Bacteriocins are commonly used by bacteria to kill neighboring cells that compete for resources. Although most bacteriocins are secreted, the aquatic, oligotrophic bacterium Caulobacter crescentus produces a two-peptide bacteriocin, CdzC/D, that remains attached to the outer membranes of cells, enabling contact-dependent killing of cells lacking the immunity protein CdzI. The receptor for CdzC/D has not previously been reported. Here, we describe a genetic screen for mutations that confer resistance to CdzC/D. One locus identified, perA, encodes a pentapeptide repeat protein that resides in the outer membrane of target cells, where it may act as the direct receptor for CdzC/D. Collectively, our results provide new insight into bacteriocin function and diversity.
KEYWORDS: Caulobacter crescentus, bacteriocins, contact-dependent inhibition
ABSTRACT
The Cdz bacteriocin system allows the aquatic oligotrophic bacterium Caulobacter crescentus to kill closely related species in a contact-dependent manner. The toxin, which aggregates on the surfaces of producer cells, is composed of two small hydrophobic proteins, CdzC and CdzD, each bearing an extended glycine-zipper motif, that together induce inner membrane depolarization and kill target cells. To further characterize the mechanism of Cdz delivery and toxicity, we screened for mutations that render a target strain resistant to Cdz-mediated killing. These mutations mapped to four loci, including a TonB-dependent receptor, a three-gene operon (named zerRAB for zipper envelope resistance), and perA (for pentapeptide envelope resistance). Mutations in the zerRAB locus led to its overproduction and to potential changes in cell envelope composition, which may diminish the susceptibility of cells to Cdz toxins. The perA gene is also required to maintain a normal cell envelope, but our screen identified mutations that confer resistance to Cdz toxins without substantially affecting the cell envelope functions of PerA. We demonstrate that PerA, which encodes a pentapeptide repeat protein predicted to form a quadrilateral β-helix, localizes primarily to the outer membrane of cells, where it may serve as a receptor for the Cdz toxins. Collectively, these results provide new insights into the function and mechanisms of an atypical, contact-dependent bacteriocin system.
IMPORTANCE Bacteriocins are commonly used by bacteria to kill neighboring cells that compete for resources. Although most bacteriocins are secreted, the aquatic, oligotrophic bacterium Caulobacter crescentus produces a two-peptide bacteriocin, CdzC/D, that remains attached to the outer membranes of cells, enabling contact-dependent killing of cells lacking the immunity protein CdzI. The receptor for CdzC/D has not previously been reported. Here, we describe a genetic screen for mutations that confer resistance to CdzC/D. One locus identified, perA, encodes a pentapeptide repeat protein that resides in the outer membrane of target cells, where it may act as the direct receptor for CdzC/D. Collectively, our results provide new insight into bacteriocin function and diversity.
INTRODUCTION
In the wild, bacteria are often in competition with other cells and other species for scarce resources and nutrients. To help survive and proliferate in such environments, many bacteria produce and secrete toxins that kill nearby competitors. One common class of toxins are bacteriocins, small, ribosomally synthesized toxins that are typically diffusible (1). Although they serve as powerful antimicrobials in some environments, these toxins can be rapidly swept away in other environments, particularly aquatic settings, limiting the benefit to a producing cell. In addition, the production of such a “public good” in a population can lead to the rise of “cheaters” that exploit the bacteriocin without producing it (2, 3).
Perhaps as a means of avoiding these issues with diffusible toxins, many bacteria also deploy contact-dependent inhibition systems in which toxins are either displayed on the cell surface or directly delivered by a producer cell into a neighboring cell (4, 5). The two primary examples of such contact-dependent systems involve type V transporters that localize contact-dependent inhibition (CDI) toxins to the outer membrane of some Gram-negative bacteria (6) and type VI secretion systems that directly inject a variety of toxins into neighboring cells (7).
Recently, we discovered and characterized a new type of contact-dependent killing involving an atypical two-peptide bacteriocin in the aquatic oligotrophic alphaproteobacterium Caulobacter crescentus (8). The Cdz toxin (contact-dependent inhibition by glycine zipper proteins) is composed of two small hydrophobic proteins, CdzC and CdzD, that harbor extended glycine zipper repeat motifs (GXXXG tandem repeats). These proteins are secreted via a type I secretion system, but unlike most bacteriocins that are secreted by type I systems, CdzC and CdzD accumulate on the surface of producer cells to mediate contact-dependent killing of target cells. The mechanism of CdzC/D aggregation has not been characterized but likely shares some similarity to other proteins and toxins harboring glycine zippers, such as the amyloid β peptide in Alzheimer’s disease (9). The exact mechanism of toxin delivery into target cells also remains elusive.
Initial evidence suggested that the Cdz toxins may target the inner membrane like many other bacteriocins (8) that can increase membrane permeability to ions by directly forming pores in the membrane (1) or by associating with integral membrane proteins and altering their structure to cause leakage of ions and other cellular contents (10, 11). CdzC and CdzD are likely active in the inner membrane, since the immunity protein CdzI that protects producer cells from self-intoxication is a small, integral inner membrane protein (8). In addition, both CdzC and CdzD bear weak sequence similarity to the diffusible, pore-forming bacteriocins thermophilin 13 from Streptococcus thermophilus (12) and microcin MccE492 from Klebsiella pneumoniae (13). However, these and other pore-forming bacteriocins are typically amphipathic (14), whereas CdzC and CdzD are comprised almost entirely of hydrophobic residues in their predicted transmembrane regions. In competition with a Cdz producer, target cells become permeable to the viability dye propidium iodide (PI), indicating compromised membrane integrity (8). However, this permeabilization could be an indirect effect of cell death by a toxin with a different direct cellular target (15).
How Cdz toxins attach to and target other cells remains unclear. Many bacteriocins have specific receptors on the surface of cells that they use to gain access to the inner membrane (1). Similarly, CDI toxins bind specific cellular receptors to engage target cells and mediate the delivery of their toxic C-terminal regions (16). To investigate the mechanism(s) by which Cdz toxins from C. crescentus target and kill cells lacking the CdzI immunity protein, we conducted a genetic screen to identify mutations that allow ΔcdzCDI target cells (lacking the CdzC/D toxins and the immunity protein CdzI) to escape Cdz-mediated killing. We identified mutations in four loci. One encodes a TonB-dependent receptor, which Cdz may use, along with other, as yet unidentified outer membrane channels, to get across the outer membrane and into the periplasm of target cells. We also found mutations that upregulate a three-gene operon, zerRAB (now named zipper envelope resistance), likely to counteract the effects of Cdz toxins by altering cell envelope properties. Most notably, we identify loss-of-function mutations in a pentapeptide repeat protein now called PerA (pentapeptide envelope resistance gene A) that normally resides in the outer membrane of cells, where we hypothesize it may serve as a direct receptor for the Cdz toxins. Collectively, our results provide important new insights into the mechanisms by which Cdz toxins mediate contact-dependent inhibition.
RESULTS
A screen for mutations that render cells resistant to Cdz-mediated killing.
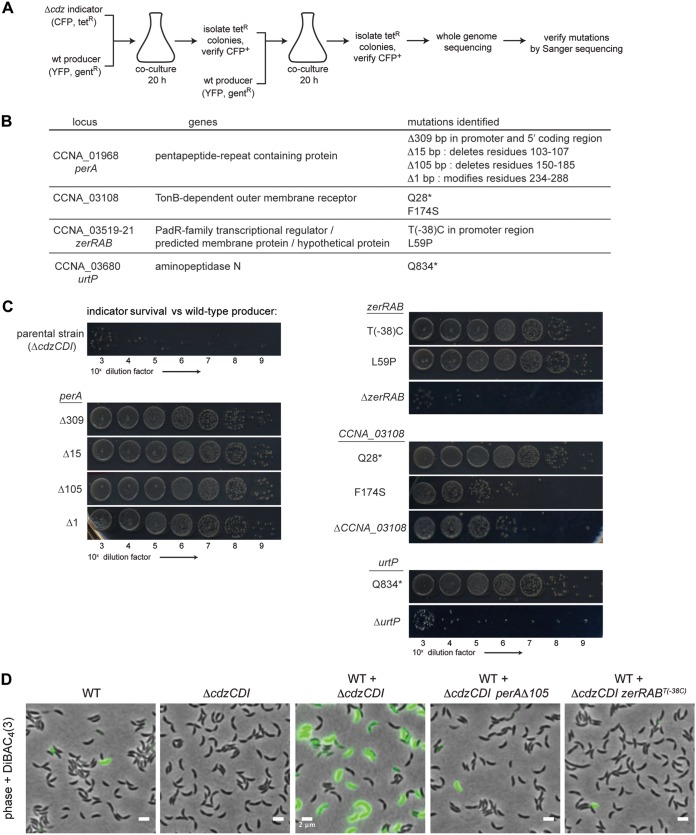
When a ΔcdzCDI target strain is cocultured with a wild-type C. crescentus producer for 20 h, the target population loses up to 7 logs of viability by CFU counts, with only ∼1,000 CFU/ml surviving (8). When surviving colonies were restreaked and again competed against the wild-type producer, roughly half exhibited resistance to Cdz-mediated killing and retained full viability in coculture. We took advantage of this observation to identify spontaneous mutations in a target strain that render it resistant to Cdz toxins. The genetic selection outlined in Fig. 1A used an indicator (target) and producer strain that each harbored two different markers, one a fluorescent protein and one an antibiotic resistance cassette. After two rounds of competition, target strain isolates that were still Tetr and CFP+ were submitted for whole-genome sequencing to identify putatively causal mutations (Fig. 1B). Reads were mapped to the reference parental genome and mutations identified using the software package breseq (17), which can detect single nucleotide variants, small insertions or deletions, and larger chromosomal rearrangements. All candidate causal mutations identified were verified by Sanger sequencing (Fig. 1B) and resistance was verified for each mutant strain in competition with a wild-type producer (Fig. 1C).
FIG 1.
Identification of mutations that confer resistance to Cdz-mediated killing. (A) Experimental design of the resistance mutation screen. (B) Summary of resistance mutations identified in the screen. *, stop codon. (C) Survival of the ΔcdzCDI parental indicator strain, the spontaneous resistant strains, or strains with complete gene deletions of the identified resistance genes, each in competition with a wild-type Cdz producer. (D) DiBAC4(3) staining of the strains indicated, 30 min after strains were mixed at an OD600 of 0.85.
Previously, we showed that CdzC/D-mediated killing leads to a disruption of cell membrane integrity after several hours in competition, as judged by propidium iodide staining (8). To confirm that the mutant strains identified in our screen were resistant to membrane disruption by CdzC/D, we grew two of the strains in competition with a wild-type producer strain and then assessed membrane potential using the fluorescent anionic lipophilic dye DiBAC4(3), which can only enter cells with depolarized membranes (Fig. 1D). As a control, we first confirmed that when stationary-phase wild-type producer cells are mixed at an equal ratio with a ΔcdzCDI indicator strain, approximately half of the cells were DiBAC positive 30 min after mixing, indicating that Cdz causes rapid membrane depolarization (loss of the proton gradient across the inner membrane). In contrast, when resistant strains were grown with a wild-type producer, nearly every cell remained DiBAC negative after 30 min, supporting the conclusion that the selected mutant strains are indeed resistant to membrane depolarization (Fig. 1D) and Cdz-mediated killing (Fig. 1C).
In total, nine mutations conferring resistance were identified in four genetic loci (Fig. 1B and C). For one locus, a nonsense mutation was identified in the gene CCNA_03680 (urtP), which encodes a putative ABC-2 family transporter with a periplasmic zinc-dependent aminopeptidase domain (18). We made a markerless deletion of urtP in a ΔcdzCDI strain and tested it in competition with the wild-type producer (Fig. 1C). The ΔurtP strain was fully sensitive to Cdz killing, indicating that the Q834* resistance allele does not display a loss-of-function phenotype and showing that this gene is not required for Cdz toxicity. We did not investigate this gene further, but its resistance phenotype may result from decreased expression of a putative Cdz surface receptor PerA, as discussed below.
For the other three loci, we identified at least two independent mutations. Two single-nucleotide substitutions were identified in CCNA_03108, which encodes a TonB-dependent receptor, with one mutation introducing an F174S substitution and the other leading to a premature stop codon at residue 28, Q28*, of an otherwise 1,112-residue protein. TonB-dependent receptors are outer membrane channels that facilitate the import of specific substrates, powered by the TonB complex, which couples the import process with the proton gradient across the inner membrane (19). Many bacteriocins hijack TonB-dependent receptors to get translocated across the outer membranes of Gram-negative bacteria (20). The Cdz toxins may use the TonB-dependent receptor encoded by CCNA_03108 for access to the periplasm or cytoplasm of target cells. To determine whether the resistance mutations we selected were null alleles, we made a ΔCCNA_03108 ΔcdzCDI strain and carried out a competition with a wild-type producer (Fig. 1C). The ΔCCNA_03108 ΔcdzCDI strain displayed levels of resistance that were intermediate to those seen with the selected Q28* allele (as well as other selected alleles shown in Fig. 1C) and the parental ΔcdzCDI strain. This result indicates that CCNA_03108 is unlikely to be the only, or even primary, receptor for CdzC/D. However, after, or concurrent with, the binding of CdzC/D to a primary surface receptor, CCNA_03108 could help mediate transport across the outer membrane, possibly along with another of the 65 TonB-dependent outer membrane proteins in Caulobacter. Intermediate resistance may also have arisen if CCNA_03108 is a secondary receptor or coreceptor of CdzC/D, or possibly if it promotes cell-cell attachment. Alternatively, mutations in this TonB-dependent receptor may indirectly affect the ability of Cdz toxins to kill cells and disrupt membrane permeability.
We identified two resistance mutations mapping to an operon of three genes, CCNA_03519-03521, which we renamed zerRAB for zipper envelope resistance. Four resistance mutations mapped to the gene CCNA_01968, which we renamed perA, for pentapeptide envelope resistance A, which encodes a previously uncharacterized pentapeptide repeat-containing protein. The resistance mutations mapping to zerRAB and perA are further characterized below.
Increased expression of the zerRAB operon confers resistance to Cdz.
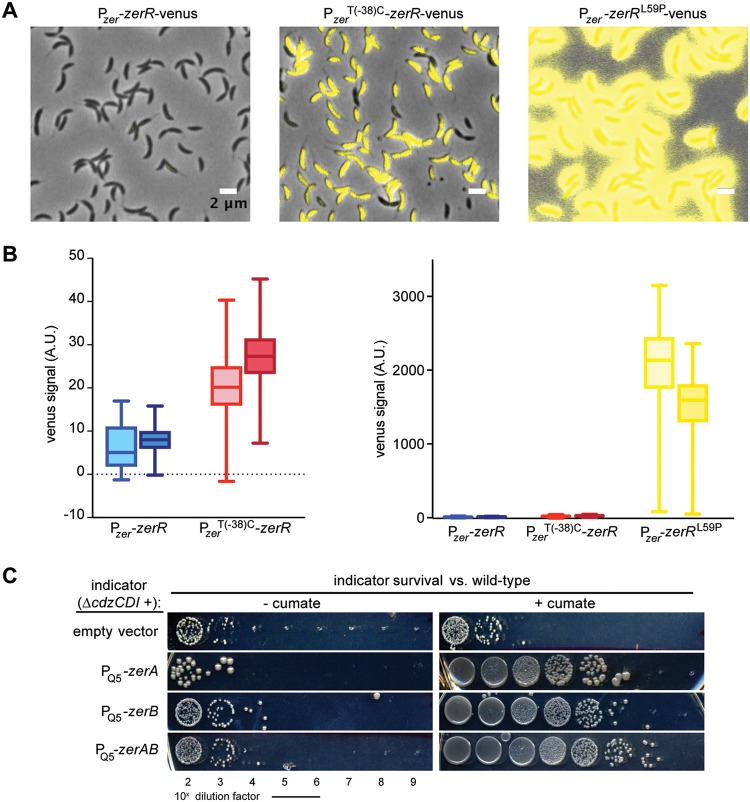
Two of the strains with mutations mapping to the zerRAB operon had an identical mutation, which arose independently in separate competition experiments, a T(−38)C nucleotide substitution within the predicted promoter for this operon, 38 nucleotides upstream of the annotated transcription start site (21). The other mutation produces a nonsynonymous L59P substitution in ZerR, a predicted transcriptional repressor of the PadR family. These mutants did not display a substantial growth defect relative to the wild type in rich medium (see Fig. S1 in the supplemental material). To determine whether these resistance mutations were loss-of-function mutations, we made a markerless deletion of the three genes. The ΔzerRAB ΔcdzCDI strain was fully sensitive to killing by a Cdz producer (Fig. 1C). Thus, we hypothesized that the mutations identified in our screen may, in fact, increase expression of the zerRAB operon. To test this hypothesis, we first constructed a transcriptional reporter in which the promoter region and coding sequence of zerR were placed upstream of the coding sequence for Venus fluorescent protein. We then grew cells harboring this construct, or variants containing the two mutations identified, to exponential or early stationary phase and examined expression levels by epifluorescence microscopy (Fig. 2A and B). The fluorescence in individual cells was substantially higher in the strains harboring the Cdz resistance mutations compared to an isogenic wild-type reporter strain, particularly for the ZerR(L59P) mutant.
FIG 2.
Increased expression of the zerRAB operon leads to Cdz resistance. (A) Venus-based transcriptional reporter in a wild-type or zer mutant background, as indicated, for cells grown to early stationary phase. (B) Quantification of the Venus fluorescence intensity in each reporter strain (in arbitrary units [A.U.]) in exponential (OD600 = 0.1) or stationary phase (OD600 ∼ 0.9 to 1). Three frames and a minimum of 500 cells were quantified. The box indicates first to third quartile range; the whiskers indicate the 1 to 99 percentile range. (C) Survival of a ΔcdzCDI indicator strain harboring the indicated construct driven by the cumate-inducible promoter PQ5 integrated on the chromosome, in competition with a wild-type Cdz producer and grown either with or without cumate, as indicated. Spots are 10-fold serial dilutions.
To pinpoint the gene(s) in the zerRAB operon responsible for resistance to Cdz toxins, we made strains in which each gene was individually overexpressed or with zerAB both overexpressed. Each construct was controlled by PQ5, a cumate-inducible promoter (22), on the chromosome and expressed in a ΔcdzCDI background. Expression was induced in early exponential phase for 12 h prior to mixing with a Cdz producer. Expression of either zerA or zerB alone led to an ∼4-log increase in survival in competition with a wild-type producer strain, with the overexpression of zerAB together sufficient to reduce Cdz-mediated killing by almost 5 log units (Fig. 2C). These results demonstrated that the overexpression of either ZerA or ZerB, or both, can protect cells. The fact that expressing ZerA or ZerB alone did not provide as much resistance as expressing ZerAB together suggests that the relative stoichiometry of ZerA and ZerB may be important for full protection or that these proteins have complementary activities.
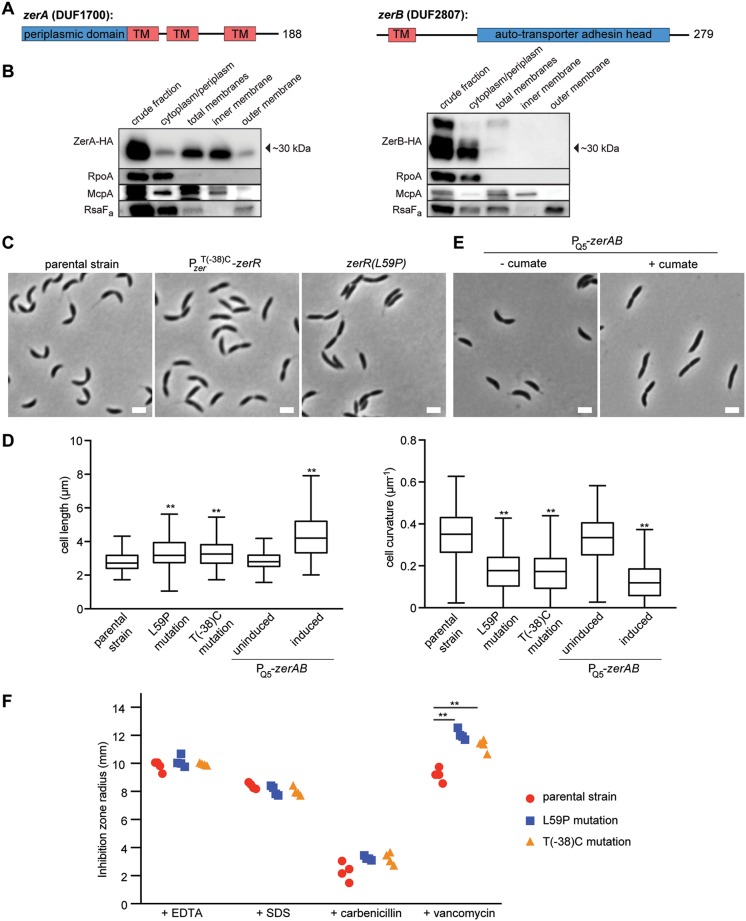
To further understand the role of ZerAB in Cdz toxin resistance, we sought to better characterize their physiological functions. ZerA and ZerB are predicted, based on TMHMM, to have three and one transmembrane helices, respectively, with each also containing a predicted periplasmic domain (Fig. 3A). For ZerB, this periplasmic domain has homology to trimeric autotransporter adhesin heads. This family of proteins typically adopts a β-roll fold and has characteristic repeats centered around GSG motifs that allow tight β turns (23, 24). In some bacteria, these proteins have a role in cell-cell adhesion and in attachment to host cells, like canonical autotransporter adhesins. However, previously characterized autotransporter adhesins are large extracellular proteins that also include an outer membrane β-barrel anchor and a long stalk (23), whereas ZerB has only the adhesin head domain and is predicted to be anchored to the inner membrane.
FIG 3.
ZerA and ZerB reside in the inner membrane and affect cell envelope properties. (A) Schematic of the predicted domain structure of ZerA and ZerB. TM, transmembrane domain predicted by TMHMM. Numbers to the right indicate number of residues in each protein. (B) Western blots of the indicated cell fractions from a culture of cells producing HA-tagged ZerA or ZerB from the chromosome under the cumate-inducible promoter and grown to stationary phase. RpoA, McpA, and RsaFa serve as controls for the cytoplasmic, inner membrane, and outer membrane fractions, respectively. (C) Phase microscopy of the ΔcdzCDI parental strain and the isolated resistant strains harboring the mutations indicated. Scale bars, 2 μm. (D) Quantification of cell length and curvature for the strains indicated in panels C and E. A minimum of 200 cells per strain were quantified. **, P < 0.005 (ANOVA, compared to the parental strain). (E) Phase microscopy of a ΔcdzCDI indicator strain harboring the indicated construct driven by the cumate-inducible promoter PQ5 on the chromosome. (F) Susceptibilities of the zerRAB mutants to envelope-acting compounds: radius of inhibition around a disk impregnated with 10 μl of the indicated substance (1 M EDTA, 10% SDS, 50 mg/ml carbenicillin, 100 mg/ml vancomycin) and placed on a lawn of the indicated strain on soft agar. Data from each of four replicates are shown. **, P < 0.005 (multiple-comparison ANOVA).
To determine the subcellular localization of ZerA and ZerB, we performed cell fractionation experiments on cells producing hemagglutinin (HA)-tagged variants of each protein (Fig. 3B). As controls for the fractionation procedure, we verified by immunoblotting that RpoA, a subunit of RNA polymerase, was found predominantly in the cytoplasmic fraction; the chemoreceptor McpA was found in the total membrane and inner membrane fractions; and RsaFa, a protein involved in S-layer production, was found primarily in the total membrane and outer membrane fractions. ZerA-HA was found primarily in the total and inner membrane fractions. For ZerB-HA, a faint band running at the approximate position predicted for full-length ZerB was seen in the total membrane fraction. The absence of a full-length ZerB in the inner membrane fraction was likely a result of protein loss during the fractionation procedure. We also observed a stronger but faster running band in the cytoplasmic/periplasmic fraction, suggesting that ZerB is processed into a smaller form that lacks the N-terminal transmembrane domain and is predominantly cytoplasmic or periplasmic. Taking all these results together, we conclude that ZerA and ZerB are likely both inner membrane proteins, with ZerB being rapidly cleaved into the cytosol or periplasm. Alternatively, the predicted transmembrane domain at the N terminus of ZerB could be a secretion signal for a solely periplasmic protein.
To probe the function of ZerA and ZerB in Caulobacter, we first examined the morphology of the two resistant strains we had isolated, harboring either the T(–38C) mutation in the zerRAB promoter or the zerR(L59P) mutation. Although these mutants had growth rates comparable to the wild type in culture (Fig. S1), cells from each resistant strain appeared longer and had noticeably reduced cell curvature compared to an isogenic parental strain, with the vast majority of cells in each case lacking the characteristic crescent shape of C. crescentus (Fig. 3C and D). Overexpressing zerAB from the chromosome caused a similar morphological phenotype (Fig. 3D and E).
The changes in cellular morphology observed likely reflect changes in peptidoglycan composition or structure. As noted above, the CdzC/D toxins kill target cells by disrupting membrane integrity, so changes to the composition of the cell membrane or cell wall (e.g., the degree of peptidoglycan cross-linking or turnover) in cells overproducing ZerA and ZerB could explain their increased resistance to Cdz toxins. To test whether increased levels of ZerAB affected the cell envelope, we tested the sensitivity of the T(–38C) and zerR(L59P) strains to the detergent sodium dodecyl sulfate (SDS) and the cation chelator EDTA but observed no differences relative to the parental ΔcdzCDI strain (Fig. 3F). In addition, the mutants and the parental strain had comparable susceptibility to the cell wall targeting β-lactam carbenicillin. However, the two zerRAB mutants isolated in our screen each showed increased sensitivity to vancomycin (Fig. 3F), which inhibits a peptidoglycan synthesis step in the periplasm. This finding supports the notion that the mutations identified that increase zerAB expression may render cells resistant to CdzC/D by altering the normal composition or structure of the cell wall.
The pentapeptide repeat protein PerA is required for cdz toxin-dependent killing.
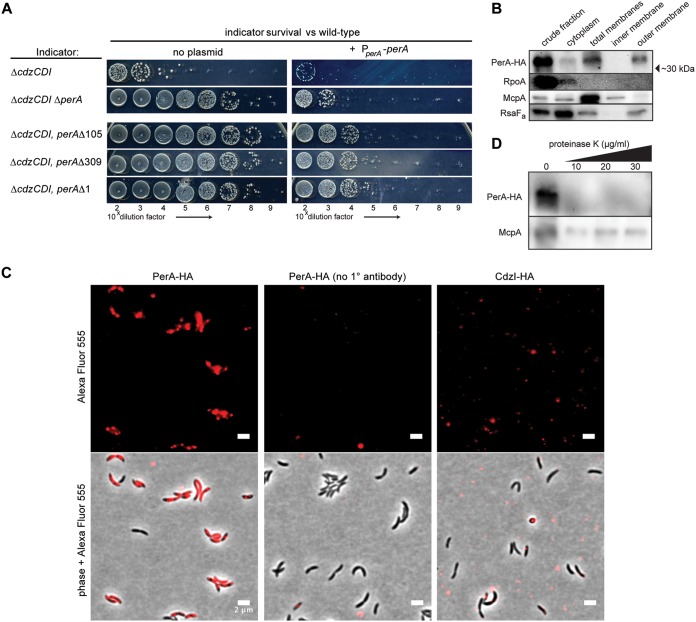
We obtained four independent resistant strains with mutations mapping to the gene perA, a hypothetical pentapeptide repeat protein of unknown function (Fig. 1C). One of the resistance mutations was a deletion of the entire promoter and 5′ region of the perA gene. The other mutations were smaller in-frame deletions (5 or 35 residues) in the core of the coding region and a frameshift leading to a truncation at the C terminus (missing residues 234 to 288). To determine whether the resistance mutations identified were loss-of-function alleles, we introduced an ectopic copy of the wild-type perA gene into each of the resistant strains and tested them in competition with a wild-type producer (Fig. 4A). In each case, the presence of a wild-type copy of perA restored ∼5 log units of sensitivity to the wild-type producer. The remaining 1 to 2 log units of sensitivity could indicate that the perA expression construct used did not fully restore PerA expression.
FIG 4.
PerA is a surface-exposed, outer membrane protein required for Cdz sensitivity. (A) Survival of the indicated strains in competition with a wild-type Cdz producer. (B) Western blots of the indicated cell fractions from a culture of cells producing HA-tagged PerA. RpoA, McpA, and RsaFa serve as controls for the cytoplasmic, inner membrane, and outer membrane fractions, respectively. (C) Immunofluorescence of ΔmanB ΔrsaA cells, expressing an HA-tagged PerA or CdzI, as indicated. The middle panels are for cells in which no primary antibody was added. (D) Western blots of proteinase K-treated cells expressing HA-tagged PerA.
To test whether a perA null strain also conferred resistance to killing by Cdz toxins, we constructed a strain in which the entire perA coding region was deleted. The perA gene was previously suggested to be essential for viability based on a saturating transposon insertion screen in rich medium (25). However, we were able to make a viable perA deletion strain, although this mutation caused a significant growth defect, as discussed below. Despite this growth defect, ΔperA ΔcdzCDI cells survived in competition with a Cdz producer (Fig. 4A). Introduction of a full-length copy of perA resensitized the ΔperA strain to killing. Taken together, these results confirm that a loss of PerA function leads to resistance to Cdz toxins.
PerA has a predicted secretion leader sequence and, according to an outer membrane proteomics study, is associated with the outer membrane (26). To further probe the subcellular localization of PerA, we performed cell fractionation, as done for ZerAB, on a strain producing an HA-tagged variant of PerA. This analysis indicated that PerA indeed localizes almost exclusively to the outer membranes of cells (Fig. 4B). To corroborate these results, we used immunofluorescence microscopy on a strain expressing HA-tagged PerA and lacking manB, a gene required for exopolysaccharide production (27), and rsaA, which encodes the proteinaceous S-layer capsule (28). In this strain, we clearly detected PerA on the surface of unpermeabilized cells (Fig. 4C). As expected, no signal was detected without addition of the anti-HA antibody. In addition, no cell-associated signal was detected for a strain expressing HA-tagged CdzI, which localizes to the inner membrane (8), indicating that cells were not being inadvertently permeabilized during the immunofluorescence procedure. To further test whether PerA is surface exposed, we treated whole cells with low concentrations of proteinase K and then performed Western blots on whole-cell lysates. Notably, such treatments led to a nearly complete loss of signal with the anti-HA antibody, implying that the proteinase K had degraded surface-exposed PerA-HA. As a control, we confirmed that McpA, a known inner membrane protein, was still detected by Western blotting after proteinase K treatment (Fig. 4D). Taken all together, our results strongly support the conclusion that PerA resides in the outer membrane, facing the extracellular environment, where it could act as a receptor for the Cdz toxins.
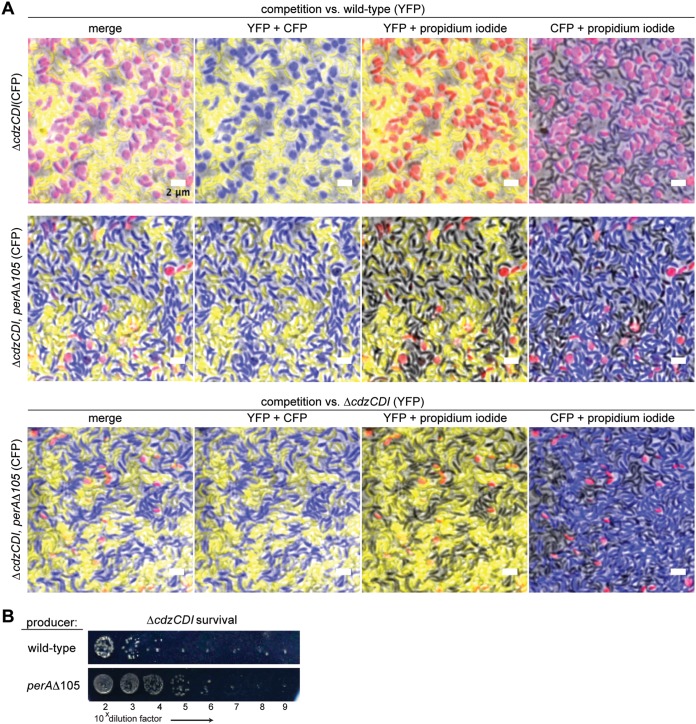
Pentapeptide repeat proteins like PerA are predicted to form β-helix structures (29, 30). Notably, the CdiA toxins in some E. coli strains and related species also form right-handed β-helical proteins that promote adhesion to target cells to mediate contact-dependent inhibition by CDI systems (23, 31). To determine whether PerA mediates cell-cell attachment in C. crescentus, we sought to test whether enforced cell-cell contact between producer and target cells would bypass the requirement for PerA, i.e., if PerA is required to mediate cell contact, a perA mutant should no longer confer resistance to Cdz-mediated killing when cells are forced into direct contact. To test this possibility, we conducted competition experiments on agarose pads, where cells grow and form a dense multilayer lawn. We used different fluorescent proteins to distinguish the strains, and conducted competitions in the presence of propidium iodide (PI) as a marker of cell death. After 3 days in coculture, the agarose pads were blotted onto a coverslip and imaged by fluorescence microscopy.
In a competition between a wild-type producer (YFP+) and a ΔcdzCDI indicator/target strain (CFP+), the vast majority of target cells had been killed by day three (Fig. 5A). In sharp contrast, a competition of the wild type with a ΔcdzCDI indicator also harboring the resistance mutation perAΔ105 did not show significant levels of cell death despite the enforced cell-cell contact. This result strongly suggests that PerA is not mediating cell-cell adhesion as the main means of promoting killing by Cdz toxins. However, CdzC/D toxicity could require specific molecular interactions between producer and target cells and not just close physical proximity. In particular, PerA could, like the β-helical adhesin Ag43 in uropathogenic E. coli (32), mediate a homotypic interaction between producer and target cells that then enables successful transfer of the CdzC/D toxin. To test this possibility, we sought to determine whether the perAΔ105 allele, which yielded high resistance to target cells (Fig. 1C), would affect the ability of producer cells to kill a ΔcdzCDI indicator strain (Fig. 5B). If PerA were mediating cell adhesion via a homotypic interaction between producer and target cells, then the perAΔ105 allele should eliminate killing if present in either cell type during an infection. We found that the survival of the indicator strain increased by ∼2 log units when mixed with a perAΔ105 producer compared to a wild-type producer, but survival of the indicator was still ∼5 log units lower that seen for a perAΔ105 indicator mixed with a wild-type producer. These results suggest that PerA likely does not contribute to Cdz-dependent killing by promoting a homotypic interaction between producer and indicator cells and instead is specifically required in the target cell, possibly as a receptor or coreceptor for CdzC/D.
FIG 5.
PerA does not promote attachment or cohesion of producer and target cells. (A) The strains listed on the left, each expressing CFP, were competed against a wild-type Cdz producer expressing YFP on agarose pads with propidium iodide (PI). After 3 days, the agarose pads were blotted onto coverslips and imaged for CFP, YFP, and PI. (B) Survival of the indicator strain ΔcdzCDI, in competition with the indicated Cdz producer.
PerA may act as the CdzC/D surface receptor.
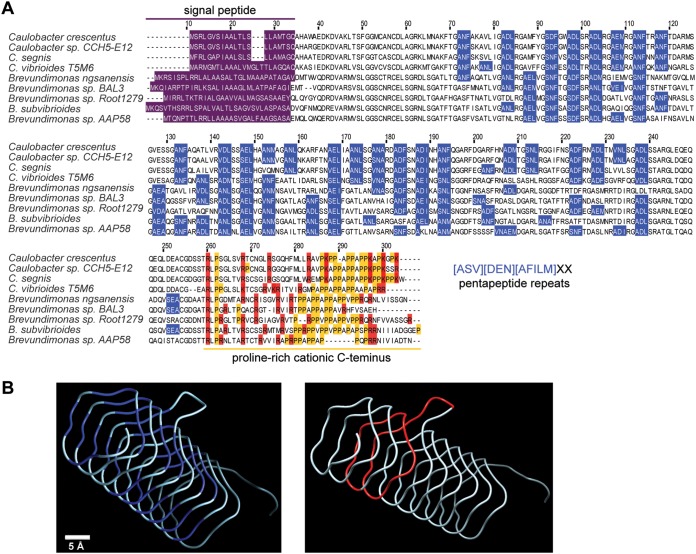
To test whether the mutations identified in perA render cells resistant to Cdz toxins by altering the cell envelope, as suggested for zerRAB, we sought to characterize the PerA protein and its molecular-level function. PerA is relatively well conserved in many close relatives of Caulobacter, with more distantly related homologs found throughout the α-proteobacteria. C. crescentus PerA has 25 repeats of the [ASV][DEN][AFILM]XX motif, where “X” is any amino acid (Fig. 6A). Each occurrence of the repeat motif is thought to be one side of a right-handed quadrilateral β-helix fold. PerA is predicted to have a highly regular structure with one excursion, a small protruding loop (Fig. 6B). The predicted core of the helix is mostly composed of hydrophobic residues, while the outside surface has polar and charged residues. The other striking feature of PerA, which contrasts with other pentapeptide repeat proteins, is a conserved, proline-rich, and positively charged C-terminal region (Fig. 6A).
FIG 6.
PerA is a pentapeptide repeat protein. (A) Multiple sequence alignment of PerA homologs from C. crescentus and other close relatives. Blue boxes represent instances of the first three residues of the pentapeptide repeat motif. Prolines are indicated in yellow and positively charged residues at the C terminus in red. (B) Representation of the predicted three-dimensional structure for the pentapeptide repeat domain (excluding the secretion leader sequence and the C terminus), made by threading the C. crescentus PerA sequence onto PDB structure 4YFOA. (Left) Pentapeptide repeats are highlighted in blue. (Right) The 35-residue in-frame deletion in the perAΔ105 allele is highlighted in red.
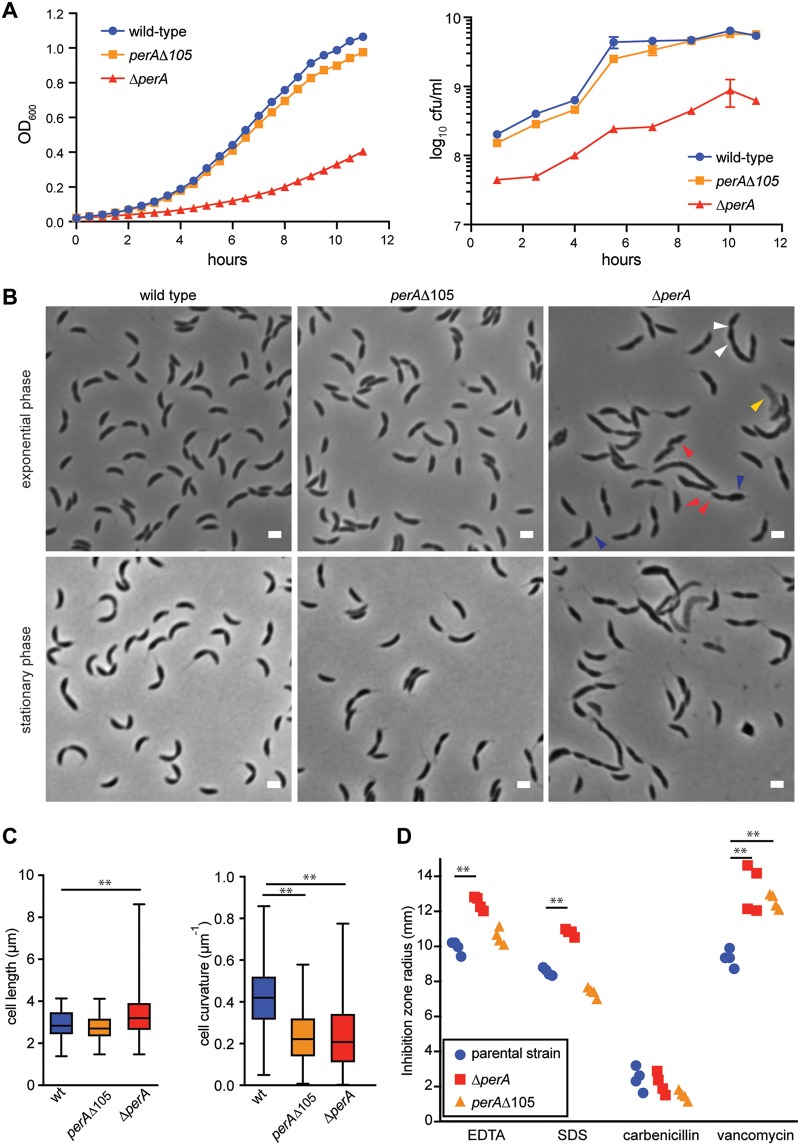
A strain lacking perA exhibited a significant growth defect in a rich medium, with slower colony formation on plates (3 days instead of 2) and an ∼35% lower growth rate during exponential phase in culture (Fig. 7A). The ΔperA strain exhibited evidence of cell lysis in a fraction of the population, with significant morphological aberrations in virtually all other cells (Fig. 7B). The surface of cells appeared irregular, with evidence of blebbing and some regions of increased cell width. In addition, rather than being crescent shaped, the cells were relatively straight, and some cells were filamentous, often with several constriction sites (Fig. 7B and C). The ΔperA strain also exhibited sensitivity to several external stressors, including EDTA, SDS, and vancomycin (Fig. 7D).
FIG 7.
PerA is required for cell-envelope homeostasis and may serve as a Cdz toxin receptor. (A) Growth curves (left) and CFU curves (right) of the indicated strains in rich medium. Data points represent the means of three independent cultures; error bars indicate standard deviations. (B) Phase micrographs of the indicated strains in exponential (OD600 = 0.25) and stationary (OD600 ∼ 0.9 to 1) phases. For the ΔcdzCDI ΔperA image, red arrowheads point at membrane blebs, blue arrowheads indicate cells with increased cell width, white arrowheads show pinching sites in a filamentous cell, and yellow arrowheads indicate “ghosts” of lysed cells. (C) Quantification of cell morphology for the strains shown, in stationary phase. A minimum of 500 cells and three frames were quantified. Scale bars, 2 μm. (D) Susceptibilities of the perA mutants to cell envelope stressors: radius of inhibition around a disk impregnated with 10 μl of the indicated substance (1 M EDTA, 10% SDS, 50 mg/ml carbenicillin, 100 mg/ml vancomycin) and placed on a lawn of the indicated strain on soft agar. Data from each of four replicates are shown. **, P < 0.005 (multiple-comparison ANOVA).
The defects of the perA deletion strain might reflect changes in the cell envelope, which could, as suggested for zerRAB, explain the Cdz resistance of cells harboring loss-of-function mutations in perA. Notably, however, the resistant strain carrying the perAΔ105 allele did not exhibit the same severe growth defect (Fig. 7A) or morphological anomalies (Fig. 7B and C) as the ΔperA strain or the other perA mutants identified (Fig. S2), although it did exhibit decreased cell curvature. The perAΔ105 strain also did not show significant sensitivity to EDTA or SDS as with the ΔperA strain (Fig. 7D). In sum, the perAΔ105 allele, which deletes roughly two complete turns of the β-helix and part of the predicted protruding loop (Fig. 6B), leads to a mostly functional protein, with respect to growth and morphology, while rendering cells highly resistant to Cdz toxins. Thus, this allele appears to largely uncouple the role of PerA in cellular morphology from its role in Cdz-dependent killing, suggesting that the perA mutants identified in our screen do not confer Cdz resistance simply by altering cell envelope composition. Instead, given its surface-exposed location in the outer membrane of cells and the strong resistance of the perAΔ105 strain, we suggest that PerA is a primary receptor for the Cdz toxins. We cannot, however, fully rule out the possibility that modest changes in the cell envelope of perAΔ105 cells somehow change the susceptibility of cells to Cdz toxins or change the presentation of the bona fide receptor.
DISCUSSION
The recently described Cdz bacteriocin system from the oligotrophic aquatic bacterium Caulobacter crescentus differs from other characterized bacteriocins in terms of sequence (hydrophobic core with an extended glycine zipper) and especially in its ability to aggregate on the surface of cells and kill other cells in a contact-dependent manner (8). However, similar to many characterized bacteriocins, the main mechanism by which Cdz kills target cells is likely through membrane permeabilization leading to a loss of the proton gradient across the inner membrane. Binding of bacteriocins to target cells typically requires a receptor, but no receptor for the Cdz toxins was previously identified (8). Here, using a screen for spontaneous mutations that yield resistance to Cdz toxins we identified PerA, an outer membrane protein, as a strong candidate for the Cdz receptor. We also identified a locus, zerRAB, that can, when overexpressed, protect cells, probably by modifying the cell envelope of cells or by rendering cells resistant in some other indirect manner.
Our screen for mutations that render cells resistant to the effects of Cdz toxins identified two independent mutations in the zerRAB operon. ZerAB are unlikely to serve as a receptor for the Cdz toxins since the mutations identified led to an upregulation of ZerAB; for a receptor we would have expected a loss-of-function or null mutation. In addition, the ZerA and ZerB proteins are localized to the inner membrane and likely the periplasm, respectively, of cells, and the Cdz receptor is presumably located in the outer membrane. Moreover, ZerAB are not well conserved, even in the alphaproteobacteria. Thus, we hypothesize that the mutations leading to upregulation of ZerAB protect against Cdz toxins by modulating cell envelope composition and, consequently, cell shape (Fig. 3C to F). Notably, for other pore-forming bacteriocins, common mechanisms of resistance also often involve changes in membrane composition, charge, or fluidity, or changes in peptidoglycan layer properties, including the thickness, charge, and degree of cross-linking (33, 34). Overexpressing ZerAB could also potentially render cells resistant to Cdz toxins by changing the trafficking, distribution, or levels of an outer membrane receptor.
We hypothesize that PerA is a primary receptor for Cdz toxins given that it resides in the outer membrane and multiple loss-of-function mutations were identified in our screen for Cdz resistance. PerA is unrelated to and quite different from the direct receptors of other characterized bacteriocins, which are usually integral outer membrane proteins or lipopolysaccharide (1, 35, 36). PerA is found in the outer membranes of Caulobacter cells (Fig. 4B to D), but based on the polar and charged surfaces of PerA, it is unlikely to be an integral outer membrane protein. Instead, we hypothesize that the proline-rich cationic C terminus anchors PerA to the negatively charged lipopolysaccharide (LPS) surface, or enables binding to an integral outer membrane protein. Whatever the case, our results are consistent with PerA being an exposed, surface receptor for Cdz toxins on target cells, with the toxins then imported across the outer membrane via an integral outer membrane protein such as a TonB-dependent receptor, as occurs with most Gram-negative bacteriocins. The TonB-dependent receptor CCNA_03108 identified here may be one such importer for the Cdz toxins but is likely not the only outer membrane protein used for import, since a null allele conferred only modest levels of resistance (Fig. 1C).
Importantly, a direct physical interaction between PerA and the Cdz toxins has not yet been demonstrated and will likely be difficult given the insoluble nature of the Cdz toxins (8). It is also formally possible that PerA is only indirectly involved in Cdz toxicity and somehow affects the presentation or activity of the bona fide receptor, although no other outer membrane proteins were identified in our screen. However, our screen was certainly not saturated since only a single mutant was found in one of the genes (CCNA_03680). Thus, additional genes that affect sensitivity or resistance to Cdz may remain to be discovered.
The receptors of toxins and bacteriocins are often important to the viability or survival of an organism under some growth or stress conditions such that producer cells can effectively rely on their presence on target cells (36, 37). The C. crescentus perA gene was originally suggested to be essential for viability (25). We found that it is formally dispensable in rich medium, but cells lacking perA grow significantly more slowly than a wild-type strain and exhibit a range of morphological defects (Fig. 7) suggesting it plays an important role in cells that harbor it. In particular, cells lose their characteristic crescent shape, becoming straight or bent at sharper angles than normal, possibly reflecting an abnormal cell wall.
PerA is annotated as a pentapeptide repeat protein, a member of a family of proteins characterized by the occurrence of multiple tandem repeats of the motif [STAV][D/N][L/F]XX, where “X” can be any amino acid (30, 38). Crystal structures show that these repeats adopt a regular right-handed quadrilateral β-helix fold (Rfr fold) with few excursions from the main structural element (29, 39). This protein family is ubiquitous in bacteria and particularly abundant in cyanobacteria, which often carry up to 40 variants per genome (38). Some of these pentapeptide repeat proteins from cyanobacteria contribute to heterocyst differentiation, glycolipid localization in the cell envelope, and the regulation of manganese uptake (40–42), but their precise molecular functions remain largely obscure. Interestingly, some gammaproteobacteria and Gram-positive bacteria harbor pentapeptide repeat proteins that reduce susceptibility to antibiotics from the quinolone family (gyrase inhibitors), possibly by acting as structural DNA mimics that competitively bind DNA gyrase (29, 43, 44). Some pentapeptide repeat proteins also confer immunity to modified peptide bacteriocins that inhibit gyrase (45–47). These pentapeptide repeat proteins are required in the bacteriocin producer cell to prevent self-intoxication. In contrast, in C. crescentus PerA confers susceptibility rather than resistance.
Most previously characterized pentapeptide repeat proteins are cytosolic (38, 48, 49), whereas PerA resides in the outer membrane. In addition to potentially serving as the receptor for Cdz toxins, PerA also seems to affect the cell envelope. How an extracellular protein affects cell shape and the cell envelope is unclear. In C. crescentus curvature is mainly determined by crescentin filaments, which associate in the cytosol with the inner membrane to regulate incorporation of new glycan strands into the peptidoglycan (50). However, it has also been previously shown that an altered form of O-antigen in LPS leads to loss of cell curvature, but the mechanism responsible is not known (51).
Although PerA is important for normal growth in rich medium, RNA-seq experiments (8, 21) indicate that the gene is expressed at relatively low levels under these conditions. Under cationic metal stress (Cu2+, Zn2+, and UO22+), perA expression is strongly upregulated as part of a putative envelope stress response mediated by the two-component system UzcRS (52). Thus, PerA may be particularly important for survival under various challenging conditions and could, in principle, make cells under these conditions particularly susceptible to Cdz toxins.
The mechanism by which a mutation in the ABC-transporter aminopeptidase urtP confers resistance to Cdz may also be through modulation of PerA levels. UrtP is a 1,195-residue protein that was recently shown to span the inner membrane, with an ∼600-residue C-terminal portion facing the periplasmic side (18). UrtP is predicted to bind zinc and catalyze the hydrolysis of polypeptides, with the neighboring CCNA_03681 (urtA) acting as the ATPase for the ABC transporter. UrtAP was suggested to be a negative regulator of the UzcRS two-component signaling pathway, which induces perA expression (18). The Q834* allele of urtP, which, unlike the urtP deletion, yields high levels of Cdz resistance, could be affecting PerA levels to produce resistance.
Concluding remarks.
Most of the well-studied bacteriocin systems belong to a limited group of bacterial clades and environments. Moreover, interbacterial antagonism systems remain relatively poorly characterized in nutrient-poor, aquatic environments like those where C. crescentus thrives. Interestingly, although divergent in delivery mechanism and protein sequence, Cdz appears to have a common inhibitory mechanism with many other small bacteriocins that disrupt membrane integrity and cause loss of the proton gradient across the inner membrane. However, the receptor for CdzC/D appears to be substantially different in C. crescentus, likely being the pentapeptide repeat protein PerA found in the outer membranes of target cells, or possibly an outer membrane component regulated by PerA. Further investigations into PerA and the Cdz system promise to provide new insights into this atypical bacteriocin system and, more broadly, the diversity of mechanisms used by bacteria to inhibit each other in contact-dependent ways.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All oligonucleotides, strains, and plasmids used in this study are listed in Tables S1, S2, and S3 in the supplemental material, respectively, along with details of how each strain was constructed. E. coli strains were grown at 37°C in Luria-Bertani medium. C. crescentus strains were grown at 30°C in peptone-yeast extract (PYE). Media were supplemented, as necessary, with a final concentration of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) or 50 μM cumate (4-isopropylbenzoic acid stock in 100% ethanol) to induce expression from the Plac and PQ5 promoters, respectively. Antibiotics were used at the following concentrations (liquid/solid media for E. coli; liquid/solid media for C. crescentus [in mg/ml]): oxytetracycline (12/12; 1/2), kanamycin (30/50; 5/25), and gentamicin (15/20; not applicable/5).
Competition experiments.
Coculture competition experiments in liquid culture were conducted as described previously (8). Briefly, producer and indicator strains were grown individually in liquid medium to early stationary phase (optical density at 600 nm [OD600] ∼ 0.85), mixed at equal volumes, and cocultures were incubated for 16 to 18 h. The CFU of each strain were determined by serial dilutions and plating on PYE supplemented with either tetracycline or gentamicin to select for the indicator or producer, respectively. Competitive indexes of the indicator were calculated as the ratio of CFU of each strain at the endpoint, relative to the ratio at the time of mixing. For competitions with indicator strains expressing zerA, zerB, or zerAB, we used strains carrying a chromosomal insertion at the vanillate locus of zerA, zerB, or zerAB expressed by the cumate-inducible promoter PQ5 and the cumate repressor gene (22). Each strain was grown to exponential phase, diluted to an OD600 of 0.01, and the culture was supplemented with 50 μM cumate. Cells were mixed with the producer strain after 13 h of induction.
For competitions on agarose pads, cells were grown in medium supplemented with IPTG. At an OD600 of ∼0.9, indicator and producer strains were mixed at a 1:1 volume ratio, diluted 1:3 in PYE, and 10 μl was spread on a 24- by 24-mm, 1.5% low-melting-point agarose pad of 900 μl of PYE supplemented with IPTG and 1 μM PI. After the cell droplet was dried, the pads were incubated in a humid chamber at 30°C for 3 days in the dark. At t = 0 and at every 24-h interval, the cells were imaged. For time points after 24 h, the pads were blotted several times against a 24- by 60-mm coverslip to remove excess cells, and the coverslip blots were imaged, choosing frames were a monolayer of cells was visible.
Resistance mutation screening and mapping.
Competition experiments were conducted in liquid culture as described above, but plating on selective media was done after 20 h. Isolated indicator colonies were restreaked on selective plates. These isolates were subjected to a second round of competition, and those that exhibited full resistance were imaged as described below after IPTG induction in liquid medium to verify presence of the expected fluorescent protein marker. Whole-genome sequencing of the parental and mutant resistant strains was done as previously described (53). Mutations were mapped to the reference genome using breseq (17). Genomic regions corresponding to the mapped mutations were amplified by PCR, and mutations were verified by Sanger sequencing. The strain initially isolated carrying the perAΔ105 mutation had an additional mutation in the genome and so was reconstructed by allelic replacement in a clean wild-type or ΔcdzCDI background (Table S2) for additional characterization steps such as growth curves and competition experiments.
Membrane depolarization analysis.
Each strain was grown separately to stationary phase and then mixed at an equal volume ratio. A 200-μl aliquot was collected 30 min after mixing and stained with bis-[1,3-dibutylbarbiturate] trimethine oxonol [DiBAC4(3)] as described previously (54), with modifications. Briefly, cells were collected by centrifugation and resuspended in 200 μl of M2 salts, followed by centrifugation and resuspension in 200 μl of M2 salts with 1 μg/ml DiBAC4(3). After incubation with shaking at 30°C for 10 min, cells were collected by centrifugation and resuspended in 400 μl of M2 salts medium and spotted on agarose pads for fluorescence microscopy via a GFP channel.
Chemical sensitivity disk assays.
Soft agar chemical sensitivity assays were done as described by (55), with some modifications. Each strain was grown to exponential phase in PYE and 200 μl of cells at an OD600 of 0.25 were added to 4 ml of warm PYE with 0.5% agar. The mix was homogenized and poured on top of a PYE agar plate to solidify. Each 6-mm sterile filter paper disk was impregnated with 10 μl of the tested compound, air dried, and placed on top of the solidified soft agar. Zones of inhibition were measured after 24 h of incubation at 30°C. The distance between the disk and the edge of bacterial lawn growth was quantified using Fiji (56).
Fluorescence microscopy.
To evaluate cellular morphology following the overexpression of zerA, zerB, or zerAB, we used strains carrying the respective construct under the control of the cumate-inducible promoter as a chromosomal insertion at the vanillate locus (57). Cells were grown to an OD600 of ∼0.2, supplemented with cumate, and incubated for 4 h prior to imaging. Phase and fluorescence microscopy was carried out as previously described (8). The following emission/excitation filters were used: YFP, 500/25 and 535/30m; CFP, 436/25 and 480/40; mCherry, 560/40 and 630/75; and GFP, S470/40 and 520/40. Image processing was done using Fiji (56). Image segmentation and quantification of cell attributes was done using the MicrobeJ plug-in (58).
Triton X-100 fractionation and Western blotting.
Induction of HA-tagged ZerA or ZerB was carried out in the same way as for competition experiments. Samples for fractionation were collected after 13 h. Induction of HA-tagged PerA was done by supplementing an exponential-phase culture at an OD600 of ∼0.15 with 10 μM cumate and then collecting samples after 6 h. Triton X-100 cell fractionation and immunoblotting were carried out as described previously (8).
Proteinase K treatment of whole cells.
Cells were grown to an OD600 of ∼0.2 and supplemented with 10 μM cumate to induce the expression of HA-tagged PerA. Samples were collected after 7 h of induction, pelleted and resuspended in phosphate-buffered saline (PBS). Proteinase K was added to a final concentration of 10, 20, or 30 μg/ml; incubated at 30°C for 30 min; and resuspended in PBS with SigmaFAST protease inhibitor cocktail (EDTA-free; Sigma). After an additional wash in PBS with protease inhibitor, cells were pelleted and resuspended in SDS sample buffer for SDS-PAGE and Western blotting.
Immunofluorescence.
For immunofluorescence, cells were grown in the same way as described for proteinase K treatment, and immunofluorescence was carried out as described previously (8). To reduce background, one additional wash step with fresh PYE was added after incubation in primary antibody, and two additional wash steps with fresh PYE were added after incubation in secondary antibody.
Alignment visualization and three-dimensional model prediction.
Protein structures were predicted by threading using RaptorX (59) and visualized using Chimera (60). Multiple sequence alignments were conducted using ClustalW (61) and visualized using Jalview (62).
Supplementary Material
ACKNOWLEDGMENTS
We thank the labs of R. Sauer, A. Grossman, L. Shapiro, J. Smit, and B. Imperiali for various reagents. We acknowledge S. Levine at the MIT BioMicroCenter for guidance with Illumina sequencing. We are grateful to members of the Laub laboratory for discussions and to L. Matano, J. M. Swiecicki, and G. Lozano for discussions and comments on the manuscript.
This study was supported by an NIH grant (R01GM082899) to M.T.L., who is also an Investigator of the Howard Hughes Medical Institute; by an HHMI International Predoctoral Fellowship to L.G.-B.; and by a National Science Foundation Graduate Research Fellowship to K.G.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00538-18.
REFERENCES
- 1.Cotter PD, Ross RP, Hill C. 2013. Bacteriocins: a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 2.Gerardin Y, Springer M, Kishony R. 2016. A competitive trade-off limits the selective advantage of increased antibiotic production. Nat Microbiol 1:16175. doi: 10.1038/nmicrobiol.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkup BC, Riley MA. 2004. Antibiotic-mediated antagonism leads to a bacterial game of rock–paper–scissors in vivo. Nature 428:412–414. doi: 10.1038/nature02429. [DOI] [PubMed] [Google Scholar]
- 4.Aoki SK, Poole SJ, Hayes CS, Low DA. 2011. Toxin on a stick: modular CDI toxin delivery systems play roles in bacterial competition. Virulence 2:356–359. doi: 10.4161/viru.2.4.16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho BT, Dong TG, Mekalanos JJ. 2014. A view to a kill: the bacterial type VI secretion system. Cell Host Microbe 15:9–21. doi: 10.1016/j.chom.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- 7.Hood RD, Singh P, Hsu F, Güvener T, Carl MA, Trinidad RRS, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Bayona L, Guo MS, Laub MT. 2017. Contact-dependent killing by Caulobacter crescentus via cell surface-associated glycine zipper proteins. Elife 6:e24869. doi: 10.7554/eLife.24869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonte V, Dostal V, Roberts CM, Gonzales P, Lacor PN, Lacor P, Velasco PT, Magrane J, Dingwell N, Fan EY, Silverman MA, Stein GH, Link CD. 2011. A glycine zipper motif mediates the formation of toxic β-amyloid oligomers in vitro and in vivo. Mol Neurodegener 6:61. doi: 10.1186/1750-1326-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bieler S, Silva F, Soto C, Belin D. 2006. Bactericidal activity of both secreted and nonsecreted microcin E492 requires the mannose permease. J Bacteriol 188:7049–7061. doi: 10.1128/JB.00688-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF. 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc Natl Acad Sci U S A 104:2384–2389. doi: 10.1073/pnas.0608775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marciset O, Jeronimus-Stratingh MC, Mollet B, Poolman B. 1997. Thermophilin 13, a nontypical antilisterial poration complex bacteriocin, that functions without a receptor. J Biol Chem 272:14277–14284. doi: 10.1074/jbc.272.22.14277. [DOI] [PubMed] [Google Scholar]
- 13.de Lorenzo V, Pugsley AP. 1985. Microcin E492, a low-molecular-weight peptide antibiotic which causes depolarization of the Escherichia coli cytoplasmic membrane. Antimicrob Agents Chemother 27:666–669. doi: 10.1128/AAC.27.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rea MC, Ross RP, Cotter PD, Hill C. 2011. Classification of bacteriocins from Gram-positive bacteria, p 29–53. In Drider D, Rebuffat S (ed), Prokaryotic antimicrobial peptides. Springer, New York, NY. [Google Scholar]
- 15.Shi L, Günther S, Hübschmann T, Wick LY, Harms H, Müller S. 2007. Limits of propidium iodide as a cell viability indicator for environmental bacteria. Cytometry 71A:592–598. doi: 10.1002/cyto.a.20402. [DOI] [PubMed] [Google Scholar]
- 16.Ruhe ZC, Nguyen JY, Xiong J, Koskiniemi S, Beck CM, Perkins BR, Low DA, Hayes CS. 2017. CdiA effectors use modular receptor-binding domains to recognize target bacteria. mBio 8:e00290-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deatherage DE, Barrick JE. 2014. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq, p 165–188. In Sun L, Shou W (ed), Engineering and analyzing multicellular systems. Springer, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park DM, Wesley Overton K, Jiao Y. The UzcRS two-component system in Caulobacter crescentus integrates regulatory input from diverse auxiliary regulators. Mol Microbiol, in press. doi: 10.1111/mmi.14180. [DOI] [PubMed] [Google Scholar]
- 19.Krewulak KD, Vogel HJ. 2011. TonB or not TonB: is that the question? Biochem Cell Biol 89:87–97. doi: 10.1139/o10-141. [DOI] [PubMed] [Google Scholar]
- 20.Rebuffat S. 2011. Bacteriocins from Gram-negative bacteria: a classification?, p 55–72. In Drider D, Rebuffat S (ed), Prokaryotic antimicrobial peptides. Springer, New York, NY. [Google Scholar]
- 21.Schrader JM, Zhou B, Li G-W, Lasker K, Childers WS, Williams B, Long T, Crosson S, McAdams HH, Weissman JS, Shapiro L. 2014. The coding and noncoding architecture of the Caulobacter crescentus genome. PLoS Genet 10:e1004463. doi: 10.1371/journal.pgen.1004463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaczmarczyk A, Vorholt JA, Francez-Charlot A. 2013. Cumate-inducible gene expression system for sphingomonads and other Alphaproteobacteria. Appl Environ Microbiol 79:6795–6802. doi: 10.1128/AEM.02296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nummelin H, Merckel MC, Leo JC, Lankinen H, Skurnik M, Goldman A. 2004. The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel β-roll. EMBO J 23:701–711. doi: 10.1038/sj.emboj.7600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczesny P, Linke D, Ursinus A, Bär K, Schwarz H, Riess TM, Kempf VAJ, Lupas AN, Martin J, Zeth K. 2008. Structure of the head of the Bartonella adhesin BadA. PLoS Pathog 4:e1000119. doi: 10.1371/journal.ppat.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christen B, Abeliuk E, Collier JM, Kalogeraki VS, Passarelli B, Coller JA, Fero MJ, McAdams HH, Shapiro L. 2011. The essential genome of a bacterium. Mol Syst Biol 7:528. doi: 10.1038/msb.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y, Johnson HM, Bazemore-Walker CR. 2012. Improved enrichment and proteomic identification of outer membrane proteins from a Gram-negative bacterium: focus on Caulobacter crescentus. Proteomics 12:251–262. doi: 10.1002/pmic.201100288. [DOI] [PubMed] [Google Scholar]
- 27.Gandham L, Nomellini JF, Smit J. 2012. Evaluating secretion and surface attachment of SapA, an S-layer-associated metalloprotease of Caulobacter crescentus. Arch Microbiol 194:865–877. doi: 10.1007/s00203-012-0819-9. [DOI] [PubMed] [Google Scholar]
- 28.Ford MJ, Nomellini JF, Smit J. 2007. S-layer anchoring and localization of an S-layer-associated protease in Caulobacter crescentus. J Bacteriol 189:2226–2237. doi: 10.1128/JB.01690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah S, Heddle JG. 2014. Squaring up to DNA: pentapeptide repeat proteins and DNA mimicry. Appl Microbiol Biotechnol 98:9545–9560. doi: 10.1007/s00253-014-6151-3. [DOI] [PubMed] [Google Scholar]
- 30.Vetting MW, Hegde SS, Fajardo JE, Fiser A, Roderick SL, Takiff HE, Blanchard JS. 2006. Pentapeptide repeat proteins. Biochemistry 45:1–10. doi: 10.1021/bi052130w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruhe ZC, Townsley L, Wallace AB, King A, Van der Woude MW, Low DA, Yildiz FH, Hayes CS. 2015. CdiA promotes receptor-independent intercellular adhesion: BamA-independent CdiA-mediated adhesion. Mol Microbiol 98:175–192. doi: 10.1111/mmi.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heras B, Totsika M, Peters KM, Paxman JJ, Gee CL, Jarrott RJ, Perugini MA, Whitten AE, Schembri MA. 2014. The antigen 43 structure reveals a molecular Velcro-like mechanism of autotransporter-mediated bacterial clumping. Proc Natl Acad Sci U S A 111:457–462. doi: 10.1073/pnas.1311592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastos Mdo C, Coelho ML, Santos OC. 2015. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 161:683–700. doi: 10.1099/mic.0.082289-0. [DOI] [PubMed] [Google Scholar]
- 34.Guest RL, Raivio TL. 2016. Role of the Gram-negative envelope stress response in the presence of antimicrobial agents. Trends Microbiol 24:377–390. doi: 10.1016/j.tim.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 35.McCaughey LC, Grinter R, Josts I, Roszak AW, Waloen KI, Cogdell RJ, Milner J, Evans T, Kelly S, Tucker NP, Byron O, Smith B, Walker D. 2014. Lectin-like bacteriocins from Pseudomonas spp. utilize d-rhamnose containing lipopolysaccharide as a cellular receptor. PLoS Pathog 10:e1003898. doi: 10.1371/journal.ppat.1003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Zamaroczy M, Chauleau M. 2011. Colicin killing: foiled cell defense and hijacked cell functions, p 255–287. In Drider D, Rebuffat S (ed), Prokaryotic antimicrobial peptides. Springer, New York, NY. [Google Scholar]
- 37.Cotter PD. 2014. An ‘Upp’-turn in bacteriocin receptor identification: identifying bacteriocin receptors. Mol Microbiol 92:1159–1163. doi: 10.1111/mmi.12645. [DOI] [PubMed] [Google Scholar]
- 38.Ni S, Sheldrick G, Benning M, Kennedy M. 2009. The 2Å resolution crystal structure of HetL, a pentapeptide repeat protein involved in regulation of heterocyst differentiation in the cyanobacterium Nostoc sp. strain PCC 7120. J Struct Biol 165:47–52. doi: 10.1016/j.jsb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Vetting MW, Hegde SS, Hazleton KZ, Blanchard JS. 2007. Structural characterization of the fusion of two pentapeptide repeat proteins, Np275 and Np276, from Nostoc punctiforme: resurrection of an ancestral protein. Protein Sci 16:755–760. doi: 10.1110/ps.062637707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anwari K, Poggio S, Perry A, Gatsos X, Ramarathinam SH, Williamson NA, Noinaj N, Buchanan S, Gabriel K, Purcell AW, Jacobs-Wagner C, Lithgow T. 2010. A modular BAM complex in the outer membrane of the α-proteobacterium Caulobacter crescentus. PLoS One 5:e8619. doi: 10.1371/journal.pone.0008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black K, Buikema WJ, Haselkorn R. 1995. The hglK gene is required for localization of heterocyst-specific glycolipids in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol 177:6440–6448. doi: 10.1128/jb.177.22.6440-6448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandler LE, Bartsevich VV, Pakrasi HB. 2003. Regulation of manganese uptake in Synechocystis 6803 by RfrA, a member of a novel family of proteins containing a repeated five-residues domain. Biochemistry 42:5508–5514. doi: 10.1021/bi027113a. [DOI] [PubMed] [Google Scholar]
- 43.Gibello A, Díaz de Alba P, Blanco MM, Machuca J, Cutuli MT, Rodríguez-Martínez JM. 2014. Lactococcus garvieae carries a chromosomally encoded pentapeptide repeat protein that confers reduced susceptibility to quinolones in Escherichia coli producing a cytotoxic effect. Res Microbiol 165:590–599. doi: 10.1016/j.resmic.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Montero C, Mateu G, Rodriguez R, Takiff H. 2001. Intrinsic resistance of Mycobacterium smegmatis to fluoroquinolones may be influenced by new pentapeptide protein MfpA. Antimicrob Agents Chemother 45:3387–3392. doi: 10.1128/AAC.45.12.3387-3392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cociancich S, Pesic A, Petras D, Uhlmann S, Kretz J, Schubert V, Vieweg L, Duplan S, Marguerettaz M, Noëll J, Pieretti I, Hügelland M, Kemper S, Mainz A, Rott P, Royer M, Süssmuth RD. 2015. The gyrase inhibitor albicidin consists of p-aminobenzoic acids and cyanoalanine. Nat Chem Biol 11:195–197. doi: 10.1038/nchembio.1734. [DOI] [PubMed] [Google Scholar]
- 46.Garrido M, Herrero M, Kolter R, Moreno F. 1988. The export of the DNA replication inhibitor microcin B17 provides immunity for the host cell. EMBO J 7:1853. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholz R, Molohon KJ, Nachtigall J, Vater J, Markley AL, Sussmuth RD, Mitchell DA, Borriss R. 2011. Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J Bacteriol 193:215–224. doi: 10.1128/JB.00784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knodler LA, Vallance BA, Hensel M, Jäckel D, Finlay BB, Steele-Mortimer O. 2004. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes: association of SPI-2 effectors with DRMs. Mol Microbiol 49:685–704. doi: 10.1046/j.1365-2958.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- 49.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. 2009. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev 22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabeen MT, Charbon G, Vollmer W, Born P, Ausmees N, Weibel DB, Jacobs-Wagner C. 2009. Bacterial cell curvature through mechanical control of cell growth. EMBO J 28:1208–1219. doi: 10.1038/emboj.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cabeen MT, Murolo MA, Briegel A, Bui NK, Vollmer W, Ausmees N, Jensen GJ, Jacobs-Wagner C. 2010. Mutations in the lipopolysaccharide biosynthesis pathway interfere with crescentin-mediated cell curvature in Caulobacter crescentus. J Bacteriol 192:3368–3378. doi: 10.1128/JB.01371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park DM, Overton KW, Liou MJ, Jiao Y. 2017. Identification of a U/Zn/Cu responsive global regulatory two-component system in Caulobacter crescentus: a metal responsive TCS in Caulobacter. Mol Microbiol 104:46–64. doi: 10.1111/mmi.13615. [DOI] [PubMed] [Google Scholar]
- 53.Jonas K, Liu J, Chien P, Laub M. 2013. Proteotoxic stress induces a cell-cycle arrest by stimulating lon to degrade the replication initiator DnaA. Cell 154:623–636. doi: 10.1016/j.cell.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smit J, Sherwood CS, Turner RF. 2000. Characterization of high density monolayers of the biofilm bacterium Caulobacter crescentus: evaluating prospects for developing immobilized cell bioreactors. Can J Microbiol 46:339–349. doi: 10.1139/w99-145. [DOI] [PubMed] [Google Scholar]
- 55.Ryan KR, Taylor JA, Bowers LM. 2010. The BAM complex subunit BamE (SmpA) is required for membrane integrity, stalk growth and normal levels of outer membrane β-barrel proteins in Caulobacter crescentus. Microbiol Read Engl 156:742–756. doi: 10.1099/mic.0.035055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thanbichler M, Iniesta AA, Shapiro L. 2007. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res 35:e137. doi: 10.1093/nar/gkm818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ducret A, Quardokus EM, Brun YV. 2016. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1:16077. doi: 10.1038/nmicrobiol.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kallberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. 2012. Template-based protein structure modeling using the RaptorX web server. Nat Protoc 7:1511–1522. doi: 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera A visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 61.Thompson JD, Gibson TJ, Higgins DG. 2002. Multiple sequence alignment using ClustalW and ClustalX, p 2.3.1–2.3.22. In Baxevanis AD, Petsko GA, Stein LD, Stormo GD (ed), Current protocols in bioinformatics. John Wiley & Sons, Inc., Hoboken, NJ. [DOI] [PubMed] [Google Scholar]
- 62.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview version 2: a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.