Abstract:
Extracorporeal membrane oxygenation (ECMO) has become an integral treatment option for patients as a bridge to transplant, management of post cardiotomy cardiogenic shock, and for rescue after cardiopulmonary arrest. Significant strides in ECMO technology and management cannot, however, replace the importance of maintaining and following a comprehensive safety checklist. We herein report a case of massive air entrainment from an inadvertently disconnected port of a central venous catheter (CVC) in the neck which culminated in an airlock of the ECMO circuit. Ascertaining the relative position of the tip of the CVC with respect to the venous cannula on chest X-ray, tightly securing all its ports, and appraising and educating the health-care team can prevent this rare but devastating complication of fatal air embolism.
Keywords: extracorporeal membrane oxygenation, bridge to transplant, post cardiotomy syndrome, cardiopulmonary arrest, central venous catheter, massive air entrainment
OVERVIEW
Over the last decade, extracorporeal membrane oxygenation (ECMO) has become an essential lifesaving measure providing patients with cardiopulmonary support over an extended period until myocardial or pulmonary and/or end-organ recovery (1). Although significant advancements such as heparin-coated circuits, flow probes, and miniaturized circuitry have been made in ECMO technology, certain complications remain inherent to its use. Of them, complications such as pump failure, exsanguinating bleeding, accidental decannulation, cardiac arrest, and massive air embolism (MAE) need to be addressed expeditiously. Depending on the amount and rate at which air enters the body, air embolism could be in the form of gaseous microemboli (GME) causing neurocognitive dysfunction or MAE having fatal outcomes (2). Experimentally, in large animal studies, the pulmonary microvasculature has been shown to filter microbubbles and withstand air volumes upto .3 mL/kg/min (3). Beyond this threshold, they spill over to systemic circulation, and hence, it is the MAE which is life threatening.
Air entrainment in the circuit could be due to breakage in tubings, migration, disconnection or venous decannulation, or through intravascular access lines during patient-care activities such as delivery of medications. Transthoracic or transesophageal echo may be diagnostic and should be considered early. The objectives of treatment are to remove the source of air embolism, support circulation, and allow cardiopulmonary recovery (4).
CASE DESCRIPTION
A 40-year-old female without significant comorbidities underwent laser therapy for subglottic stenosis by an otolaryngologist at our institution. Her postoperative course was complicated by fever, cough, and difficulty breathing 1 week later for which she was admitted at the local community hospital. She was started on supportive treatment and antibiotics. A chest X-ray revealed that she had developed left lower lobe consolidation. However, her clinical condition deteriorated further, culminating in respiratory failure requiring intubation and mechanical ventilation. Despite respiratory support, her hemodynamics continued to worsen, and inotropic support was initiated. Anticipating imminent cardiopulmonary arrest, our team was called for assessment and consideration of ECMO insertion. At the time of preparing her ECMO cannulation, the patient went into cardiac arrest and cardiopulmonary resuscitation (CPR) was initiated. Emergency venoarterial ECMO was instituted by inserting a 17-Fr right femoral arterial cannula (Bio-Medicus Medtronic, Fridley, MN) and 25-Fr right femoral vein multi-stage cannula (Bio-Medicus Medtronic) with a circuit using a ROTAFLOW centrifugal pump (Maquet, Rastatt, Germany) and QUADROX oxygenator (Maquet). The distal superficial femoral artery (SFA) ipsilateral to the arterial cannula was accessed using a micropuncture needle followed by a .018-inch nitinol wire (Cook Medical, Inc., Bloomington, IN). A 6-Fr DPC (Cook Medical, Inc.) was advanced into the mid-SFA. The catheter was attached to the side port of the arterial cannula using a 6-inch extension tubing with an intervening three-way stopcock. The cannulae position was checked by chest X-ray and abdomen. Pre and postmembrane oxygenator as well patient blood gases which were noted to be satisfactory. The patient was transferred to our institution for further management. After arrival in our intensive care unit and initial stabilization, we suddenly noticed a significantly large amount of air in the venous cannula causing the ECMO circuit to shut off. We immediately clamped the arterial and venous cannula, placed in the head-down position, provided full ventilation, and inotropic support to maintain hemodynamics and changed the entire ECMO circuit expeditiously as we have a primed circuit in case of emergency situations.
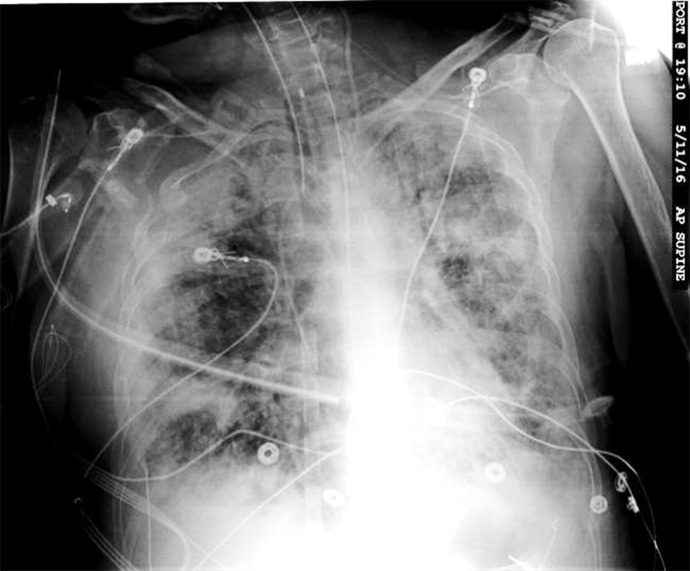
On further exploring the cause of MAE, we noted that one of the ports of the central venous catheter (CVC) in the left neck for delivery of medications was inadvertently left open. On chest X-ray, the tip of this CVC was noticed to be adjacent to the venous cannula (Figure 1). Thereby, the atmospheric air was accidently suctioned through this CVC into the venous cannula and further into the ECMO circuit causing it to be deprimed and stop the pump. Over the next 2 days, her course was further complicated by right lower limb ischemia requiring multiple fasciotomies. She was not hemodynamically stable to have any form of imaging for the brain. During her second fasciotomy, she had another cardiac arrest and eventually the family decided to withdraw care on the 5th day.
Figure 1.
X-ray chest of the patient on venoarterial ECMO showing the tip of the central venous catheter adjacent to the venous cannula which extends up the superior vena cava.
COMMENTS
Traditionally, gaseous and particle microemboli have been demonstrated as a major cause for neuropsychological dysfunction after cardiopulmonary bypass (CPB) procedure in pediatric and adult cardiac patients. A direct correlation exists between the number of intravascular microemboli detected and the incidence of postoperative brain injury, patient mortality, and morbidity (2). Cerebral microemboli have been implicated in the etiology of neuropsychological dysfunction, with several investigators demonstrating worse cognitive outcome for patients with greater numbers of emboli (5). However, it has been only recently that quantification of gaseous embolic episodes has been demonstrated during VA-ECMO use and shown to rival with that seen on CPB. Intravenous injections account for an estimated 68% of GME and 88% of GME volume, whereas care involving movement account for an estimated 6% of GME and 3% of volume (6). Patients with increased perfusionist interventions and, therefore, increased GME had significantly worse performances in tests on learning, memory, attention, and concentration (7,8). For ethical reasons, it is impossible to have clinical studies on MAE and most of data are from animal experiments. Fracasso et al. demonstrated in an experimental model that retrograde air embolism could be caused by a disconnected catheter and is even more likely if the neck is in the vertical position (9). Air flow of up to 300 mL/min could be obtained from the CVC with the flow in CPB circuit of 2.3 L/min, and a linear relationship has been observed between the flow in the circuit and air embolism (10).
When gas emboli are detected in the circuit, it is prudent to check for cannula migration, integrity of the ECMO circuit, breakage in tubing or disconnection, and patient vascular access sites and ports being properly sealed. ECMO patients requiring renal replacement therapy pose another specific challenge. Use of an in-line hemofilter or introduction of a continuous renal replacement therapy device into the ECMO circuit have resulted in complications in patients without alternate access for continuous veno-venous hemodialysis when the ports on the ECMO circuit have been used for this purpose (11). Accidental dislodgement of the caps after the completion of procedure or during transport to computed tomography (CT) scan or catheterization laboratory is another source. Therefore, such access has been frowned upon unless no other viable alternatives are available, and even then, the entire team must ensure safety.
Hypothermia, supranormal blood pressure (BP), enhanced cardiac output, hyperoxia, and hyperbaric oxygen treatment are the therapeutic options for postoperative management of cerebral air embolism (12). Perfluorocarbons have been shown in experimental studies to extend the tolerance of the brain to ischemic damage from an air embolism by increased availability of oxygen to ischemic areas and indirect reduction in size of air bubbles through denitrogenation (13). Although GME are more common than MAE, it is the latter which is responsible for fatal outcomes. To this end, we have modified our ECMO checklist and made the following recommendations:
1. Apart from checking the cannula position after ECMO insertion, it should be a dictum to also ascertain the relative position of the tip of CVC catheter with respect to the venous cannula.
2. In case of proximity between the CVC and venous cannula, readjustment in the position of CVC must be carried out on priority basis.
3. Insertion of 16-Fr CVC of 16 cm length in the neck.
4. Continued education of the health-care team members.
There is a need to step up further simulation-based training programs so as to better prepare for such emergencies.
In health-care team–based practice involving surgeons, intensivists, other physicians, perfusionists, nurses, respiratory therapists, and technicians, it is essential that safe patient practices be implemented by every discipline and that they complement the other team members.
Medical errors have become the third leading cause of death in United States (14). Is it possible to have a health-care system devoid of accidents? The place to look up this answer is the safety culture in the aviation industry. The industry also goes to great lengths to learn from faults that have caused accidents in the past, to ensure that they are not repeated. That is why the process to investigate aircraft incidents is a coordinated effort by all appropriate parties. These methods have proven to be an invaluable tool for the evolution of aviation safety and need to be applied to the health-care sector as well where complex systems and hierarchies have the potential to create risks.
To summarize, the risk of air entrainment from CVC into the ECMO must always be borne in mind. Maintenance of an adequate checklist and diligently following it along with continued education of team members can help avoid this complication.
REFERENCES
- 1.ELSO. 2017. ELSO guidelines. Available at: https://www.elso.org/Portals/0/ELSO Guidelines For Adult Respiratory Failure 1_4.pdf, Page5. Accessed May 22, 2018.
- 2.Whitaker DC, Stygall J, Hope-Wynne C, et al. . A prospective clinical study of cerebral microemboli and neuropsychological outcome comparing vent-line and auto-venting arterial line filters: Both filters are equally safe. Perfusion. 2006;21:83–6. [DOI] [PubMed] [Google Scholar]
- 3.van Hulst RA, Klein J, Lachmann B. Gas embolism: Pathophysiology and treatment. Clin Physiol Funct Imaging. 2003;23:237–46. [DOI] [PubMed] [Google Scholar]
- 4.Rawlins R, Momin A, Platts D, et al. . Traumatic cardiogenic shock due to massive air embolism. A possible role for cardiopulmonary bypass. Eur J Cardio Thorac Surg. 2002;22:845–6. [DOI] [PubMed] [Google Scholar]
- 5.Borger MA, Ivanov J, Weisel RD, et al. . Stroke during coronary bypass surgery: Principal role of cerebral macroemboli. Eur J Cardio Thorac Surg. 2001;19:627–32. [DOI] [PubMed] [Google Scholar]
- 6.Fracasso T, Karger B, Schmidt PF, et al. . Retrograde venous cerebral air embolism from disconnected central venous catheter: An experimental model. J Forensic Sci. 2011;56(Suppl 1):S101–4. [DOI] [PubMed] [Google Scholar]
- 7.Borger MA, Peniston CM, Weisel RD, et al. . Neuropsychologic impairment after coronary bypass surgery: Effect of gaseous microemboli during perfusionist interventions. J Thorac Cardiovasc Surg. 2001;121:743–9. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez RA, Williams KA, Babaev A, et al. . Effect of perfusionist technique on cerebral embolization during cardiopulmonary bypass. Perfusion. 2005;20:3–10. [DOI] [PubMed] [Google Scholar]
- 9.Jiao Y, Gipson KE, Bonde P, et al. . Quantification of postmembrane gaseous microembolization during venoarterial extracorporeal membrane oxygenation. Asaio J. 2018;64:31–7. [DOI] [PubMed] [Google Scholar]
- 10.Hogetveit JO, Saatvedt K, Norum H, et al. . Central venous catheters may be a potential source of massive air emboli during vascular procedures involving extracorporeal circulation: An experimental study. Perfusion. 2011;26:341–6. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Yu RG, Yin NN, et al. . Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: A systematic review. Crit Care. 2014;18:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tovar EA, Del Campo C, Borsari A, et al. . Postoperative management of cerebral air embolism: Gas physiology for surgeons. Ann Thorac Surg. 1995;60:1138–42. [DOI] [PubMed] [Google Scholar]
- 13.Menasche P, Pinard E, Desroches AM, et al. . Fluorocarbons: A potential treatment of cerebral air embolism in open-heart surgery. Ann Thorac Surg. 1985;40:494–7. [DOI] [PubMed] [Google Scholar]
- 14.Makary MA, Daniel M. Medical error-the third leading cause of death in the US. BMJ. 2016;353:i2139. [DOI] [PubMed] [Google Scholar]