Abstract
Background:
Asymptomatic bacteriuria (ASB) is common in neonates, preschool children, pregnant women, elderly, diabetics, catheterized patients, and patients with abnormal urinary tracts or renal diseases. Though there is currently no consensus on treatment of ASB in various population groups, it is advisable to treat the same in patients with diabetes mellitus (DM).
Aims:
To determine the prevalence of ASB in patients with type 2 DM and to study the spectrum of uro-pathogens causing ASB along with their antibiotic susceptibility profile.
Settings and Design:
This prospective, observational study was conducted in the department of Medicine of a tertiary care teaching hospital.
Methods:
The study was conducted on 100 patients with type 2 DM. Urine wet mount and gram stain examination was done for all to detect the presence of pus cells and bacteria in urine. Antibiotic sensitivity testing was performed in patients with significant bacteriuria to determine the sensitivity profile of isolated uro-pathogens. The data were analyzed to determine the association between diabetes and ASB.
Results:
ASB was common among diabetics, as evident by a prevalence of 21%. Presence of ASB showed positive correlation with poor glycemic control. Escherichia coli (E. coli) was the most common organism causing ASB followed by Candida, Pseudomonas, Klebsiella, and Citrobacter. E. coli isolated from study patients was most sensitive to imipenem and nitrofurantoin (NFT).
Conclusions:
ASB is common among diabetics, with poor glycemic control being a significant risk factor. E. coli is the most common organism causing ASB in diabetics, and it is most sensitive to imipenem and NFT.
Keywords: Antibiotic susceptibility, asymptomatic bacteriuria, type 2 diabetes mellitus, uro-pathogens
Introduction
Diabetes mellitus (DM) refers to a group of common metabolic disorders that share the phenotype of hyperglycemia. It is associated with decrease in production and utilization of insulin, resulting in body's inability to utilize nutrients properly.[1]
The worldwide prevalence of DM has risen dramatically over the past two decades, from an estimated 30 million cases in 1985 to 382 million in 2013. Based on the current trends, the International Diabetes Federation projects that 592 million individuals will have diabetes by the year 2035.[2] The rising incidence of DM and the sheer number of people with DM living in India have given this country the dubious distinction of being the “Diabetes Capital” of the world.[3]
The reason for greater frequency of infections in DM patients include incompletely defined abnormalities in cell-mediated immunity and phagocyte function associated with hyperglycemia as well as diminished vascularization. Pneumonia, urinary tract infections (UTIs), and skin and soft tissue infections are all more common in the diabetic population. UTIs (either lower urinary tract or pyelonephritis) are the result of common bacterial agents such as Escherichia coli, although several yeast species (Candida and Torulopsis glabrata) are also commonly observed to cause UTI in diabetics. Bacteriuria occurs frequently in individuals with diabetic cystopathy. Poor glycemic control is a common factor in individuals with these infections.[4]
Asymptomatic bacteriuria (ASB) is common in neonates, preschool children, pregnant women, elderly people, diabetics, catheterized patients, and patients with abnormal urinary tracts or renal diseases. Though there is currently no consensus on treatment of ASB in these population groups, it is advisable to treat ASB in patients with DM. Since data on ASB among diabetic patients from India are scarce, there is a need for studying the prevalence and impact of ASB in diabetic patients in Indian setup. Hence, this study was undertaken to determine the prevalence of ASB in type 2 diabetic patients, document the organism responsible for it, and study the antimicrobial sensitivity pattern of these organisms. This can guide the treating physicians including primary care and family physicians to screen for ASB in diabetic patients, send urine samples for culture and sensitivity, and initiate appropriate empirical therapy before the results of antimicrobial sensitivity are received. Family physicians should incorporate the testing of urine for routine microscopy and culture in the clinical care protocols of all diabetic patients. Besides, there is a need to address the emerging multidrug resistance among uro-pathogens causing ASB and UTI among diabetics. This may be a result of antibiotic abuse, which is widely prevalent in the community and calls for urgent participation of family physicians with respect to rational use of antibiotics. There is also an utmost need for continuous scrutiny of antibiotic sensitivity profile of micro-organisms causing UTIs among diabetics in the hospital and the community.
Materials and Methods
It was a prospective observational study conducted in the Department of Medicine of a tertiary care teaching hospital. In total, 100 patients, 50 males and 50 females aged 25–65 years, fulfilling the American Diabetes Association (ADA) criteria for DM were enrolled for the study. Permission was sought from the institutional ethics committee and written informed consent was taken from each subject before enrolling him/her for the study.
The study excluded patients with symptomatic UTIs, renal failure, obstructive uropathy, indwelling catheters, pregnant females, immuno-compromised patients, and patients who had received antimicrobial drugs during the previous 2 weeks.
Apart from routine biochemical investigations, such as complete blood count, blood urea, serum creatinine, fasting blood sugar, and viral markers, HbA1C was done in all the subjects. Clean voided, mid-stream urine samples were collected from all the patients and processed following standard guidelines. Urine wet mount and gram stain examination was done for the presence of pus cells and bacteria. In patients with significant bacteriuria, antibiotic susceptibility was done following clinical laboratory standards institute (CLSI) guidelines.[5] For diagnosing ASB in females, two consecutive specimens with isolation of the same species in quantitative counts of at least 100,000 colony forming units (CFUs)/mL of urine were considered, whereas in males, a single specimen with one bacterial species isolated in a quantitative count of at least 100,000 CFUs/mL was considered.
Results
In total, 100 diabetic patients, 50 males and 50 females aged 25–65 years, were included. Among these, 21 patients were found to be ASB positive, and the remaining 79 were ASB negative. ASB was more common in females, with 15 (71.43%) out of total 21 ASB-positive patients being females.
The most common micro-organism isolated from ASB-positive patients was E. coli, accounting for 10 out of 21 cases (48%), followed by Candida in 7 cases (33%), Pseudomonas aeruginosa (P. aeruginosa) in 2 (9.4%), and 1 case each (4.8%) positive for Klebsiella pneumoniae (K. pneumoniae) and Citrobacter freundii (C. freundii) [Graph 1].
Graph 1.
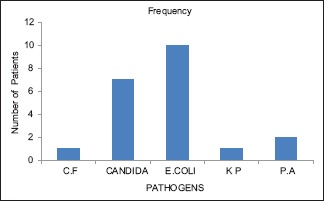
Distribution of various pathogens among ASB-positive patients. ASB = Asymptomatic bacteriuria, CF = Citrobacter freundii, KP = Klebsiella pneumoniae, PA = Pseudomonas aeruginosa
Among the 15 ASB-positive female patients, 6 were positive for Candida, 5 were positive for E. coli, 2 for P. aeruginosa, and 1 each for C. freundii and K. pneumoniae, whereas among the six ASB-positive male patients, E. coli was found to be positive in five patients, and one was positive for Candida [Table 1].
Table 1.
Distribution of various pathogens in ASB-positive patients according to gender
| Pathogen | CF | Candida | E. coli | KP | PA | Total |
|---|---|---|---|---|---|---|
| Sex | ||||||
| F | 1 | 6 | 5 | 1 | 2 | 15 |
| M | 0 | 1 | 5 | 0 | 0 | 6 |
| Total | 1 | 7 | 10 | 1 | 2 | 21 |
ASB: Asymptomatic bacteriuria, CF: Citrobacter freundii, KP: Klebsiella pneumoniae, PA: Pseudomonas aeruginosa
The prevalence of ASB was found to be more in patients with poor glycemic control having HbA1C >7%. Among the 21 ASB-positive patients, 15 (71.43%) were found to have HbA1c >7% and 6 patients (28.57%) had HbA1c ≤7%. Among the 79 ASB-negative patients, 70 (88.6%) had HbA1c ≤7%, and only 9 (11.4%) were found to have HbA1c >7% [Table 2].
Table 2.
Comparison of HbA1c levels with presence of ASB in Type 2 DM
| Growth | Total | ||
|---|---|---|---|
| No growth | Growth | ||
| HBA1C | |||
| ≤7 | 70 (88.6%) | 6 (28.57%) | 81 |
| >7 | 9 (11.4%) | 15 (71.43%) | 19 |
| Total | 79 | 21 | 100 |
ASB: Asymptomatic bacteriuria
E. coli was isolated from 10 ASB-positive patients. All of the 10 isolates were sensitive to imipenem. Out of these 10 isolates, 9 were also sensitive to nitrofurantoin (NFT), with only one isolate being resistant to it. Sensitivity to colistin was observed in seven isolates, with three being resistant to it. Less number of E. coli isolates were found sensitive to amikacin (4 of the 10 isolates), piperacillin–tazobactam and norfloxacin (2 isolates each) and cefotaxime and ceftriaxone (1 isolate each) [Table 3].
Table 3.
Antibiotic sensitivity pattern of E. coli in ASB-positive patients
| E. coli isolates | ||
|---|---|---|
| Sensitive | Resistant | |
| Cefotaxime | 1 | 9 |
| Ceftriaxone | 1 | 9 |
| Nitrofurantoin | 9 | 1 |
| Norfloxacin | 2 | 8 |
| Amikacin | 4 | 6 |
| Piperacillin-tazobactam | 2 | 8 |
| Imipenem | 10 | 0 |
| Colistin | 7 | 3 |
ASB: Asymptomatic bacteriuria
P. aeruginosa was isolated from two ASB-positive patients. Both isolates were sensitive to amikacin, piperacillin-tazobactam and imipenem, and were resistant to cefotaxime, ceftriaxone, NFT, norfloxacin, and colistin [Table 4].
Table 4.
Antibiotic sensitivity patterns of Pseudomonas aeruginosa, Klebsiella pneumonia and Citrobacter freundii in ASB-positive patients
| Antibiotic | Pseudomonas aeruginosa | Klebsiella pneumoniae | Citrobacter freundii | |||
|---|---|---|---|---|---|---|
| Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | |
| Cefotaxime | 0 | 2 | 0 | 1 | 0 | 1 |
| Ceftriaxone | 0 | 2 | 0 | 1 | 0 | 1 |
| Nitrofurantoin | 0 | 2 | 0 | 1 | 0 | 1 |
| Norfloxacin | 0 | 2 | 0 | 1 | 0 | 1 |
| Amikacin | 2 | 0 | 0 | 1 | 0 | 1 |
| Piperacillin-tazobactam | 2 | 0 | 0 | 1 | 0 | 1 |
| Imipenem | 2 | 0 | 1 | 0 | 0 | 1 |
| Colistin | 0 | 2 | 0 | 1 | 1 | 0 |
K. pneumoniae was isolated from one ASB-positive patient. The isolate was sensitive only to imipenem and was resistant to cefotaxime, ceftriaxone, NFT, norfloxacin, amikacin, piperacillin–tazobactam, and colistin [Table 4].
C. freundii was isolated from one ASB-positive patient. The isolate was sensitive only to colistin and was resistant to cefotaxime, ceftriaxone, NFT, norfloxacin, amikacin, piperacillin–tazobactam, and imipenem [Table 4].
Discussion
DM is a syndrome with disordered metabolism and inappropriate hyperglycemia due to either a deficiency of insulin secretion or to a combination of insulin resistance and inadequate insulin secretion to compensate for the resistance. DM has been commonly associated with UTI. The mechanism of pathogenesis for this association has not been fully elucidated. However, it is suggested that high glucose concentration in urine may favor the growth of pathogenic microorganisms, either in the form of symptomatic UTI or ASB.
The distinction between symptomatic UTI and ASB has major clinical implications. Both UTI and ASB denote the presence of bacteria in the urinary tract, usually accompanied by white blood cells and inflammatory cytokines in the urine. However, ASB occurs in the absence of symptoms attributable to bacteria in the urinary tract. The diagnosis of ASB requires presence of ≥105 bacterial CFUs/mL, except in catheter-associated disease, in which ≥102 CFUs/mL is the cutoff.
In our study, among the 100 diabetic patients (50 females and 50 males), 21% had ASB of which 15 (71.43%) were females and 6 (28.57%) were males. Similar findings were seen in a study conducted by Venkatesan et al., in which they found prevalence of ASB to be 32% in 100 diabetic patients (50 males and 50 females), of which 20 (62.5%) were females and 12 (37.5%) were males.[6] Another study conducted by Vishwanath et al. showed that ASB was present in four (4%) out of 100 patients with type 2 DM.[7]
In this study, among the 21 ASB-positive patients, the prevalence of ASB was found to be more in patients with poor glycemic control having HbA1C >7%. Out of 21 ASB-positive patients, 15 (71.43%) were found to have HbA1c >7% and 6 patients (28.57%) had HbA1c ≤7%. In a similar study, the mean duration of diabetes and mean HBA1c levels were significantly higher in ASB positive as compared with ASB-negative patients.[8]
Another study conducted by Bonadio et al.[9] on 228 diabetic women found that prevalence of ASB was 17.5% and the presence of higher HbA1c levels was a significant risk factor for ASB in women with type 2 DM. Ajay Adhikaree et al. in their study included 116 diabetic adults and observed that overall prevalence of ASB was 10.3% and patients with poor glycemic control had higher prevalence of ASB in comparison to those with good glycemic control.[10]
In this study, E. coli was the most common organism isolated in urine cultures, found in 10 (47.6%) cases, followed by Candida in seven (33.3%), Pseudomonas in two (9.5%), Klebsiella in one (4.76%), and Citrobacter in one case (4.76%). In a previous study by Simkhada et al.[11] on 100 diabetic patients (53 females and 47 males), E. coli was the most common organism isolated, found in 11 (52.38%) cases, followed by Klebsiella in three (14.28%), Pseudomonas, Proteus, and Enterococcus faecalis in two (9.52%) each, and Acinetobacter in one (4.76%) case. In another study conducted by Manish Rijal et al., out of 467 (253 females and 214 males) diabetic patients, significant microbial growth was found in 86 (18.42%) samples, whereas 381 (81.58%) samples showed no growth. In that same study, the most commonly isolated organism was E. coli (47.7%), followed by K. pneumoniae (18.6%), Staphylococcus aureus (12.8%), Proteus mirabilis (9.3%), Enterococcus fecalis (5.8%), Staphylococcus saprophyticus (2.3%), and Streptococcus pyogenes (2.3%).[12]
Venkatesan et al. also observed that E. coli (37.5%) was the most prevalent organism isolated from urine cultures in their study, followed by K. pneumoniae (18.7%), Enterococcus faecalis (15.6%), Staphylococcus aureus (9.4%), Pseudomonas (6.3%), Proteus (6.3%), Coagulase-negative staphylococci (3.1%) and Candida sp. (3.1%).[6] In another study conducted by Adhikaree et al., E. coli was the most frequently isolated pathogen (75%), followed by Klebsiella (16.7%) and Staphylococcus aureus.[10]
Bissong et al.[13] in their study found that out of 265 participants, including 154 diabetics and 111 nondiabetics, ASB was detected in 33.2% of participants (38.3% in diabetics and 26.1% in nondiabetics). Coagulase-negative staphylococci were the predominant organisms (36.3%) isolated. Other isolates included Klebsiella (15.9%), Candida (13.7%), E. coli (10.8%) and Serratia (10.8%).
In this study, antibiotic sensitivity of various pathogens was assessed and it was found that imipenem was the most effective antibiotic with 13 isolates being sensitive to it (61.9%), followed by NFT, which was sensitive in nine isolates (42.8%), colistin in eight (38.1%), amikacin in six (28.6%), and piperacillin/tazobactum in four isolates (19.04%), whereas ceftriaxone and cefotaxime were the most resistant antibiotics. A similar study was conducted by Boyko et al., who concluded that among the antibiotics, aminoglycosides (34%), NFT (21%), and gatifloxacin (14%) had excellent activity against the isolates and could be used for empirical treatment.[14] Lyamuya concluded that all isolates showed low level of resistance against amoxicillin/clavulanic acid, ciprofloxacin, ceftriaxone, gentamycin, and trimethoprim–sulfamethoxazole and high level of resistance was observed against tetracyclines.[15] Venkatesan et al. observed that E. coli, the most prevalent organism (37.5%) in their study, was most sensitive to NFT, amikacin, and gentamicin. Most of the organisms isolated in this study were resistant to nalidixic acid, ciprofloxacin, cefotaxime, and ampicillin. They recommended that amikacin, gentamicin, and NFT can be considered as ideal first-line drugs of choice for treating ASB in diabetics.[6] In the study by Adhikaree et al., E. coli was the most frequently isolated pathogen (75%), followed by Klebsiella (16.7%), and E. coli was most sensitive to NFT and imipenem, while Klebsiella was most sensitive to ciproflocin/norfloxacin, cotrimoxazole, and imipenem.[10]
Above observations and previous literature suggest that ASB is common among diabetics, with female predominance. Also, poor glycemic control (i.e., higher HbA1c level) is associated with a higher prevalence of ASB in diabetics. This emphasizes the importance of strict glycemic control in preventing ASB. In view of various factors such as emerging drug resistance, variable drug susceptibility pattern in different geographical areas and changing prevalence of uro-pathogens in ASB cases, antibiotic therapy should be formulated based on the antibiotic susceptibility results. Based on the available antibiotic sensitivity patterns of the commonly isolated pathogens, appropriate empirical therapy for ASB can be instituted timely to reduce complications, such as pyelonephritis and other upper UTIs. However, further studies are needed to substantiate the efficacy of antimicrobial therapy in preventing complications secondary to ASB in diabetic patients.
Strength of the study
Few studies from India have focused on presence of ASB in type 2 DM patients in spite of it being an important public health issue due to sheer number of diabetics in India. This study highlighted the significant prevalence of ASB in type 2 diabetics especially among those having poor glycemic control, thus reinforcing the importance of strict glycemic control in preventing ASB and symptomatic UTIs in diabetic patients. Careful monitoring of glycemic status, regular screening for ASB in diabetics, and judicial use of antibiotics should be practiced by all especially by primary care physicians to prevent ASB- related complications in diabetics.
Limitations of the study
This was a single center study with a small sample size.
Conclusions
ASB is common among patients with type 2 diabetes, with poor glycemic control being a significant risk factor for its development. This emphasizes the importance of strict glycemic control in preventing ASB. Also, E. coli is the most common organism causing ASB in diabetics and it is most sensitive to imipenem and NFT. The high level of resistance to routinely used antibiotics is a matter of great concern. Careful monitoring of glycemic status, regular screening for ASB in diabetics, and judicial use of antibiotics by primary care physicians can help resolve ASB-related complications in diabetics. Based on the available antibiotic sensitivity patterns of the commonly isolated pathogens, appropriate empirical therapy for ASB can be instituted timely to reduce complications, such as pyelonephritis and other upper UTIs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Londhe A, Naik M, Shinde V, Patel P, Wyavahare S. Study of diastolic dysfunction in asymptomatic type 2 diabetes mellitus. Int J Curr Med Appl Sci. 2016;9:101–6. [Google Scholar]
- 2.Dustan D, Zimmet P, Welborn T, Courtan M, Cameron A, Sicriee R, et al. The rising prevalence of diabetes and impaired glucose tolerance: The Australian Diabetes, obesity and lifestyle study. Diabetes Care. 2002;25:829–34. doi: 10.2337/diacare.25.5.829. [DOI] [PubMed] [Google Scholar]
- 3.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 4.Soo PB, Lee SJ, Wha KY, Sik HJ, Kim J, Chang SG, et al. Outcome of nephrectomy and kidney-preserving procedures for the treatment of emphysematous pyelonephritis. Scand J Urol Nephrol. 2006;40:332–8. doi: 10.1080/00365590600794902. [DOI] [PubMed] [Google Scholar]
- 5.Hamden HZ, Kubbara E, Adam AM, Hassan OS, Suliman SO. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Ann Clin Microbiol Antimicrob. 2015;14:1–14. doi: 10.1186/s12941-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesan KD, Chander S, Loganathan K, Victor K. Study on asymptomatic bacteriuria in diabetic patients. International Journal of Contemporary Medical Research. 2017;4:480–3. [Google Scholar]
- 7.Vishwanath S, Sarda R, D'souza AO, Mukhopadhyay C. Asymptomatic bacteriuria among patients with diabetes mellitus at a tertiary care center. National Journal of Laboratory Medicine. 2013;2:16–9. [Google Scholar]
- 8.Adly NN, Ragab YM, Hashem AM, Ahmady AK. Effect of diabetes on occurrence of urinary tract infection and asymptomatic bacteriuria among diabetic and ketoacidosis patients in Egypt. Int Res J Microbiol. 2015;6:27–36. [Google Scholar]
- 9.Bonadio M, Boldrini E, Forotti G, Matteucci E, Vigna A, Mori S, et al. Asymptomatic bacteriuria in women with diabetes: Influence of metabolic control. Clin Infect Dis. 2004;38:41–5. doi: 10.1086/381755. [DOI] [PubMed] [Google Scholar]
- 10.Adhikaree A, Kohli SC, Pokhrel DR, Bhatta D. Asymptomatic bacteriuria in diabetic adults. J Lumbini Med Coll. 2016;3:25–9. [Google Scholar]
- 11.Simkhada R. Urinary tract infection and antibiotic sensitivity pattern among diabetics. Nepal Med Coll J. 2013;15:1–4. [PubMed] [Google Scholar]
- 12.Rijal M, Neupane B, Bhandari P, Aryal S. Asymptomatic bacteriuria in elderly patients with diabetes attending a tertiary care center. J Trop Dis. 2015;3:1–3. [Google Scholar]
- 13.Bissong ME, Fon PN, Tabe-Bessong FO, Akenji TN. Asymptomatic bacteriuria in diabetes mellitus patients in South west Cameroon. Afr Health Sci. 2013;13:661–6. doi: 10.4314/ahs.v13i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol. 2005;161:557–64. doi: 10.1093/aje/kwi078. [DOI] [PubMed] [Google Scholar]
- 15.Lyamuya EF, Moyo SJ, Komba EV, Haule M. Prevalence, antimicrobial resistance and associated risk factors for bacteriuria in diabetic women in Dar es Salaam, Tanzania. Afr J Microbiol Res. 2011;5:683–9. [Google Scholar]


