The intestinal microbiota is a diverse microbial ecosystem that provides numerous benefits to humans. The factors that govern its establishment and stability are just beginning to be elucidated. Identification and characterization of antimicrobial toxins produced by its members and their killing range are essential to understanding the role of antagonism in community composition and stability. Here, we identify a fifth antimicrobial toxin produced by a single Bacteroides fragilis strain and identify its target. The finding of such a large number of toxins that antagonize competing members suggests that this feature substantially contributes to the fitness of these bacteria. In addition, these toxins may have applications in genetically engineered gut bacteria to allow engraftment or to antagonize a potentially pathogenic member.
KEYWORDS: antagonism, bacteriocins, bacteroides, MACPF, microbiota, pore-forming toxins
ABSTRACT
Bacteroidales are the most abundant Gram-negative bacteria of the healthy human colonic microbiota, comprising nearly 50% of the colonic bacteria in many individuals. Numerous species and strains of gut Bacteroidales are present simultaneously at high concentrations in this ecosystem. Studies are revealing that gut Bacteroides has numerous antibacterial weapons to antagonize closely related members. In this study, we identify a new diffusible antibacterial toxin produced by Bacteroides fragilis 638R, designated BSAP-4. This is the fifth antibacterial toxin produced by this strain and the second toxin of this strain with a membrane attack complex/perforin domain (MACPF). We identify the target molecule of sensitive cells as a β-barrel outer membrane protein (OMP) with calycin-like domains. As with other MACPF toxins, the gene encoding the target in sensitive strains is in the same genetic region as bsap-4 in producing strains. A comparison of B. fragilis strains showed there are two sensitive variants of this OMP that are 87% similar to each other and 50% similar to the resistant OMP. Unlike other MACPF toxins, there are numerous B. fragilis strains that harbor the resistant OMP without bsap-4. Several OMP variants from strains that are BSAP-4 resistant under the conditions of our assay confer BSAP-4 sensitivity to Bacteroides thetaiotaomicron when constitutively expressed. Using a reporter assay, we show that the BSAP-4 receptor gene is differentially expressed in sensitive and resistant strains leading to apparent BSAP-4 resistance under the conditions of our assay, despite harboring the BSAP-4 target gene.
IMPORTANCE The intestinal microbiota is a diverse microbial ecosystem that provides numerous benefits to humans. The factors that govern its establishment and stability are just beginning to be elucidated. Identification and characterization of antimicrobial toxins produced by its members and their killing range are essential to understanding the role of antagonism in community composition and stability. Here, we identify a fifth antimicrobial toxin produced by a single Bacteroides fragilis strain and identify its target. The finding of such a large number of toxins that antagonize competing members suggests that this feature substantially contributes to the fitness of these bacteria. In addition, these toxins may have applications in genetically engineered gut bacteria to allow engraftment or to antagonize a potentially pathogenic member.
INTRODUCTION
Over the last several years, the predominant Gram-negative bacteria of the human colon, the Bacteroidales, have been shown to secrete antimicrobial toxins that antagonize closely related strains and species. These antimicrobial toxins include those secreted by type VI secretion systems (T6SSs) as well as diffusible toxins. The genetic architecture 3 (GA3) T6SSs are present exclusively in Bacteroides fragilis and have been studied in different B. fragilis strains (1–3). These GA3 T6SS loci encode toxic effectors and immunity proteins in the two divergent regions of these loci (4). Of the GA3 T6SS loci analyzed to date, each encodes two toxic effectors (1, 2), some of which have been shown to antagonize nearly all Bacteroidales species analyzed, including those of distinct families (1).
Strains of numerous Bacteroides species also secrete diffusible antimicrobial toxins; the majority of those analyzed have membrane attack complex/perforin (MACPF) domains. These domains are found in many eukaryotic proteins and are involved in various functions, including immunity, defense, and development (reviewed in reference 5). The first MACPF toxin identified in Bacteroides, BSAP-1, is produced by approximately 44% of B. fragilis strains, has a signal peptidase II cleavage site, and is thus a lipoprotein that is secreted via outer membrane vesicles and likely kills by pore formation (6). BSAP-1 targets a β-barrel outer membrane protein (OMP) of sensitive B. fragilis strains (7). B. fragilis strains typically either contain the bsap-1 gene and produce BSAP-1 or lack the gene and are sensitive to the toxin. This property is due to the fact that bsap-1 is present in the producer’s genome in the same location as the gene encoding the target OMP of sensitive strains. In the producer’s genome, there is a gene adjacent to bsap-1 that encodes an ortholog of the target OMP molecule that is likely functionally equivalent but distinct enough from the sensitive OMP that it does not serve as a target for BSAP-1.
In addition to BSAP-1, BSAP-2 and BSAP-3, produced by some Bacteroides uniformis and Bacteroides vulgatus/Bacteroides dorei strains, respectively, are also MACPF toxins (7, 8). Unlike BSAP-1, these toxins target the lipopolysaccharide (LPS) glycan (core or O-antigen) of sensitive strains. Similar to bsap-1, the genes for these toxins are present in the same genetic region as the target gene(s) in sensitive strains, in this case, in the LPS glycan biosynthesis loci. Each Bacteroides species has a predominant LPS glycan genetic locus, typically without a MACPF gene, whereas a minority of strains have a MACPF gene and replacements of a few glycosyltransferase genes in these loci. The genetic features suggest that these MACPF toxin genes were acquired with new glycosyltransferase genes that replaced those of the predominant LPS glycan type, sufficiently altering the glycan so it no longer serves as target for the toxin. We identified at least one strain from nine Bacteroides species with a MACPF domain-encoding gene in its LPS glycan region, along with glycosyltransferase gene replacements at the predominant glycan locus of the species. In addition to BSAP-2 and BSAP-3, we confirmed that two other MACPF proteins encoded in LPS glycan regions are toxins that antagonize species-matched strains with the predominant LPS glycan type (8). Like the BSAP-1 target, the LPS glycan target of BSAP-2 is important for gut colonization, demonstrating why genes encoding the targets of MACPF toxins are replaced rather than lost in the toxin-producing strain. MACPF domain proteins are widely distributed in the phylum Bacteroidetes, including in diverse members that live in soil and marine environments (6). As the target molecules of the MACPF toxins are species specific, all MACPF antibacterial toxins that have been identified to date target strains of the same or very closely related species, such as B. vulgatus and B. dorei (9).
In addition to BSAP-1, some B. fragilis strains secrete a diffusible antibacterial molecule that is very similar to human ubiquitin (BfUbb) (10). Like the MACPF toxins, this small protein also antagonizes strains of the same species; however, many strains that do not harbor the ubb gene are also resistant to it. The mechanism of action of BfUbb has not yet been reported.
B. fragilis strain 638R has served as our model strain for study of many of these antimicrobial molecules. BSAP-1 and BfUbb are both produced by this strain. In addition, we identified the two toxic effectors of the GA3 T6SS of this strain, Bfe1 and Bfe2 (1). In our previous analysis of BfUbb, we created a mutant where both bsap-1 and ubb were deleted. Here, we tested a panel of 34 B. fragilis strains and found that some strains are still antagonized by a diffusible secreted molecule produced by this double deletion mutant. In this study, we identified this fifth antimicrobial molecule of this strain and its target in sensitive cells.
RESULTS
Identification of an additional diffusible toxin produced by B. fragilis 638R.
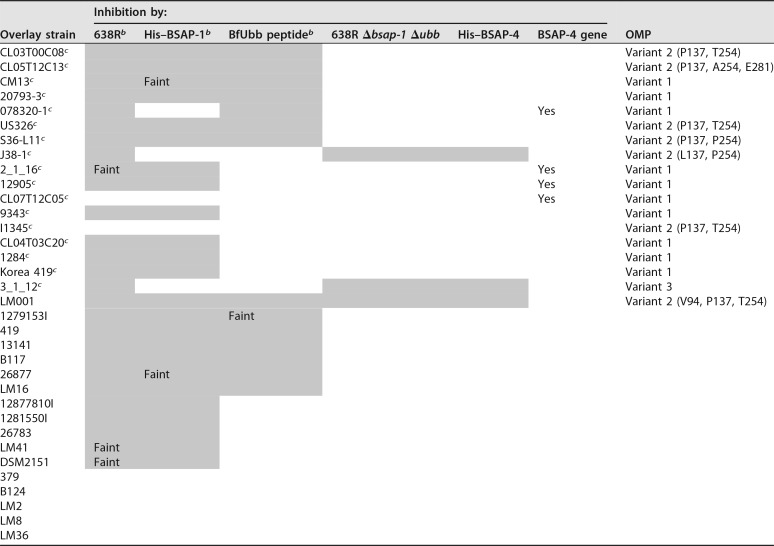
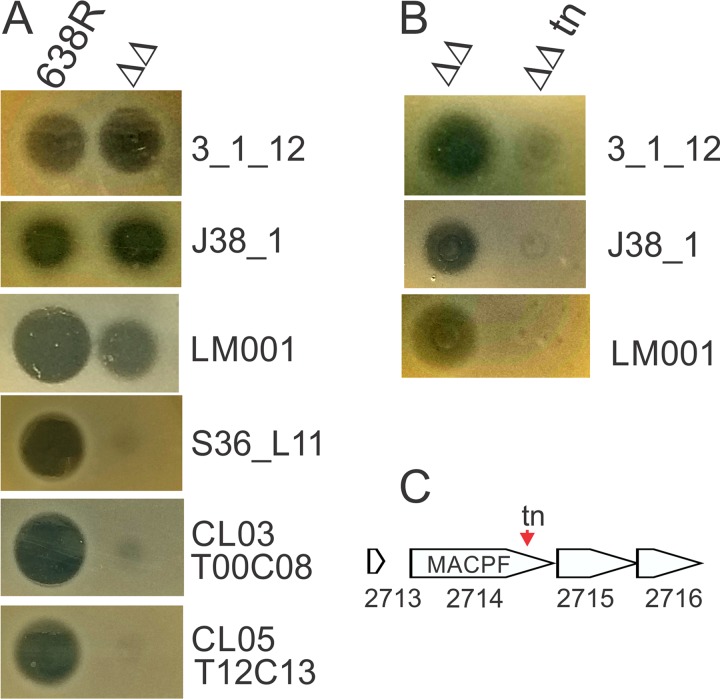
A B. fragilis 638R mutant in which both previously identified diffusible toxin-encoding genes, BF638R_1646 (bsap-1) and BF638R_3923 (ubb), are deleted no longer inhibits the growth of several B. fragilis strains in the in vitro spot overlay assay (10). We analyzed a panel of 34 B. fragilis strains and found that three of these B. fragilis strains are growth inhibited to some extent by a molecule secreted by this double deletion mutant (Table 1; Fig. 1), though the zones of inhibition are not as strong compared to those produced by the BSAP-1 and BfUbb toxins. To identify this antimicrobial molecule, we performed transposon mutagenesis using the 638R Δbsap-1 Δubb mutant background strain. A transposon mutant was identified that was severely attenuated in its ability to inhibit the growth of all three B. fragilis strains (Fig. 1B). The transposon insertion site mapped to bp 1263 of gene BF638R_2714, which encodes a protein with a MACPF domain (Fig. 1C).
TABLE 1.
Ability of B. fragilis 638R, 638R Δbsap-1, 639R Δubb, 638R Δbsap-1 Δubb, and His–BSAP-4 to inhibit the growth of B. fragilis strainsa
Data regarding growth inhibition of strains and mutants are from when assays were performed using BHIS plates. Shading indicates that the strain is growth inhibited by the strain/toxin.
bData were recently reported (10) but are shown here for comparative purposes.
cGenome sequence available.
FIG 1.
Identification of a third secreted diffusible antimicrobial molecule of B. fragilis 638R. (A) Agar overlay assays showing the ability of diffusible molecules from wild-type 638R or the double deletion mutant 638R Δbsap-1 Δubb (ΔΔ) to inhibit the growth of B. fragilis strains (strains used in overlays listed on the right). (B) Agar overlay showing a transposon mutant (tn) in background strain 638R Δbsap-1 Δubb that attenuates its antimicrobial activity against all three sensitive strains. (C) The transposon insertion site in BF638R_2714.
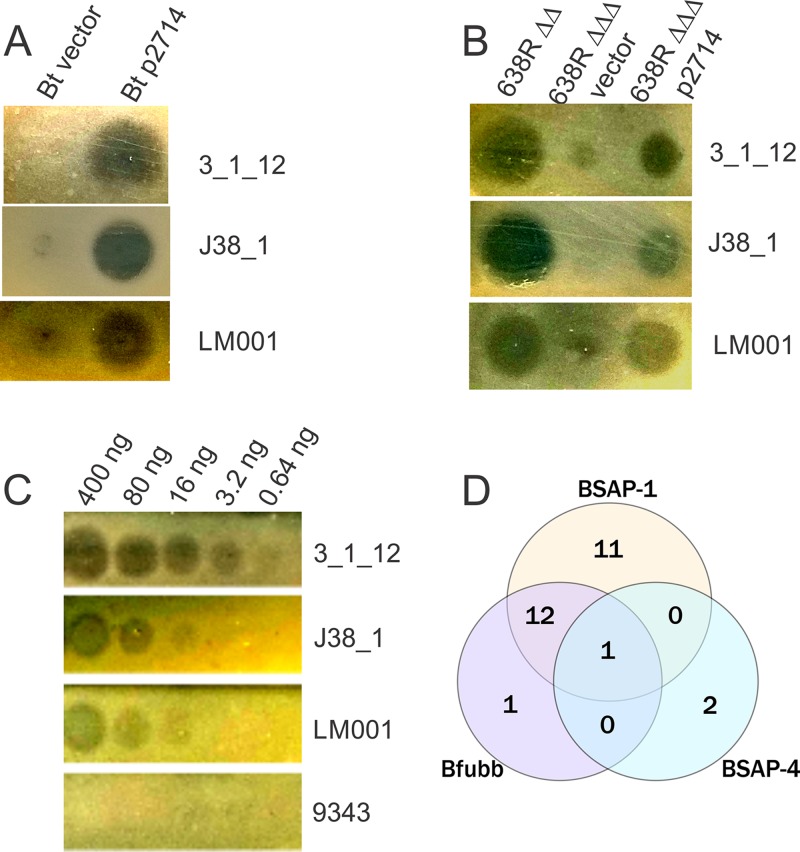
To confirm that BF638R_2714 confers this toxin activity, we used pMCL177 (6), a previously constructed plasmid with BF638R_2714 cloned into a Bacteroides expression vector, and placed it in trans in the heterologous species Bacteroides thetaiotaomicron VPI-5482. This gene conferred antimicrobial toxin activity to B. thetaiotaomicron when tested against the three sensitive strains (Fig. 2A). To further confirm that this MACPF protein is the inhibitory factor produced by the Δbsap-1 Δubb strain, we made an internal deletion mutant of BF638R_2714 in the 639R Δbsap-1 Δubb mutant background and found that this triple deletion mutant was attenuated in its ability to inhibit the growth of the three B. fragilis strains. The addition of BF638R_2714 in trans to the triple deletion mutant partially restored this growth inhibitory activity (Fig. 2B). As final proof that BF638R_2714 encodes a toxin, we created an N-terminal His-tagged fusion replacing the SpII signal sequence of the MACPF protein with the His tag. We found that this purified protein targets all three sensitive B. fragilis strains (Fig. 2C) but none of the other 31 strains shown in Table 1. We named this MACPF antimicrobial protein BSAP-4.
FIG 2.
Confirmation that BF638R_2714 encodes toxin activity. (A) Placement of BF638R_2714 in trans (p2714) in B. thetaiotaomicron VPI-5482 conferred growth inhibitory activity against all three B. fragilis strains. (B) Deletion of BF638R_2714 in the 638R Δbsap-1 Δubb (ΔΔ) background, resulting in 638RΔΔΔ, abrogated toxin activity that is partially restored when the gene is added in trans. (C) Purified His-tagged 2714 demonstrates toxin activity in a dose-dependent manner against the three sensitive B. fragilis strains but not against resistant strain NCTC 9343. (D) Venn diagram of the sensitivity of the 34 B. fragilis strains analyzed in this study against each of the three diffusible toxins produced by B. fragilis 638R.
Of the panel of 34 B. fragilis strains, we previously showed that 24 are targeted by BSAP-1, 14 strains are targeted by BfUbb (10), and three by BSAP-4. Of the three targeted by BSAP-4, two strains are targeted only by BSAP-4, whereas strain LM001 is targeted by all three toxins (Table 1; Fig. 2D). Only seven of these B. fragilis strains are not targeted by any of these three diffusible toxins of strain 638R.
Identification of the BSAP-4 target.
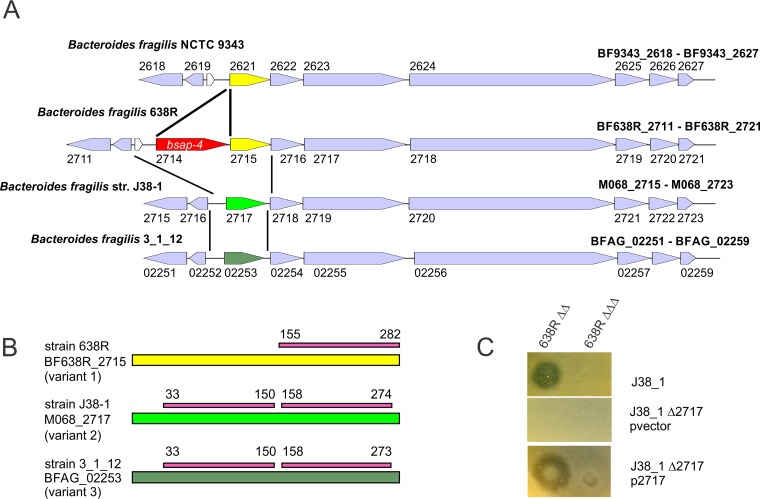
Based on our previous findings that the BSAP-1, -2, and -3 genes are found in the same genetic region as the receptor gene in sensitive strains, we analyzed the genetic region adjacent to bsap-4 and aligned this region in all the sequenced strains shown in Table 1. Based on genetic organization, bsap-4 may be part of an eight-gene operon (Fig. 3A), as the largest intergenic gap in this region is 84 bp. Several of the genes in this region are similar to heme uptake and macromolecular transport proteins. The gene immediately downstream of bsap-4 encodes a β-barrel outer membrane protein of the calycin superfamily with β-barrel structures (11). This gene is divergent in B. fragilis genomes, with extensive DNA identity (89% to 99%) resuming downstream of this gene (Fig. 3A). Although divergent, these variant genes from different B. fragilis genomes all encode proteins with a calycin-like domain(s). We predicted that the variant of this calycin-like OMP in sensitive strains may be the BSAP-4 receptor. We analyzed the sequences of this gene and its products from all 17 sequenced B. fragilis strains listed in Table 1 and found three major variants of the calycin-like OMP among these strains (Fig. 3A). Notably, in 10 of these 17 strains, this protein is identical or nearly identical to that of 638R (>99% identical) (variant 1), but only four of these strains have the adjacent BSAP-4 gene. All 10 strains, however, are resistant to BSAP-4 (Table 1). Of the three strains of B. fragilis sensitive to BSAP-4, two have sequenced genomes available, and we PCR amplified and sequenced this region from the third strain (B. fragilis LM001). Strains J38-1 and LM001 have a second variant of this OMP that is 50% similar to the variant 1 OMP. Strain 3_1_12 has a third variant of this OMP (variant 3) that is 53% similar to that of 638R (variant 1) and 87% similar to that of strains LM001 and J38-1 (variant 2). Interestingly, variant 2 and 3 OMPs, which are more similar to each other than to the variant 1 OMP, have two repeated calycin-like domains (Pfam PF13944.5), whereas the variant 1 OMP has only a single copy of this domain (Fig. 3B). To determine if the variant 2 OMP is necessary for BSAP-4 sensitivity, we made a clean deletion mutant of M068_2717 in strain J38-1 (variant 2), as strains LM001 and 3_1_12 are resistant to the two antibiotics used for genetic manipulation in Bacteroides. Deletion of M068_2717 renders the strain resistant to BSAP-4, and sensitivity is restored when the gene was added to this mutant in trans (Fig. 3C).
FIG 3.
BSAP-4 receptor analysis. (A) ORF maps of the four distinct genetic variants in the bsap-4 or corresponding genetic regions of sequenced B. fragilis strains. Genes highlighted in purple are conserved between genomes. Red indicates the bsap-4 MACPF gene. Yellow and green genes identify the three OMP variants. Lines between genomes delineate the extent of the major divergences. (B) Each of the three OMP variant proteins shown as a line with the extent of the calycin-like domains (PF13944.5) shown above in pink with the amino acid positions of the domains indicated. (C) Agar overlay assays showing that the deletion of the variant 2 OMP gene in strain J38-1 (J38-1 Δ2717) renders the strain resistant to BSAP-4 and that sensitivity to BSAP-4 is restored when the OMP gene is added to the mutant in trans (p2717).
Heterologous expression of diverse variant OMP genes and phenotypic analysis.
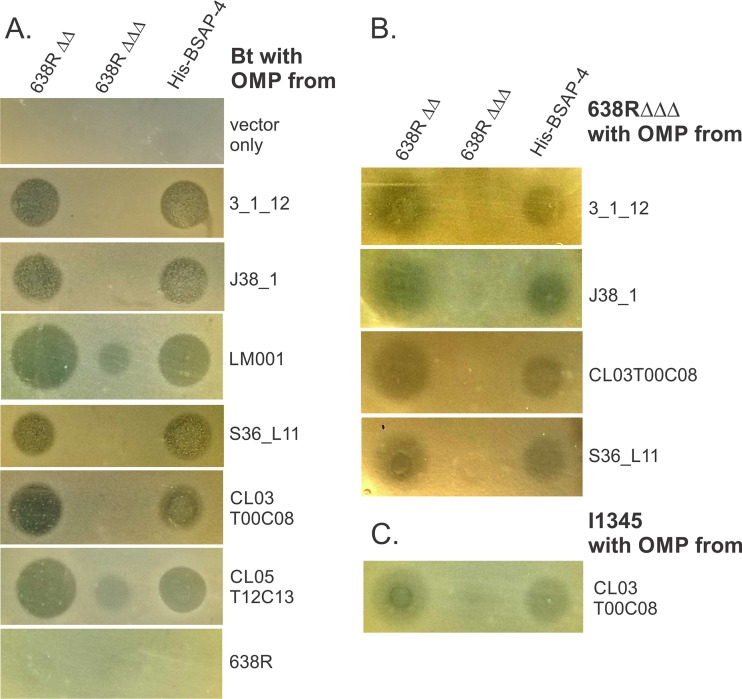
The variant 2 OMP is found in both BSAP-4-sensitive and -resistant B. fragilis strains (Table 1). An alignment of the DNA sequences of this gene from the six sequenced variant 2 strains listed in Table 1 revealed that the DNA upstream and downstream of the gene (100 bp analyzed) is identical between strains. However, there are minor nucleotide variants within the genes resulting in single-amino-acid differences between these proteins. There are four positions that are variable between these seven proteins. The protein from sensitive strain J38-1 has an L at position 137, whereas the six other proteins, including that from sensitive strain LM001, have a P at this position. There are three different residues at position 254 (P, T, or A), which do not segregate based on strain sensitivity, as J38-1 has a P at this position and LM001 has a T. As these amino acid differences do not correlate with strain sensitivity, we sought to determine whether each of these variant 2 OMPs could render B. thetaiotaomicron sensitive to BSAP-4. All five variant 2 OMPs, as well as the variant 1 OMP from 638R and the variant 3 OMP from strain 3_1_12 were cloned into a Bacteroides expression vector. When placed in trans in B. thetaiotaomicron, all variant 2 OMPs and the variant 3 OMP conferred BSAP-4 sensitivity to this strain, whereas the variant 1 OMP from strain 638R did not (Fig. 4A). These data show that each of these variant 2 OMPs is able to serve as a BSAP-4 target when expressed from a constitutive promoter in B. thetaiotaomicron. We also transferred several of these OMP variants into the 638R mutant deleted for all three diffusible toxins (638RΔΔΔ). Similar to the result in B. thetaiotaomicron, each variant rendered the 638RΔΔΔ strain somewhat sensitive to BSAP-4, although the zones of inhibition were not as sharp and clear as those resulting from the expression of these target genes in B. thetaiotaomicron (Fig. 4B). In addition, we placed the variant 2 OMP from strain CL03T00C08, which is identical to the OMP from strain I1345, into the wild-type I1345 background. This strain, which under the conditions of our assay does not appear to be growth inhibited by BSAP-4, becomes growth inhibited when its own OMP is expressed from a constitutive promoter. Therefore, there must be factors other than amino acid differences in the variant 2 OMPs that render a B. fragilis strain sensitive to BSAP-4.
FIG 4.
Analysis of the growth inhibition of strains when distinct variant 1, 2, and 3 OMP genes are constitutively expressed in trans. (A) A variant 1 OMP (638R), a variant 3 OMP (3_1_12), and each of the five distinct variant 2 OMPs were cloned into a vector for constitutive expression and placed in trans in B. thetaiotaomicron. Compared to vector alone, the variant 3 and all variant 2 OMPs conferred BSAP-4 sensitivity to B. thetaiotaomicron, but the variant 1 OMP did not. (B) The same analysis as in panel A except that the OMP-encoding plasmids were transferred to 638R Δbsap-1 Δubb Δbsap-4 (638RΔΔΔ). (C) Constitutive expression of the CL03T00C08 OMP, which is identical to that of the background strain I1345, confers a degree of BSAP-4 sensitivity to this otherwise resistant strain.
Reporter analysis of the variant 2 OMP promoter in BSAP-4-sensitive and -resistant strains.
The data suggest that variant 2 OMP genes are differentially expressed in BSAP-4-sensitive and -resistant B. fragilis strains. The promoter regions upstream of the variant 2 OMP genes of sensitive and resistant strains are identical for the first 266 bp, and there is only a single base pair difference between strains in the entire 301-bp upstream intergenic region. However, this single base pair change does not correlate with BSAP-4 sensitivity or resistance. Therefore, the promoter sequence is not likely a factor in expression differences. To determine the expression levels of this promoter in sensitive and resistant strains with variant 2 OMPS, we cloned the entire 301-bp intergenic region upstream of the variant 2 OMP gene of resistant strain I1345 into a NanoLuc reporter plasmid (12). This plasmid is pNBU2 based (13) and integrates into Bacteroides chromosomes at an att site (13). Among B. fragilis strains with variant 2 OMPs, there is one BSAP-4-sensitive strain (J38-1) and two BSAP-4-resistant strains (S36_L11 and I1345) that are erythromycin sensitive, allowing for selection of the integrants. The pNBU2 plasmid itself or plasmid pMM553 that has the promoter of the housekeeping sigma factor of B. thetaiotaomicron cloned upstream of the NanoLuc gene (12) were also integrated into these three B. fragilis strains to serve as negative or positive controls, respectively.
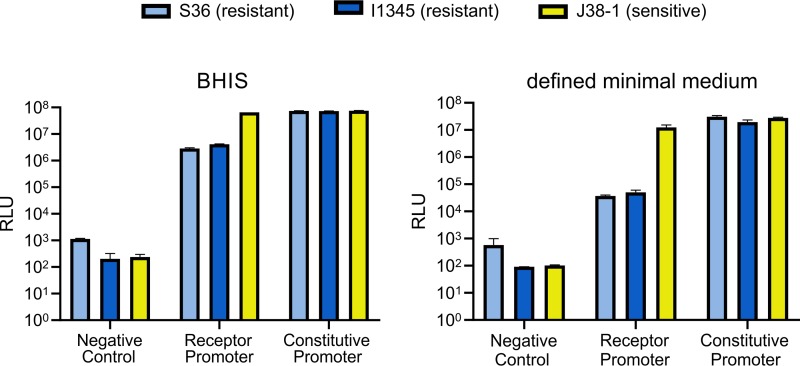
We measured relative luciferase units (RLU) when these nine constructs were grown in supplemented brain heart infusion (BHIS) broth, the same medium used for the plate overlay assays, or in a defined M9-based minimal medium. When grown in BHIS broth, the RLU from the variant 2 OMP promoter in the BSAP-4-sensitive strain J38-1 were 23-fold and 16-fold higher than luciferase units from BSAP-4-resistant strains S36-L11 and I1345, respectively (Fig. 5; see also Table S1 in the supplemental material). Therefore, the same promoter in different B. fragilis strains is differentially regulated. The results are even more striking when these strains are grown in a defined minimal medium. Under these conditions, the RLU produced by the variant 2 OMP promoter in the BSAP-4-sensitive strain J38-1 is 336-fold and 247-fold greater than in strains S36_L11 and I1345, respectively (Fig. 5; Table S1). These data confirm that there are differences in these background strains unrelated to the promoter sequence that dictate differential expression of the OMP genes and therefore whether a variant 2 OMP carrying strain is sensitive or resistant to BSAP-4.
FIG 5.
NanoLuc reporter data of the promoter activity of the variant 2 OMP (BSAP-4 receptor) in sensitive and resistant strains under two growth conditions. For each experiment, the negative control is the vector without the NanoLuc gene. The experimental group has the promoter for the variant 2 OMP gene cloned upstream of the NanoLuc gene, and the positive control is the B. thetaiotaomicron housekeeping sigma factor promoter cloned upstream of the NanoLuc gene. RLU, relative luciferase units. Each bar is the average from biological triplicates, and error bars show the standard errors of the means. Data are shown for the B. fragilis J38-1 strain (BSAP-4 sensitive) and for two strains that are resistant to BSAP-4.
Genetic analysis of the BSAP-4 or corresponding genetic region in B. fragilis strains.
The detection of three different OMP variants, and that a resistant OMP (variant 1) can be present without bsap-4, prompted us to determine the frequency of each of these four genetic heterogeneities (variant 1 OMP gene with bsap-4, variant 1 OMP gene without bsap-4, variant 2 OMP gene, and variant 3 OMP gene) in sequenced B. fragilis strains. As shown in Table S2, of the 118 sequenced B. fragilis genomes, 50 strains harbor a variant 2 OMP, 38 harbor BSAP-4 and the variant 1 OMP, 27 harbor the variant 1 OMP but not BSAP-4, and 4 strains harbor the variant 3 OMP. Therefore, unlike our findings with BSAP-1, -2, and -3, nearly 23% of strains do not harbor BSAP-4 and yet are resistant to it due to the presence of a resistant receptor ortholog.
DISCUSSION
MACPF domain proteins are a major family of antibacterial toxins of gut Bacteroides and may also mediate competition in diverse Bacteroidetes species. Bacteroides MACPF toxins are the first MACPF toxins shown to be produced by bacteria that kill bacteria. BSAP-4 and BSAP-1 are the only proteins of B. fragilis 638R with MACPF domains. During our original identification of BSAP-1, we noted that some B. fragilis strains were still inhibited by the bsap-1 deletion mutant, and at that time, we tested whether BF638R_2714 (BSAP-4) may be conferring this additional toxin activity (6). We did not detect toxin activity by BF638R_2714 against four B. fragilis strains that were still inhibited by Δbsap-1 and have since shown that they are targeted by BfUbb (10). Therefore, our inability to detect toxin activity by BF638R_2714 was because none of the strains previously tested for inhibition are sensitive to this MACPF protein.
There are two major types of molecules that these MACPF toxins target on sensitive cells: β-barrel OMPs and glycan molecules of LPS. BSAP-1 and BSAP-4 are the only two MACPF toxins identified to date that recognize β-barrel OMPs, albeit distinct molecules, on target cells. However, these MACPF toxins share very little similarity to each other (42% similarity). The same is true of the MACPF toxins that target LPS glycan molecules. There are no obvious clues in the sequences of these proteins to suggest what their cellular targets may be. Instead, we have found that the target molecules can typically be identified based on the location of the MACPF gene and the products encoded by the surrounding gene(s) that differ from those in sensitive strains.
There are a few features about the BSAP-4 toxin system that are distinct from the previously characterized MACPF toxins of Bacteroides. The first is that B. fragilis strains can have a gene encoding the resistant variant of the target without the MACPF toxin gene. Second, there are two sensitive variants of the target OMP that confer sensitivity. These variants are 87% similar to each other, and unlike the resistant variant 1 OMP, they each have two tandem calycin-like domains, which may contribute to recognition by the BSAP-4 toxin. In addition, the variant 2 BSAP-4 receptor gene is differentially regulated in strains, rendering some strains that likely have the potential to be inhibited by BSAP-4 to appear resistant. It will be interesting to identify growth conditions under which this OMP is turned on in these resistant strains. It is possible that this regulation is an adaptation to selective pressure by this toxin.
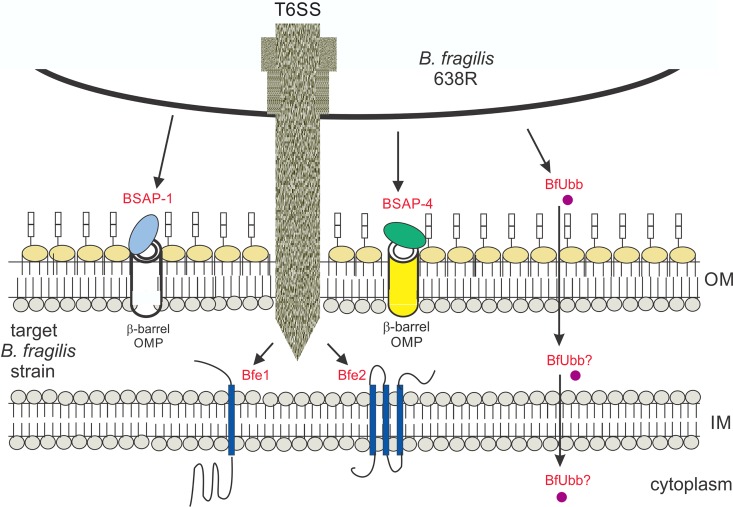
The large number of antibacterial toxins produced by B. fragilis suggests that the ability of these bacteria to antagonize other members is important for competitive fitness in the mammalian gut (1–3, 7). A schematic of the five antibacterial toxins produced by B. fragilis 638R is shown in Fig. 6. Most B. fragilis strains have a GA3 T6SS that is able to kill numerous gut Bacteroidales species. Such a broad-spectrum killing system should allow these organisms to compete with other Bacteroidales members that are able to harvest a greater range of nutrients. All four of the identified diffusible toxins of B. fragilis, namely, BSAP-1, BSAP-4, BfUbb, and a MACPF toxin of B. fragilis strain J38-1 that recognizes an LPS glycan (8), target strains of the same species. Indeed, these are the organisms with which they compete most for nutrients and space. Our accumulating data suggest that of all the human gut Bacteroides species, B. fragilis strains may produce the largest arsenal of antimicrobial toxins. As each of the three diffusible toxins of 638R target a different subset of B. fragilis strains, the accumulation of toxin genes allows for a greater range of antagonism. In addition, if more than one toxin targets the same competing strain, it may allow for more rapid or robust antagonism of that strain. Lastly, a larger repertoire of toxins may allow for antagonism under different environmental conditions if toxins and receptor molecules are regulated by various conditions in the human gut.
FIG 6.
Schematic of the five antibacterial toxins produced by B. fragilis 638R. Toxins are designated in red font. Three toxins are actively secreted from B. fragilis 638R and two are delivered by a type VI secretion system (T6SS). The toxic effectors of the T6SS (Bfe1 and Bfe2) have transmembrane domains and are predicted to insert into the cytoplasmic membrane of the target cell. Their mechanism of toxicity has not been described. BSAP-1 and BSAP-4 are MACPF domain proteins and bind different β-barrel OMPs on the surfaces of sensitive cells and are predicted to form large pores in the outer membrane. The ubiquitin-like protein functions by an unknown mechanism and its localization and target in sensitive cells has not been reported.
MATERIALS AND METHODS
All oligonucleotides used in this study are listed in Table S3 in the supplemental material.
Bacterial strains and growth conditions.
Bacteroides strains used in this study were previously described and are listed in Table 1. Bacteroides strains were grown anaerobically at 37°C in supplemented basal medium (14) or supplemented brain heart infusion (BHIS) medium for liquid cultures or on BHIS plates. Defined minimal medium has M9 salts with added glucose, l-cysteine, CaCl2, MgSO4, hemin, vitamin K, FeSO4, and vitamin B12. Antibiotics (erythromycin, 5 μg/ml; gentamicin, 200 μg/ml) were added as indicated in “Construction of deletion mutants," below. Escherichia coli strains [DH5α, BL21(DE3) and S17 λ pir] were grown in L broth or on L plates supplemented with antibiotics (carbenicillin, 100 μg/ml; kanamycin, 100 μg/ml) when appropriate.
Agar spot test for growth inhibition analysis.
Diffusible antimicrobial activity was assayed using the agar spot test (15). Bacterial cells were scraped from petri dishes into 500 μl of phosphate-buffered saline (PBS) and resuspended to an approximate density of 3 × 109 cells/ml, and 5 μl was spotted onto BHIS plates. These plates were incubated overnight to allow secretion of antimicrobial molecules into the medium. Cells were removed using a cotton swab, and remaining cells were killed by exposing the plate to chloroform vapor for 15 min. Alternatively, His–BSAP-4 was spotted directly onto plates. Strains to be tested for growth inhibition were grown to mid-log phase, added to 4 ml of top agar, and poured over the prepared plates. These agar overlay plates were incubated overnight, and zones of clearing were analyzed after ∼20 h. In some instances, the gamma value was adjusted for the image.
Transposon mutagenesis.
Bacteroides fragilis 638R Δbsap-1 Δubb was the background strain for transposon mutagenesis using plasmid pSAM_BcellWH2 (16). Transposon mutants were screened for loss of inhibitory activity against B. fragilis 3_1_12 using the agar overlay assay. The transposon insertion site was identified by arbitrary PCR amplification using a primer within the transposon with an arbitrary primer followed by a second round of amplification with two primers as previously described (17). Amplicons were purified from agarose gels and sequenced using an oligonucleotide directed outward from the transposon cassette.
Cloning and heterologous expression of genes.
Genes for expression in Bacteroides were PCR amplified and cloned into expression vector pFD340 (18) either by restriction digest cloning or assembly using NEBuilder (New England Biolabs) (Table S3). In these constructs, transcription initiates from a constitutive vector-borne promoter. Sequence-confirmed clones were conjugally transferred from E. coli DH5α into Bacteroides strains using helper plasmid RK231.
Construction of deletion mutants.
Nonpolar deletion mutants were constructed using Bacteroides suicide vector pKNOCK-bla-ermGb. Approximately 2,500-bp flanking regions upstream and downstream of the gene to be deleted were PCR amplified and cloned using NEBuilder (New England Biolabs) into the suicide vector linearized with BamHI, which was then transformed into E. coli S17 λ pir. A plasmid confirmed to have the correct assembly of the segments was conjugally transferred into the appropriate B. fragilis strain, and cointegrates were selected on BHIS plates containing gentamicin and erythromycin. Cointegrates were passaged three times and then plated on BHIS plates. Double cross-outs were identified by replica plating onto BHIS plates with erythromycin, and mutants were identified by PCR followed by Sanger sequencing.
Cloning and purification of His-tagged BSAP-4.
BF638R_2714 was PCR amplified using primers designed to omit the first 84 bp of the open reading frame (ORF) to eliminate its 28-amino-acid N-terminal signal sequence (Table S3). The amplified fragment was digested with NdeI and BamHI and ligated into the pET16b vector (Novagen) to introduce an N-terminal His tag. Transformants of E. coli BL21(DE3) were confirmed by Sanger sequencing. Expression of the recombinant protein was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside), and the recombinant protein was purified using the Probond nickel-nitrilotriacetic acid (NTA) purification system (Thermo Fisher Scientific) according to the manufacturer’s instructions for native protein purification. Eluted fractions were dialyzed against PBS, and the protein concentration was quantified using a Qubit fluorometer (Thermo Fisher Scientific).
Construction of NanoLuc plasmid and luciferase assay.
We started with a previously constructed NanoLuc-containing plasmid designated pMM553 (12) that utilizes the pNBU2 plasmid (13) with the promoter from the B. thetaiotaomicron housekeeping sigma factor BT1311 (19) cloned upstream of the NanoLuc gene. Using NEBuilder (NEB) and the primers listed in Table S3, we replaced the BT1311 promoter of pMM553 with the 301-bp region of the variant 2 OMP promoter of B. fragilis I1345. This construct, as well as pNBU2 and pMM553, were each individually conjugally transferred to three B. fragilis strains, J38-1, S36_L11, and I1345, where they integrate into the chromosomes at an att site (13). For luciferase assays, these nine strains were swabbed from a fresh plate and resuspended to an optical density at 600 nm (OD600) of approximately 0.8, and then 150 μl was added to 1.35 ml of medium and grown for 4 h (BHIS broth) or 6 h (defined minimal medium). The final OD600 values of the cultures following growth in a given medium were standardized so that equivalent cell numbers were analyzed per condition. The cells were collected by centrifugation and lysed using Bugbuster protein extraction reagent (MilliporeSigma, Burlington, MA). Equal volumes of lysed cells and Nano-Glo luciferase assay reagent (Promega Corp., Madison, WI) were combined in half-area white 96-well plates (Greiner Bio-One, Monroe, NC), and luminescence was measured using a SpectraMax L microplate reader (Molecular Devices, LLC., San Jose, CA) with a 1-s integration time using noncorrective photon counting. Biological triplicates were performed for all assays.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R01AI093771 from the NIH/National Institute of Allergy and Infectious Diseases and BRICS, contract number HR0011-15-C-0094.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We thank the BEI for providing some of the strains used in this study.
We thank J. Gordon for supplying pSAM_BcellWH2, E. Martens for supplying pKNOCK-bla-ermGb, and M. Mimee for supplying pMM553. We thank S. Von for technical assistance. We also thank L. Garcia-Bayona and M. Chatzidaki-Livanis for helpful discussions.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00577-18.
REFERENCES
- 1.Chatzidaki-Livanis M, Geva-Zatorsky N, Comstock LE. 2016. Bacteroides fragilis type VI secretion systems use novel effector and immunity proteins to antagonize human gut Bacteroidales species. Proc Natl Acad Sci U S A 113:3627–3632. doi: 10.1073/pnas.1522510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wexler AG, Bao Y, Whitney JC, Bobay LM, Xavier JB, Schofield WB, Barry NA, Russell AB, Tran BQ, Goo YA, Goodlett DR, Ochman H, Mougous JD, Goodman AL. 2016. Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc Natl Acad Sci U S A 113:3639–3644. doi: 10.1073/pnas.1525637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht AL, Casterline BW, Earley ZM, Goo YA, Goodlett DR, Bubeck Wardenburg J. 2016. Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep 17:1281–1291. doi: 10.15252/embr.201642282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyne MJ, Roelofs KG, Comstock LE. 2016. Type VI secretion systems of human gut Bacteroidales segregate into three genetic architectures, two of which are contained on mobile genetic elements. BMC Genomics 17:58. doi: 10.1186/s12864-016-2377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosado CJ, Kondos S, Bull TE, Kuiper MJ, Law RH, Buckle AM, Voskoboinik I, Bird PI, Trapani JA, Whisstock JC, Dunstone MA. 2008. The MACPF/CDC family of pore-forming toxins. Cell Microbiol 10:1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatzidaki-Livanis M, Coyne MJ, Comstock LE. 2014. An antimicrobial protein of the gut symbiont Bacteroides fragilis with a MACPF domain of host immune proteins. Mol Microbiol 94:1361–1374. doi: 10.1111/mmi.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roelofs KG, Coyne MJ, Gentyala RR, Chatzidaki-Livanis M, Comstock LE. 2016. Bacteroidales secreted antimicrobial proteins target surface molecules necessary for gut colonization and mediate competition in vivo. mBio 7:e01055-16. doi: 10.1128/mBio.01055-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laclare McEneany V, Coyne MJ, Chatzidaki-Livanis M, Comstock LE. 2018. Acquisition of MACPF domain-encoding genes is the main contributor to LPS glycan diversity in gut Bacteroides species. ISME J 12:2919–2928. doi: 10.1038/s41396-018-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen RM, Marmolin ES, Justesen US. 2013. Species differentiation of Bacteroides dorei from Bacteroides vulgatus and Bacteroides ovatus from Bacteroides xylanisolvens—back to basics. Anaerobe 24:1–3. doi: 10.1016/j.anaerobe.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Chatzidaki-Livanis M, Coyne MJ, Roelofs KG, Gentyala RR, Caldwell JM, Comstock LE. 2017. Gut symbiont Bacteroides fragilis secretes a eukaryotic-like ubiquitin protein that mediates intraspecies antagonism. mBio 8:e01902-17. doi: 10.1128/mBio.01902-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flower DR. 1993. Structural relationship of streptavidin to the calycin protein superfamily. FEBS Lett 333:99–102. doi: 10.1016/0014-5793(93)80382-5. [DOI] [PubMed] [Google Scholar]
- 12.Mimee M, Tucker AC, Voigt CA, Lu TK. 2015. Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the murine gut microbiota. Cell Syst 1:62–71. doi: 10.1016/j.cels.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Shoemaker NB, Wang GR, Salyers AA. 2000. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J Bacteriol 182:3559–3571. doi: 10.1128/JB.182.12.3559-3571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pantosti A, Tzianabos AO, Onderdonk AB, Kasper DL. 1991. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect Immun 59:2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avelar KE, Pinto LJ, Antunes LC, Lobo LA, Bastos MC, Domingues RM, Ferreira MC. 1999. Production of bacteriocin by Bacteriodes fragilis and partial characterization. Lett Appl Microbiol 29:264–268. doi: 10.1046/j.1365-2672.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu M, McNulty NP, Rodionov DA, Khoroshkin MS, Griffin NW, Cheng J, Latreille P, Kerstetter RA, Terrapon N, Henrissat B, Osterman AL, Gordon JI. 2015. Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 350:aac5992. doi: 10.1126/science.aac5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein BA, Tenorio EL, Lazinski DW, Camilli A, Duncan MJ, Hu LT. 2012. Identification of essential genes of the periodontal pathogen Porphyromonas gingivalis. BMC Genomics 13:578. doi: 10.1186/1471-2164-13-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CJ, Rogers MB, McKee ML. 1992. Heterologous gene expression in Bacteroides fragilis. Plasmid 27:141–154. doi: 10.1016/0147-619X(92)90014-2. [DOI] [PubMed] [Google Scholar]
- 19.Vingadassalom D, Kolb A, Mayer C, Rybkine T, Collatz E, Podglajen I. 2005. An unusual primary sigma factor in the Bacteroidetes phylum. Mol Microbiol 56:888–902. doi: 10.1111/j.1365-2958.2005.04590.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.