Abstract
Oxidative stress plays an important role in various neurological disorders. Milk fat globule-epidermal growth factor-factor 8 (MFG-E8) is a regulatory protein for microglia. However, its involvement in microglial oxidative stress has not been established. In this study, we observed microglial oxidative stress in response to lipopolysaccharide (LPS) both in vitro and in vivo. LPS induced significant elevation of TNF-α, IL-6, MDA, and ROS and reduction of GSH and SOD in the mouse brains and primary microglia, which were reversed by MFG-E8 pretreatment. MFG-E8 induced the expression of Nrf-2 and HO-1 that was reduced by LPS incubation. Moreover, LPS-increased Keap-1 expression was reversed by MFG-E8. But the above tendencies were not seen when MFG-E8 was applied alone. The current study established the involvement of MFG-E8 in antioxidant effects during neuroinflammation. It may achieve the effects through the regulation of Keap-1/Nrf-2/HO-1 pathways.
1. Introduction
Neuroinflammation plays an important role in the development of a set of neurological disorders, for example, cerebral infarction, Alzheimer's Disease (AD), and multiple sclerosis [1–4]. Microglia account for 5% of the total cells in the brain, and they are key cells in balancing neuroinflammation. Once activated, they would produce a set of factors, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and reactive oxygen species (ROS) [5–7]. ROS accumulates from impaired degradation and/or excessive production in cells, in response to insults [8]. Microenvironment in the central nervous system turned imbalanced, and subsequent neuronal damage occurs [9]. Therefore, it is believed that effective modulation of microglial oxidative stress can be a therapeutic option for neurological disorders.
Milk fat globule-epidermal growth factor-factor 8 (MFG-E8) is a protein widely expressed throughout the body. It is initially identified in the mammary epithelia during lactation [10] and is attracting attention for its existence in the brain. MFG-E8 could modulate microglia activities through its connection with α V β 3/5 integrin [11]. Many studies have indicated its anti-inflammatory role in AD and ischemic stroke models [11, 12], reprograming microglia from a M1 (inflammatory) to a M2 phenotype (anti-inflammatory) [13]. However, the effects of MFG-E8 on microglial oxidant responses have not been established.
We investigate here the involvement of MFG-E8 in microglial oxidative stress. The findings would help establish a novel framework of a therapeutic option for neuroinflammation.
2. Materials and Methods
All the experiments and procedures were approved by the Animal Ethics Committee of Sun Yat-sen University and conducted in accordance with the Declaration of Helsinki (1964).
2.1. Chemical Agents and Antibodies
The lipopolysaccharide (LPS) and Glutathione (GSH) assay kits were purchased from Sigma-Aldrich Chemical Company (St. Louis, USA). DAPI was from Thermo Fisher Scientific. Mouse recombinant MFG-E8 protein was from R&D Systems (Minneapolis, USA). F12-Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), and penicillin/streptomycin came from Life Technologies (New York, USA). The Iba-1 antibody was from Wako (Fuji, Japan). The TRIzol reagent was purchased from Invitrogen (Waltham, USA). Kelch-like ECH-associated protein 1 (Keap-1), Nuclear factor E2-related factor 2 (Nrf-2), Heme oxygenase 1 (HO-1), and GAPDH antibodies were purchased from Cell Signaling Technology (Beverly, USA). The Malondialdehyde (MDA) kit, Superoxide Dismutase (SOD) kit, ROS assay kit, TNF-α ELISA kit, and IL-6 ELISA kit were purchased from Beyotime (Shanghai, China).
2.2. Animal Treatment
Eight-week-old wild-type male C57 mice (25-30 g) were from the Animal Center of Sun Yat-sen University. Mice were kept in an environmental-controlled room (22°C ± 3°C, 12 h light-dark cycle, relative humidity of 60 ± 5%, and free access to food and drinking water were preserved). They were randomly divided into the following groups: (1) Control, daily intraperitoneal PBS injection for 7 days as a vehicle; (2) MFG-E8, daily intraperitoneal PBS (2 ml) and daily intracerebroventricular MFG-E8 (1 μg) injection; (3) LPS, daily intraperitoneal PBS (2 ml)+LPS (250 μg/kg) injection for 7 days; and (4) LPS+MFG-E8, daily intraperitoneal LPS (250 μg/kg) and daily intracerebroventricular MFG-E8 (1 μg) for 7 days. At the 8th day, the mouse brains were quickly collected after cardiac perfusion with PBS. The left or right hemispheres of the brain were randomly chosen for immunofluorescence staining and for the activity of ROS, MDA, SOD, and GSH, as well as for the detection in quantitative real-time PCR and western blot analysis.
2.3. Primary Microglial Culture and Treatment
Primary microglia were prepared using the brains of 1-3-day-old neonatal C57 mice, which were from the Animal Center of Sun Yat-sen University. Microglia were isolated and purified using a modified protocol reported in our previous studies [1, 13]. Microglia were treated with LPS (100 ng/ml) or MFG-E8 (100 ng/ml) for 1 h before sample collection. For the combined treatment, microglia were treated with MFG-E8 (100 ng/ml) for 1 h before LPS incubation.
2.4. Quantitative Real-Time PCR
Total RNA was extracted from brain tissues in each group using the TRIzol reagent, according to the standard instructions. Reverse transcriptase and quantitative PCR were performed on One-Step RT-PCR System (Applied Biosystems). The total RNA template (500 ng) was reverse-transcribed into cDNA using a PrimeScript RT Master Mix kit. Then, 2 ml of cDNA solution was turned into real-time polymerase chain reaction in a 20 μl reaction volume with 10 μl SYBR Premix Ex Taq II, 0.8 μl PCR forward primer (0.4 mM), 0.8 μl PCR reverse primer (0.4 mM), and 6 μl ddH2O. Triplicate reactions were done for every sample. The primers of genes designed for the study are shown in Table 1.
Table 1.
Primer sequences for real-time PCR.
| Forward 5′-3′ | Reverse 5′-3′ | |
|---|---|---|
| NQO1 | ATTGTACTGGCCCATTCAGA | GGCCATTGTTTACTTTGAGC |
| SOD1 | ATCCACTTCGAGCAGAAG | TTCCACCTTTGCCCAAGT |
| SOD2 | AGCGGTCGTGTAAACCTCA | AGACATGGCTGTCAGCTTC |
| GST-Ya | AAGCCAGGACTCTCACTA | AAGGCAGTCTTGGCTTCT |
| GST-Yc | GGAAGCCAGTCCTTCATTACT | CGTCATCAAAAGGCTTCCTCT |
| β-Actin | GTCAGAAGGACTCCTATGTG | CTCATTGTAGAAGGTGTGGT |
2.5. MDA Assessment
For MDA determination, 100 μl of cell suspension or tissue homogenate was mixed with 1 ml of solution containing thiobarbituric acid. The mixture was incubated at 95°C for 40 min and then cooled down using tap water. The samples were centrifuged at 4000 rpm for 10 min at room temperature. The absorbance of the supernatant was measured at 532 nm. The concentration of MDA was expressed as nanomoles of MDA per milligram of protein.
2.6. SOD Analysis
SOD activity was measured by using commercial kits according to the manufacturer's instructions (Nanjing JianCheng Bioengineering Institute, Nanjing, China). Briefly, for SOD activity determination, 20 μl of cell suspension or tissue homogenate was mixed with 220 μl of reaction solution, and the mixture was incubated at 37°C for 30 min. The absorbance was measured at 450 nm, and the results were recorded for analysis.
2.7. ROS Assay
The level of intracellular ROS was assessed using a ROS assay kit. The brain homogenate in each group was rendered as single cell suspension according to the protocol of Villalba et al. [14]. Then, cell suspension was added into a 96-well plate and incubated with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) for 1 h. Primary cells were seeded in the 96-well plate and added with DCFH-DA for 1 h after treatment. Then, the fluorescent signal was assessed with an excitation at 488 nm and emission at 525 nm using a microplate reader. Fluorescent intensity was normalized to control wells for analysis.
2.8. GSH Detection
The GSH content was determined according to the manufacturer's protocol. Generally, GSH reacted with 5,5′-dithio-bis-2-nitrobenzoic acid to produce a yellow chromophore, and the absorbance was measured at 412 nm using a UV spectrometer.
2.9. Immunofluorescence Staining
For in vivo samples, they were fixed with 4% paraformaldehyde at 4°C for 24 h. The brains were dehydrated in a gradient sucrose solution (10%, 20%, and 30%), each at 4°C for 24 h. Brain sections were cut with a thickness of 10 μm and then stored in cryoprotectant solution at 30°C until use. Sections were rinsed using PBS with 0.3% Triton X-100 for 3 times (each for 10 min) at room temperature and blocked in a solution with 5% normal goat serum for 1 h. After washing 3 times, sections were incubated with the Iba-1 antibody (1 : 500) for 24 h in a humidified chamber at 4°C. Then FITC-conjugated secondary antibody (1 : 200) was used to incubate samples for 1 h. Sections were coverslipped with glycerol and observed using a fluorescence microscope (Carl Zeiss, Germany).
For in vitro staining, microglia were seeded at 0.8 × 106 on 1.5 mm2 coverslips for 24 h. After treatment, cells were fixed with ice-cold 4% paraformaldehyde for 20 min at 4°C. They were then air-dried followed by blocking and permeabilizing with 1% BSA and 0.1% Triton X-100 in PBS for 30 min. Then, cells were incubated with the Iba-1 antibody (1 : 500) for 24 h in a humidified chamber at 4°C. After washing three times using 0.3% Triton X-100 in PBS, the FITC-conjugated secondary antibody (1 : 200) was used to incubate samples for 1 h. Coverslips were transferred onto glass slides after 10 min staining with DAPI. Images were acquired by a fluorescence microscope (Carl Zeiss, Germany).
Images were quantitatively analyzed for microglial morphology using a grid-crossing method, modified based on the study by Luckoff et al. [15]. Briefly, cell morphology was analyzed by counting the number of grid-crossing points per cell in each group.
2.10. Western Blot Analysis
Microglia were scraped and lysed in a RIPA buffer at 4°C for 1 h. The total intracellular protein was extracted and quantified by a BCA kit according to the protocol from the suppliers. Cell lysates were solubilized with a SDS sample buffer (40 μg/lane) and separated by 10% SDS-PAGE (110 V, 75 min). Proteins were then transferred to a 0.45 μm PVDF membrane at 60 V for 1 h. After that, the membrane was blocked using TBST with 3% BSA. The following incubation with antibodies (anti-Keap-1, 1 : 1000; anti-Nrf-2, 1 : 1000; anti-HO-1, 1 : 1000; and anti-GAPDH, 1 : 500) was applied for 24 h at 4°C. Then, horseradish peroxidase- (HRP-) conjugated secondary antibodies were subsequently adopted for 1 h at room temperature and detected with the enhanced chemiluminescence (ECL) plus detection system. The density of each band was quantified by Quantity One Software. The density ratio represented the relative intensity of each band against GAPDH and normalized those in controls.
2.11. Statistical Analysis
Data in this study were depicted as the mean ± SD and analyzed using Statistical Package for the Social Sciences (SPSS) version 21.0. One-way analysis of variance (ANOVA) was used to analyze variables with the least significant difference (LSD). The level of statistical significance was set as P < 0.05.
3. Results
3.1. Effects of MFG-E8 on LPS-Induced Microglial Activation
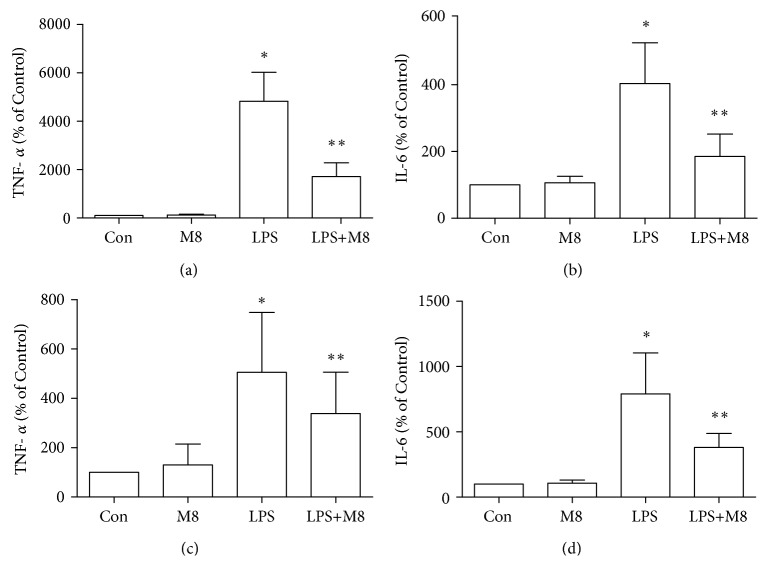
Microglial activation was observed through staining with an anti-Iba-1 antibody. As shown in in vivo results, microglia in controls showed a ramified form, while it turned, after LPS exposure, into an amoeba shape with a larger cell body and thicker processes, consistent with a deramified and amoeboid morphology (Figure 1). Brain sections from mice treated with MFG-E8 before LPS exhibited similar morphology with controls. We also found the consistent changes in each group in vitro (Figure 2). We further used the grid-crossing analysis method to quantify the morphology of cells (Supplementary Figure 1). It showed that cells in controls occupied more grids than cells treated with LPS, and the tendency by LPS was significantly reversed by MFG-E8 pretreatment. Production of inflammatory factors is a symbol of microglial activation. We assessed the levels of TNF-α and IL-6 after treatment (Figure 3). LPS induced higher levels of TNF-α and IL-6 in the brains (Figures 3(a) and 3(b)) and cellular culture (Figures 3(c) and 3(d)), which were inhibited by MFG-E8 preincubation. However, no obvious changes of in vivo and in vitro morphology and inflammatory factor production were seen when MFG-E8 was applied alone.
Figure 1.
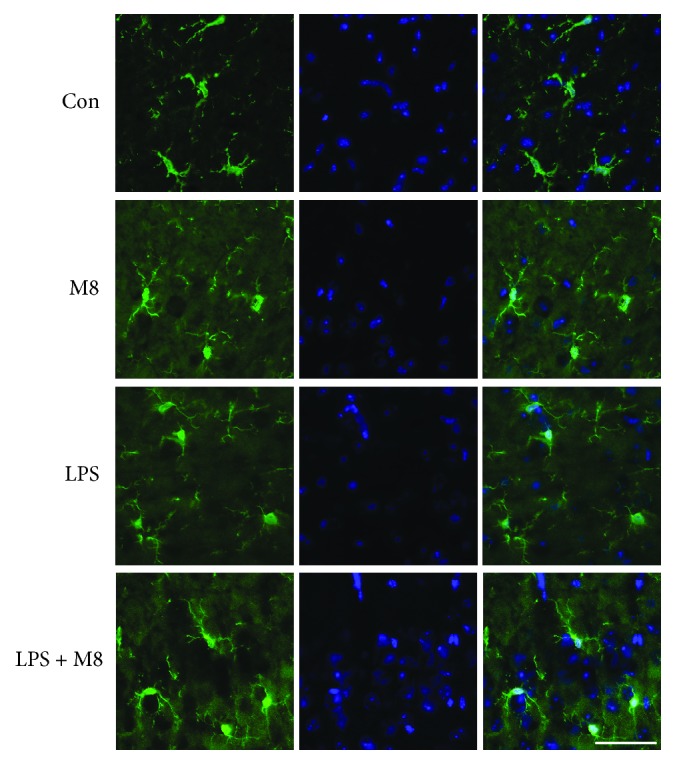
MFG-E8 inhibited LPS-induced microglial activation in vivo. Representative immunofluorescent staining images of Iba-1 (green) and DAPI (blue) in the cerebral cortex of mice of Control (n = 10), MFG-E8 (n = 10), LPS (n = 10), and LPS+MFG-E8 (n = 10). Bar = 50 μm. Con: Control; M8: MFG-E8.
Figure 2.
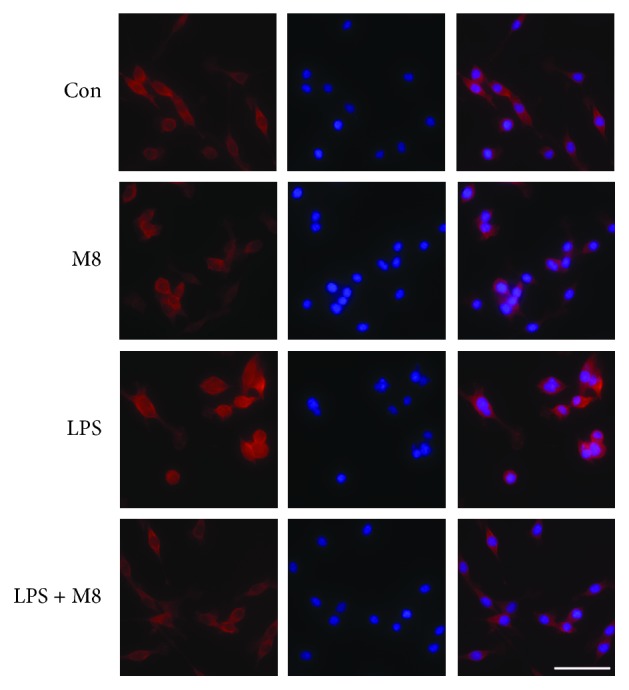
MFG-E8 inhibited LPS-induced microglial activation in vitro (n = 3). Representative immunofluorescent staining images of Iba-1 (red) and DAPI (blue) in primary microglia of Control, MFG-E8, LPS, and LPS+MFG-E8. Bar = 20 μm. Con: Control; M8: MFG-E8.
Figure 3.
Effects of MFG-E8 on production of TNF-α and IL-6 in the cerebral cortex (a, b) and primary microglia (c, d) of Control, MFG-E8, LPS, and LPS+MFG-E8. Data were expressed as the mean ± SD (in vivo, n = 10; in vitro, n = 3). ∗ P < 0.05 versus the Control group; ∗∗ P < 0.05 versus the LPS group. Con: Control; M8: MFG-E8.
3.2. Effects of MFG-E8 on LPS-Induced Oxidative Stress In Vivo
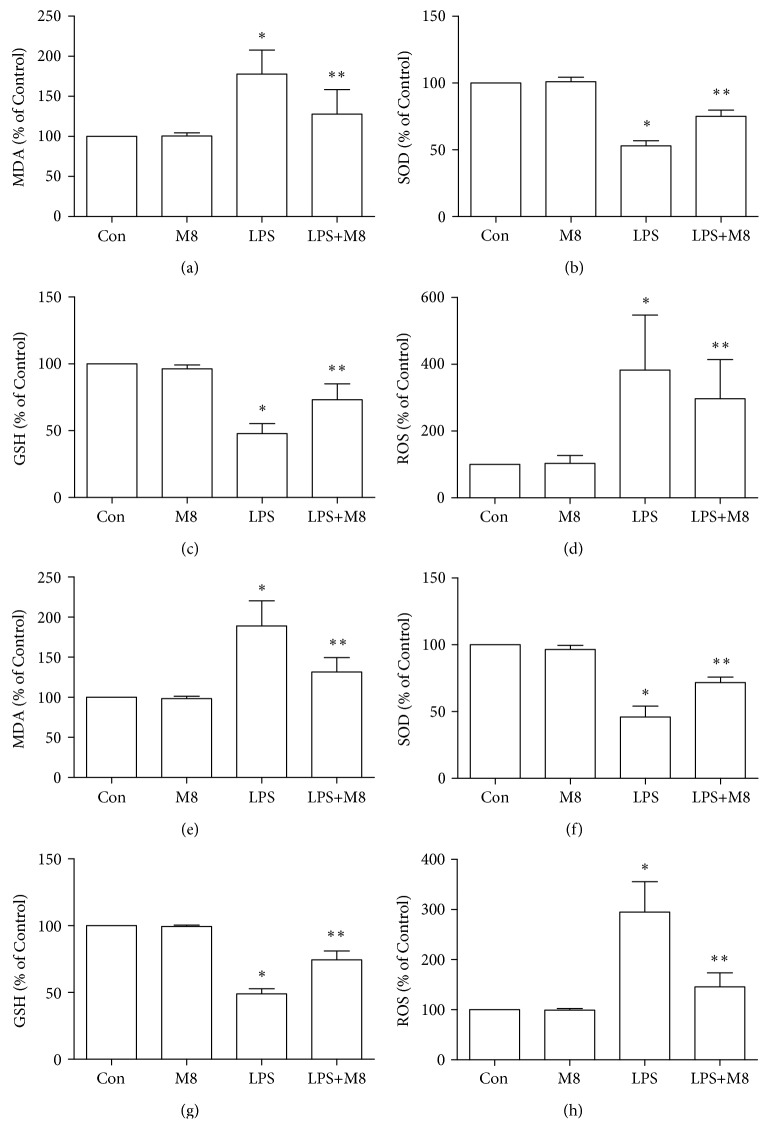
Administration of LPS significantly increased MDA and ROS as compared to controls, whereas MFG-E8 reversed the increase of the MDA and ROS contents (Figures 4(a) and 4(d)). Antioxidative effects were evaluated by analyzing the parameters of SOD and GSH (Figures 4(b) and 4(c)). When LPS was applied, the SOD and GSH levels were suppressed significantly, which were blocked by MFG-E8. Also, MFG-E8 in the absence of LPS did not alter the trends of MDA, ROS, SOD, and GSH in the brain, compared with control mice.
Figure 4.
Effects of MFG-E8 on oxidative stress markers in the cerebral cortex (a–d) and primary microglia (e–h) of Control, MFG-E8, LPS, and LPS+MFG-E8. Data were expressed as percentage of Control. Data were expressed as the mean ± SD (in vivo, n = 10; in vitro, n = 3). ∗ P < 0.05 versus the Control group; ∗∗ P < 0.05 versus the LPS group. Con: Control; M8: MFG-E8.
3.3. Effects of MFG-E8 on Microglial Oxidative Stress in Response to LPS In Vitro
We assessed here oxidative stress in primary microglia. LPS induced a significant increase of MDA and ROS (Figures 4(e) and 4(h)) and decreased production of SOD (Figure 4(f)) and GSH (Figure 4(g)), compared with controls. However, MFG-E8 pretreatment tended to reverse the above changes against LPS. Moreover, the single use of MFG-E8 did not change the contents of MDA, ROS, SOD, and GSH in microglia, when compared with control cells.
3.4. Effects of MFG-E8 on Keap-1/Nrf-2/HO-1 Pathways
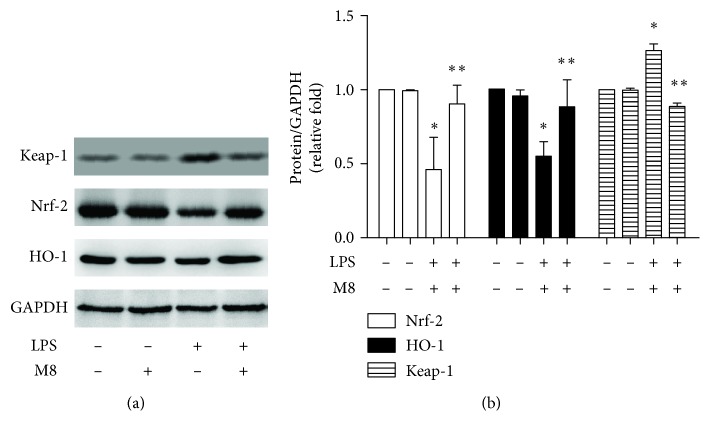
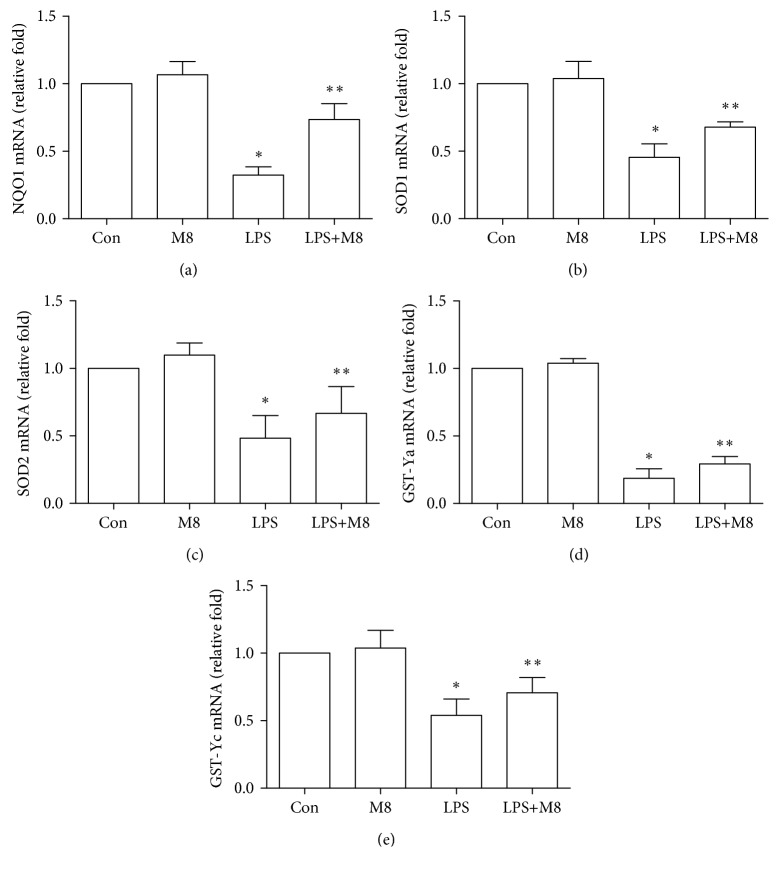
Keap-1/Nrf-2/HO-1 is an important pathway in cellular oxidative stress. We first assessed the expression of Keap-1, Nrf-2, and HO-1 in microglia. It indicated higher Keap-1 expression and lower levels of Nrf-2 and HO-1 in cells with LPS than controls (Figures 5(a) and 5(b)). However, MFG-E8 pretreatment significantly blocked the above changes by LPS. MFG-E8 treatment alone did not change the expression of these proteins. We also evaluated Nrf-2-regulated genes (Figure 6) and found that mRNAs of NQO1, SOD1, SOD2, GST-Ya, and GST-Yc were significantly suppressed by LPS, which were markedly reversed by MFG-E8.
Figure 5.
MFG-E8 reversed LPS-induced downregulation of Nrf-2/HO-1. (a) Representative western blot images of Nrf-2, HO-1, and GAPDH. (b) Quantitative analysis of each group was conducted. Fold changes of Nrf-2 (white bar) and HO-1 (black bar) expression were shown. Data were expressed as the mean ± SD (n = 3). ∗ P < 0.05 versus the Control group; ∗∗ P < 0.05 versus the LPS group. Con: Control; M8: MFG-E8.
Figure 6.
mRNA levels of NQO1, SOD1, SOD2, GST-Ya, and GST-Yc in primary microglia treated with LPS and MFG-E8. Data were expressed as the mean ± SD (n = 3). ∗ P < 0.05 versus the Control group; ∗∗ P < 0.05 versus the LPS group. Con: Control; M8: MFG-E8.
4. Discussion
The current study evaluated the effects of MFG-E8 on microglial oxidative responses against LPS. We found oxidative stress in the mouse brain, and microglia were alleviated by MFG-E8 treatment, suggesting its involvement in neuroprotection. But MFG-E8 alone did not induce changes of oxidative responses. The specific mechanisms need further clarification.
Microglial cells are primary participants in neuroinflammation. Resting microglia exhibit surveillance function by sensing the extracellular changes with their branches [16]. In brain injury, microglia transformed into a specific morphology with motile processes and long branches, ensuring their activity in the sensation of external stimuli [17]. In this study, the cortex was chosen for both in vivo observation and in vitro microglial purification. We used the method previously used in 2017 [13] to provide primary microglia for in vitro treatment. This protocol ensures the high production of microglia and the inflammatory features in response to external factors. Studies indicated that cells from cortex regions present increased Iba-1 immunoreactivity after LPS challenge, which is usually used as a marker to detect microglial morphological changes [18, 19]. In the current study, LPS induced microglia into an activation morphology with an amoeba shape. MFG-E8 reversed the shape changes by LPS. To better demonstrate the immunostaining results, we used a grid-crossing method to quantify cellular morphology. This method calculated the grids covered per cell in each group and discovered more girds in MFG-E8-pretreated cells than in LPS-stimulated cells. We speculated that it was attributed to the role of MFG-E8 on a microglial cytoskeleton. It is believed that MFG-E8 is a cytoskeletal rearrangement enhancer for microglial phagocytosis [20, 21]. Neniskyte and Brown [11] demonstrated that MFG-E8 is an essential factor for microglial phagocytosis. Cells from MFG-E8-knockout animals exhibit deficits in phagocytic ability. Subsequent signaling pathways in microglia, which were associated with cytoskeletal rearrangement, for example, DOCK180/Rac-1 [22], get stimulated.
Microglial production of inflammatory factors is concomitant with the morphological changes during activation. In the previous study, we found that MFG-E8 pretreatment significantly reversed Aβ-induced microglial activation along with an upregulation of IL-1β, IL-6, and TNF-α [1, 13]. An anti-inflammatory phenotype (M2) was induced by MFG-E8 [13]. This is also confirmed by assessing TNF-α and IL-6 in LPS-treated cells in the study. Moreover, activated microglia show an imbalance in production and clearance of oxidative factors [23]. We first observed MDA and ROS overproduction in both the brain and microglial samples treated with LPS. Antioxidative factors, GSH and SOD, were decreased in the same samples. MFG-E8 pretreatment then suppressed the oxidative changes led by LPS, implying the role of MFG-E8 in oxidative balance. Nrf-2 is a pleiotropic protein and a key antioxidant sensor. The activation of this protein is crucial for cellar hemostasis [24–27]. Under physiological conditions, Nrf-2 is bound to its natural inhibitor Keap-1 in the cytoplasm. By insults and stimuli, Nrf-2 would be released by Keap-1, translocate into the nucleus, and mediate the transcription of HO-1, leading to resistance to oxidative stress [28]. The upregulation of Nrf-2 and HO-1, as well as the inhibition of Keap-1 by MFG-E8, indicated that MFG-E8 achieved its antioxidant effects via the Nrf-2/HO-1 pathway. Nrf-2-regulated genes, including NQO1, SOD1, SOD2, GST-Ya, and GST-Yc, were also increased by MFG-E8 against LPS, confirming the regulatory role of MFG-E8 on the Nrf-2 pathway. This broadened the understanding of MFG-E8, which is a promising therapeutic agent for neurological disorders.
Interestingly, MFG-E8 alone did not induce changes in the microglial state, while its antiactivation role was only seen in LPS-treated samples. Therefore, MFG-E8 might exhibit its protective effects in an inflammation environment, but not under normal conditions. Once injury occurs, the balanced environment changed along with an inflammatory change. In this case, MFG-E8 started to act its role on microglia. The underlying mechanisms should be explored in the future.
5. Conclusion
The current study tries to establish the antioxidant role of MFG-E8 on microglia. MFG-E8 is involved in antioxidant effects during neuroinflammation. It may achieve the effects through the regulation of Keap-1/Nrf-2/HO-1 pathways.
Acknowledgments
This study was supported by grants from the Talent Scientific Research Start-up Foundation of Yijishan Hospital, Wannan Medical College (Grant No. YR201603), and the National Natural Science Foundation of China (Grant No. 81701061).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There were no conflicts of interest among the authors.
Supplementary Materials
Supplementary Figure 1: grid-crossing analysis of microglia morphology in animals and primary microglia.
References
- 1.Shi X., Zheng Z., Li J., et al. Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: involvement of ERK1/2 and p38 signaling pathways. Neuroscience Letters. 2015;594:105–110. doi: 10.1016/j.neulet.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Xu X., Cai X., Zhu Y., et al. MFG-E8 inhibits Aβ-induced microglial production of cathelicidin-related antimicrobial peptide: a suitable target against Alzheimer’s disease. Cellular Immunology. 2018;331:59–66. doi: 10.1016/j.cellimm.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Bogie J. F. J., Stinissen P., Hendriks J. J. A. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathologica. 2014;128(2):191–213. doi: 10.1007/s00401-014-1310-2. [DOI] [PubMed] [Google Scholar]
- 4.Huang X., Liao W., Huang Y., et al. Neuroprotective effect of dual specificity phosphatase 6 against glutamate-induced cytotoxicity in mouse hippocampal neurons. Biomedicine & Pharmacotherapy. 2017;91:385–392. doi: 10.1016/j.biopha.2017.04.096. [DOI] [PubMed] [Google Scholar]
- 5.Voet S., Prinz M., van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends in Molecular Medicine. 2019;25(2):112–123. doi: 10.1016/j.molmed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Sawicki C. M., Kim J. K., Weber M. D., et al. Microglia promote increased pain behavior through enhanced inflammation in the spinal cord during repeated social defeat stress. The Journal of Neuroscience. 2019;39(7):1139–1149. doi: 10.1523/JNEUROSCI.2785-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J., Min J. S., Kim B., et al. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neuroscience Letters. 2015;584:191–196. doi: 10.1016/j.neulet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Xu P., Liu Q., Xie Y., et al. Breast cancer susceptibility protein 1 (BRCA1) rescues neurons from cerebral ischemia/reperfusion injury through NRF2-mediated antioxidant pathway. Redox Biology. 2018;18:158–172. doi: 10.1016/j.redox.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng Y. T., Hsu Y. Y., Shih Y. T., Lo Y. C. Paeonol attenuates microglia-mediated inflammation and oxidative stress–induced neurotoxicity in rat primary microglia and cortical neurons. Shock. 2012;37(3):312–318. doi: 10.1097/SHK.0b013e31823fe939. [DOI] [PubMed] [Google Scholar]
- 10.Raymond A., Ensslin M. A., Shur B. D. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. Journal of Cellular Biochemistry. 2009;106(6):957–966. doi: 10.1002/jcb.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neniskyte U., Brown G. C. Lactadherin/MFG-E8 is essential for microglia-mediated neuronal loss and phagoptosis induced by amyloid β . Journal of Neurochemistry. 2013;126(3):312–317. doi: 10.1111/jnc.12288. [DOI] [PubMed] [Google Scholar]
- 12.Cheyuo C., Aziz M., Yang W. L., Jacob A., Zhou M., Wang P. Milk fat globule-EGF factor VIII attenuates CNS injury by promoting neural stem cell proliferation and migration after cerebral ischemia. PLoS One. 2015;10(4, article e0122833) doi: 10.1371/journal.pone.0122833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi X., Cai X., Di W., et al. MFG-E8 selectively inhibited Aβ-induced microglial M1 polarization via NF-κB and PI3K-Akt pathways. Molecular Neurobiology. 2017;54(10):7777–7788. doi: 10.1007/s12035-016-0255-y. [DOI] [PubMed] [Google Scholar]
- 14.Villalba M., Martínez-Serrano A., Börner C., Blanco P., Satrústegui J. NMDA-induced increase in [Ca2+]i and 45Ca2+ uptake in acutely dissociated brain cells derived from adult rats. Brain Research. 1992;570(1-2):347–353. doi: 10.1016/0006-8993(92)90600-E. [DOI] [PubMed] [Google Scholar]
- 15.Luckoff A., Scholz R., Sennlaub F., Xu H., Langmann T. Comprehensive analysis of mouse retinal mononuclear phagocytes. Nature Protocols. 2017;12(6):1136–1150. doi: 10.1038/nprot.2017.032. [DOI] [PubMed] [Google Scholar]
- 16.Benarroch E. E. Microglia: multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81(12):1079–1088. doi: 10.1212/WNL.0b013e3182a4a577. [DOI] [PubMed] [Google Scholar]
- 17.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 18.Wohleb E. S., Fenn A. M., Pacenta A. M., Powell N. D., Sheridan J. F., Godbout J. P. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37(9):1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norden D. M., Trojanowski P. J., Villanueva E., Navarro E., Godbout J. P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64(2):300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fricker M., Neher J. J., Zhao J. W., Thery C., Tolkovsky A. M., Brown G. C. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. The Journal of Neuroscience. 2012;32(8):2657–2666. doi: 10.1523/JNEUROSCI.4837-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendricks L., Aziz M., Yang W. L., et al. Milk fat globule-epidermal growth factor-factor VIII–derived peptide MSP68 is a cytoskeletal immunomodulator of neutrophils that inhibits Rac1. Journal of Surgical Research. 2017;208:10–19. doi: 10.1016/j.jss.2016.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akakura S., Singh S., Spataro M., et al. The opsonin MFG-E8 is a ligand for the αvβ5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Experimental Cell Research. 2004;292(2):403–416. doi: 10.1016/j.yexcr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Kato T. A., Hyodo F., Yamato M., Utsumi H., Kanba S. Redox and microglia in the pathophysiology of schizophrenia. Yakugaku Zasshi. 2015;135(5):739–743. doi: 10.1248/yakushi.14-00235-4. [DOI] [PubMed] [Google Scholar]
- 24.Di W., Shi X., Lv H., et al. Activation of the nuclear factor E2-related factor 2/anitioxidant response element alleviates the nitroglycerin-induced hyperalgesia in rats. The Journal of Headache and Pain. 2016;17(1):p. 99. doi: 10.1186/s10194-016-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bautista-Exposito S., Penas E., Frias J., Martinez-Villaluenga C. Pilot-scale produced fermented lentil protects against t-BHP-triggered oxidative stress by activation of Nrf2 dependent on SAPK/JNK phosphorylation. Food Chemistry. 2019;274:750–759. doi: 10.1016/j.foodchem.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Takemoto Y., Inaba S., Zhang L., Tsujikawa K., Uezumi A., Fukada S. I. Implication of basal lamina dependency in survival of Nrf2-null muscle stem cells via an antioxidative-independent mechanism. Journal of Cellular Physiology. 2019;234(2):1689–1698. doi: 10.1002/jcp.27040. [DOI] [PubMed] [Google Scholar]
- 27.Thomas N. S., George K., Selvam A. A. A. Anticancer mechanism of troxerutin via targeting Nrf2 and NF-κB signalling pathways in hepatocarcinoma cell line. Toxicology in Vitro. 2019;54:317–329. doi: 10.1016/j.tiv.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Mohan S., Gupta D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomedicine & Pharmacotherapy. 2018;108:1866–1878. doi: 10.1016/j.biopha.2018.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: grid-crossing analysis of microglia morphology in animals and primary microglia.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.