors). Bottom panel: Corresponding capillary electrophoresis tracing (FR1) also shows a dominant clonal peak and a small peak on the far right around 350 bp, which corresponds to the small sequence (rank, 5 and 6) 301 bp in length, V5-51_01;J6. In this case, the background is shifted down and begins at sequence rank 9 (highlighted orange). Corresponding flow cytometry identifies two distinct abnormal B-cell populations, 5.2% (λ restricted) and 7.9% (κ restricted) of the total white blood cells. N, no; Y, yes.
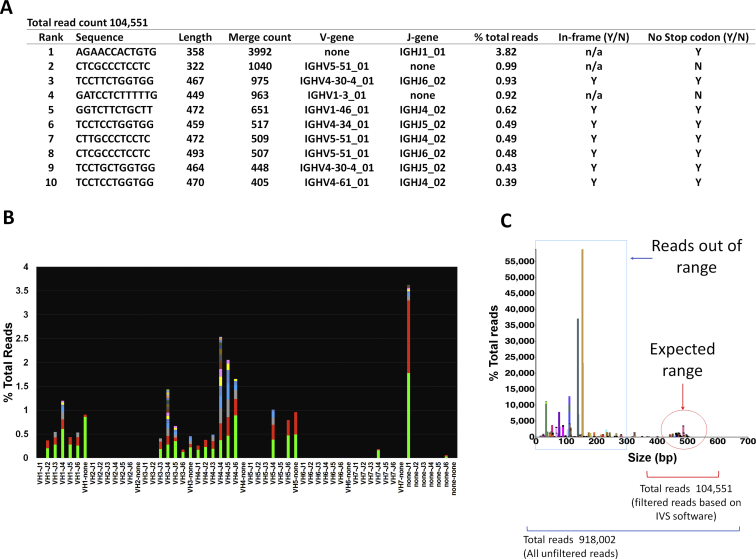
Supplemental Figure S6.
Example of a case generating a relatively high number of reads but unsuitable for diagnosis. A: The merged read summary output shows a dominant nonspecific sequence at 3.82% and a background with low variability. When a high number of sequences are seen with a V or J segment that cannot be categorized (denoted as none), this generally represents miss-priming events. B: Snapshot of the V-J sequence frequency graph using LymphoTrack MiSeq Software version 2.3.1 shows a prominent sequence within a polyclonal background with low variability. C: Plotting of all unfiltered sequences using the Memorial Sloan Kettering LymphoClone viewer shows that most sequences are short, truncated sequences outside of the expected range, confirming suboptimal sequencing. N, no; n/a, not applicable; Y, yes.
Supplemental Figure S7.
Next-generation sequencing (NGS) clonality detection in common B-cell lymphomas. Comparison of NGS clonality detection in common B-cell lymphomas in this study compared with previously published results using BIOMED-2 primers (Report of the BIOMED-2 Concerted Action BHM4-CT98-3936).7 In all B-cell malignancy subsets, except mantle cell lymphoma (MCL), the detection rate of the NGS assay with FR1 and leader primers alone surpasses the detection of standardized assays using BIOMED-2 primers targeting FR1, FR2, and FR3 combined. In MCL, the detection is 100% for both. CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma.
References
- 1.van Zelm M.C., van der Burg M., de Ridder D., Barendregt B.H., de Haas E.F., Reinders M.J., Lankester A.C., Revesz T., Staal F.J., van Dongen J.J. Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J Immunol. 2005;175:5912–5922. doi: 10.4049/jimmunol.175.9.5912. [DOI] [PubMed] [Google Scholar]
- 2.Dik W.A., Pike-Overzet K., Weerkamp F., de Ridder D., de Haas E.F., Baert M.R., van der Spek P., Koster E.E., Reinders M.J., van Dongen J.J., Langerak A.W., Staal F.J. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201:1715–1723. doi: 10.1084/jem.20042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langerak A.W., Groenen P.J., Bruggemann M., Beldjord K., Bellan C., Bonello L., Boone E., Carter G.I., Catherwood M., Davi F., Delfau-Larue M.H., Diss T., Evans P.A., Gameiro P., Garcia Sanz R., Gonzalez D., Grand D., Hakansson A., Hummel M., Liu H., Lombardia L., Macintyre E.A., Milner B.J., Montes-Moreno S., Schuuring E., Spaargaren M., Hodges E., van Dongen J.J. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012;26:2159–2171. doi: 10.1038/leu.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trainor K.J., Brisco M.J., Story C.J., Morley A.A. Monoclonality in B-lymphoproliferative disorders detected at the DNA level. Blood. 1990;75:2220–2222. [PubMed] [Google Scholar]
- 5.van Dongen J.J., Langerak A.W., Bruggemann M., Evans P.A., Hummel M., Lavender F.L., Delabesse E., Davi F., Schuuring E., Garcia-Sanz R., van Krieken J.H., Droese J., Gonzalez D., Bastard C., White H.E., Spaargaren M., Gonzalez M., Parreira A., Smith J.L., Morgan G.J., Kneba M., Macintyre E.A. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 6.Boone E., Verhaaf B., Langerak A.W. PCR-based analysis of rearranged immunoglobulin or T-cell receptor genes by GeneScan analysis or heteroduplex analysis for clonality assessment in lymphoma diagnostics. Methods Mol Biol. 2013;971:65–91. doi: 10.1007/978-1-62703-269-8_4. [DOI] [PubMed] [Google Scholar]
- 7.Evans P.A., Pott C., Groenen P.J., Salles G., Davi F., Berger F., Garcia J.F., van Krieken J.H., Pals S., Kluin P., Schuuring E., Spaargaren M., Boone E., Gonzalez D., Martinez B., Villuendas R., Gameiro P., Diss T.C., Mills K., Morgan G.J., Carter G.I., Milner B.J., Pearson D., Hummel M., Jung W., Ott M., Canioni D., Beldjord K., Bastard C., Delfau-Larue M.H., van Dongen J.J., Molina T.J., Cabecadas J. Significantly improved PCR-based clonality testing in B-cell malignancies by use of multiple immunoglobulin gene targets: Report of the BIOMED-2 Concerted Action BHM4-CT98-3936. Leukemia. 2007;21:207–214. doi: 10.1038/sj.leu.2404479. [DOI] [PubMed] [Google Scholar]
- 8.Liu H., Bench A.J., Bacon C.M., Payne K., Huang Y., Scott M.A., Erber W.N., Grant J.W., Du M.Q. A practical strategy for the routine use of BIOMED-2 PCR assays for detection of B- and T-cell clonality in diagnostic haematopathology. Br J Haematol. 2007;138:31–43. doi: 10.1111/j.1365-2141.2007.06618.x. [DOI] [PubMed] [Google Scholar]
- 9.Boyd S.D., Marshall E.L., Merker J.D., Maniar J.M., Zhang L.N., Sahaf B., Jones C.D., Simen B.B., Hanczaruk B., Nguyen K.D., Nadeau K.C., Egholm M., Miklos D.B., Zehnder J.L., Fire A.Z. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med. 2009;1:12ra23. doi: 10.1126/scitranslmed.3000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotrova M., Muzikova K., Mejstrikova E., Novakova M., Bakardjieva-Mihaylova V., Fiser K., Stuchly J., Giraud M., Salson M., Pott C., Bruggemann M., Fullgrabe M., Stary J., Trka J., Fronkova E. The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL. Blood. 2015;126:1045–1047. doi: 10.1182/blood-2015-07-655159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langerak A.W., Bruggemann M., Davi F., Darzentas N., van Dongen JJM, Gonzalez D., Cazzaniga G., Giudicelli V., Lefranc M.P., Giraud M., Macintyre E.A., Hummel M., Pott C., Groenen P., Stamatopoulos K., EuroClonality-NGS Consortium High-throughput immunogenetics for clinical and research applications in immunohematology: potential and challenges. J Immunol. 2017;198:3765–3774. doi: 10.4049/jimmunol.1602050. [DOI] [PubMed] [Google Scholar]
- 12.Hung S.S., Meissner B., Chavez E.A., Ben-Neriah S., Ennishi D., Jones M.R., Shulha H.P., Chan F.C., Boyle M., Kridel R., Gascoyne R.D., Mungall A.J., Marra M.A., Scott D.W., Connors J.M., Steidl C. Assessment of capture and amplicon-based approaches for the development of a targeted next-generation sequencing pipeline to personalize lymphoma management. J Mol Diagn. 2018;20:203–214. doi: 10.1016/j.jmoldx.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Scherer F., Kurtz D.M., Newman A.M., Stehr H., Craig A.F., Esfahani M.S., Lovejoy A.F., Chabon J.J., Klass D.M., Liu C.L., Zhou L., Glover C., Visser B.C., Poultsides G.A., Advani R.H., Maeda L.S., Gupta N.K., Levy R., Ohgami R.S., Kunder C.A., Diehn M., Alizadeh A.A. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med. 2016;8:364ra155. doi: 10.1126/scitranslmed.aai8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaque J.P., Martinez N., Batlle-Lopez A., Perez C., Montes-Moreno S., Sanchez-Beato M., Piris M.A. B-cell lymphoma mutations: improving diagnostics and enabling targeted therapies. Haematologica. 2014;99:222–231. doi: 10.3324/haematol.2013.096248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin S., Hwang I.S., Kim J., Lee K.A., Lee S.T., Choi J.R. Detection of immunoglobulin heavy chain gene clonality by next-generation sequencing for minimal residual disease monitoring in B-lymphoblastic leukemia. Ann Lab Med. 2017;37:331–335. doi: 10.3343/alm.2017.37.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D., Emerson R.O., Sherwood A., Loh M.L., Angiolillo A., Howie B., Vogt J., Rieder M., Kirsch I., Carlson C., Williamson D., Wood B.L., Robins H. Detection of minimal residual disease in B lymphoblastic leukemia by high-throughput sequencing of IGH. Clin Cancer Res. 2014;20:4540–4548. doi: 10.1158/1078-0432.CCR-13-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeKosky B.J., Ippolito G.C., Deschner R.P., Lavinder J.J., Wine Y., Rawlings B.M., Varadarajan N., Giesecke C., Dorner T., Andrews S.F., Wilson P.C., Hunicke-Smith S.P., Willson C.G., Ellington A.D., Georgiou G. High-throughput sequencing of the paired human immunoglobulin heavy and light chain repertoire. Nat Biotechnol. 2013;31:166–169. doi: 10.1038/nbt.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gawad C., Pepin F., Carlton V.E., Klinger M., Logan A.C., Miklos D.B., Faham M., Dahl G., Lacayo N. Massive evolution of the immunoglobulin heavy chain locus in children with B precursor acute lymphoblastic leukemia. Blood. 2012;120:4407–4417. doi: 10.1182/blood-2012-05-429811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan A.C., Gao H., Wang C., Sahaf B., Jones C.D., Marshall E.L., Buno I., Armstrong R., Fire A.Z., Weinberg K.I., Mindrinos M., Zehnder J.L., Boyd S.D., Xiao W., Davis R.W., Miklos D.B. High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment. Proc Natl Acad Sci U S A. 2011;108:21194–21199. doi: 10.1073/pnas.1118357109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan A.C., Zhang B., Narasimhan B., Carlton V., Zheng J., Moorhead M., Krampf M.R., Jones C.D., Waqar A.N., Faham M., Zehnder J.L., Miklos D.B. Minimal residual disease quantification using consensus primers and high-throughput IGH sequencing predicts post-transplant relapse in chronic lymphocytic leukemia. Leukemia. 2013;27:1659–1665. doi: 10.1038/leu.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y.C., Kipling D., Leong H.S., Martin V., Ademokun A.A., Dunn-Walters D.K. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood. 2010;116:1070–1078. doi: 10.1182/blood-2010-03-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker B.A., Wardell C.P., Johnson D.C., Kaiser M.F., Begum D.B., Dahir N.B., Ross F.M., Davies F.E., Gonzalez D., Morgan G.J. Characterization of IGH locus breakpoints in multiple myeloma indicates a subset of translocations appear to occur in pregerminal center B cells. Blood. 2013;121:3413–3419. doi: 10.1182/blood-2012-12-471888. [DOI] [PubMed] [Google Scholar]
- 23.Wren D., Walker B.A., Bruggemann M., Catherwood M.A., Pott C., Stamatopoulos K., Langerak A.W., Gonzalez D., EuroClonality-NGS consortium Comprehensive translocation and clonality detection in lymphoproliferative disorders by next-generation sequencing. Haematologica. 2017;102:e57–e60. doi: 10.3324/haematol.2016.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faham M., Zheng J., Moorhead M., Carlton V.E., Stow P., Coustan-Smith E., Pui C.H., Campana D. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120:5173–5180. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Lopez J., Lahuerta J.J., Pepin F., Gonzalez M., Barrio S., Ayala R., Puig N., Montalban M.A., Paiva B., Weng L., Jimenez C., Sopena M., Moorhead M., Cedena T., Rapado I., Mateos M.V., Rosinol L., Oriol A., Blanchard M.J., Martinez R., Blade J., San Miguel J., Faham M., Garcia-Sanz R. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawstron A.C., Fazi C., Agathangelidis A., Villamor N., Letestu R., Nomdedeu J. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia. 2016;30:929–936. doi: 10.1038/leu.2015.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawstron A.C., Gregory W.M., de Tute R.M., Davies F.E., Bell S.E., Drayson M.T., Cook G., Jackson G.H., Morgan G.J., Child J.A., Owen R.G. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125:1932–1935. doi: 10.1182/blood-2014-07-590166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood B., Wu D., Crossley B., Dai Y., Williamson D., Gawad C., Borowitz M.J., Devidas M., Maloney K.W., Larsen E., Winick N., Raetz E., Carroll W.L., Hunger S.P., Loh M.L., Robins H., Kirsch I. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood. 2018;131:1350–1359. doi: 10.1182/blood-2017-09-806521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R., Advani R., Ghielmini M., Salles G.A., Zelenetz A.D., Jaffe E.S. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syed M.H., Nafa K., Baldi T., Zehir A., Cheng D.T., Ladanyi M., Arcila M.E. MSK-LYMPHOCONE: data analysis pipeline and tools for immune repertoire analysis. J Mol Diagn. 2015;17:804a. [Google Scholar]
- 31.Roshal M., Flores-Montero J.A., Gao Q., Koeber M., Wardrope J., Durie B.G.M., Dogan A., Orfao A., Landgren O. MRD detection in multiple myeloma: comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood Adv. 2017;1:728–732. doi: 10.1182/bloodadvances.2016003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartram J., Mountjoy E., Brooks T., Hancock J., Williamson H., Wright G., Moppett J., Goulden N., Hubank M. Accurate sample assignment in a multiplexed, ultrasensitive, high-throughput sequencing assay for minimal residual disease. J Mol Diag. 2016;18:494–506. doi: 10.1016/j.jmoldx.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez D., Balanzategui A., Garcia-Sanz R., Gutierrez N., Seabra C., van Dongen J.J., Gonzalez M., San Miguel J.F. Incomplete DJH rearrangements of the IgH gene are frequent in multiple myeloma patients: immunobiological characteristics and clinical implications. Leukemia. 2003;17:1398–1403. doi: 10.1038/sj.leu.2402964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.